Abstract
Melanin-concentrating hormone (MCH) is an important regulator of food intake, glucose metabolism, and adiposity. However, the mechanisms mediating these actions remain largely unknown. We used pharmacological and genetic approaches to show that the sirtuin 1 (SIRT1)/FoxO1 signaling pathway in the hypothalamic arcuate nucleus (ARC) mediates MCH-induced feeding, adiposity, and glucose intolerance. MCH reduces proopiomelanocortin (POMC) neuronal activity, and the SIRT1/FoxO1 pathway regulates the inhibitory effect of MCH on POMC expression. Remarkably, the metabolic actions of MCH are compromised in mice lacking SIRT1 specifically in POMC neurons. Of note, the actions of MCH are independent of agouti-related peptide (AgRP) neurons because inhibition of γ-aminobutyric acid receptor in the ARC did not prevent the orexigenic action of MCH, and the hypophagic effect of MCH silencing was maintained after chemogenetic stimulation of AgRP neurons. Central SIRT1 is required for MCH-induced weight gain through its actions on the sympathetic nervous system. The central MCH knockdown causes hypophagia and weight loss in diet-induced obese wild-type mice; however, these effects were abolished in mice overexpressing SIRT1 fed a high-fat diet. These data reveal the neuronal basis for the effects of MCH on food intake, body weight, and glucose metabolism and highlight the relevance of SIRT1/FoxO1 pathway in obesity.
Introduction
Melanin-concentrating hormone (MCH) is a 19-amino acid neuropeptide predominantly expressed in the lateral hypothalamic area that plays a pivotal role in the regulation of energy homeostasis (1,2). The central infusion of MCH induces feeding (3), and overexpression of MCH in transgenic (Tg) mice leads to obesity (4). Conversely, pharmacological inhibition of MCHR1 reduces appetite, body weight, and adiposity (5–7). In line with this, the lack of MCH causes hypophagia and leanness (8); attenuates leptin deficiency–induced obesity (9,10), diet-induced obesity (DIO) (11), and aging-associated increases in body weight and insulin resistance (12); and protects from hepatosteatosis (13). Independent of its actions on feeding and body weight, MCH induces insulin resistance (4,14), and MCH-expressing neurons are stimulated by glucose and involved in the control of peripheral glucose homeostasis (15). In addition, MCH neurons are both necessary and sufficient for sensing the nutrient value of sucrose, indicating that these neurons play a critical role in establishing nutrient preference (16). MCH also favors lipid storage in white adipose tissue (WAT) and the liver through the sympathetic nervous system (SNS) and para-SNS, respectively (17).
MCH binds to MCH receptor 1 (MCHR1) (18), and MCHR1-deficient mice are lean, hypophagic, and resistant to DIO (19,20). MCHR1 and MCH projections are widely distributed throughout the brain (21–26), suggesting that MCH is implicated in a large variety of functions. The complexity of the MCH system raises the possibility that multiple mechanisms underlie the biological actions of this neuropeptide. In line with this, MCH-induced food intake is blocked by different anorexigenic factors such as α-melanocyte–stimulating hormone (27,28), glucagon-like peptide 1 (28), and neuropeptide Y antagonism (29).
On the other hand, sirtuin 1 (SIRT1) is a highly conserved NAD+-dependent deacetylase that is activated in response to calorie restriction and acts as a cellular sensor to detect energy availability and regulate metabolism in a wide variety of tissues (30–32). Hypothalamic SIRT1 controls energy balance (33,34), and these actions are at least partially mediated by the melanocortin system (35,36). The lack of SIRT1 in proopiomelanocortin (POMC) neurons leads to increased weight gain (37), while its deficiency in agouti-related peptide (AgRP) neurons leads to a lean phenotype (36).
Although the anabolic action of MCH was first shown nearly 20 years ago (3) and its physiological relevance is beyond any doubt, the neuronal circuits controlling this action remain largely unknown. We describe that MCH requires a SIRT1/FoxO1/POMC signaling pathway within the hypothalamic arcuate nucleus (ARC) to modulate feeding, adipocyte lipid metabolism, and glucose metabolism.
Research Design and Methods
Animals and Surgery
Eight- to ten-week-old Sprague-Dawley male rats, male C57/BL6 wild-type (WT) mice, mice with moderate overexpression of SIRT1 (SIRT1 Tg) under the control of its own promoter, Pomc-Cre:ROSA-tdTomato mice, and AgRP-Cre mice were housed in individual cages under conditions of controlled temperature (23°C) and illumination (12-h light/12-h dark cycle). Animals were allowed ad libitum access to water and standard laboratory chow or a high-fat diet (HFD) (60% by energy) (D12492; Research Diets, New Brunswick, NJ). All experiments and procedures involved in this study were reviewed and approved by the ethics committee of Universidad de Compostela, the institutional ethics committees for the care and use of experimental animals of the Universities of Lille, and the University of Iowa Animal Research Committee in accordance with European Union normative for the use of experimental animals.
Patch-Clamp Recordings
Whole-cell patch-clamp recordings were performed in current-clamp mode as previously described (38) (see Supplementary Information).
Intracerebroventricular Infusions
Intracerebroventricular infusions in rats and mice were conducted as previously described (39) (see Supplementary Information).
Stereotaxic Microinjection of Lentiviral Expression Vectors
Lentiviral vectors expressing green fluorescent protein (GFP) and inhibiting SIRT1 (shSIRT1), FoxO1 (shFoxO1), MCHR1 (shMCHR1), POMC (shPOMC) (Sigma-Aldrich), and γ-aminobutyric acid receptor (GABA-R) (shGABA-R) genes or scrambled sequences were injected bilaterally into the ARC (anterior to bregma [AP] −2.85 mm, lateral to the sagittal suture [L] ± 0.3 mm, and ventral from the surface of the skull [V] −10.2 mm), with a microliter syringe (17,40–42). The viral particles (1 μL, 3.1 × 106 plaque-forming units/mL) were infused over 5 min, and the injector was kept in place for an additional 5 min. GFP fluorescence, visualized under the microscope, was used as a marker of effective transduction of the lentivirus at the injection site. Dissection of the ARC was performed by micropunches under the microscope, as previously reported (43,44). The specificity of the ARC dissection was confirmed by analyzing the mRNA of specific markers, namely POMC and AgRP, the expression of which was 80% higher in the ARC compared with the ventromedial hypothalamus (VMH). To inhibit the expression of SIRT1 specifically in POMC neurons, we injected into the ARC adeno-associated virus (AAV)8-hSyn-DIO (double-floxed inverted orientation)-GFP or AAV8-hSyn-shSIRT1-DIO-GFP of mice expressing Cre IRES POMC neurons. POMC-IRES-Cre mice were anesthetized and placed in a stereotaxic frame (Kopf Instruments). Specific infection of AAV in POMC neurons was evaluated by immunohistochemistry. In all experimental settings, body weight and food intake were recorded until 2–3 weeks after the surgery, and then we performed the acute or chronic MCH treatments.
Western Blot Analysis and Real-Time PCR
Western blot and real-time PCR were performed as described previously (17) (see Supplementary Information).
