Abstract
Aims
Stroke is often a devastating event among patients with heart failure with reduced ejection (HFrEF). In COMMANDER HF, rivaroxaban 2.5 mg b.i.d. did not reduce the composite of first occurrence of death, stroke, or myocardial infarction compared with placebo in patients with HFrEF, coronary artery disease (CAD), and sinus rhythm. We now examine the incidence, timing, type, severity, and predictors of stroke or a transient ischaemic attack (TIA), and seek to establish the net clinical benefit of treatment with low-dose rivaroxaban.
Methods and results
In this double-blind, randomized trial, 5022 patients who had HFrEF(≤40%), elevated natriuretic peptides, CAD, and who were in sinus rhythm were treated with rivaroxaban 2.5 mg b.i.d. or placebo in addition to antiplatelet therapy, after an episode of worsening HF. The primary neurological outcome for this post hoc analysis was time to first event of any stroke or TIA. Over a median follow-up of 20.5 (25th–75th percentiles 20.0–20.9) months, 150 all-cause stroke (127) or TIA (23) events occurred (ischaemic stroke in 82% and haemorrhagic stroke in 11% of stroke events). Overall, 47.5% of first-time strokes were either disabling (16.5%) or fatal (31%). Prior stroke, low body mass index, geographic region, and the CHA2DS2-VASc score were predictors of stroke/TIA. Rivaroxaban significantly reduced the primary neurological endpoint of all-cause stroke or TIA compared with placebo by 32% (1.29 events vs. 1.90 events per 100 patient-years), adjusted for the time from index HF event to randomization and stratified by geographic region (adjusted hazard ratio 0.68, 95% confidence interval 0.49–0.94), with a number needed to treat of 164 patients per year to prevent one stroke/TIA event. The principal safety endpoint of fatal bleeding or bleeding into a critical space, occurred at a similar rate on rivaroxaban and placebo (0.44 events vs. 0.55 events per 100 patient-years).
Conclusions
Patients with HFrEF and CAD are at risk for stroke or TIA in the period following an episode of worsening heart failure in the absence of atrial fibrillation. Most strokes are of ischaemic origin and nearly half are either disabling or fatal. Rivaroxaban at a dose of 2.5 mg b.i.d. reduced rates of stroke or TIA compared with placebo in this population.
Trial Registration
COMMANDER HF (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure); ClinicalTrials.gov NCT01877915.
Keywords: Heart failure, Oral anticoagulation, Stroke, Thrombotic, Transient ischaemic attack
Introduction
Stroke is a devastating occurrence in patients with heart failure with reduced ejection (HFrEF).1,2 The sequelae of stroke include a marked decline in health-related quality of life, higher healthcare utilization, and increased cost of care.3 Although atrial fibrillation (AF) has been the traditional target population for stroke risk reduction, patients with HF and sinus rhythm face elevated risk of stroke compared with the general population.4,5 Important gaps exist in our contemporary understanding of stroke risk in this unique population, since prior studies used historical information, relied on administrative claims data, and did not include patients on current guideline-mandated medical therapies.6
Since stroke as an endpoint has been challenging to study or safely modify in HFrEF and sinus rhythm,7–10 contemporary guidelines do not support a routine strategy of anticoagulation in patients with HFrEF in the absence of AF or other compelling indication.11,12 Non-vitamin K antagonist oral anticoagulants (NOACs) are approved for use in patients with AF or in the treatment or prevention of venous thromboembolism. COMMANDER HF (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure) did not demonstrate significant reduction in the composite primary endpoint of death, myocardial infarction, or stroke with addition of rivaroxaban at a dose of 2.5 mg b.i.d. compared with placebo in patients with HFrEF, coronary artery disease (CAD), and sinus rhythm receiving antiplatelet therapy and standard HF therapy.13 However, rivaroxaban did appear to reduce risk of stroke (a component of the primary endpoint).13
In this post hoc analysis of COMMANDER HF, among patients after a recent episode of worsening chronic HFrEF, sinus rhythm, and CAD, we set out to comprehensively explore (i) the incidence, timing, type, and severity of stroke or a transient ischaemic attack (TIA); (ii) clinical predictors of the occurrence of stroke or TIA; and (iii) the net clinical benefit of treatment with low-dose rivaroxaban compared with placebo on prevention of stroke or TIA.
