Abstract
Novel biomarkers of type 2 diabetes (T2D) and response to preventative treatment in individuals with similar clinical risk may highlight metabolic pathways that are important in disease development. We profiled 331 metabolites in 2,015 baseline plasma samples from the Diabetes Prevention Program (DPP). Cox models were used to determine associations between metabolites and incident T2D, as well as whether associations differed by treatment group (i.e., lifestyle [ILS], metformin [MET], or placebo [PLA]), over an average of 3.2 years of follow-up. We found 69 metabolites associated with incident T2D regardless of treatment randomization. In particular, cytosine was novel and associated with the lowest risk. In an exploratory analysis, 35 baseline metabolite associations with incident T2D differed across the treatment groups. Stratification by baseline levels of several of these metabolites, including specific phospholipids and AMP, modified the effect that ILS or MET had on diabetes development. Our findings highlight novel markers of diabetes risk and preventative treatment effect in individuals who are clinically at high risk and motivate further studies to validate these interactions.
Introduction
Clinical interventions can successfully prevent progression to type 2 diabetes (T2D) in individuals at high risk; however, not everyone responds. The Diabetes Prevention Program (DPP) was a randomized control trial that demonstrated that lifestyle changes and metformin pharmacotherapy reduced diabetes progression in individuals with impaired glucose metabolism (1). Subgroup analyses suggested that clinical factors such as age, BMI, fasting plasma glucose (FPG), and 2-h oral glucose tolerance tests (OGTTs) were associated with differences in diabetes risk reduction between lifestyle (ILS), metformin (MET), and placebo (PLA) treatment groups (1).
Metabolomics—the study of small molecules involved in cellular processes—leverages high-throughput technologies to identify biomarkers of risk and treatment response. Previous studies in population-based cohorts have demonstrated that certain metabolites are persistently associated with diabetes decades before clinical diagnosis—including higher levels of branched-chain (BCAAs) and aromatic (AAAs) amino acids (2–5) and specific triacylglycerol (TAG) species (4,6) or lower levels of glycine and glutamine (5,7)—even after adjustment for clinical risk factors. The majority of individuals in these cohorts, however, had normal glucose homeostasis. Metabolomics has also been leveraged to study the effects of exercise and metformin but mostly in small studies focused on healthy individuals or those with overt diabetes (8–12). There is little information regarding metabolite profiles associated with behavioral or pharmacological interventions on preventing diabetes.
A prior analysis in the DPP profiled a limited number of metabolites in plasma samples from 427 diabetes case-control pairs matched on sex, hypertension, diabetes propensity score, and treatment group. Previously established associations of BCAA, AAA, and glutamine/glutamate with diabetes incidence were confirmed but attenuated after adjustment for clinical risk factors. Four novel metabolite associations with incident disease persisted after adjustment; namely, betaine and serine concentrations were inversely associated with diabetes, and methionine sulfoxide and propionylcarnitine were positively associated. These findings suggested that there are unique biomarkers of diabetes development in this population at high risk. Betaine levels also increased significantly after a mean 3.2 years of ILS, and this increase was associated with a reduction in incident diabetes, demonstrating that plasma metabolites can be associated with preventative intervention response (13).
In the current study, we profiled an expanded number of metabolites in >2,000 stored baseline plasma samples from all three treatment groups of the DPP. Our main goals were twofold: 1) to identify a unique metabolomic profile associated with progression to T2D in this population at high risk using a larger sample size—including validation of our previous findings—and 2) to explore baseline metabolite interactions with the effects of lifestyle changes or metformin. Ultimately, novel associations could implicate metabolic pathways involved in disease development and inform future discoveries in biomarkers for preventative intervention response.
Research Design and Methods
The DPP
The DPP was a multicenter randomized control trial with a median follow-up of 3.2 years among individuals at high risk for diabetes. Inclusion and exclusion criteria were published previously (14). At baseline, participants had impaired oral glucose tolerance (75-g OGTT of 140–199 mg/dL), a fasting glucose of 95–125 mg/dL (<125 mg/dL in American Indians), and BMI ≥24 kg/m2 (≥22 kg/m2 in Asians). More than two-thirds of study participants were female, and 45% were from racial/ethnic minorities (15). A total of 3,234 participants recruited were randomized to three interventions (1,079 to ILS, 1,073 to MET, and 1,082 to PLA). Those in ILS targeted a goal weight loss of ≥7% of initial body weight achieved through a low-calorie, low-fat diet and moderate-intensity exercise for a minimum of 150 min per week. Participants in MET received 850 mg twice daily. Published study results showed a reduction in incident diabetes of 58% with ILS and 31% with MET compared with PLA after mean follow-up time of 2.8 years (1). Study protocols were approved by the institutional review board of each clinical center, and all participants provided written consent.
Outcome Measures
The study outcome was T2D incidence over an average follow-up of 3.2 years determined by an annual OGTT or semiannual FPG. A diagnosis of T2D was defined by the 1997 American Diabetes Association criteria of at least one FPG ≥126 mg/dL (7.0 mmol/L) or plasma glucose ≥200 mg/dL (11 mmol/L) after a 75-g OGTT (16). The diagnosis was confirmed with repeat testing within 6 weeks.
Metabolite Sample Selection and Analysis
For this analysis, a total of 2,015 baseline fasting plasma samples from all three treatment groups were obtained, aliquoted, and stored at −80°C during the DPP. Selection was based on these criteria: a nested case control subset for cardiovascular disease (CVD) and cancer (n = 1,008), a randomly selected subset from all three treatment groups (n = 1,007), and sufficient sample volume for metabolite profiling.
Three platforms were used (17,18): for assessment of amino acids and amines, hydrophilic interaction chromatography (Waters; Milford, MA) was coupled to a Q Exactive mass spectrometer (MS) in positive ion mode (Thermo Fisher Scientific, Waltham, MA). For assessment of lipids, C8 chromatography (Waters) was coupled to a Q Exactive MS in positive ion mode. For assessment of organic acids, amide chromatography (Waters) was coupled to an Agilent 6490 triple quadrupole MS (Agilent Technologies, Santa Clara, CA) using negative ion mode electrospray ionization. Quality control measures included monitoring of isotope-labeled internal standards in each sample and the use of pooled plasma reference samples inserted every 10–20 study samples to serve as a reference to standardize within and across batches. Separate pooled plasma injections were included every 20 injections to gauge the effectiveness of the normalization and to determine the coefficient of variation of each metabolite. Of the 365 metabolites measured, 331 (91%) qualified for inclusion based on <10% sample missingness and an assay CV <25% (Supplementary Table 1).
Data Analysis
We included data from the masked phase of DPP collected from 1996–2001 corresponding to the data lock of 31 July 2001 and an average follow-up of 3.2 years. To correct for nonrandom missingness and bias in treatment effects on diabetes incidence, we employed inverse probability weighting (IPW) using a two-step approach (19). First, a propensity score for each participant was created by fitting logistic regression models separately for individuals with and without incident diabetes to model the availability of metabolite data as outcome and to adjust for treatment group, sex, hypertension, age randomized, race/ethnicity, fasting glucose, and BMI. Then the inverse of the propensity scores were used as weights in Cox (IPW-Cox) and linear (IPW linear) models detailed below. A similar approach was used in a previous report (20). The number of diabetes cases was similar between the nested CVD and cancer case-control (19%) and random (18%) sample subsets over the study period. CVD and cancer outcomes were outside the focus of our analyses and so are not included.
For determination of whether baseline metabolites are associated with diabetes incidence in the DPP, IPW-Cox models pooled data from all treatment groups with adjustment for treatment and traditional diabetes risk factors (baseline age, sex, race/ethnicity, BMI, FPG, and hypertension status) using weights from the logistic models. These were the same adjustments used in the previous smaller case-control DPP metabolomics study (13). The metabolites were transformed as z scores in the models, and associations were expressed as hazard ratio (HR) per 1 SD of the baseline metabolite distribution. The 14 metabolites associated with diabetes incidence in the prior smaller DPP metabolite study were included in this current study for validation (13) and were not considered for multiplicity adjustment. In the 317 metabolites assessed for discovery, we applied Benjamini-Hochberg false discovery rate (FDR) adjustment with a threshold for statistical significance of <0.05 (21). Models were also used to further adjust for clinical laboratory–measured HDL, log-transformed triglycerides, and statin use.
IPW-Cox models were used to conduct a hypothesis-generating exploratory analysis stratified by treatment group with the same adjustments as above (baseline age, sex, race/ethnicity, BMI, FPG, and hypertension status) to assess whether the metabolite-diabetes associations differed among the three treatment groups. The test of homogeneity was conducted to identify metabolite associations with incident diabetes that differed by treatment using Benjamini-Hochberg FDR adjustment for multiplicity using an overall significance level of 0.05.