Statistical Analysis and Data Presentation
Data are expressed as mean ± SEM. Protein data were expressed in relation (%) to control (vehicle) or GFP-treated rats/mice. Sympathetic nerve activity (SNA) was expressed as a percentage change from baseline. Statistical significance was determined by Student t test when two groups were compared or one-way ANOVA and post hoc one-tailed Bonferroni test when more than two groups were compared. A P < 0.05 was considered significant.
Data and Resource Availability
The data that support the findings of this study are available from the corresponding author upon request.
Results
Central MCH Stimulates FoxO1 and Inhibits POMC Protein Levels via MCHR in the ARC
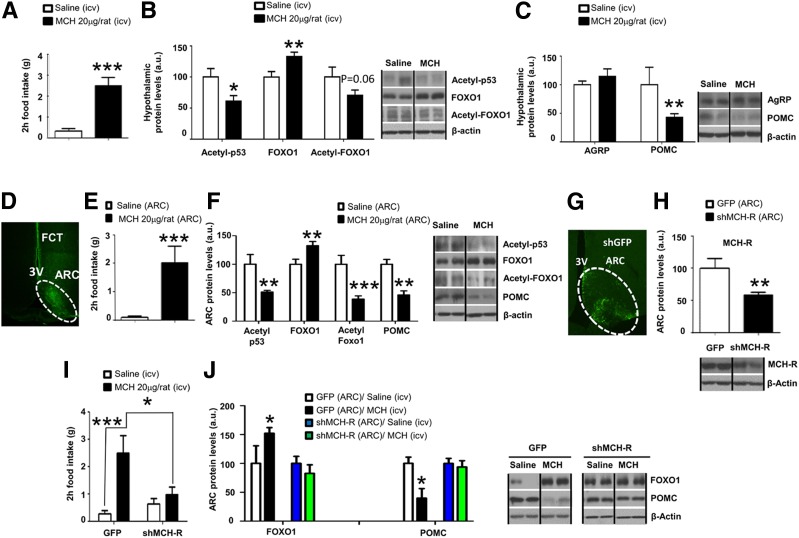
As expected, an acute intracerebroventricular bolus of MCH increased feeding after 2 h of injection in satiated Sprague-Dawley rats (Fig. 1A). Intracerebroventricular MCH-treated rats showed unchanged levels of hypothalamic pAMPK, p-mTOR, and enzymes involved in fatty acid metabolism (Supplementary Fig. 1A). However, MCH diminished acetyl-p53 levels, a surrogate marker of SIRT1 activity, and therefore decreased acetyl-FoxO1 levels while raising FoxO1 protein levels in the hypothalamus (Fig. 1B). Additionally, we found that central MCH significantly decreased POMC protein levels, whereas no changes were observed in neuropeptide Y, AgRP, or CART (cocaine- and amphetamine-regulated transcript) (Fig. 1C and Supplementary Fig. 1B). Remarkably, these molecular changes induced by MCH are observed also at the mRNA level (Supplementary Fig. 2A–C). Moreover, our results show the specificity of MCH-induced changes in FoxO1 and SIRT1 protein levels in the ARC, because those effects were not found in other hypothalamic areas such as the VMH or the lateral hypothalamic area (Supplementary Fig. 2D–G). Of note, the specificity of the isolation of hypothalamic nuclei was corroborated by measuring POMC and steroidogenic factor-1 in the ARC and in the VMH (Supplementary Fig. 2H and I).
Figure 1.
Central MCH stimulates hypothalamic FoxO1 levels and downregulates POMC protein levels through MCHR in the ARC. Central effects of intracerebroventricular MCH administration (20 µg/rat) on food intake (A), hypothalamic protein levels of acetyl-p53, FoxO1, acetyl-FoxO1 (B), and hypothalamic protein levels of AgRP and POMC (C) in rats after 2 h. D: FITC staining in the hypothalamic ARC. Food intake (E) and ARC protein levels (F) of acetyl-p53, FoxO1, acetyl-FoxO1, and POMC in rats 2 h after injection of MCH directly in the ARC. G: GFP expression in the hypothalamic ARC after stereotaxic injection of shMCHR1 lentivirus. H: Protein levels of MCHR in the ARC of rats stereotaxically injected with scrambled or shMCHR1 lentiviruses. I: Effect of intracerebroventricular MCH on food intake in rats stereotaxically injected with scrambled or shMCHR1 lentiviruses into the ARC. J: Protein levels of FoxO1 and POMC in the hypothalamic ARC of rats stereotaxically injected with scrambled or shMCHR1 lentiviruses in the ARC and intracerebroventricular (icv) MCH. β-Actin was used to normalize protein levels. Dividing lines indicate spliced bands from the same gel. a.u., arbitrary units. Values are mean ± SEM of 7–10 animals per group. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 vs. controls.
We combined ARC microinjection of vehicle or MCH with FITC that allowed us to control the diffusion of the treatment within the hypothalamus (Fig. 1D). Consistent with bulk brain delivery of MCH, explicit targeting of MCH to the ARC stimulated food intake after 2 h (Fig. 1E) and decreased acetyl-p53, acetyl-FoxO1, and POMC while increasing FoxO1 protein levels within the ARC (Fig. 1F). To elucidate the specific contribution of ARC MCHR1 to the hyperphagic effect of MCH, we used lentivirus encoding an shRNA that silences MCHR1. Infection efficiency was assessed 2 weeks later by the expression of GFP in the ARC (Fig. 1G) and by the decreased protein levels of MCHR1 in the ARC (Fig. 1H). Inhibition of ARC MCHR1 blunted the orexigenic effect of intracerebroventricular MCH (Fig. 1I) and blocked MCH effects on FoxO1 and POMC protein levels in the ARC (Fig. 1J). These results indicate that MCH requires MCHR1 in the ARC to induce feeding and to regulate FoxO1 and POMC in this hypothalamic nucleus.
