Abstract
Optogenetics is an emerging field, which uses light and molecular genetics to manipulate the activity of live cells by expressing light-sensitive proteins. With the discovery of bacteriorhodopsin, a light-sensitive bacterial protein, in 1971 Oesterhelt and Stoeckenius laid the pavement of optogenetics. However, the cross-integration of different disciplines is a little more than a decade old. The toolbox contains fluorescent sensors and optogenetic actuators that enable visualization of signaling events and manipulation of cellular activities, respectively. Neuropathic pain is pain caused either by damage or disease that affects the somatosensory system. The exact mechanism for neuropathic pain is not known, however proposed mechanisms include immune reactions, ion channel expressions, and inflammation. Current regimen for the disease provides about 50% relief for only 40–60% of patients. Recent in vivo and in vitro studies demonstrate the potential therapeutic applications of optogenetics by manipulating the activity of neurons. This review summarizes the basic concept, therapeutic applications for neuropathy, and potential of optogenetics to reach from bench to bedside in the near future.
Keywords: Neuropathic pain, optogenetics, treatment
INTRODUCTION
Neuropathic pain (NP) is pain caused either by damage or disease that affects the somatosensory nervous system centrally (multiple sclerosis, spinal cord injury, post-stroke, etc.) or peripherally (polyneuropathies related to diabetes, chemotherapy, alcoholism, infection, etc.) [1]. Patients suffering from neuropathy present with lancinating, shooting, and/or burning pain along with tingling sensation, which substantially impairs their quality of life [2,3]. A single etiology or specific lesion cannot explain this heterogeneous set of conditions. A multidisciplinary approach to treatment is often the most successful, which includes drugs (antidepressant and antiepileptic drugs), anesthetic techniques, and surgical interventions (in patients with refractory NP) [4,5]. Opioids have been considered a good choice for treatment; however, they fail to reduce the pain and are controversial [6]. Other drugs include acetyl-L-carnitine (ALC), alpha-lipoic-acid (ALA), cannabis products, botulinum toxin, and angiotensin type 2 receptor antagonists [5,6]. A precise treatment regimen is still not available; however, blockers of voltage-gated sodium channels and of the alpha-2-delta-1 subunit of calcium channels demonstrate a significant therapeutic potential [7,8]. Moreover, these centrally acting drugs are lipophilic and nonspecific with a narrow therapeutic window, thus restraining their prolonged use in pain management. Considering the pitfalls of conventional neuropathy treatments, a novel approach to the treatment of chronic pain is required, which would regulate the excitability of nociceptors. The importance of nociceptors, immune cells, and G protein-coupled receptors (GPCRs) is well established in the pathology of NP [2,3].
In general, new technical advancements and interdisciplinary treatment methods offer more therapeutic effectiveness. One such interdisciplinary approach is optogenetics. Optogenetics is a versatile technique in which engineers, clinicians, and basic science researchers converge their knowledge of genetics and optics to achieve gain or loss of function in specific cells of living tissue. Besides in vitro and in vivo manipulation of neuronal excitability and network activity, various optogenetic tools make it possible to target intracellular processes in particular organelles [9]. This light-sensitive technology has revolutionized the study of neuroscience with single-cell and millisecond-precision control of neurons [9] in living systems. This review article discusses the scientific landscape of the decade-old technique in NP.
BASIC CONCEPT OF OPTOGENETICS
As the name suggests, optogenetics is the integration of two fields, i.e., optics and genetics. The optic part is associated with illumination of a specific light spectrum, whereas the genetic part is associated with the expression of a modified opsin protein in cells. The basic concept of optogenetics is to manipulate cellular activity by transducing electrical currents generated by light-sensitive proteins [10,11]. Figure 1 shows the three major optogenetic tools, which include: 1) light-activated proteins, 2) light, and 3) mode of delivery (important for both of the first two tools).
FIGURE 1.
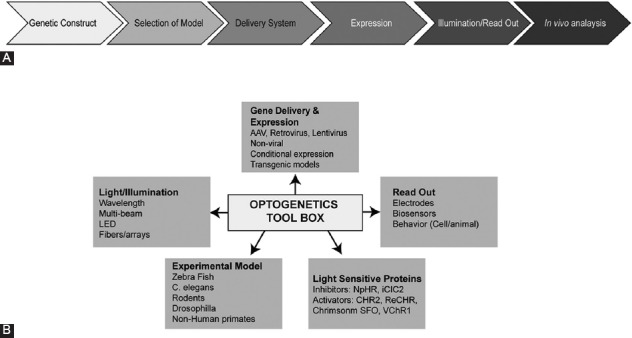
Flowchart of (A) experimental steps and (B) various tools in optogenetics.