We also pursued an exploratory analysis to identify metabolites that modified the effects of lifestyle changes and metformin on diabetes incidence. Metabolites found to have significant heterogeneity among treatment groups from the previous analysis were considered in these analyses. HRs of the treatment effects were estimated in subgroups defined by quartiles of metabolite concentration levels and testing for homogeneity among quartiles was conducted using a significance level of 0.05.
In selected metabolites, we graphically depict the hazard rates across the biomarker levels expressed as z scores using the estimates derived from the treatment-stratified IPW-Cox models. Estimates of the absolute risk gradient associated with metabolite concentrations across a range of z values (±1.96 or 2 SD) were used to describe the hazard rate for a participant with metabolite value equal to the group mean (z score = 0). The point on each line indicates the expected hazard rate for a subject at the mean value for the group as estimated in life table analysis.
All analyses were conducted using SAS (9.2; Cary, NC) and R (version 3.5.1; R Foundation for Statistical Computing).
Data and Resource Availability
The data sets generated during and/or analyzed for the current study will be available in the National Institute of Diabetes and Digestive and Kidney Diseases repository at https://repository.niddk.nih.gov/studies/dppos/.
Results
Clinical characteristics of the metabolite profiling subcohort and complete DPP cohort were similar (Table 1). With the IPW adjustment (see research design and methods), baseline traits of the original DPP cohort and of the treatment groups were balanced in the metabolite profiling subcohort except for small differences in BMI and the percentage of female participants in PLA compared with the other groups. Mean values of baseline FPG, 2-h glucose after a 75-g OGTT, and BMI were elevated in all groups and consistent with the original study population at high risk.
Table 1.
Baseline characteristics of DPP subjects, individuals included in the metabolite profiling subcohort, and the DPP cohort and treatment arms after IPW
| DPP | IPW | |||||
|---|---|---|---|---|---|---|
| Whole cohort | Metabolite subcohort | Metabolite subcohort | ILS | MET | PLA | |
| N | 3,234 | 2,015 | 3,228 | 1,077 | 1,069 | 1,083 |
| ILS group, n (%) | 1,079 (33.4) | 685 (34.0) | 1,077 (33.4) | |||
| MET group, n (%) | 1,073(33.2) | 669 (33.2) | 1,069 (33.1) | |||
| PLA group, n (%) | 1,082 (33.4) | 661 (32.8) | 1,083 (33.5) | |||
| Age, years | 51 (11) | 53 (10) | 51 (13) | 51 (14) | 51 (13) | 50 (13) |
| Sex, n (%) | ||||||
| Female | 2,191 (67.7) | 1,336 (66.3) | 2,158 (66.9) | 706 (65.5)* | 664 (62.2)* | 789 (72.8)* |
| Race/ethnicity, n (%) | ||||||
| Caucasian | 1,768 (54.7) | 1,158 (57.5) | 1,768 (54.7) | 587 (54.5) | 607 (56.8) | 574 (53.1) |
| African American | 645 (19.9) | 376 (18.7) | 639 (19.8) | 194 (18.0) | 215 (20.1) | 230 (21.3) |
| Hispanic | 508 (15.7) | 264 (13.1) | 508 (15.4) | 173 (16.1) | 166 (15.5) | 169 (15.6) |
| Asian | 142 (4.4) | 93 (4.6) | 139 (4.3) | 60 (5.5) | 41 (3.9) | 38 (3.5) |
| American Indian | 171 (5.3) | 124 (6.1) | 173 (5.4) | 63 (5.9) | 39 (3.7) | 71 (6.5) |
| BMI, kg/m2 | 34.0 (6.7) | 33.3 (6.20) | 34.0 (8.6) | 33.6 (7.9)* | 33.9 (8.3)* | 34.6 (9.5)* |
| Circumference, cm | 105.1 (14.5) | 104.3 (14.0) | 105.1 (18.3) | 104.8 (18.2) | 105.6 (18.8) | 105 (18.1) |
| Smoking, n (%) | 226 (7.0) | 123 (6.1) | 211 (6.5) | 50 (4.7) | 77 (7.2) | 84 (7.8) |
| Hypertensive, n (%) | 925 (28.6) | 624 (31) | 938 (29.1) | 296 (27.5) | 317 (29.6) | 325 (30.0) |
| Lipid-lowering medication, n (%) | 160 (4.9) | 115 (5.7) | 158 (4.9) | 45 (4.2) | 62 (5.8) | 51 (4.7) |
| Statin medications | 137 (4.2) | 101 (5.0) | 139 (4.3) | 36 (3.3) | 57 (5.3) | 46 (4.3) |
| Nonstatin medications | 23 (0.7) | 14 (0.7) | 19 (0.6) | 9 (0.9) | 6 (0.5) | 4 (0.4) |
| Family history of diabetes, n (%) | 2,243 (69.4) | 1,367 (67.9) | 2,213 (68.6) | 737 (68.5) | 714 (66.8) | 762 (70.5) |
| FPG, mg/dL | 106.5 (8.3) | 106.4 (8.30) | 106.6 (10.67) | 106.4 (10.4) | 106.6 (10.6) | 106.7 (11.0) |
| 2-h plasma glucose, mg/dLǂ | 164 (17.0) | 165 (17.2) | 165 (21.8) | 164.6 (21.2) | 164.3 (21.9) | 165.1 (22.3) |
| Triglycerides, mg/dL | 141 (99, 201) | 143 (101, 200) | 143 (101, 199) | 138 (98, 197) | 142 (101, 199) | 147 (104, 205) |
| HDL cholesterol, mg/dL | 45.6 (11.8) | 46.6 (12.2) | 46.6 (12.2) | 46.3 (15.8) | 46 (14.3) | 45.4 (15.6) |
| Fasting insulin, μU/mL | 24 (16, 33) | 23 (16, 32) | 24 (16, 34) | 24 (16, 33) | 24 (16, 35) | 24 (17, 33) |
| HbA1c, % | 5.9 (0.5) | 5.9 (0.5) | 5.9 (0.6) | 5.9 (0.6) | 5.9 (0.6) | 5.9 (0.6) |
| HbA1c, mmol/mol | 41 (4.3) | 41 (5.4) | 41 (7.0) | 41 (7.0) | 41 (7.0) | 41 (6.8) |
Data shown are n (%) for all categorical variables and mean (SD) for all quantitative traits except triglycerides and fasting insulin, which are presented as median (interquartile range). Following implementation of the IPW, the demographic characteristics of the DPP were restored in the IPW metabolite profiling subcohort and were balanced across treatment groups, except for sex and BMI.
*P values <0.05. ǂ2-h plasma glucose determined after a 75-g oral glucose load.
Baseline Metabolite Levels Predict Incident Diabetes
IPW-Cox models were used to determine the association between baseline metabolite level and incident T2D with adjustment for age, sex, race/ethnicity, hypertension, FPG, and BMI. Out of 331 metabolites included in the analysis, 69 were associated with diabetes incidence with an FDR q value <0.05 in the entire metabolite profiling subcohort independent of treatment group randomization (Figs. 1 and 2).
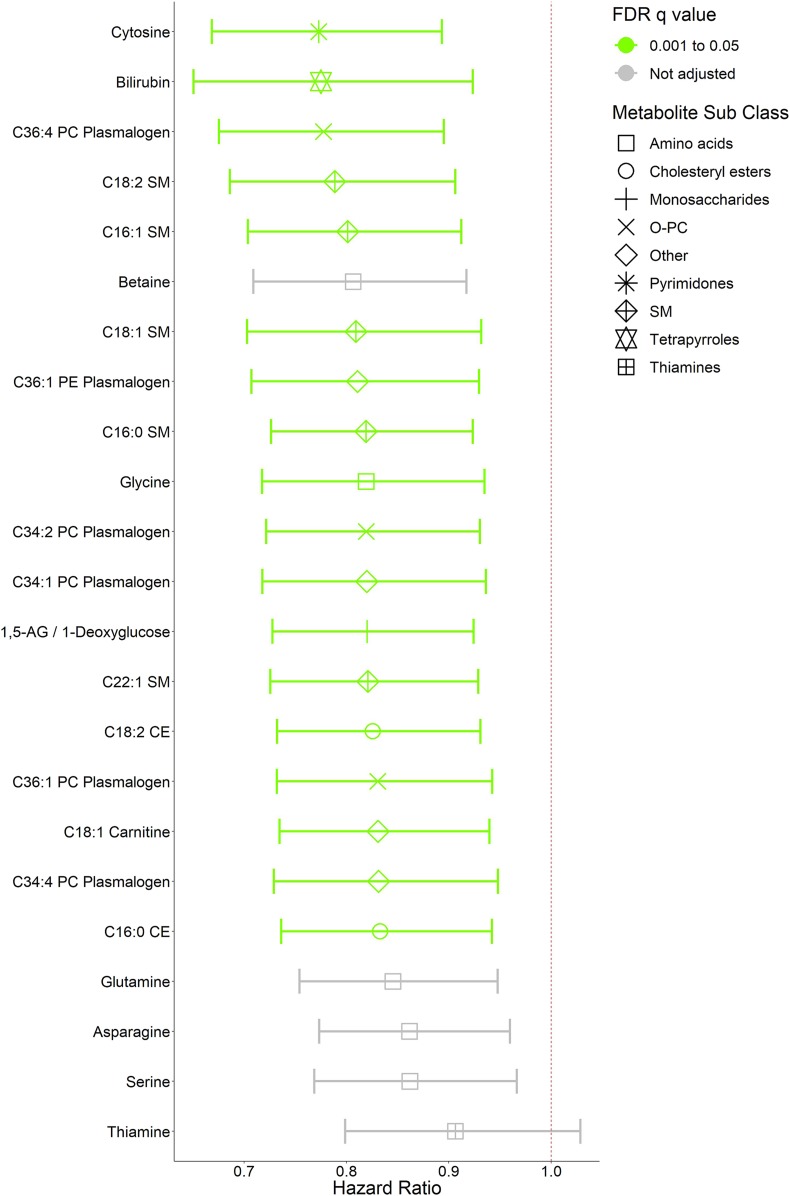
Figure 1.