MCH Reduces the Activity of POMC Neurons
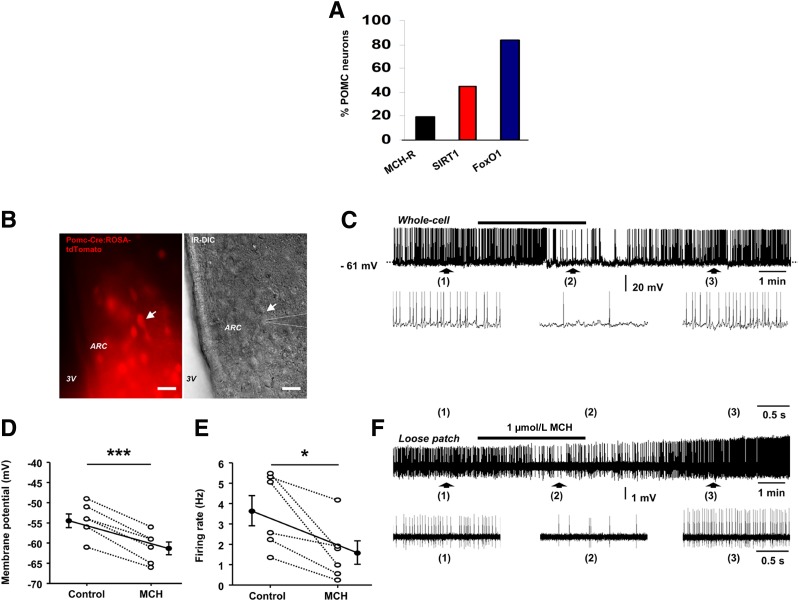
By using FACS sorting and single-cell RNA sequencing of 163 POMC EGFP neurons, we found that 19% of POMC neurons express MCHR1, 45% of POMC neurons express SIRT1, and 84% of POMC neurons express FoxO1 (GEO Database repository, GEO Accession: GSE92707) (Fig. 2A). Since electrical activity of ARC POMC neurons changes across the hunger-satiety cycle and selective sustained opto- or chemogenetic stimulation of these cells promotes satiety (45), we performed whole-cell current-clamp recordings from fluorescent-labeled cells in acute brain slices from Pomc-Cre:ROSA-tdTomato mice (Fig. 2B) to investigate whether MCH-induced hyperphagia is also paralleled by decreased electrical activity of anorexigenic ARC POMC neurons. We found that bath application of 1 μmol/L MCH (46) reversibly reduced the spontaneous firing rate of 50% of ARC POMC neurons (6 of 12 cells from four mice) by 58.92 ± 11.39% (Fig. 2C and D), an effect that was accompanied by a membrane hyperpolarization of 6.83 ± 0.65 mV (n = 6 cells from four mice) (Fig. 2C and D). MCH had no effect on the six other cells tested (data not shown). Furthermore, the MCH-induced inhibitory effect on POMC neuronal firing persisted in the loose patch-clamp configuration (in two of two cells from two mice) (Fig. 2E and F), indicating that it was not a consequence of dilution of the intracellular compartment by whole-cell dialysis. These results show that MCH inhibits the activity of ARC POMC neurons, an effect that together with the aforementioned MCH-induced downregulation of POMC gene expression in the ARC converges toward an inhibition of the anorexigenic POMC signaling.
Figure 2.
MCH inhibits the activity of POMC neurons in the ARC. A: FACS sorting and single-cell RNA sequencing of POMC-EGFP neurons showing MCHR, SIRT1, and FoxO1 expression (GEO Database repository, GEO Accession: GSE92707). B: Left, a spontaneously fluorescent ARC POMC neuron (arrow) from a Pomc-Cre:ROSA-tdTomato mouse was identified for patch-clamp recording; right, infrared differential interference contrast (IR-DIC) of the same image showing a patched pipette (dotted lines) placed on the cell membrane of the identified POMC neuron (arrow). Scale bars = 50 μm. C: Whole-cell current-clamp recording showing that MCH reversibly decreased the spontaneous firing activity of the POMC neuron patched in B. Note that the inhibitory effect was accompanied by a membrane hyperpolarization. Denoted regions of the recording are shown underneath with an expanded time scale. D: Average membrane potential of ARC POMC neurons in control conditions and in the presence of MCH (n = 6 cells from four mice). ***P ≤ 0.001 by paired t test. E: Average firing rate of ARC POMC neurons in control conditions and in the presence of MCH (n = 6 cells from four mice). *P ≤ 0.05 by paired t test. F: Trace showing that MCH reduced the spontaneous firing activity of another ARC POMC neuron recorded in loose patch configuration. Pooled data are shown as mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 vs. controls.
Central MCH Requires POMC but Not AgRP to Stimulate Feeding
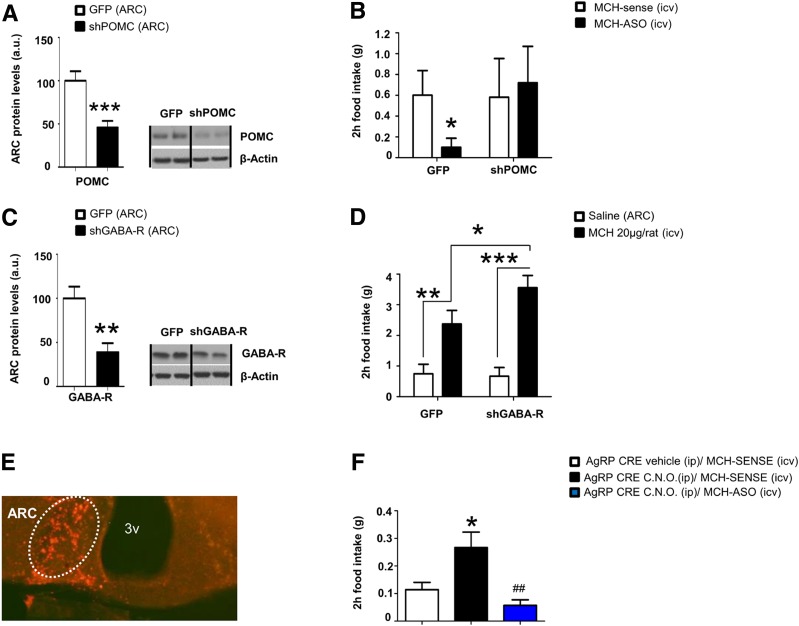
Since MCH decreases POMC levels and POMC activity, we hypothesized that MCH-antisense oligonucleotides (ASO), which are known to suppress feeding and to decrease MCH protein levels (data not shown), might require an upregulation of POMC to exert their anorexigenic action. Thus, we next injected into the ARC a lentivirus encoding shRNA to silence POMC (Fig. 3A) prior to the intracerebroventricular administration of MCH-ASO and found that the hypophagic action of MCH-ASO was blunted (Fig. 3B).
Figure 3.
Central MCH requires POMC but not AgRP to stimulate feeding. A: POMC protein levels in the ARC. B: Effect of intracerebroventricular (icv) MCH-ASO on food intake in rats stereotaxically injected with scrambled or shPOMC lentiviruses in the ARC. C: GABA-R protein levels in the ARC. D: Effect of intracerebroventricular MCH on food intake in rats stereotaxically injected with scrambled or shGABA-R lentiviruses in the ARC. E: mCherry expression in the hypothalamic ARC after stereotaxic injection of hSYN-DIO-Hm3D(Gq)-mCherry adenoviral vector. F: Effect of intracerebroventricular MCH-ASO on food intake in AgRP-CRE mice stereotaxically injected with hSYN-DIO-Hm3D(Gq)-mCherry adenoviral vector in the ARC prior to intraperitoneal administration of clozapine N-oxido (C.N.O.). β-Actin was used to normalize protein levels. Dividing lines indicate spliced bands from the same gel. a.u., arbitrary units; ip, intraperitoneal. Values are mean ± SEM of 8–10 animals per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. controls; #P ≤ 0.05 vs. AgRP-CRE CNO MCH sense.