Opsins capture light and use it to either actively pump ions across the cell membrane or to open the channels, which allows the passive flow of ions across the cell membrane. These proteins are ubiquitously present in all organisms, including eukaryotes and prokaryotes. Engineering the expression of opsins in non-light-sensitive cells enables rapid optical control of specific cellular processes. Therefore, by applying the optogenetic approach a rapid activation and silencing of expressed proteins can be achieved with no use of chemicals [9,11]. Microbial opsins, an important class of light-sensitive proteins, are categorized based on membrane potential. Channelrhodopsins (ChRs) from Chlamydomonas reinhardtii depolarize the neuronal membrane with light, whereas the second class of opsins, halorhodopsin from Natronomonas pharaonis (NpHR), causes hyperpolarization. Proton pumps such as bacteriorhodopsin, proteorhodopsin, and archaerhodopsin also promote the hyperpolarization of the neuronal membrane by extruding protons from the cytoplasm. The co-expression of depolarizing and hyperpolarizing opsins due to varying illumination patterns allows creating a bidirectional channel in cells [10,12]. Chimeric receptors that mimic various signaling cascades represent another class of light-activated opsins. These include light-activated membrane-integrated GPCRs (e.g., OptoXR) and hybrid receptors with the extracellular domain of rhodopsin and the intracellular domain of specific adrenergic receptors (AR) such as opto-α1-AR (Gq-coupled human α1A-AR) and opto-β2-AR (Gs-coupled hamster β2-AR) [10,12,13]. Properties like i) ion specificity, ii) subcellular localization, iii) sensitivity to light, iv) fast kinetics, v) possibility for bidirectional control, and vi) simple structure make opsins an ideal tool in the field of optogenetics.
The second tool in the optogenetic toolbox is the light wavelength to activate a specific opsin. The differential wavelength specificity and sensitivity of opsins allow the co-expression of different proteins in one cell and make simultaneous activation and deactivation feasible. Therefore, two different proteins can be used antagonistically or synergistically. For example, ChR2 is activated by ~460 nm light and NpHR is activated by ~570 nm light. Engineered opsins with red-shifted activation wavelengths make it easy to deliver the proteins to challenging sites, such as deep in the brain, with minimal invasiveness. Red-shifted activation wavelengths help reducing the light scattering and, therefore, allow deeper light penetration. ChRs are light-gated, non-specific cation channels, activated by blue-green wavelength from 450 nm to 545 nm [14]. Such blue-green wavelengths fail to penetrate neural tissue as they are absorbed by flavins, hemoglobin, and melanin. Furthermore, blue-green wavelengths scatter more strongly than yellow-red wavelengths. To overcome this, red-shifted ChRs with spectral peaks near or above 600 nm are engineered, which include the cation channels (activation wavelengths) as follows: red-activatable ChR (ReaChR) (~590–630); ChR-1/Volvox (C1V1; E122T mutation; ChR1/VChR1 chimera) [~600 nm]; Chrimson (~660 nm); fast red-activatable ChR (bReaChES) [~590 nm]; Volvox ChR (VChR1; similar to ChR-1) [~531 nm], and the chloride pump jaws (~635 nm). Moreover, the ChR2 gene has been modified to produce a “bistable” channel also known as step-function opsin (SFO). This channel is opened by one wavelength and closed by a different wavelength [9,15].
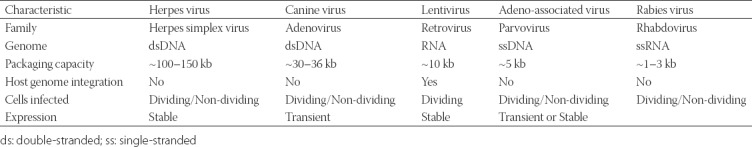
Due to the blood–brain barrier (BBB), the delivery of proteins to the brain is difficult. One of the delivery methods is the transfection of lentivirus or adeno-associated virus (AAV) that is packaged with an opsin gene through direct brain infusion. This viral-mediated gene delivery system is one of the most commonly used methods. Table 1 summarizes the properties of viruses used in optogenetics. Viruses are ideal vehicles for gene delivery as they i) naturally penetrate into host cells and ii) exploit the host transcription machinery for the expression of viral genes. Another method is the use of transgenic mice to create a uniform expression of an opsin in a specific group of cells; however, this method is limited by weak transcriptional promoter activity in some cells, making it difficult to express opsins in a cell-specific manner. Even though these gene-based methods are noninvasive, they are limited by a high maintenance cost and significant time required for the development of animal models. Transcranial focused ultrasound (FUS) technology, a noninvasive method targeting the BBB, is another technology used for the delivery of opsins. This technique involves a systemic injection of a mixture of lipid-based ultrasound contrast agents and opsins [16,17].