Baseline metabolites associated with decreased risk of incident diabetes in the DPP. The pooled HRs and 95% CIs are shown for baseline metabolite associations with decreased risk of incident diabetes for every 1-SD increase in metabolite concentration in the entire metabolite profiling subcohort over an average follow-up of 2.8 years. Metabolites are arranged in increasing-HR order and color coded according to FDR q value. All metabolites with FDR q < 0.05 were included. Glutamine, serine, and asparagine were previously associated with decreased risk of incident diabetes in a smaller matched case-control metabolite profiling study in the DPP. They were included in the analysis for validation but were not included in the FDR q adjustment. Association with FDR q < 0.05, lightest green; unadjusted, gray. Weighted Cox models used are adjusted for treatment group, age, sex, race/ethnicity, hypertension status, baseline FPG, and baseline BMI. Symbols represent metabolite subclasses. Phospholipid notation denotes: total number of carbon atoms:total number of double bonds.
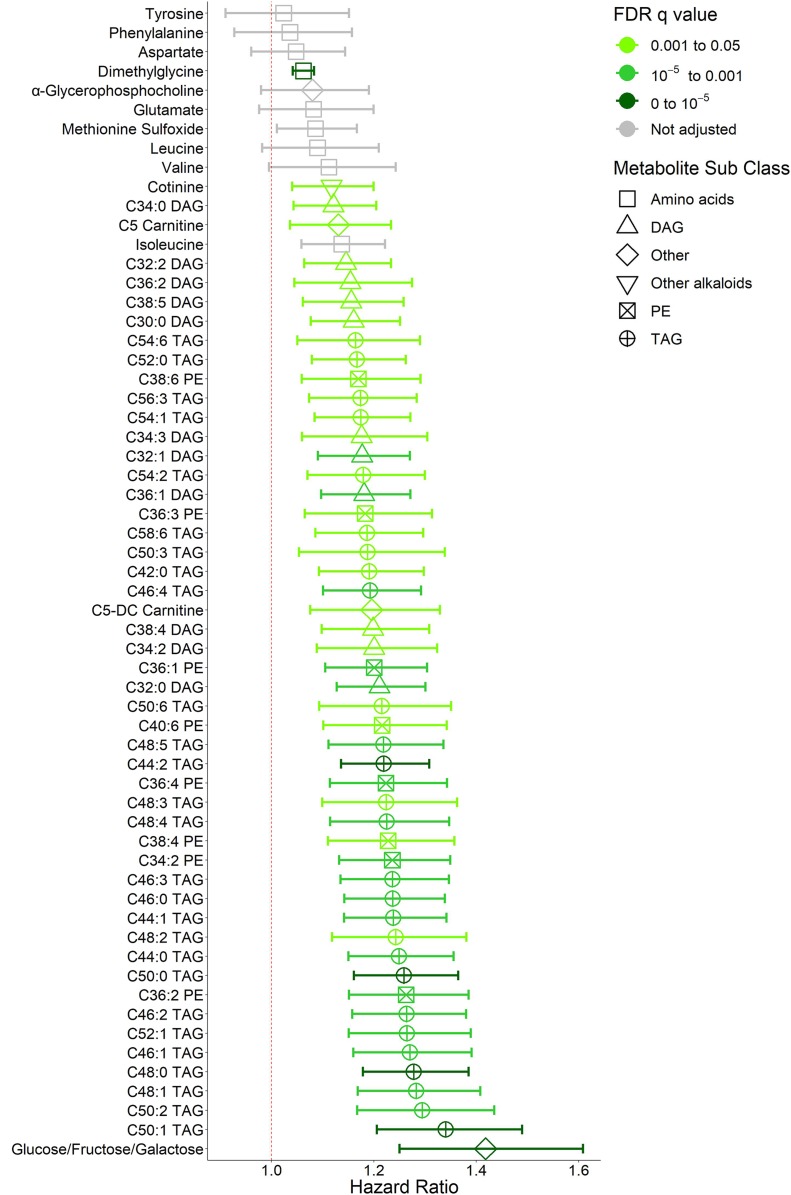
Figure 2.
Baseline metabolites associated with increased risk of incident diabetes in the DPP. The pooled HRs and 95% CIs are shown for baseline metabolite associations with increased risk of incident diabetes for every 1-SD increase in metabolite concentration in the entire metabolite profiling subcohort over an average follow-up of 2.8 years. Metabolites are arranged in increasing-HR order and color coded according to FDR q value. All metabolites with FDR q < 0.05 were included. Tyrosine, phenylalanine, glutamate, α-glycerophosphocholine, methionine sulfoxide, leucine, valine, and isoleucine were associated with increased risk of incident diabetes in a smaller matched case-control metabolite profiling study in the DPP. They were included in the analysis for validation but were not included in the FDR q adjustment. Association with FDR q < 10−5, darkest green; q < 10−3, medium green; q < 0.05, lightest green; unadjusted, gray. Weighted Cox models used are adjusted for treatment group, age, sex, race/ethnicity, hypertension status, baseline FPG, and baseline BMI. Symbols represent metabolite subclasses. Phospholipids notation denotes: total number of carbon atoms:total number of double bonds.
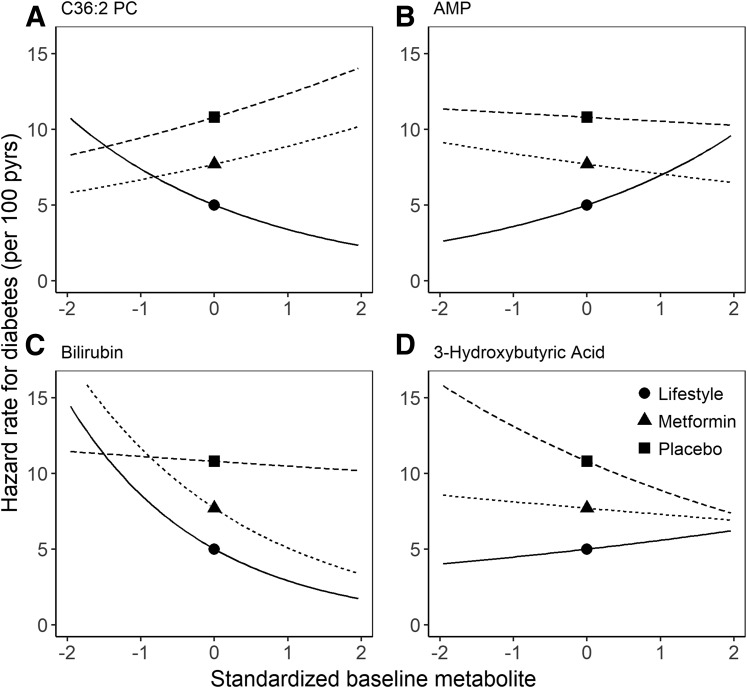
Figure 3.
Treatment-specific hazard rates for incident diabetes across standardized baseline metabolite concentrations. Treatment-specific HRs for incident diabetes in ILS (●), MET (▲), and PLA (▪) are shown for an individual with metabolite value equal to the group mean. The associated curves represent the absolute risk gradient across standardized baseline metabolite concentrations. Phospholipid notation denotes total number of carbon atoms:total number of double bonds. pyrs, person-years.