To rule out the potential role of AgRP neurons in the actions of MCH, we performed two additional studies. First, since AgRP neurons modulate POMC activity through the release of GABA, we silenced GABA-R in the ARC of rats to test the possible regulation of AgRP neurons over POMC neuronal activity (Fig. 3C). The knockdown of ARC GABA-R did not alter MCH-induced hyperphagia (Fig. 3D). Second, using DREADDs (Designer Receptor Exclusively Activated by Designer Drugs) technology, we stimulated AgRP neurons in mice and found a clear stimulation of feeding, but the chemogenetic stimulation of AgRP neurons did not prevent the hypophagic action of the MCH-ASO (Fig. 3E and F). Thus, POMC but not AgRP is required for the orexigenic action of MCH.
Central MCH Requires the Interaction Between SIRT1 and FoxO1 to Stimulate Feeding
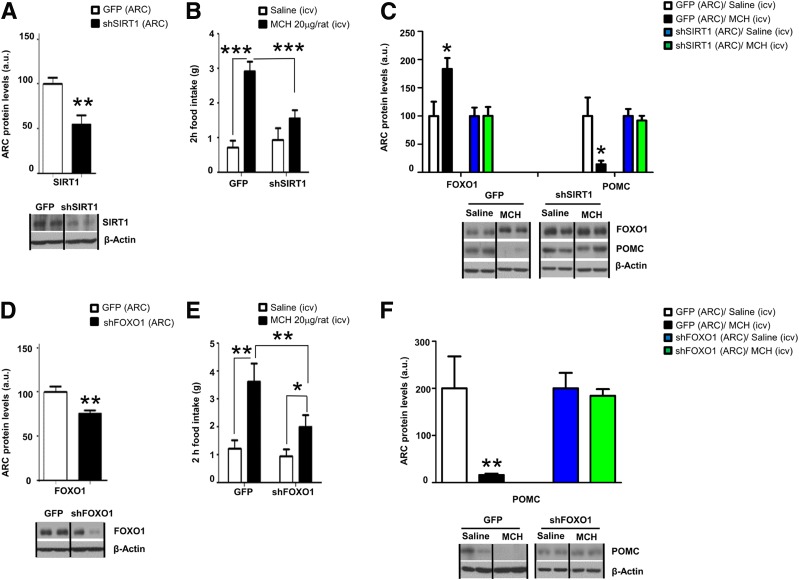
We next assessed whether pharmacological or genetic blockade of SIRT1 interferes with the orexigenic action of MCH. Intracerebroventricular Ex527, a selective SIRT1 inhibitor, administered 20 min before intracerebroventricular MCH blunted the orexigenic action of MCH (Supplementary Fig. 3A). Genetically inhibiting SIRT1 expression in the ARC via a lentivirus encoding an shRNA that silences SIRT1 (Fig. 4A) blunted the orexigenic effect of intracerebroventricular MCH (Fig. 4B), and blocked MCH-induced changes in FoxO1 and POMC protein levels in the ARC (Fig. 4C). Of note, the titer and volume of lentiviruses encoding shSIRT1 used for these manipulations did not cause alterations in physiological body weight or food intake (Supplementary Fig. 3B and C). To evaluate the role of FoxO1 as the downstream mediator of SIRT1-dependent MCH orexigenic action, we administered into the ARC a lentivirus encoding an shRNA that silences FoxO1 (Fig. 4D). Inhibition of ARC FoxO1 partially blocked the orexigenic effect of intracerebroventricular MCH (Fig. 4E) and reversed the effects of MCH on POMC protein levels in the ARC (Fig. 4F).
Figure 4.
Central MCH requires SIRT1 and FoxO1 in the hypothalamic ARC to stimulate feeding. A: SIRT1 protein levels in the ARC. B: Effect of intracerebroventricular (icv) MCH on food intake. C: Protein levels of FoxO1 and POMC in the hypothalamic ARC of rats stereotaxically injected with scrambled or shSIRT1 lentiviruses in the ARC and intracerebroventricular MCH. D: FoxO1 protein levels in the ARC. E: Effect of intracerebroventricular MCH on food intake in rats. F: Protein levels of POMC in the hypothalamic ARC of rats stereotaxically injected with scrambled or shFoxO1 lentiviruses in the ARC and intracerebroventricular MCH. β-Actin was used to normalize protein levels. Dividing lines indicate spliced bands from the same gel. a.u., arbitrary units. Values are mean ± SEM of 8–10 animals per group. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 vs. controls.
ARC SIRT1 and FoxO1 Are Necessary for Central MCH to Promote Adipocyte Lipid Storage and Glucose Intolerance
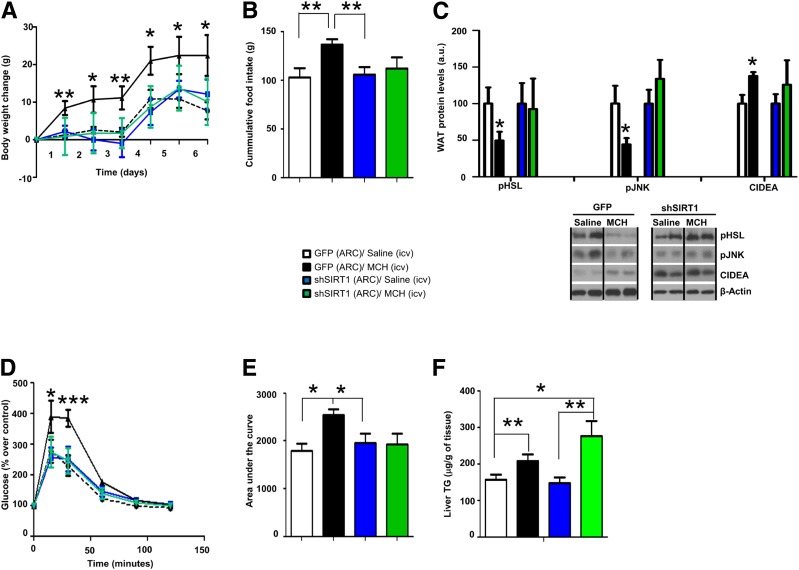
In order to study the role of the SIRT1/FoxO1 pathway in central actions of MCH on adipocyte metabolism and glucose metabolism, we administered into the ARC a lentivirus encoding a shRNA SIRT1 together with GFP or adenovirus expressing GFP scrambled shRNA (control). Two weeks later, rats in each group received intracerebroventricular MCH or vehicle (saline) for one week. While chronic central infusion of MCH significantly induced weight gain and food intake, these effects were abolished in rats in which SIRT1 was silenced in the ARC (Fig. 5A and B). Consistent with the increased weight gain and previous report (17), MCH decreased WAT protein levels of hormone-sensitive lipase (pHSL) and c-Jun N-terminal kinase (pJNK) and increased CIDEA (Fig. 5C). ARC SIRT1 silencing abolished these effects evoked by MCH in WAT (Fig. 5C). In order to test whether the actions of central MCH on WAT were mediated by thermogenesis or browning of WAT, we performed an immunohistochemistry of UCP-1 in WAT and brown adipose tissue (BAT). Consistent with the previous study (17), MCH did not affect UCP-1 levels (Supplementary Fig. 4). Since MCH also impairs glucose tolerance (4,14), we next sought to investigate whether ARC SIRT1 is involved in the effects of central MCH on glucose metabolism. Indeed, we found that the chronic central infusion of MCH caused glucose intolerance, but this action was blunted in rats that have SIRT1 genetically inhibited in the ARC (Fig. 5D and E). In order to assess whether hypothalamic SIRT1 was also an essential mediator of the hepatic actions of MCH, we measured the hepatic triglyceride content. In agreement with previous reports (13,17), the central treatment with MCH augmented the amount of triglyceride in the liver, and this effect was still persistent when ARC SIRT1 was downregulated (Fig. 5F), indicating that SIRT1 does not mediate the central actions of MCH on hepatic lipid metabolism.