TABLE 1.
Commonly used viral delivery systems in optogenetics
The delivery of light into deep brain regions in a controlled fashion is another challenge in optogenetics. For in vitro experiments, a laser or light-emitting diode (LED) can be directly coupled to a microscope. Tapered optical fibers (TOFs), which are smaller compared to flat-cleaved optical fibers, are the best to deliver light over large brain volumes or spatially confined sub-regions [9]. Direct implantation of small LED bulbs in the thinned skull above the target region can be used for the stimulation of superficial layers of the cortex. Because the light density drops to as low as 1% with 1 mm tissue penetration, sensitive methods for penetrating the tissues were developed [18]. For example, fiber-optic-based interface with LED or laser diode systems coupled to lightweight flexible optic fibers that deliver light to deeper brain tissue was developed. Coupled with optogenetic technology, this method allows to simultaneously stimulate and record activity in specific groups of neurons in freely moving animals. Recent advances in light delivery, like implementation of closed-loop circuits and optrodes, allow wireless light stimulation and real-time control, therefore, simulating more natural environment [19,20].
OPTOGENETICS IN NEUROPATHY
Optogenetics offers selective activation with a millisecond temporal precision compared to electrical stimulation, which is more generalized and non-selective. Such light-sensitive cellular stimulation eliminates the crucial step of placing the electrodes in the brain that has a relatively homogeneous group of neurons [21]. Although initial in vivo studies have been performed in transgenic mouse models, most of them were restricted to in vitro preparations and were not applied to freely moving animals. In an early study, Wang and Zylka demonstrated the power of optogenetics in causal dissection of pain circuitry [22]. They showed functional connectivity in the substantia gelatinosa layer in a transgenic mouse line that expressed a stimulatory opsin in a nociceptor subtype expressing Mas-related GPCR member D [22]. In another study, Daou et al. [23] demonstrated the role of optogenetics in pain inhibition using a binary genetic approach, to deliver ChR2 channels to peripheral nociceptors in Nav1.8-Cre transgenic mouse line. In their experiment, the pain was elicited noninvasively and remotely in animals (without the implantation of optical devices) [23]. AAV serotype 6 expressing ChR protein successfully controlled NP in vivo. AAV virus has advantage compared to other vectors [24]. In vivo stimulation of yellow light-sensitive third-generation chloride pump halorhodopsin (eNpHR3.0) channel successfully prevented pain in a NP model and, therefore, highlighted the therapeutic potential of optogenetics [25,26].
The real challenge in optogenetics is to pinpoint damaged nociceptive neurons. Therefore, understanding the genetics in pathological neurons compared to normal functioning neural circuits is pertinent. A complete transcriptome of the trigeminal ganglia (TG) and the dorsal root ganglia (DRG) of adult mice has been analyzed to get an insight into the expression of ion channels and GPCRs in physiological and pathophysiological conditions [27-29]. This analysis revealed that the conventional strategies, which inhibit individual ion channels or inflammatory processes, would not be as useful given the complex etiology of pathological changes in ganglia.
Because NP affects not only patients but also their families, there is an urgent need to find the treatment. Researchers are developing alternative treatment approaches like designer receptors exclusively activated by a designer drug (DREADD). Synthetic ligands such as clozapine N-oxide (CNO) manage NP by activating these designer receptors. Except the modulation by synthetic chemicals and not by endogenous ligands, DREADDs behave similar to endogenous GPCRs and, therefore, interact efficiently with downstream intracellular signaling components [30-32]. Due to the similarities to GPCRs, testing how DREADDs affect the GPCR downstream signaling is encouraged.