We identified higher concentrations of several TAG, diacylglycerol (DAG), and phosphatidylethanolamine (PE) species that were associated with diabetes incidence (Fig. 1), consistent with prior studies (4,6). C50:1 (total number of carbon atoms:total number of double bonds) TAG conferred the greatest risk of all lipids, with an HR of 1.34 for incident T2D per 1-SD increase in concentration (95% CI 1.21–1.49, FDR q = 7.80 × 10−6). A composite measurement of glucose, fructose, and galactose—metabolites of identical mass that cannot be differentiated on the platform—was positively associated with diabetes risk even after adjustment for baseline FPG levels (HR 1.42 [95% CI 1.25–1.61], FDR q = 7.80 × 10−6). Metabolites associated with lower diabetes incidence at higher concentrations that have previously been reported included bilirubin (0.78 [0.65–0.92], FDR q = 3.43 × 10−2 [Fig. 2]), sphingomyelins (SMs), cholesterol esters (CEs), phosphatidylcholine (PC) plasmalogens, and glutamine (22,23). Carnitines were both positively (C5 dicarboxyl carnitine HR 1.20 [95% CI 1.08–1.33], FDR q = 1.26 × 10−2, and C5 carnitine 1.13 [1.04–1.23], FDR q = 4.25 × 10−2) and inversely (C18:1 carnitine 0.83 [0.73–0.94], FDR q = 2.79 × 10−2) associated with diabetes incidence. Twenty-eight of these associations remained significant after further adjustments for clinically measured HDL and log-transformed triglycerides (Supplementary Table 2) without significant changes after further adjustment for statin use.
Higher levels of cytosine and C36:1 PE plasmalogen were found to have novel associations with lower diabetes incidence in our expanded population. Cytosine had the greatest associated risk reduction (23% reduction per 1-SD increase in concentration) of all measured metabolites (95% CI 0.67–0.89, FDR q = 8.32 × 10−3). This relationship remained significant after further adjustment for clinically measured HDL and log-transformed triglycerides (Supplementary Table 2) and statin use. C36:1 PE plasmalogen, a phospholipid with an ethanolamine head and distinctive vinyl-ether bond, was associated with 18% lower diabetes incidence per SD increase in concentration (FDR q = 2.48 × 10−2). Of the 14 metabolites included for validation, all associations were directionally consistent with previous findings in the DPP, but only six associations remained at least nominally significant (P < 0.05) after multivariable adjustment (Supplementary Table 1). These included the positive associations of isoleucine (HR 1.14 [95% CI 1.06–1.22], P = 4.38 × 10−3) and methionine sulfoxide (1.09 [1.01–1.17], P = 0.02) and inverse associations of betaine (0.81 [0.71–0.92], P = 1.06 × 10−3), glutamine (0.85 [0.75–0.95], P = 3.94 × 10−3), asparagine (0.86 [0.77–0.96], P = 6.77 × 10−3), and serine (0.86 [0.77–0.97], P = 0.01).
Due to potential biases introduced by our sample selection scheme, we evaluated metabolite-diabetes associations in the whole metabolite cohort versus the 1,007 random samples. Overall, there was a high degree of concordance despite a halving of the sample size and diabetes cases (Supplementary Fig. 1). However, a few metabolites had stronger associations in the random samples, shown in Supplementary Table 3, including specific carnitine (e.g., C5-DC, C4-OH, etc.), TAG (e.g., C56:3 and C56:2), and CE (e.g., C22:6, C22:5, etc.) species.
Differences in Metabolite Associations With Diabetes Incidence by Treatment Group
For assessment of whether baseline metabolite associations with diabetes incidence differed between treatment groups, associations of the 331 known metabolites profiled were assessed using treatment group–specific IPW-Cox models. Thirty-five metabolite-diabetes associations were found in a general test for heterogeneity to differ across the three treatment groups (Table 2), many of which were phospholipids. Among individuals randomized to ILS, higher levels of PCs, CEs, SMs, PEs, phosphatidylinositols, and lysophosphatidylethanolamines (LPEs) were associated with decreased diabetes incidence. Conversely, higher levels of AMP were associated with increased incidence (HR 1.39 [95% CI 1.19–1.64], FDR q = 0.002) along with higher levels of xanthosine (1.25 [1.05–1.49], FDR q = 0.07) and the conjugated bile acids, glycocholic acid ( [1.10–1.54], FDR q = 0.02) and taurocholic acid (1.45 [1.14–1.84], FDR q = 0.03). Higher levels of bilirubin were associated with lower incidence in individuals randomized to MET (0.66 [0.52–0.83], FDR q = 0.008). 1-methylnicotinamide, a biologically inactive metabolite of nicotinamide (1.23 [1.08–1.39], FDR q = 0.02); indole-3-propanoic acid, a gut microbiome metabolite (1.35 [1.20–1.52], FDR q = 0.00003); and methylthioadenosine, a nucleoside involved in methionine and purine salvage (1.21 [1.08–1.36], FDR q = 0.01), were associated with increased incidence in MET.
Table 2.
Baseline metabolite associations with incident diabetes that differed* across treatment groups
| Baseline metabolite | HR (95% CI) per 1 SD | Phomogeneity for treatment group | ||
|---|---|---|---|---|
| PLA | ILS | MET | ||
| AMP | 0.98 (0.85, 1.12) | 1.39 (1.19, 1.64) | 0.92 (0.74, 1.14) | 0.0010 |
| C36:2 PC | 1.14 (0.97, 1.34) | 0.68 (0.53, 0.87) | 1.15 (0.94, 1.41) | 0.0012 |
| C18:2 CE | 1.07 (0.87, 1.32) | 0.65 (0.52, 0.81) | 0.78 (0.64, 0.94) | 0.0040 |
| C34:0 PI | 1.15 (1.00, 1.33) | 0.66 (0.49, 0.89) | 0.99 (0.84, 1.17) | 0.0044 |
| C38:2 PE | 1.06 (0.88, 1.27) | 0.60 (0.45, 0.80) | 0.97 (0.76, 1.24) | 0.0046 |
| C44:13 PE plasmalogen | 1.10 (0.91, 1.32) | 0.58 (0.41, 0.81) | 0.96 (0.80, 1.14) | 0.0049 |
| 1-pethylnicotinamide | 0.79 (0.60, 1.02) | 0.97 (0.78, 1.20) | 1.23 (1.08, 1.39) | 0.0052 |
| C34:2 PC | 1.24 (1.02, 1.50) | 0.75 (0.56, 0.99) | 1.26 (1.03, 1.56) | 0.0063 |
| C36:4 PC | 1.12 (0.95, 1.32) | 0.64 (0.46, 0.88) | 1.09 (0.89, 1.34) | 0.0080 |
| Indole-3-propanoic acid | 1.32 (0.65, 2.67) | 0.76 (0.53, 1.07) | 1.35 (1.20, 1.52) | 0.0089 |
| C32:2 PC | 1.27 (1.07, 1.51) | 0.74 (0.54, 1.02) | 1.21 (0.99, 1.47) | 0.0128 |
| C16:0 CE | 1.02 (0.85, 1.22) | 0.61 (0.45, 0.83) | 0.79 (0.65, 0.97) | 0.0143 |
| C14:0 SM | 1.08 (0.91, 1.27) | 0.70 (0.54, 0.90) | 0.87 (0.72, 1.06) | 0.0167 |
| C3 carnitine | 1.54 (1.22, 1.94) | 0.98 (0.79, 1.21) | 1.25 (1.03, 1.51) | 0.0168 |
| Bilirubin | 0.97 (0.78, 1.21) | 0.58 (0.38, 0.90) | 0.66 (0.52, 0.83) | 0.0227 |
| Taurocholic acid | 0.98 (0.85, 1.13) | 1.45 (1.14, 1.84) | 1.09 (0.97, 1.22) | 0.0232 |
| C16:1 SM | 0.95 (0.76, 1.19) | 0.60 (0.45, 0.79) | 0.90 (0.73, 1.10) | 0.0239 |
| C20:4 LPE | 0.96 (0.80, 1.15) | 0.73 (0.53, 1.00) | 1.17 (0.99, 1.37) | 0.0250 |
| C34:3 PC | 1.08 (0.90, 1.30) | 0.71 (0.50, 0.99) | 1.22 (0.99, 1.50) | 0.0250 |
| Glycocholic acid | 0.97 (0.85, 1.11) | 1.30 (1.10, 1.54) | 1.06 (0.96, 1.17) | 0.0256 |
| C22:5 CE | 1.01 (0.83, 1.22) | 0.68 (0.51, 0.90) | 0.74 (0.60, 0.91) | 0.0295 |
| 3-hydroxybutyric acid | 0.82 (0.67, 1.02) | 1.12 (0.99, 1.26) | 0.95 (0.81, 1.11) | 0.0305 |
| C36:3 PC | 1.10 (0.92, 1.33) | 0.77 (0.56, 1.04) | 1.26 (1.03, 1.54) | 0.0319 |
| Methylthioadenosine | 1.03 (0.77, 1.38) | 0.91 (0.75, 1.10) | 1.21 (1.08, 1.36) | 0.0334 |
| C22:1 SM | 0.97 (0.80, 1.18) | 0.61 (0.46, 0.82) | 0.87 (0.72, 1.06) | 0.0338 |
| Xanthosine | 0.91 (0.76, 1.10) | 1.25 (1.05, 1.49) | 1.01 (0.87, 1.16) | 0.0379 |
| C18:2 LPE | 0.99 (0.83, 1.17) | 0.64 (0.42, 0.99) | 1.17 (0.97, 1.41) | 0.0381 |
| C18 carnitine | 1.16 (0.96, 1.40) | 0.74 (0.51, 1.05) | 0.89 (0.72, 1.09) | 0.0384 |
| C16:0 ceramide (D18:1) | 1.19 (0.99, 1.42) | 0.77 (0.58, 1.02) | 1.01 (0.84, 1.21) | 0.0387 |
| C38:2 PC | 1.07 (0.91, 1.25) | 0.78 (0.60, 1.01) | 1.18 (0.97, 1.44) | 0.0403 |
| C34:1 PC | 1.01 (0.84, 1.20) | 0.87 (0.67, 1.12) | 1.24 (1.06, 1.46) | 0.0409 |
| C34:4 PC | 1.20 (1.00, 1.44) | 0.79 (0.58, 1.07) | 1.21 (1.00, 1.45) | 0.0440 |
| C36:1 PC | 0.97 (0.83, 1.14) | 0.82 (0.66, 1.03) | 1.17 (0.99, 1.38) | 0.0442 |
| Pyroglutamic acid | 0.95 (0.81, 1.12) | 1.21 (1.07, 1.37) | 1.00 (0.82, 1.22) | 0.0447 |
| C36:0 PC | 0.98 (0.81, 1.17) | 0.66 (0.51, 0.86) | 0.92 (0.75, 1.13) | 0.0481 |
Metabolites are arranged by increasing Phomogeneity value across treatment groups. The HRs are expressed as incident diabetes risk for each SD increase in baseline metabolite levels within a treatment group. FDR q values <0.05 are in boldface type. Phospholipid notation denotes: total number of carbon atoms:total number of double bonds. PI, phosphatidylinositol.