Methods
COMMANDER HF trial
The design14 and primary findings13 of COMMANDER HF have been previously described. In brief, COMMANDER HF was a global, multicentre, double-blind, randomized clinical trial that evaluated the safety and efficacy of rivaroxaban compared with placebo among patients with chronic HFrEF (≤40%) with recent episode of worsening HF within 21 days and underlying CAD. Participants were randomized 1:1 to receive low-dose rivaroxaban 2.5 mg b.i.d. or matching placebo, in addition to standard care at the discretion of the treating physician. Subjects that had prior stroke within 90 days of randomization were excluded. The COMMANDER HF study protocol was approved by the ethics committees/institutional review boards of each participating site and all participants provided written informed consent for participation. The study complied with the Declaration of Helsinki.
Stroke, transient ischaemic attack, and safety
Study visits occurred at 4 weeks, 12 weeks, and every 12 weeks thereafter to determine safety and efficacy endpoints. Investigators determined key events using dedicated case report forms based on explicit event definitions and criteria. Available source documentation was reviewed by the local trial monitor and transmitted to the sponsor for independent confirmatory review using protocol-specified criteria (Supplementary material online, Table S1).
The primary neurological outcome for the present post hoc analysis is time to first all-cause stroke or TIA, defined as new, sudden, focal neurological deficit resulting from a presumed cerebrovascular cause without another identifiable cause after the study randomization. If neurological deficits lasted longer than 24 h, a stroke definition was met; if it lasted less than 24 h, a TIA was diagnosed. Strokes were further categorized based on available imaging as ischaemic, haemorrhagic, subarachnoid, or uncertain. A Modified Rankin Scale (mRS), a validated metric to determine stroke-related disability that categorizes stroke severity on a scale of 0–6 with higher scores denoting more disability (mRS 0–5) and ultimately death (mRS 6),15 was obtained between 6 and 18 weeks following a first or recurrent stroke or at the end of study, whichever occurred first.
The principal safety outcome was fatal bleeding or bleeding into a critical space with a potential for causing permanent disability, which was also a site-adjudicated event. Risk of events is described using incidence rates (events per 100 patient-years of observation).
Statistical analysis
All patients included in the intention-to-treat analytic set (randomized participants with signed valid informed consent) were assessed in this post hoc analysis of stroke or TIA events which were characterized by timing, type, and severity. The timing of stroke/TIA was calculated by adding the time from worsening HF episode (index event) to randomization, to the time from randomization to incident stroke/TIA during follow-up, or until the global trial end date (GTED). The time-course of stroke risk was described from the time of the index episode of worsening HF only in the placebo arm. The incremental incidence rate and its 95% confidence interval for each time segment were derived using the bootstrap method (10 000 resamples) and Kaplan–Meier cumulative risk estimates.
Baseline clinical profiles of those experiencing any stroke/TIA during the follow-up duration (up-to-GTED) were compared with patients who were free from stroke/TIA during the study. Given significant treatment effects of rivaroxaban, a risk prediction model for the stroke or TIA event was built among patients in the placebo arm alone. Pre-specified variables5,16 which were tested in stroke or TIA prediction models included: age, geographic region, race, body mass index, New York Heart Association classification, timing from episode of worsening HFrEF, prior stroke, hypertension, diabetes mellitus, and ejection fraction. Univariate and multivariate analyses were performed. Final model discrimination was determined using the concordance statistic (C-statistic). We quantified optimism in model estimates of C-statistics using a bootstrap resampling approach. Optimism estimates, averaged across 100 bootstrap samples, were subtracted from the naïve estimate of model discrimination. The percentile-corrected interval of the C-statistic was calculated by subtracting the 2.5 and 97.5 percentiles of optimism estimates from the naïve estimates.
To account for death as a competing risk event, the similar model selection process was repeated using a covariate-adjusted proportional sub-distribution hazard model (Fine and Gray17) to identify key independent predictors of stroke or TIA. This competing risk regression model identified similar predictors as the Cox proportional hazards model, and as such the latter approach is presented for simplicity. An established score for AF (CHA2DS2-VASc) was also assessed in risk prediction of stroke/TIA.
The overall treatment effects were determined by Cox proportional hazards models, accounting for time from index HF event to randomization as a covariate and stratified by geographic region. We performed interaction analyses to determine if the efficacy and safety of rivaroxaban was modified by baseline dual antiplatelet therapy or the CHA2DS2-VASc score.