The light-sensitive proteins are generally introduced to targeted mammalian neurons using viral gene delivery approaches [12,33]. However, the limitations of the viral vector delivery system in humans are well known [33,34]. A newer noninvasive technology, transcranial FUS, targets the BBB by systemic injection of lipid-based microbubbles (a mixture composed of ultrasound contrast agents) containing molecules of interest [17,35,36]. Considering the noninvasive nature of the FUS technique, viral vectors encoding various light-activated protein channels can be delivered in vivo, allowing an entirely noninvasive neural stimulation.
Optogenetics allows us to manipulate and monitor the neuronal circuit functions, and it has been successfully implicated in behavioral studies including sensory perception [31], pain [37], and social interactions [38]. A recent advancement in optogenetics has revealed that specific components of higher brain functions can neither be linked to single transmitter systems nor to specific brain locations [1,39], suggesting that a multitasking optogenetic sensor has to be designed. With these advancements in the field of optogenetics and promising results from recent experiments in non-human primates [40,41], we anticipate that the therapeutic application of optogenetics will be available in clinics soon.
Spatially targeted, genetic strategies for sustained inhibition of nociceptors are an example of the translation of laboratory research into the clinic. A recent report demonstrated the advantage of a step-function inhibitory ChR, named SwiChR, over the normal ChR by overcoming the required constant illumination [26]. SwiChR inhibits pain for long periods of time with infrequent transdermal delivered light pulses, reducing the required light exposure by >98% and, therefore, resolving a long-standing limitation in optogenetic inhibition [26,37,38].
OPTOGENETICS: BEYOND NEUROSCIENCE
Although first introduced in neuroscience, optogenetics has been applied to other fields as well, including cardiovascular research, cancer research, and hepatology. Recent developments in stem cell research demonstrate a potential of a combined approach for the treatment of different diseases. The approach combining optogenetics and stem cells has opened up treatment strategies for neurodegenerative and cardiovascular diseases. Engineered stem cells expressing exogenous light-activated opsins have been used in recent years. A functional analysis of human embryonic stem cells (ESC)-derived neurons in a Parkinson’s disease mouse model that was implanted with a fiber-optic cannula revealed that light induces selective silencing of transplanted neurons in mice [42]. Bruegmann et al. demonstrated the induction of optically derived currents using genetically modified mouse ECS-derived cardiomyocytes expressing ChR2 variant [43]. Some of the studies have also used hESC for the activation of cardiomyocytes by optogenetics [44,45]. In addition, optogenetics has been widely studied in other research fields such as diabetes research [46], immunotherapy [47], retinal gene therapy, and ophthalmology [48].
CONCLUDING REMARKS AND FUTURE PROSPECTIVE
Chronic pain is one of the major health issues worldwide. Besides affecting the patient’s functionality, it also impacts the patient’s family; therefore, the treatment of such disorders would be beneficial for both. Selective targeting of neuronal systems enables the inhibition of chronic pain. Future studies should aim to delineate the influence of an individual’s genotype on the predisposition to pain chronicity and the response to therapeutic treatments, considering that the sensitivity to painful stimuli and the response to drugs vary from one person to another. In addition, a better understanding of epigenetic regulation of the disease could also lead to innovations and, therefore, should be among the major focuses of future therapeutic approaches.
Although optogenetics is an exciting approach for future treatment of chronic pain, there are still many unanswered questions. For example, it is not clear if the treatment with light will be short-term or long-term? Furthermore, what would be the appropriate treatment regimen in terms of dosage? Another concern is what the route of administration would be and how light-sensitive proteins will be introduced in human subjects. As stated earlier, the major obstacle is the controlled delivery of light to cells; however, recent studies have shown some progress in combating with this problem, using microscopy-based cell analyses and flow cytometry [16,49]. Another powerful approach represents a technique that combines optogenetics and the clustered regularly interspaced short palindromic repeats (CRISPR)–Cas system. These technologies will facilitate testing of optogenetic tools in various cellular models to attain a proof of principle.
RetroSense Therapeutics (now acquired by Allergan) delivered the first-in-human optogenetic therapy for advanced retinitis pigmentosa, which was followed by RPE65 rAAV by Spark therapeutics, an FDA-approved therapy for retinal dystrophy in December 2017 [50]. In such conditions, photoreceptors in the retina gradually die off, which eventually leads to blindness. The RetroSense therapy RST-001 delivers an adenovirus virus containing the ChR2 gene in the eye of patients to create new photosensors in retinal cells. This clinical trial is in Phase I/II and is still enrolling the subjects (https://clinicaltrials.gov/ct2/home). Till date, no results have been revealed from this clinical trial. The exact type of vision which will be generated by modified retinal cells remains unclear because a diverse group of retinal ganglion cells are stimulated.