Differential effects across treatment groups are identified using a test for homogeneity in HR across treatment groups with P < 0.05 from weighted Cox models with development of diabetes as outcome and metabolite as the exposure of interest with adjustment for age, sex, race/ethnicity, hypertension status, baseline FPG, and baseline BMI.
Lifestyle Change and Metformin Effects on Diabetes Incidence in Subgroups Defined by Metabolite Quartiles
To explore the relationship that baseline levels of metabolites had with treatment effect, we compared the HRs associated with treatment effect stratified across quartiles of metabolite concentrations. Treatment effects in pairwise group comparisons were found to differ across concentration quartiles in 15 of these metabolites (P < 0.05 for homogeneity across metabolite quartiles within treatment effects [Table 3]). The majority of these associations were in phospholipids.
Table 3.
HR (95% CI) of treatment effects in subgroups defined by quartiles of metabolites found to have heterogeneity among treatment groups in the association of metabolite–incident diabetes
| Baseline metabolite | Quartile | ILS vs. PLA | MET vs. PLA | ILS vs. MET |
|---|---|---|---|---|
| AMP | 1 | 0.30 (0.16, 0.58) | 0.94 (0.57, 1.56) | 0.31 (0.17, 0.57) |
| 2 | 0.41 (0.24, 0.71) | 0.63 (0.37, 1.09) | 0.68 (0.37, 1.27) | |
| 3 | 0.52 (0.31, 0.90) | 0.78 (0.49, 1.24) | 0.65 (0.38, 1.12) | |
| 4 | 0.57 (0.33, 0.96) | 0.56 (0.33, 0.95) | 1.03 (0.62, 1.72) | |
| C36:2 PC | 1 | 0.75 (0.43, 1.31) | 0.71 (0.42, 1.20) | 1.02 (0.60, 1.76) |
| 2 | 0.40 (0.23, 0.71) | 0.62 (0.37, 1.04) | 0.56 (0.32, 0.98) | |
| 3 | 0.42 (0.25, 0.72) | 0.66 (0.40, 1.09) | 0.91 (0.52, 1.59) | |
| 4 | 0.23 (0.12, 0.43) | 0.82 (0.53, 1.28) | 0.25 (0.14, 0.47) | |
| C18:2 CE | 1 | 0.94 (0.58, 1.53) | 0.91 (0.56, 1.45) | 1.04 (0.65, 1.66) |
| 2 | 0.32 (0.18, 0.56) | 0.76 (0.49, 1.17) | 0.41 (0.23, 0.72) | |
| 3 | 0.34 (0.19, 0.60) | 0.46 (0.27, 0.80) | 0.76 (0.40, 1.43) | |
| 4 | 0.30 (0.15, 0.57) | 0.73 (0.42, 1.25) | 0.39 (0.19, 0.77) | |
| C38:2 PE | 1 | 0.64 (0.40, 1.04) | 0.61 (0.38, 0.98) | 1.13 (0.68, 1.87) |
| 2 | 0.48 (0.27, 0.85) | 0.68 (0.41, 1.14) | 0.75 (0.43, 1.29) | |
| 3 | 0.40 (0.22, 0.70) | 0.62 (0.36, 1.08) | 0.58 (0.31, 1.07) | |
| 4 | 0.23 (0.11, 0.45) | 0.97 (0.60, 1.57) | 0.22 (0.11, 0.43) | |
| C44:13 PE plasmalogen | 1 | 1.03 (0.61, 1.75) | 1.06 (0.66, 1.68) | 0.97 (0.60, 1.57) |
| 2 | 0.43 (0.27, 0.69) | 0.52 (0.32, 0.84) | 0.78 (0.46, 1.32) | |
| 3 | 0.36 (0.20, 0.67) | 0.75 (0.44, 1.28) | 0.47 (0.25, 0.87) | |
| 4 | 0.25 (0.13, 0.47) | 0.74 (0.47, 1.17) | 0.35 (0.18, 0.67) | |
| C34:2 PC | 1 | 0.49 (0.29, 0.81) | 0.45 (0.26, 0.76) | 1.08 (0.61, 1.93) |
| 2 | 0.70 (0.36, 1.33) | 1.13 (0.63, 2.03) | 0.58 (0.33, 1.02) | |
| 3 | 0.45 (0.26, 0.78) | 0.64 (0.39, 1.04) | 0.76 (0.44, 1.31) | |
| 4 | 0.26 (0.15, 0.47) | 0.77 (0.49, 1.20) | 0.33 (0.18, 0.6) | |
| C36:4 PC | 1 | 0.71 (0.42, 1.21) | 0.77 (0.47, 1.27) | 0.98 (0.58, 1.66) |
| 2 | 0.46 (0.27, 0.77) | 0.56 (0.35, 0.91) | 0.80 (0.47, 1.38) | |
| 3 | 0.38 (0.21, 0.67) | 0.59 (0.34, 1.03) | 0.61 (0.34, 1.1) | |
| 4 | 0.23 (0.13, 0.44) | 0.93 (0.60, 1.42) | 0.26 (0.14, 0.49) | |
| Bilirubin | 1 | 0.50 (0.31, 0.79) | 0.57 (0.36, 0.90) | 0.96 (0.60, 1.56) |
| 2 | 0.99 (0.57, 1.73) | 1.30 (0.78, 2.15) | 0.65 (0.39, 1.07) | |
| 3 | 0.21 (0.10, 0.44) | 0.73 (0.44, 1.20) | 0.30 (0.14, 0.61) | |
| 4 | 0.27 (0.14, 0.52) | 0.52 (0.29, 0.94) | 0.56 (0.28, 1.12) | |
| C20:4 LPE | 1 | 0.45 (0.27, 0.73) | 0.44 (0.27, 0.72) | 1.06 (0.60, 1.90) |
| 2 | 0.52 (0.29, 0.92) | 0.70 (0.42, 1.18) | 0.72 (0.41, 1.27) | |
| 3 | 0.66 (0.36, 1.20) | 1.14 (0.68, 1.90) | 0.59 (0.33, 1.04) | |
| 4 | 0.25 (0.13, 0.47) | 0.77 (0.47, 1.27) | 0.31 (0.17, 0.57) | |
| C34:3 PC | 1 | 0.61 (0.37, 1.00) | 0.54 (0.33, 0.88) | 1.22 (0.71, 2.09) |
| 2 | 0.32 (0.17, 0.60) | 0.81 (0.48, 1.36) | 0.38 (0.20, 0.72) | |
| 3 | 0.53 (0.30, 0.92) | 0.70 (0.43, 1.16) | 0.76 (0.43, 1.34) | |
| 4 | 0.28 (0.16, 0.50) | 0.87 (0.56, 1.36) | 0.30 (0.16, 0.54) | |
| 3-hydroxybutyric acid | 1 | 0.29 (0.17, 0.52) | 0.56 (0.34, 0.91) | 0.50 (0.28, 0.90) |
| 2 | 0.79 (0.43, 1.44) | 0.86 (0.49, 1.51) | 0.95 (0.55, 1.63) | |
| 3 | 0.28 (0.16, 0.49) | 0.61 (0.39, 0.96) | 0.42 (0.24, 0.73) | |
| 4 | 0.72 (0.39, 1.31) | 1.06 (0.61, 1.84) | 0.68 (0.39, 1.22) | |
| C36:3 PC | 1 | 0.47 (0.27, 0.80) | 0.48 (0.29, 0.80) | 1.06 (0.56, 2.04) |
| 2 | 0.65 (0.37, 1.12) | 0.86 (0.50, 1.45) | 0.72 (0.43, 1.22) | |
| 3 | 0.36 (0.21, 0.61) | 0.60 (0.37, 0.97) | 0.63 (0.36, 1.09) | |
| 4 | 0.32 (0.17, 0.59) | 1.02 (0.65, 1.61) | 0.30 (0.16, 0.55) | |
| C22:1 SM | 1 | 0.75 (0.47, 1.22) | 0.74 (0.47, 1.18) | 1.02 (0.62, 1.68) |
| 2 | 0.32 (0.19, 0.54) | 0.60 (0.38, 0.95) | 0.57 (0.33, 0.99) | |
| 3 | 0.29 (0.15, 0.56) | 0.59 (0.35, 1.01) | 0.47 (0.25, 0.86) | |
| 4 | 0.40 (0.21, 0.75) | 0.83 (0.49, 1.40) | 0.48 (0.26, 0.91) | |
| C18:2 LPE | 1 | 0.70 (0.40, 1.22) | 0.74 (0.44, 1.24) | 0.97 (0.56, 1.69) |
| 2 | 0.44 (0.25, 0.77) | 0.55 (0.33, 0.91) | 0.82 (0.46, 1.46) | |
| 3 | 0.41 (0.24, 0.70) | 0.56 (0.34, 0.93) | 0.76 (0.44, 1.31) | |
| 4 | 0.26 (0.13, 0.52) | 1.21 (0.77, 1.89) | 0.22 (0.11, 0.43) | |
| C34:1 PC | 1 | 0.48 (0.28, 0.81) | 0.37 (0.21, 0.63) | 1.25 (0.67, 2.35) |
| 2 | 0.40 (0.22, 0.70) | 0.67 (0.40, 1.11) | 0.64 (0.35, 1.16) | |
| 3 | 0.53 (0.31, 0.93) | 1.18 (0.74, 1.87) | 0.46 (0.28, 0.78) | |
| 4 | 0.37 (0.21, 0.65) | 0.86 (0.54, 1.37) | 0.39 (0.22, 0.69) |
Metabolites included in this table were 1) associated with different treatment effects across treatment groups with a Phomogeneity for treatment group <0.05 and 2) associated with differences across metabolite quartiles within treatment effects with a Phomogeneity value <0.05 in at least one pairwise treatment group comparison. HR and Phomogeneity values across metabolite quartiles within treatment effects <0.05 are in boldface type. The HR is expressed as diabetes risk for each pairwise treatment group comparison for the given baseline metabolite concentration quartile. All weighted Cox models were adjusted for age, sex, race/ethnicity, hypertension status, baseline FPG, and baseline BMI. Phospholipid notation denotes: total number of carbon atoms:total number of double bonds.