Figure 5.
SIRT1 in the ARC is essential for MCH-induced food intake, WAT lipid storage, and glucose intolerance. Body weight change (A), cumulative food intake (B), WAT protein levels of pHSL, pJNK, and CIDEA (C), glucose tolerance test (D), area under the curve (E), and hepatic triglyceride (TG) content (F) in rats that received shSIRT1 or GFP scrambled lentiviruses in the ARC followed by chronic intracerebroventricular (icv) MCH (10 µg/day/rat) for 1 week. β-Actin was used as loading control. Dividing lines indicate spliced bands from the same gel. a.u., arbitrary units. Values are mean ± SEM of 7–10 animals per group. *P ≤ 0.05 and **P ≤ 0.01 vs. controls.
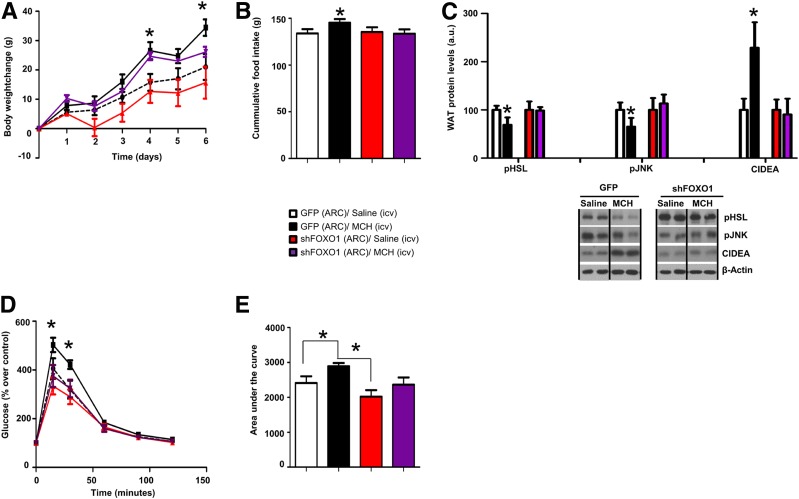
Next, we evaluated the role of the transcription factor FoxO1 as the downstream mediator of SIRT1-dependent MCH action on adipocyte metabolism and glucose intolerance, following a setting similar to that aforementioned for SIRT1. As expected, chronic central infusion of MCH induced significant weight gain (Fig. 6A) and hyperphagia (Fig. 6B), decreased WAT pHSL and pJNK levels, increased WAT CIDEA (Fig. 6C), and caused glucose intolerance (Fig. 6D and E). Notably, all of these MCH-induced effects were blunted in rats where FoxO1 was downregulated in the ARC (Fig. 6A–E) independently of BAT thermogenesis or browning of WAT (Supplementary Fig. 4).
Figure 6.
FoxO1 in the ARC is essential for MCH-induced food intake, WAT lipid storage, and glucose intolerance. Body weight change (A), cumulative food intake (B), WAT protein levels of pHSL, pJNK, and CIDEA (C), glucose tolerance test (D), and area under the curve (E) in rats that were microinjected with shFoxO1 or GFP scrambled lentiviruses into the ARC before intracerebroventricular (icv) MCH (10 µg/day/rat) for 1 week. β-Actin was used as the loading control. Dividing lines indicate spliced bands from the same gel. a.u., arbitrary units. Values are mean ± SEM of 7–10 animals per group. *P ≤ 0.05 vs. controls.
Inhibition of SIRT1 in POMC Neurons Compromises the MCH-Induced Feeding, Body Weight Gain, and Adiposity
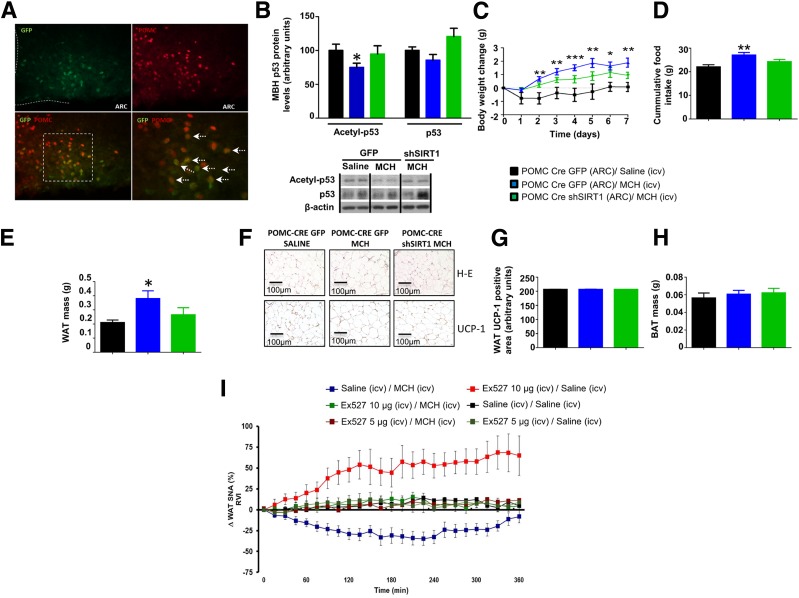
Since inhibition of SIRT1 in the ARC impairs the anabolic actions of MCH and MCH inhibits POMC neuronal activity, we hypothesized that specific inhibition of SIRT1 in POMC neurons could impair MCH function. To test this, we silenced SIRT1 specifically in POMC neurons by injecting AAV8-hSyn-shSIRT1-DIO-GFP into the MBH of POMC-IRES-Cre mice. Our results showed the specificity of the infection, because GFP staining was restricted to POMC neurons (Fig. 7A). Miniature osmotic pumps were implanted in mice 3 weeks after transfection to deliver intracerebroventricular MCH or vehicle (saline) for 1 week. Chronic central infusion of MCH induced significant reduction of MBH acetyl-p53 levels (Fig. 7B), weight gain (Fig. 7C), hyperphagia (Fig. 7D), and adiposity (Fig. 7E). Notably, all of these MCH-induced effects were blunted after inhibition of SIRT1 in POMC neurons (Fig. 7B–E). Therefore, these data suggest that SIRT1 specifically in POMC neurons mediates the anabolic actions of MCH. In addition, as we pointed out in previous experiments, the anabolic role of MCH was independent of the browning of WAT since UCP1 immunostaining was unchanged in all the studied groups (Fig. 7F–H).
Figure 7.