Gensight Biologics (https://www.gensight-biologics.com) designed a “biomimetic goggles”, which consist of a camera, microprocessor, and a digital micromirror array. This device stimulates the modified retinal cells by amplifying light signals. The company has recently treated the first subject in the first-in-man PIONEER Phase I/II clinical trial of GS030 at the Moorfields Eye Hospital in London, United Kingdom. GS030 combines a gene therapy (GS030-DP) administered through a single intravitreal injection with a wearable optronic visual stimulation device [GS030-MD] (https://www.gensight-biologics.com). The final results of this study are expected in the fourth quarter of 2020. In addition to this, Gensight Biologics is developing a treatment for geographic atrophy in dry age-related macular degeneration (AMD) using the optogenetics approach. Companies like Circuit Therapeutics (http://www.circuittx.com) and Bionic Sight (https://agtc.com/programs/bionic-sight/) are also developing new optogenetic strategies to understand the cause and treat different neurological diseases. Recently, optoPAIN, an optogenetic platform to noninvasively assess changes in pain sensitivity, has been developed and used to examine pharmacological and chemogenetic inhibition of pain [48]. Such techniques and developments hold the promise to see optogenetics in clinical setting in the near future [51,52].
The pathophysiology of NP remains enigmatic. Structural modifications and activation of nociceptors in neurons generate a high action potential and transmit signals through already existing pain transmission network. Using optogenetic tools, neuronal circuit function can be manipulated and monitored, and therefore, a therapeutic approach combining optogenetics and gene therapy can be explored in the near future.
ACKNOWLEDGMENTS
We would like to thank Science Pen Group for helping in proofreading the article.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Song C, Knöpfel T. Optogenetics enlightens neuroscience drug discovery. Nat Rev Drug Discov. 2016;15:97–109. doi: 10.1038/nrd.2015.15. https://doi.org/10.1038/nrd.2015.15. [DOI] [PubMed] [Google Scholar]
- 2.Baron R. Mechanisms of disease:Neuropathic pain - a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. https://doi.org/10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SP, Mao J. Neuropathic pain:Mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. https://doi.org/10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 4.Pessoa BL, Escudeiro G, Nascimento OJ. Emerging treatments for neuropathic pain. Curr Pain Headache Rep. 2015;19:56. doi: 10.1007/s11916-015-0530-z. https://doi.org/10.1007/s11916-015-0530-z. [DOI] [PubMed] [Google Scholar]
- 5.Schestatsky P, Vidor L, Winckler PB, Araújo TG, Caumo W. Promising treatments for neuropathic pain. Arq Neuropsiquiatr. 2014;72:881–8. doi: 10.1590/0004-282x20140157. https://doi.org/10.1590/0004-282X20140157. [DOI] [PubMed] [Google Scholar]
- 6.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults:A systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73. doi: 10.1016/S1474-4422(14)70251-0. https://doi.org/10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczudlik A, Dobrogowski J, Wordliczek J, Stępień A, Krajnik M, Leppert W, et al. Diagnosis and management of neuropathic pain:Review of literature and recommendations of the Polish Association for the Study of Pain and the Polish Neurological Society part one. Neurol Neurochir Pol. 2014;48:262–71. doi: 10.1016/j.pjnns.2014.07.011. https://doi.org/10.1016/j.pjnns.2014.07.011;https://doi.org/10.1016/j.pjnns.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento OJ, Pessoa BL, Orsini M, Ribeiro P, Davidovich E, Pupe C, et al. Neuropathic pain treatment:Still a challenge. Neurol Int. 2016;8:6322. doi: 10.4081/ni.2016.6322. https://doi.org/10.4081/ni.2016.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rost BR, Schneider-Warme F, Schmitz D, Hegemann P. Optogenetic tools for subcellular applications in neuroscience. Neuron. 2017;96:572–603. doi: 10.1016/j.neuron.2017.09.047. https://doi.org/10.1016/j.neuron.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, et al. Structural foundations of optogenetics:Determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci U S A. 2016;113:822–9. doi: 10.1073/pnas.1523341113. https://doi.org/10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyden ES. Optogenetics and the future of neuroscience. Nat Neurosci. 