For specific phospholipids—including C34:2, C34:3, C36:3, C36:4, and C38:2 PC; C18:2 and C20:4 LPE; C18:2 CE; and C38:2 PE—individuals with baseline concentrations in the highest quartile had a greater reduction in diabetes incidence with ILS compared with MET, while those in the lowest quartile had no difference (Phomogeneity <0.05). There was a similar trend across quartiles for C44:13 PE plasmalogen and C22:1 SM when ILS was compared with PLA (Phomogeneity <0.05) and for C36:2 PC and C18:2 CE when ILS was compared with both PLA and MET (Phomogeneity <0.05). For C34:1 PC, individuals in the highest quartile had lower diabetes risk when ILS was compared with MET (Phomogeneity = 0.039) and higher risk when MET was compared with PLA (Phomogeneity = 0.0012). Figure 2 provides a visual depiction of the differences in diabetes risk for an individual with a given metabolite concentration at a specific z score across the three treatment groups in selected metabolites. The treatment-specific hazard rates of C36:2 PC were plotted as an example of the phospholipids, with the hazard rates of ILS converging with those of MET and PLA at lower z scores but diverging at higher z scores as ILS hazard rates decrease (Fig. 2A).
For AMP, individuals in the lowest quartile had a 69% reduction in diabetes incidence when ILS was compared with MET (HR 0.31 [95% CI 0.17–0.57]). Individuals in the highest quartile, however, had no difference (1.03 [0.62–1.72], Phomogeneity across quartiles = 0.03). When ILS was compared with PLA, individuals in all quartiles had an overall reduction in diabetes incidence. When the treatment-specific hazard rates were plotted for AMP, the hazard rates for ILS at lower levels were much lower than for MET and PLA but gradually converged with those for MET and PLA at higher values (Fig. 2B).
Other nonlipid metabolites levels associated with differences in treatment effect included bilirubin and 3-hydroxybutyric acid. For bilirubin, when ILS was compared with PLA, individuals in the highest concentration quartile had a 73% reduction in diabetes risk compared with only a 50% reduction in those in the lowest quartile (Phomogeneity = 0.002). The plot of treatment-specific hazard rates revealed that the hazard rate for PLA remained stable while the hazards rates for MET and ILS decreased and diverged at higher concentrations (Fig. 2C). For the ketone body 3-hydroxybutyric acid, when ILS was compared with PLA, individuals in the lowest quartile had a 71% reduction in diabetes risk, but there was no difference in risk among individuals in the highest quartile (Phomogeneity = 0.013). When the treatment-specific hazard rates were plotted, the hazard rates converged at the highest z scores but diverged at lower z scores, as the hazard rate for MET remained relatively stable, while ILS and PLA changed across concentrations (Fig. 2D).
Discussion
We characterized the comprehensive baseline metabolite profile of the DPP and identified unique metabolite associations with incident diabetes—including the association of higher levels of cytosine and C36:1 PE plasmalogen with lower risk—in this multiethnic, high-risk population. We also confirmed the association of higher levels of betaine and lower levels of methionine sulfoxide with decreased diabetes risk initially described in a smaller metabolite profiling study of the DPP (13). In exploratory analyses, we identified specific metabolite subclasses associated with different diabetes risk across the three treatment groups. These data suggest that individuals at high risk with low levels of AMP or high levels of specific phospholipids (e.g., C36:2 PC and C38:2 PE) will benefit the most from intensive lifestyle modification to prevent progression to T2D. By contrast, individuals with similar clinic risk but low levels of these phospholipids or higher levels of AMP can have the same benefit from metformin therapy. These findings suggest there are unique baseline metabolites associated with T2D progression and preventative treatment effect in our population that could implicate metabolic pathways important to T2D development and serve as candidate biomarkers of preventative intervention response motivating further study.
Higher levels of cytosine and C36:1 PE plasmalogen were associated with decreased T2D incidence in our study. Cytosine is a product of pyrimidine metabolism that reduces glutamine (24). Higher levels of cytosine have been associated with improved glycemic control in people with type 1 diabetes (25) and with a low glycemic diet (26). Further studies, possibly utilizing instrumental variable analysis via Mendelian randomization, could clarify whether cytosine is a surrogate marker of glycemic control or is causally related to diabetes development. C36:1 PE plasmalogen is a phospholipid with a vinyl ether bond highly susceptible to reactive oxygen species oxidation and has been proposed to protect pancreatic β-cells from injury. C36:1 PE plasmalogen levels have been inversely associated with type 1 diabetes incidence in a pediatric population (27–29). Since DPP participants were selected to be insulin resistant, β-cell function could be a stronger predictor of diabetes progression in this population. Further studies will be needed in similar populations to confirm this association with incident diabetes and to explore the relationship with β-cell reserve.
We confirmed the associations of higher levels of betaine and serine and lower levels of methionine sulfoxide with decreased risk of incident diabetes that were first described in a smaller cohort of the DPP (13). We also validated the associations of isoleucine, glutamine, and asparagine with incident T2D previously found in population-based cohorts. Other established metabolite associations with incident T2D such as the BCAAs leucine and valine and the AAAs phenylalanine and tyrosine were not significant in this study after adjustment for clinical risk factors (2,3,5,13). Several lipid species were associated with both increased (including TAGs, DAGs, and PEs) and decreased (including SMs, PE and PC plasmalogens, and CEs) diabetes risk in the entire cohort (Fig. 1). These associations were stronger than for previously established metabolite markers discovered in population-based cohorts—such as BCAAs and AAAs—and they persisted even after adjustment for clinical measures of HDL and triglycerides (Supplementary Table 2). This complements previous findings that lipid acyl chain content provides additional information about diabetes risk beyond clinical measures of “bulk” triglycerides or cholesterol (4,6,22,30). Elevated levels of DAG, TAG, and incomplete fatty acid oxidation intermediates may induce cellular insulin resistance (31,32), and phospholipids contribute to DAG formation (33,34). However, it is still unclear whether intracellular accumulation of lipids is causal or secondary to the metabolic dysfunction associated with diabetes development. Our findings show that phospholipids as a metabolite subclass have the strongest associations with incident diabetes in these individuals at high risk and may help refine risk stratification in this population.