SIRT1 in the POMC neurons regulates MCH-induced food intake, body weight, and WAT lipid storage. A: Representative immunofluorescence showing GFP and POMC colocalization. B: MBH protein levels of acetyl-p53 and p53. Body weight change (C), cumulative food intake (D), and fat mass (E). F: Representative photomicrographs of WAT histology (hematoxylin-eosin [H-E]). WAT protein levels of UCP1 (G) and BAT mass (H). I: Effect of pretreatment with vehicle vs. SIRT1 antagonist Ex-527 (5 μg/rat and 10 μg/rat) on WAT SNA response evoked by intracerebroventricular (icv) MCH (10 µg/rat) in rats. β-Actin was used to normalize protein levels. Dividing lines indicate spliced bands from the same gel. RVI, rectified/integrated voltage. Values are mean ± SEM of 6–10 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. controls.
SIRT1 Mediates MCH-Induced Weight Gain Through the SNS
We previously demonstrated a key role of the SNS inhibition in central MCH-induced weight gain and adiposity (17). Therefore, we hypothesized that downregulation of SIRT1 in the ARC modulates the efferent SNS subserving WAT. For this, we tested the effect of intracerebroventricular MCH on WAT SNA in the absence or presence of EX-527, a SIRT1 antagonist, administered intracerebroventricularly. We found that 1) intracerebroventricular Ex527 (10 μg/rat) stimulated WAT SNA, 2) intracerebroventricular MCH decreased WAT SNA, and 3) a dose of intracerebroventricular EX527 that does not change WAT SNA per se was able to suppress the MCH-induced effect on WAT SNA (Fig. 7I).
Genetic Inhibition of Central MCH Decreases Feeding and Body Weight in WT but Not in SIRT1-Tg Mice
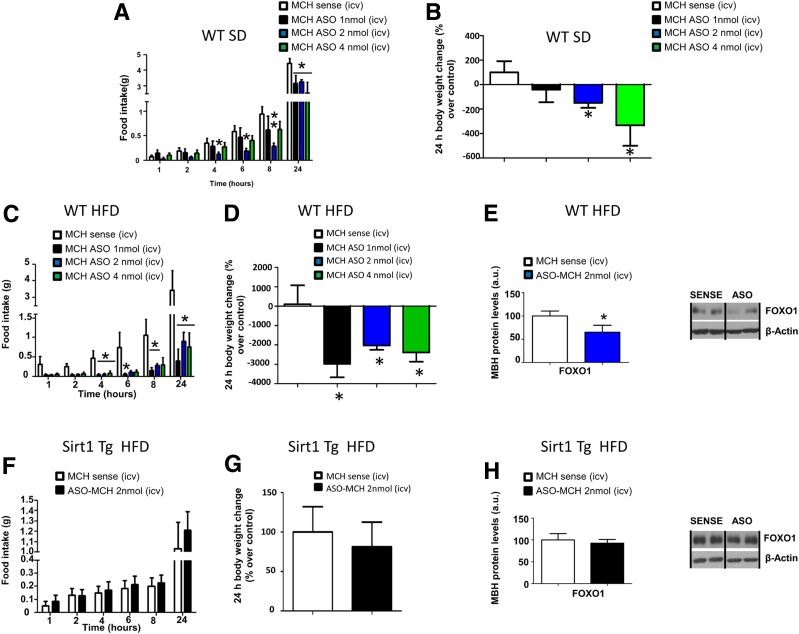
Central injection of MCH-ASO at different doses (1, 2, and 4 nmol/mouse) decreased food intake and body weight in mice fed the chow diet (Fig. 8A and B). Next, we challenged WT mice and mice overexpressing SIRT1 with a 60% HFD for 12 weeks. In WT mice fed the HFD, central administration of MCH-ASO caused a significant decrease in food intake and body weight (Fig. 8C and D), associated with a decrease in hypothalamic mediobasal FoxO1 levels (Fig. 8E). However, central MCH-ASO injection to obese SIRT1-Tg mice failed to alter feeding behavior, body weight, or hypothalamic FoxO1 levels after 24 h (Fig. 8F–H). The injection of MCH-ASO decreased MCH protein levels in both WT and SIRT1-Tg mice after 24 h (Supplementary Fig. 5A and B).
Figure 8.
Overexpression of Sirt1 blunts MCH-ASO–induced hypophagia. Effect of intracerebroventricular MCH-ASO (1, 2, and 4 nmol/mouse) on food intake (A) and body weight change (B) in WT mice fed a standard diet (SD). Effect of intracerebroventricular (icv) MCH-ASO (1, 2, and 4 nmol/mouse) on food intake (C), body weight change (D), and protein levels (E) of FoxO1 in the MBH of WT mice fed the HFD. Effect of intracerebroventricular (icv) ASO-MCH (2 nmol/mouse) on food intake (F), body weight change (G), and protein levels (H) of FoxO1 in the MBH of Sirt1-Tg mice fed an HFD. β-Actin was used to normalize protein levels. Dividing lines indicate spliced bands from the same gel. a.u., arbitrary units. Values are mean ± SEM of 6–10 animals per group. *P ≤ 0.05, **P < 0.01, and ***P < 0.001 vs. controls.
Discussion
Here, we describe for the first time that MCH inhibits the electrical activity of POMC neurons and that SIRT1/FoxO1 mediate the MCH control of food intake, adipocyte lipid storage, and glucose metabolism. The central melanocortin system interacts with SIRT1 to modulate energy homeostasis and insulin sensitivity. Pharmacological or genetic inhibition of hypothalamic SIRT1 decreases food intake and weight gain, and central administration of a specific melanocortin antagonist, SHU9119, reversed the anorectic effect of hypothalamic SIRT1 inhibition (35). Mice lacking SIRT1 in POMC neurons were more prone to DIO (37), and selective lack of SIRT1 in hypothalamic AgRP neurons decreases food intake, fat mass, and body weight (36). We therefore hypothesized that hypothalamic SIRT1 might govern the metabolic actions of MCH. We focused our attention on the ARC based on the evidence identifying this hypothalamic area as the site where MCH acts to increase food intake and adipocyte lipid deposition (17). Mechanistic studies using a pharmacological approach and viral vectors show that downregulation of SIRT1 in the ARC blunts MCH-induced feeding. Accordingly, genetic silencing of SIRT1 in the ARC blunted adipocyte lipid storage and glucose intolerance caused by chronic central infusion of MCH. Although central MCH favors hepatic lipid deposition (13,17), this action occurs in the lateral hypothalamic area (17) but remained unaltered after inhibition of SIRT1 in the ARC, indicating the specificity of the MCH-SIRT1 pathway. Therefore, these data indicate that SIRT1 is a mediator of the anabolic effects of MCH. Furthermore, the role of SIRT1 as a mediator of MCH is consistent with other reports suggesting that hypothalamic SIRT1 mediates the orexigenic action of ghrelin (36,47).