Incidence rates of first stroke or TIA across treatment arms were estimated using Kaplan–Meier analyses. Incidence rates of each subtype of stroke and TIA were described by treatment arm. Given the post hoc nature of this analysis and focus on an individual component of the primary composite endpoint of COMMANDER HF, treatment effects were further adjusted for key selected covariates (as described above).
The principle safety outcomes were assessed using a similar Cox proportional hazards models without adjusting for time from index HF event to randomization for the on-treatment period, defined as the observation period from the first dose of the study drug to 2 days after the last dose of the study drug.
The number needed to treat (NNT) to prevent 1 primary neurological outcome (first all-cause stroke or TIA) and the number needed to harm (NNH) to cause 1 principal safety outcome (fatal bleeding or bleeding into a critical space with a potential for causing permanent disability) were calculated from the rates of absolute risk reduction using annualized incidence rates. The NNT and NNH were also calculated for subgroups above and below the median (closest integer) CHA2DS2-VASc risk score.
Two-sided P-values with significance threshold of P < 0.05 were considered statistically significant, and no multiplicity adjustments were made in this post-hoc analysis. All computations were performed using SAS version 9.4.
Results
From September 2013 to October 2017, 5022 patients were enrolled in COMMANDER HF from 628 sites across 32 countries. All patients were included in this post hoc analysis. Overall, 2507 patients were randomly assigned to rivaroxaban and 2515 to placebo. COMMANDER HF participants were on average 66.4 years of age, 22.9% women, and 82.2% White. Overall, 40.9% had a history of diabetes mellitus. Over 90% were treated with aspirin at baseline and a third were on dual antiplatelet therapy. At baseline, use of background guideline-directed medical therapy for HFrEF was high.
Phenotyping stroke/transient ischaemic attack after an episode of worsening chronic heart failure with reduced ejection
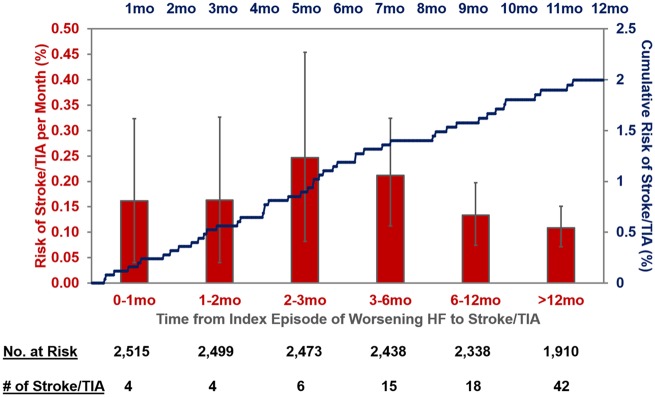
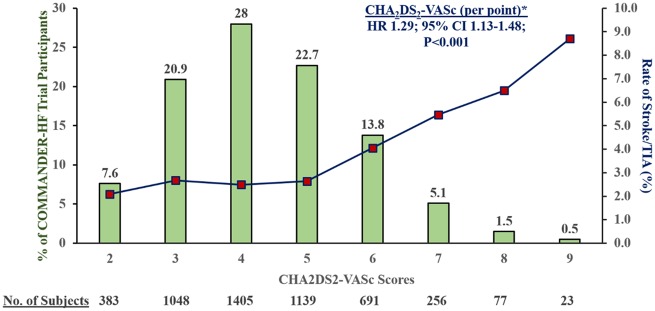
Over a median follow-up of 20.5 (25th–75th percentiles 20.0–20.9) months, 150 stroke or TIA events occurred; 5 (3.3%) occurred within 30 days and 24 (16.0%) occurred within 90 days and 49 (32.7%) by 6 months of index hospitalization. The risk of stroke/TIA calculated only in the placebo arm after an episode of worsening HFrEF remained elevated well beyond 6 months (Figure 1). Of these, 127 were first stroke events and 23 were first TIA events. Ischaemic stroke accounted for 82% of stroke events. There was limited observed heterogeneity in baseline characteristics among patients who did and did not experience a stroke/TIA during follow-up (Table 1). CHA2DS2-VASc scores were higher among patients who experienced a stroke/TIA in follow-up (median 5; 25th–75th percentiles 4–6) compared with those who did not (median 4; 25th–75th percentile: 3–5); Figure 2.
Figure 1.