In our novel exploratory analyses, we also found that specific phospholipid associations with incident diabetes differed with treatment group randomization (Table 2). In general, individuals randomized to ILS who had higher levels of these phospholipids had better outcomes. When ILS was compared with MET, individuals in the highest concentration quartiles had improved outcomes, but the outcomes were similar in individuals in the lowest quartile. Several phospholipids also had similar trends when ILS was compared with PLA. Considering these complementary trends in this metabolite subclass, certain phospholipids could be candidates for biomarkers of response to lifestyle intervention for diabetes prevention.
We also found that AMP—an allosteric nucleotide activator of the master cell energy regulator AMPK and a potential mediator of metformin’s therapeutic effect (35)—had the greatest heterogeneity of treatment effects across treatment arms. Individuals with lower levels of AMP at baseline had lower diabetes incidence when randomized to ILS. When AMP concentration was stratified into quartiles, individuals had lower risk for diabetes in all quartiles when ILS was compared with PLA (Table 3). However, when ILS was compared with MET, individuals in the lowest quartile experienced a 69% risk reduction, but this effect was completely attenuated in the highest quartile (Ptrend = 0.032). These findings suggest that while individuals overall benefit from ILS compared with PLA, those with lower levels of AMP potentially could benefit more from ILS than MET for diabetes prevention. Since AMP is central to cellular metabolism, further studies are needed to confirm these interactions.
This study has several notable limitations. Although the DPP is the largest randomized trial to date comparing lifestyle changes and metformin for diabetes prevention, due to the number of metabolites and variability in their concentrations, the study was underpowered to definitively detect heterogeneity of treatment effects. These analyses were treated as discovery analyses, and further validation of these finding are needed in an independent cohort. Finding a suitable replication cohort has its challenges, since the DPP design included two randomized intervention arms compared with placebo. The Da Qing Diabetes Prevention Study and Finnish Diabetes Prevention Study are contemporaneous randomized trials comparing lifestyle interventions with placebo that could be considered. In the Da Qing Diabetes Prevention Study, however, baseline samples are not available and only a minority of patients received both exercise and diet intervention, while a limited panel of metabolites was measured in the Finnish Diabetes Prevention Study (36–39). An orthogonal method to further investigate these associations would be to leverage available genetic data in the DPP to complete instrumental variable analysis via Mendelian randomization to determine whether these metabolite-diabetes associations are causal. Also, for improvement of the power of our primary analysis, samples from a CVD and cancer case-control study were included with randomly selected samples. While top metabolite-diabetes associations were concordant between the whole metabolite profiling cohort and random samples, some metabolites had stronger associations only in the randomly selected subcohort (Supplementary Table 3). Confounders introduced by the sampling scheme could have abrogated these associations, and further study is warranted in a suitable replication cohort. Another limitation is we were underpowered to complete subgroup analyses based on age, sex, race and ethnicity, and other baseline characteristics that would be of interest.
In summary, comprehensive metabolite profiling of baseline plasma samples from 2,015 participants in the DPP revealed novel metabolite associations with incident diabetes in this group at high risk—findings that differed from those of community-based populations. This included the novel inverse association of cytosine and C36:1 PE plasmalogen with incident diabetes. In our discovery analysis, baseline levels of several metabolites involved in the regulation of cellular metabolism including specific phospholipids and nucleotides such as AMP were associated with differences in treatment effect. Our findings suggest that baseline metabolites could highlight metabolic pathways important in the transition from impaired glucose tolerance to overt diabetes and may serve as biomarkers of disease development and response to preventative strategies, motivating further studies to fully explore these interactions.
Supplementary Material
Article Information
Acknowledgments. The DPP Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and Diabetes Prevention Program Outcomes Study (DPPOS). A complete list of centers, investigators, and staff can be found in Supplementary Data.
Funding. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) of the National Institutes of Health (U01-DKO48489) and supplemental funding by the National Cancer Institute. Further support from the NIDDK included funding to T.J.W. and R.E.G. (R01-DK081572) and training support to Z.-Z.C. (T32-DK007516), who was also supported by the National Heart, Lung, and Blood Institute (T32-HL007374). The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart, Lung, and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Merck KGaA provides medication for DPPOS. DPP/DPPOS have also received donated materials from Bristol-Myers Squibb, Parke-Davis, and LifeScan, Inc. This research was also supported, in part, by the Intramural Research Program of the NIDDK. LifeScan, Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Merck & Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation for the Advancement of Military Medicine provided support services under subcontract with the Biostatistics Research and Coordinating Center.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of Interest. The sponsors of the DPP and DPPOS study were represented on the Steering Committee and played a part in the design, completion, and publication of this work. The funding agencies were not represented on the writing group, although all members of the DPP Steering Committee had input into the contents of the manuscript. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Z.-Z.C., J.L., J.M., M.T., and R.E.G. conceived and designed the work, acquired or analyzed and interpreted data, conducted the statistical analysis, and drafted and critically revised the manuscript for important intellectual content. B.M.H.-S., C.G.L., S.D.-J., J.F.F., R.F.H., W.C.K., K.J.M., and L.P. helped draft and critically revised the manuscript for important intellectual content. J.C.F., T.J.W., and C.C. helped acquire or analyze and interpret data, critically reviewed the statistical analysis plan, and revised the manuscript for important intellectual content. All authors approved the version to be published. M.T. and R.E.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
Clinical trial reg. nos. NCT00004992 and NCT00038727, clinicaltrials.gov
M.T. and R.E.G. share senior authorship.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0236/-/DC1.
A complete list of the Diabetes Prevention Program Research Group investigators can be found in the Supplementary Data online.