The SNS connects hypothalamic centers with the WAT, and we previously demonstrated that the central MCH controls adiposity through the SNS (17). Furthermore, hypothalamic SIRT1 modulates WAT SNA (37). Our findings show that whereas intracerebroventricular MCH decreased WAT SNA, central blockade of SIRT1 blunted this effect. In line with this, the actions of ARC SIRT1 as a modulator of MCH-induced adiposity could be controlled by the administration of a β-adrenoreceptor antagonist, indicating that WAT SNA is a downstream effector of SIRT1. Overall, these results indicate that hypothalamic SIRT1 requires the SNS to modulate the actions of MCH on body fat mass. Thus, the central MCH/SIRT1 pathway represents a new neuronal circuit of the brain-WAT axis. The increased SIRT1 activity following MCH administration may at first seem counterintuitive, but there are several reports that studied the role of SIRT1 in the hypothalamus, and the results are somehow controversial. While deletion of SIRT1 in POMC neurons causes a higher sensitivity to developing obesity when mice are fed an HFD (37), we and others reported that ghrelin, a metabolic hormone, which similarly to MCH stimulates feeding and adiposity and also inhibits the SNA in the WAT, requires hypothalamic SIRT1 to exert its effects (36,47). Similarly, previous experimental evidence has shown that pharmacological inhibition of SIRT1 at the central level inhibits ghrelin-induced food intake and body weight through the regulation of the FoxO1 and the melanocortin system, including increased levels of acetyl-FoxO1 and POMC expression (35,36,48). Thus, further studies are necessary to clarify the role of hypothalamic SIRT1.
Importantly, our studies have identified FoxO1 as a critical mechanism within the ARC by which the MCH-SIRT1 pathway controls food intake, adipocyte metabolism, and glucose intolerance. Among the large list of molecules that are directly regulated by SIRT1, FoxO1 emerged as a potential candidate because of its interaction with the hypothalamic melanocortin system (49) and in mediating the metabolic effects of central SIRT1 (35). MCH administered intracerebroventricularly or directly into the ARC upregulated hypothalamic protein levels of FoxO1, whereas genetic inhibition of ARC SIRT1 abolished this effect. Similar to the results obtained with SIRT1, downregulation of FoxO1 in the ARC blunted MCH-induced feeding, adipocyte lipid storage, and glucose intolerance.
To gain insights into the pathophysiological relevance of the MCH/SIRT1/FoxO1 pathway in obesity, we injected MCH-ASO centrally, which decreased both food intake and weight gain in WT mice fed the chow diet or HFD. Intracerebroventricular MCH-ASO decreased hypothalamic levels of FoxO1 in WT mice fed the HFD. However, central MCH-ASO failed to modify food intake or body weight in mice overexpressing SIRT1. This lack of effect was associated with the inability of MCH-ASO to inhibit hypothalamic FoxO1. Finally, the interaction between the MCH system and SIRT1/FoxO1 appears to occur in POMC neurons. This is demonstrated by the fact that when we disrupted GABA signaling into the ARC neurons, which is the neurotransmitter in charge of the neural communication between AgRP and POMC neurons, central MCH was still able to stimulate feeding. Indeed, POMC neurons also receive inhibitory inputs from outside the ARC (40), but this result, together with the fact that MCH-ASO maintained their hypophagic action after the chemogenetic stimulation of AgRP neurons, suggests that AgRP neurons fail to significantly influence MCH-mediated feeding. Moreover, our functional data showed that MCH dramatically inhibits spontaneous neuronal activity in a significant subset of POMC neurons. Indeed, we obtained certain variability in the response of these neurons, which was expected due to the high heterogeneity of POMC neurons based on their molecular taxonomy, neurotransmitter, and receptor expression (50). Lastly, our hypothesis was countersigned/confirmed by the fact that the virogenetic deletion of SIRT1 specifically in POMC neurons blunted the actions of MCH. We found that in mice lacking SIRT1 in POMC neurons, MCH is less effective in inducing weight gain, feeding, and adiposity compared with mice with intact SIRT1 expression in those neurons. The incomplete blockade of MCH actions in our animal model might be explained by the fact that the adenoviral vector did not infect all MCHR-expressing POMC neurons and that some of the infected POMC neurons likely did not express MCHR. This is in concordance with the aforementioned heterogeneity of POMC neurons. Overall, our findings conclusively suggest the key involvement of these neurons in MCH-mediated effects in the ARC.
In summary, our data highlight the relevance of the MCH system as a drug target and provide a new conceptual framework on the mechanisms by which MCH modulates food intake, adipocyte lipid storage, and glucose intolerance via a SIRT1/FoxO1 in the ARC. This mechanism requires POMC but not AgRP neurons and is essential for the activity of MCH inhibitors in obesity.
Supplementary Material
Article Information
Acknowledgments. The authors thank Manuel Serrano (Centro Nacional de Investigaciones Oncológicas, Madrid, Spain) for providing SIRT1-Tg mice and Jens Bruning (Max Planck Institute for Metabolism Research, Köln, Germany) and Eleftheria Maratos-Flier (Beth Israel Deaconess Medical Center, Boston, MA) for providing AgRP-Cre, POMC-CRE, and Mchr1-cre/tdTomato transgene mice and critical reading.
Funding. O.A.-M. is funded by Instituto de Salud Carlos III (ISCIII)/Servizo Galego de Saúde (SERGAS) through research contract “Sara Borrell” (CD14/00091). M.Q. is a recipient of a postdoctoral contract from the Galician Government (Xunta de Galicia ED481B2018/004). J.C. was supported by Marie Skłodowska-Curie Actions– European Research Fellowship (H2020-MSCA-IF-2014, ID656657) and Région Hauts-de-France (program VisionAIRR). This work was supported by grants from Ministerio de Economia y Competitividad (M.L.: SAF2015-71026-R, C.D.: BFU2017-87721, R.N.: BFU2015-70664R), Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (M.L.: 2015-CP079 and 2016-PG068, R.N.: 2015-CP080, 2016-PG057), Centro singular de investigación de Galicia accreditation (2016–2019, ED431G/05), the European Regional Development Fund (ERDF), Fundación Atresmedia (M.L. and R.N.), BBVA Foundation (R.N.), and the National Science Foundation of Hungary (OTKA K101326, K100722). K.R. is supported by the National Institutes of Health National Heart, Lung, and Blood Institute (HL-084207), American Heart Association (EIA#14EIA18860041), the Veterans Affairs (BX004249), and The University of Iowa Fraternal Order of Eagles Diabetes Research Center. CIBER de Fisiopatología de la Obesidad y Nutrición (CIBERobn) is an initiative of ISCIII of Spain, which is supported by funds from Fondo Europeo de Desenvolvemento Rexional.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. O.A.-M., M.Q., J.C., D.A.M., I.K., Z.L., and K.R. made the figures. O.A.-M., M.Q., R.H.B., A.R.-P., C.F., A.S., S.C.F., M.I., and D.B. performed in vivo experiments and Western blots and collected and analyzed the data. O.A.-M., M.Q., M.J.K., S.L., M.L., Z.L., K.R., V.P., C.D., and R.N. designed the experiments and discussed the manuscript. O.A.-M., M.Q., and R.N. wrote the manuscript. J.C. performed and analyzed electrophysiological recordings from brain slices. D.A.M. and K.R. performed and analyzed the sympathetic nerve activity recording studies. I.K., V.H., L.G.-C., R.G., and Z.L. performed the immunohistochemistry images. Y.S., M.F., and M.J.C. contributed to the development of the analytical tools, reagents, and discussion. B.Y.H.L. and G.Y. performed FACS and RNA sequencing. C.D. and R.N. coordinated and directed the project and developed the hypothesis. R.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0029/-/DC1.