2015;18:1200–1. doi: 10.1038/nn.4094. https://doi.org/10.1038/nn.4094;https://doi.org/10.1038/nn1215-1862b. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–57. doi: 10.1016/j.cell.2011.12.004. https://doi.org/10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015;33:92–100. doi: 10.1016/j.tibtech.2014.11.007. https://doi.org/10.1016/j.tibtech.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–5. doi: 10.1073/pnas.1936192100. https://doi.org/10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–46. doi: 10.1038/nmeth.2836. https://doi.org/10.1038/nmeth0914-971d;https://doi.org/10.1038/nmeth0914-972;https://doi.org/10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Tang S, Wan Z, Gao Y, Cao Y, Yi J, et al. Utilization of a photoactivatable antigen system to examine B-cell probing termination and the B-cell receptor sorting mechanisms during B-cell activation. Proc Natl Acad Sci U S A. 2016;113:E558–67. doi: 10.1073/pnas.1517612113. https://doi.org/10.1073/pnas.1517612113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Kugelman T, Buch A, Herman M, Han Y, Karakatsani ME, et al. Non-invasive, focused ultrasound-facilitated gene delivery for optogenetics. Sci Rep. 2017;7:39955. doi: 10.1038/srep39955. https://doi.org/10.1038/srep39955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharf R, Tsunematsu T, McAlinden N, Dawson MD, Sakata S, Mathieson K, et al. Depth-specific optogenetic control in vivo with a scalable, high-density µLED neural probe. Sci Rep. 2016;6:28381. doi: 10.1038/srep28381. https://doi.org/10.1038/srep28381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo V, Ventalon C, De Sars V, Bradley J, Emiliani V. Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope. Neuron. 2014;84:1157–69. doi: 10.1016/j.neuron.2014.11.005. https://doi.org/10.1016/j.neuron.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon-Turcotte G, LeChasseur Y, Bories C, Messaddeq Y, De Koninck Y, Gosselin B, et al. A wireless headstage for combined optogenetics and multichannel electrophysiological recording. IEEE Trans Biomed Circuits Syst. 2017;11:1–4. doi: 10.1109/TBCAS.2016.2547864. https://doi.org/10.1109/TBCAS.2016.2547864. [DOI] [PubMed] [Google Scholar]
- 21.Gu L, Uhelski ML, Anand S, Romero-Ortega M, Kim YT, Fuchs PN, et al. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS One. 2015;10:e0117746. doi: 10.1371/journal.pone.0117746. https://doi.org/10.1371/journal.pone.0117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–9. doi: 10.1523/JNEUROSCI.3248-09.2009. https://doi.org/10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci. 2013;33:18631–40. doi: 10.1523/JNEUROSCI.2424-13.2013. https://doi.org/10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee GH, Kim SS. Therapeutic strategies for neuropathic pain:Potential application of pharmacosynthetics and optogenetics. Mediators Inflamm 2016. 2016:5808215. doi: 10.1155/2016/5808215. https://doi.org/10.1155/2016/5808215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain. 2009;5:52. doi: 10.1186/1744-8069-5-52. https://doi.org/10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer SM, Vesuna S, Ramakrishnan C, Huynh K, Young S, Berndt A, et al. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep. 2016;6:30570. doi: 10.1038/srep30570. https://doi.org/10.1038/srep30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, et al. Comprehensive RNA-seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in trigeminal ganglia. PLoS One. 2013;8:e79523. doi: 10.1371/journal.pone.0079523. https://doi.org/10.1371/journal.pone.0079523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawes JM, Antunes-Martins A, Perkins JR, Paterson KJ, Sisignano M, Schmid R, et al. Genome-wide transcriptional profiling of skin and dorsal root ganglia after ultraviolet-B-induced inflammation. PLoS One. 2014;9:e93338. doi: 10.1371/journal.pone.0093338. https://doi.org/10.1371/journal.pone.0093338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins JR, Antunes-Martins A, Calvo M, Grist J, Rust W, Schmid R, et al. A comparison of RNA-seq and exon arrays for whole genome transcription profiling of the L5 spinal nerve transection model of neuropathic pain in the rat. Mol Pain. 2014;10:7. doi: 10.1186/1744-8069-10-7. https://doi.org/10.1186/1744-8069-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrell MS, Roth BL. Pharmacosynthetics:Reimagining the pharmacogenetic approach. Brain Res. 2013;1511:6–20. doi: 10.1016/j.brainres.2012.09.043. https://doi.org/10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–83. doi: 10.1038/nature11312. https://doi.org/10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs):Chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. https://doi.org/10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- 33.Parr-Brownlie LC, Bosch-Bouju C, Schoderboeck L, Sizemore RJ, Abraham WC, Hughes SM, et al. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Front Mol Neurosci. 2015;8:14. doi: 10.3389/fnmol.2015.00014. https://doi.org/10.3389/fnmol.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sizemore RJ, Seeger-Armbruster S, Hughes SM, Parr-Brownlie LC. Viral vector-based tools advance knowledge of basal ganglia anatomy and physiology. J Neurophysiol. 2016;115:2124–46. doi: 10.1152/jn.01131.2015. https://doi.org/10.1152/jn.01131.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci U S A. 2011;108:16539–44. doi: 10.1073/pnas.1105116108. https://doi.org/10.1073/pnas.1105116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly MA, Waspe AC, Ganguly M, Hynynen K. Focused-ultrasound disruption of the blood-brain barrier using closely-timed short pulses:Influence of sonication parameters and injection rate. Ultrasound Med Biol. 2011;37:587–94. doi: 10.1016/j.ultrasmedbio.2011.01.008. https://doi.org/10.1016/j.ultrasmedbio.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol. 2014;32:274–8. doi: 10.1038/nbt.2834. https://doi.org/10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. https://doi.org/10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lammel S, Tye KM, Warden MR. Progress in understanding mood disorders:Optogenetic dissection of neural circuits. Genes Brain Behav. 2014;13:38–51. doi: 10.1111/gbb.12049. https://doi.org/10.1111/gbb.12049. [DOI] [PubMed] [Google Scholar]
- 40.Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, et al. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci. 2016;19:37–9. doi: 10.1038/nn.4192. https://doi.org/10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–32. doi: 10.1038/nmeth.2935. https://doi.org/10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 42.Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat Biotechnol. 2015;33:204–9. doi: 10.1038/nbt.3124. https://doi.org/10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. https://doi.org/10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 44.Nussinovitch U, Gepstein L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat Biotechnol. 2015;33:750–4. doi: 10.1038/nbt.3268. https://doi.org/10.1038/nbt.3268. [DOI] [PubMed] [Google Scholar]
- 45.Nussinovitch U, Shinnawi R, Gepstein L. Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins. Cardiovasc Res. 2014;102:176–87. doi: 10.1093/cvr/cvu037. https://doi.org/10.1093/cvr/cvu037. [DOI] [PubMed] [Google Scholar]
- 46.Kushibiki T, Okawa S, Hirasawa T, Ishihara M. Optogenetic control of insulin secretion by pancreatic β-cells in vitro and in vivo. Gene Ther. 2015;22:553–9. doi: 10.1038/gt.2015.23. https://doi.org/10.1038/gt.2015.23. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Hyun YM, Lim K, Lee H, Cummings RJ, Gerber SA, et al. Optogenetic control of chemokine receptor signal and T-cell migration. Proc Natl Acad Sci U S A. 2014;111:6371–6. doi: 10.1073/pnas.1319296111. https://doi.org/10.1073/pnas.1319296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobson SG, Sumaroka A, Luo X, Cideciyan AV. Retinal optogenetic therapies:Clinical criteria for candidacy. Clin Genet. 2013;84:175–82. doi: 10.1111/cge.12165. https://doi.org/10.1111/cge.12165. [DOI] [PubMed] [Google Scholar]
- 49.Brenker K, Osthof K, Yang J, Reth M. LED thermo flow - combining optogenetics with flow cytometry. J Vis Exp. 2016;118:e54707. doi: 10.3791/54707. https://doi.org/10.3791/54707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maimon BE, Diaz M, Revol ECM, Schneider AM, Leaker B, Varela CE, et al. Optogenetic peripheral nerve immunogenicity. Sci Rep. 2018;8:14076. doi: 10.1038/s41598-018-32075-0. https://doi.org/10.1038/s41598-018-32075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukerjee S, Lazartigues E. Next-generation tools to study autonomic regulation in vivo. Neurosci Bull. 2019;35:113–23. doi: 10.1007/s12264-018-0319-2. https://doi.org/10.1007/s12264-018-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song YH, Agrawal NK, Griffin JM, Schmidt CE. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev. 2018;12:30315–6. doi: 10.1016/j.addr.2018.12.011. https://doi.org/10.1016/j.addr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