Contributor Information
Collaborators: Diabetes Prevention Program Research Group, George A. Bray, Kishore Gadde, Annie Chatellier, Jennifer Arceneaux, Amber Dragg, Crystal Duncan, Frank L. Greenway, Daniel Hsia, Erma Levy, Monica Lockett, Donna H. Ryan, David Ehrmann, Margaret J. Matulik, Kirsten Czech, Catherine DeSandre, Barry J. Goldstein, Kevin Furlong, Kellie A. Smith, Wendi Wildman, Constance Pepe, Ronald B. Goldberg, Jeanette Calles, Juliet Ojito, Sumaya Castillo-Florez, Hermes J. Florez, Anna Giannella, Olga Lara, Beth Veciana, Steven M. Haffner, Helen P. Hazuda, Maria G. Montez, Kathy Hattaway, Carlos Lorenzo, Arlene Martinez, Tatiana Walker, Richard F. Hamman, Dana Dabelea, Lisa Testaverde, Denise Anderson, Alexis Bouffard, Tonya Jenkins, Dione Lenz, Leigh Perreault, David W. Price, Sheila C. Steinke, Edward S. Horton, Catherine S. Poirier, Kati Swift, Enrique Caballero, Barbara Fargnoli, Ashley Guidi, Mathew Guido, Sharon D. Jackson, Lori Lambert, Kathleen E. Lawton, Sarah Ledbury, Jessica Sansoucy, Jeanne Spellman, Steven E. Kahn, Brenda K. Montgomery, Wilfred Fujimoto, Robert H. Knopp, Edward W. Lipkin, Ivy Morgan-Taggart, Anne Murillo, Lonnese Taylor, April Thomas, Elaine C. Tsai, Dace Trence, Abbas E. Kitabchi, Samuel Dagogo-Jack, Mary E. Murphy, Laura Taylor, Jennifer Dolgoff, Debra Clark, Uzoma Ibebuogu, Helen Lambeth, Harriet Ricks, Lily M.K. Rutledge, Judith E. Soberman, Mark E. Molitch, Boyd E. Metzger, Mariana K. Johnson, Mimi M. Giles, Diane Larsen, Samsam C. Pen, David M. Nathan, Mary Larkin, Charles McKitrick, Heather Turgeon, Ellen Anderson, Laurie Bissett, Kristy Bondi, Enrico Cagliero, Kali D’Anna, Linda Delahanty, Jose C. Florez, Valerie Goldman, Peter Lou, Alexandra Poulos, Elyse Raymond, Christine Stevens, Beverly Tseng, Elizabeth Barrett-Connor, Mary Lou Carrion-Petersen, Lauren N. Claravall, Jonalle M. Dowden, Javiva Horne, Diana Leos, Sundar Mudaliar, Jean Smith, Simona Szerdi Janisch, Karen Vejvoda, F. Xavier Pi-Sunyer, Jane E. Lee, Sandra T. Foo, Susan Hagamen, David G. Marrero, Kieren J. Mather, Susie M. Kelly, Paula Putenney, Marcia A. Jackson, Gina McAtee, Ronald T. Ackermann, Carolyn M. Cantrell, Edwin S. Fineberg, Angela Hadden, Mario S. Kirkman, Erin O’Kelly Phillips, Paris J. Roach, Robert E. Ratner, Vanita Aroda, Sue Shapiro, Catherine Bavido-Arrage, Peggy Gibbs, Gabriel Uwaifo, Renee Wiggins, Mohammed F. Saad, Karol Watson, Medhat Botrous, Sujata Jinagouda, Maria Budget, Claudia Conzues, Perpetua Magpuri, Kathy Ngo, Kathy Xapthalamous, Neil H. White, Angela L. Brown, Samia Das, Prajakta Khare-Ranade, Tamara Stich, Ana Santiago, Cormarie Wernimont, Christopher D. Saudek, Sherita Hill Golden, Tracy Whittington, Frederick L. Brancati, Jeanne M. Clark, Alicia Greene, Dawn Jiggetts, Henry Mosley, John Reusing, Richard R. Rubin, Shawne Stephens, Evonne Utsey, David S. Schade, Karwyn S. Adams, Claire Hemphill, Penny Hyde, Janene L. Canady, Kathleen Colleran, Ysela Gonzales, Doris A. Hernandez-McGinnis, Carolyn King, Jill Crandall, Janet O. Brown, Gilda Trandafirescu, Elsie Adorno, Helena Duffy, Angela Goldstein, Jennifer Lukin, Helen Martinez, Dorothy Pompi, Harry Shamoon, Jonathan Scheindlin, Elizabeth A. Walker, Judith Wylie-Rosett, Trevor Orchard, Andrea Kriska, Susan Jeffries, M. Kaye Kramer, Marie Smith, Catherine Benchoff, Stephanie Guimond, Jessica Pettigrew, Debra Rubinstein, Linda Semler, Elizabeth Venditti, Valarie Weinzierl, Richard F. Arakaki, Narleen K. Baker-Ladao, Mae K. Isonaga, Nina E. Bermudez, Marjorie K. Mau, John S. Melish, Robin E. Yamamoto, William C. Knowler, Norman Cooeyate, Alvera Enote, Mary A. Hoskin, Camille Natewa, Carol A. Percy, Kelly J. Acton, Vickie L. Andre, Roz Barber, Shandiin Begay, Brian C. Bucca, Sherron Cook, Jeff Curtis, Charlotte Dodge, Matthew S. Doughty, Jason Kurland, Justin Glass, Martia Glass, Robert L. Hanson, Louise E. Ingraham, Kathleen M. Kobus, Jonathan Krakoff, Catherine Manus, Cherie McCabe, Sara Michaels, Tina Morgan, Julie A. Nelson, Christopher Piromalli, Robert J. Roy, Sandra Sangster, Miranda Smart, Darryl P. Tonemah, Rachel Williams, Charlton Wilson, Sarah Fowler, Marinella Temprosa, Michael Larsen, Tina Brenneman, Hanna Sherif, Sharon L. Edelstein, Solome Abebe, Julie Bamdad, Melanie Barkalow, Joel Bethepu, Tsedenia Bezabeh, Nicole Butler, Jackie Callaghan, Caitlin E. Carter, Costas Christophi, Gregory M. Dwyer, Mary Foulkes, Yuping Gao, Robert Gooding, Adrienne Gottlieb, Nisha Grover, Heather Hoffman, Ashley Hogan Tjaden, Kathleen Jablonski, Richard Katz, Preethy Kolinjivadi, John M. Lachin, Yong Ma, Qing Pan, Susan Reamer, Alla Sapozhnikova, Elizabeth M. Venditti, Andrea M. Kriska, Linda Semler, Valerie Weinzierl, Santica Marcovina, Greg Strylewicz, John Albers, Judith Fradkin, Sanford Garfield, Christine Lee, Edward Gregg, Ping Zhang, Jose C. Florez, David Altshuler, Liana K. Billings, Ling Chen, Maegan Harden, Robert L. Hanson, William C. Knowler, Toni I. Pollin, Alan R. Shuldiner, Kathleen Jablonski, Paul W. Franks, and Marie-France Hivert
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Wang Y, Ong C-N, et al. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016;59:2349–2359 [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Semiz S, van der Lee SJ, et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics 2017;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebholz CM, Yu B, Zheng Z, et al. doi: 10.1007/s00125-018-4573-7. Serum metabolomic profile of incident diabetes. Diabetologia 2018;61:1046–1054. [DOI] [PMC free article] [PubMed]
- 6.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breier M, Wahl S, Prehn C, et al. Immediate reduction of serum citrulline but no change of steroid profile after initiation of metformin in individuals with type 2 diabetes. J Steroid Biochem Mol Biol 2017;174:114–119 [DOI] [PubMed] [Google Scholar]
- 9.Asagami T, Abbasi F, Stuelinger M, et al. Metformin treatment lowers asymmetric dimethylarginine concentrations in patients with type 2 diabetes. Metabolism 2002;51:843–846 [DOI] [PubMed] [Google Scholar]
- 10.Huo T, Cai S, Lu X, Sha Y, Yu M, Li F. Metabonomic study of biochemical changes in the serum of type 2 diabetes mellitus patients after the treatment of metformin hydrochloride. J Pharm Biomed Anal 2009;49:976–982 [DOI] [PubMed] [Google Scholar]
- 11.Huffman KM, Slentz CA, Bateman LA, et al. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care 2011;34:174–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den OudenH, Pellis L, Rutten GEHM, et al. Metabolomic biomarkers for personalised glucose lowering drugs treatment in type 2 diabetes. Metabolomics 2016;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE; Diabetes Prevention Program Research Group . Metabolite profiles of diabetes incidence and intervention response in the Diabetes Prevention Program. Diabetes 2016;65:1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Prevention Program Research Group The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 17.Kimberly WT, O’Sullivan JF, Nath AK, et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2017;2:92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation 2018;137:841–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuelsen SO. A psudolikelihood approach to analysis of nested case-control studies. Biometrika 1997;84:379–394 [Google Scholar]
- 20.Støer NC, Samuelsen SO. Comparison of estimators in nested case-control studies with multiple outcomes. Lifetime Data Anal 2012;18:261–283 [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Royal Stat Soc B 1995;57:289–300 [Google Scholar]
- 22.Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon Y-J, Lee Y-J, Park B-J, Hong K-W, Jung D-H. Total serum bilirubin and 8-year incident type 2 diabetes mellitus: the Korean Genome and Epidemiology Study. Diabetes Metab 2018;44:346–353 [DOI] [PubMed] [Google Scholar]
- 24.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab 2016;23:27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta T, Kudva YC, Persson X-MT, et al. Impact of long-term poor and good glycemic control on metabolomics alterations in type 1 diabetic people. J Clin Endocrinol Metab 2016;101:1023–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esko T, Hirschhorn JN, Feldman HA, et al. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr 2017;105:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–466 [DOI] [PubMed] [Google Scholar]
- 28.Broniec A, Klosinski R, Pawlak A, Wrona-Krol M, Thompson D, Sarna T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic Biol Med 2011;50:892–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orešič M, Simell S, Sysi-Aho M, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med 2008;205:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang-Sattler R, Yu Z, Herder C, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 2012;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 2010;375:2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newgard CB. Metabolomics and metabolic diseases: where do we stand? Cell Metab 2017;25:43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Veen JN, Lingrell S, da Silva RP, Jacobs RL, Vance DE. The concentration of phosphatidylethanolamine in mitochondria can modulate ATP production and glucose metabolism in mice. Diabetes 2014;63:2620–2630 [DOI] [PubMed] [Google Scholar]
- 34.Ishii H, Koya D, King GL. Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med (Berl) 1998;76:21–31 [DOI] [PubMed] [Google Scholar]
- 35.Zhou G, Myers R, Li Y, et al. doi: 10.1172/JCI13505. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174. [DOI] [PMC free article] [PubMed]
- 36.Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 37.Qian X, Chen Y, Jia H-M, et al. Effects of lifestyle intervention on metabolism profile in people with impaired glucose tolerance—the China Da Qing Diabetes Prevention Study (Abstract). Diabetes 2018;67(Suppl. 1):A442.
- 38.Lindström J, Ilanne-Parikka P, Peltonen M, et al.; Finnish Diabetes Prevention Study Group . Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 39.de Mello VD, Paananen J, Lindström J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 2017;7:46337 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.