M.J.C. is currently affiliated with the Department of Neuroscience, Carleton University, Ottawa, Ontario, Canada.
References
- 1.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: the multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev 2006;27:606–620 [DOI] [PubMed] [Google Scholar]
- 2.Kawauchi H, Abe K, Takahashi A, et al. . Isolation and properties of chum salmon prolactin. Gen Comp Endocrinol 1983;49:446–458 [DOI] [PubMed] [Google Scholar]
- 3.Qu D, Ludwig DS, Gammeltoft S, et al. . A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 1996;380:243–247 [DOI] [PubMed] [Google Scholar]
- 4.Ludwig DS, Tritos NA, Mastaitis JW, et al. . Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest 2001;107:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M, Ishihara A, Gomori A, et al. . Melanin-concentrating hormone 1-receptor antagonist suppresses body weight gain correlated with high receptor occupancy levels in diet-induced obesity mice. Eur J Pharmacol 2009;624:77–83 [DOI] [PubMed] [Google Scholar]
- 6.Shearman LP, Camacho RE, Sloan Stribling D, et al. . Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol 2003;475:37–47 [DOI] [PubMed] [Google Scholar]
- 7.Mashiko S, Ishihara A, Gomori A, et al. . Antiobesity effect of a melanin-concentrating hormone 1 receptor antagonist in diet-induced obese mice. Endocrinology 2005;146:3080–3086 [DOI] [PubMed] [Google Scholar]
- 8.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 1998;396:670–674 [DOI] [PubMed] [Google Scholar]
- 9.Segal-Lieberman G, Bradley RL, Kokkotou E, et al. . Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A 2003;100:10085–10090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci 2006;26:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokkotou E, Jeon JY, Wang X, et al. . Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol 2005;289:R117–R124 [DOI] [PubMed] [Google Scholar]
- 12.Jeon JY, Bradley RL, Kokkotou EG, et al. . MCH-/- mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes 2006;55:428–434 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Ziogas DC, Biddinger S, Kokkotou E. You deserve what you eat: lessons learned from the study of the melanin-concentrating hormone (MCH)-deficient mice. Gut 2010;59:1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira-da-Silva M, De Souza CT, Gasparetti AL, Saad MJ, Velloso LA. Melanin-concentrating hormone induces insulin resistance through a mechanism independent of body weight gain. J Endocrinol 2005;186:193–201 [DOI] [PubMed] [Google Scholar]
- 15.Kong D, Vong L, Parton LE, et al. . Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab 2010;12:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domingos AI, Sordillo A, Dietrich MO, et al. . Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife 2013;2:e01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imbernon M, Beiroa D, Vazquez MJ, et al. . Central melanin-concentrating hormone influences liver and adipose metabolism via specific hypothalamic nuclei and efferent autonomic/JNK1 pathways. Gastroenterology 2013;144:636–649.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature 1999;400:265–269 [DOI] [PubMed] [Google Scholar]
- 19.Marsh DJ, Weingarth DT, Novi DE, et al. . Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A 2002;99:3240–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Hu C, Hsu CK, et al. . Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology 2002;143:2469–2477 [DOI] [PubMed] [Google Scholar]
- 21.Chee MJ, Pissios P, Maratos-Flier E. Neurochemical characterization of neurons expressing melanin-concentrating hormone receptor 1 in the mouse hypothalamus. J Comp Neurol 2013;521:2208–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol 2001;435:26–40 [DOI] [PubMed] [Google Scholar]
- 23.Bittencourt JC. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endocrinol 2011;172:185–197 [DOI] [PubMed] [Google Scholar]
- 24.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology 1989;125:2056–2065 [DOI] [PubMed] [Google Scholar]
- 25.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull 1985;15:635–649 [DOI] [PubMed] [Google Scholar]
- 26.Guyon A, Conductier G, Rovere C, Enfissi A, Nahon JL. Melanin-concentrating hormone producing neurons: activities and modulations. Peptides 2009;30:2031–2039 [DOI] [PubMed] [Google Scholar]
- 27.Ludwig DS, Mountjoy KG, Tatro JB, et al. . Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol 1998;274:E627–E633 [DOI] [PubMed] [Google Scholar]
- 28.Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes 1998;47:1687–1692 [DOI] [PubMed] [Google Scholar]
- 29.Chaffer CL, Morris MJ. The feeding response to melanin-concentrating hormone is attenuated by antagonism of the NPY Y(1)-receptor in the rat. Endocrinology 2002;143:191–197 [DOI] [PubMed] [Google Scholar]
- 30.Nogueiras R, Habegger KM, Chaudhary N, et al. . Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 2012;92:1479–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol 2012;8:287–296 [DOI] [PubMed] [Google Scholar]
- 32.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 2010;5:253–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppari R. Metabolic actions of hypothalamic SIRT1. Trends Endocrinol Metab 2012;23:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toorie AM, Nillni EA. Minireview: central Sirt1 regulates energy balance via the melanocortin system and alternate pathways. Mol Endocrinol 2014;28:1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One 2009;4:e8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich MO, Antunes C, Geliang G, et al. . Agrp neurons mediate Sirt1's action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci 2010;30:11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramadori G, Fujikawa T, Fukuda M, et al. . SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 2010;12:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clasadonte J, Scemes E, Wang Z, Boison D, Haydon PG. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 2017;95:1365–1380.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imbernon M, Sanchez-Rebordelo E, Romero-Picó A, et al. . Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology 2016;64:1086–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beiroa D, Imbernon M, Gallego R, et al. . GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014;63:3346–3358 [DOI] [PubMed] [Google Scholar]
- 41.Quiñones M, Al-Massadi O, Gallego R, et al. . Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance. Mol Metab 2015;4:961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quiñones M, Al-Massadi O, Folgueira C, et al. . p53 in AgRP neurons is required for protection against diet-induced obesity via JNK1. Nat Commun 2018;9:3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez de Morentin PB, González-García I, Martins L, et al. . Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 2014;20:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imbernon M, Sanchez-Rebordelo E, Gallego R, et al. . Hypothalamic KLF4 mediates leptin’s effects on food intake via AgRP. Mol Metab 2014;3:441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, et al. . Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 2015;4:e07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan C, Zhou J, Feng Q, et al. . Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 2013;33:3624–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velásquez DA, Martínez G, Romero A, et al. . The central sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011;60:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cyr NE, Steger JS, Toorie AM, Yang JZ, Stuart R, Nillni EA. Central Sirt1 regulates body weight and energy expenditure along with the POMC-derived peptide α-MSH and the processing enzyme CPE production in diet-induced obese male rats. Endocrinology 2015;156:961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitamura T, Feng Y, Kitamura YI, et al. . Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 2006;12:534–540 [DOI] [PubMed] [Google Scholar]
- 50.Lam BYH, Cimino I, Polex-Wolf J, et al. . Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab 2017;6:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.