Temporal pattern of risk of stroke/transient ischaemic attack after an episode of worsening chronic heart failure with reduced ejection in the placebo arm of COMMANDER HF. The total duration of time was calculated by adding the time from worsening heart failure episode to randomization to the time from randomization to stroke/transient ischaemic attack. The incremental incidence rate (red bars)) and its 95% confidence intervals (grey lines) for each time segment were derived using the bootstrap method (10 000 resamples). Kaplan–Meier cumulative risk estimates over the first 12 months after an episode of worsening heart failure are displayed in blue.
Table 1.
Baseline characteristics and medical therapies in patients experiencing stroke/transient ischaemic attack compared with patients free of stroke/transient ischaemic attack in follow-up
| Stroke/TIA |
No stroke/TIA |
|||||
|---|---|---|---|---|---|---|
| Rivaroxaban (n = 61) | Placebo (n = 89) | Total (N = 150) | Rivaroxaban (n = 2446) | Placebo (n = 2426) | Total (N = 4872) | |
| Age, mean (SD) (years) | 66.5 (9.6) | 68.3 (10.2) | 67.5 (10.0) | 66.0 (10.1) | 66.2 (10.3) | 66.4 (10.2) |
| Women, n (%) | 13 (21.3) | 24 (27.0) | 37 (24.7) | 538 (22.0) | 575 (23.7) | 1113 (22.8) |
| White race, n (%) | 45 (73.8) | 73 (82.0) | 118 (78.7) | 2018 (82.5) | 1992 (82.1) | 4010 (82.3) |
| Region, n (%) | 0.149 | |||||
| Eastern Europe | 37 (60.7) | 46 (51.7) | 83 (55.3) | 1573 (64.3) | 1568 (64.6) | 3141 (64.5) |
| North America | 1 ( 1.6) | 4 ( 4.5) | 5 ( 3.3) | 73 ( 3.0) | 71 ( 2.9) | 144 ( 3.0) |
| Asia Pacific | 13 (21.3) | 12 (13.5) | 25 (16.7) | 354 (14.5) | 354 (14.6) | 708 (14.5) |
| Latin America | 7 (11.5) | 11 (12.4) | 18 (12.0) | 222 ( 9.1) | 218 ( 9.0) | 440 ( 9.0) |
| Western Europe And South Africa | 3 ( 4.9) | 16 (18.0) | 19 (12.7) | 224 ( 9.2) | 215 ( 8.9) | 439 ( 9.0) |
| Medical history, n (%) | ||||||
| Myocardial infarction | 45 (73.8) | 61 (68.5) | 106 (70.7) | 1866 (76.3) | 1831 (75.5) | 3697 (75.9) |
| Stroke | 8 (13.1) | 18 (20.2) | 26 (17.3) | 200 ( 8.2) | 227 ( 9.4) | 427 ( 8.8) |
| Hypertension | 47 (77.0) | 74 (83.1) | 121 (80.7) | 1850 (75.6) | 1812 (74.7) | 3662 (75.2) |
| Diabetes | 29 (47.5) | 41 (46.1) | 70 (46.7) | 995 (40.7) | 987 (40.7) | 1982 (40.7) |
| Vital sign, median (IQR) | ||||||
| Systolic blood pressure (mmHg) | 123.0 (113.0, 131.0) | 128.0 (115.0, 137.0) | 125.0 (113.0, 132.0) | 122.0 (110.0, 133.0) | 122.0 (110.0, 131.0) | 122.0 (110.0, 132.0) |
| Diastolic blood pressure (mmHg) | 74.0 (70.0, 80.0) | 72.0 (67.0, 80.0) | 73.0 (67.0, 80.0) | 74.0 (69.0, 80.0) | 72.0 (68.0, 80.0) | 73.0 (68.0, 80.0) |
| Biomarkers, median (IQR) | ||||||
| BNP (pg/mL) | 607.3 (517.4, 1877.5) | 780.0 (399.4, 1380.0) | 679.0 (461.0, 1380.0) | 702.0 (389.5, 1230.0) | 686.5 (368.4, 1266.3) | 696.0 (382.3, 230.7) |
| NT-proBNP (pg/mL) | 3136.0 (1915.0, 6303.5) | 2160.5 (1237.5, 4232.5) | 2435.0 (1417.5, 5306.5) | 2806.0 (1932.0, 6360.0) | 2890.0 (1502.0, 6267.0) | 2851.5 (1511.5, 6303.5) |
| D-dimer (μg/L) | 335.0 (270.0, 685.0) | 455.0 (265.0, 950.0) | 390.00 (267.5, 710.0) | 360.0 (215.0, 680.0) | 360.0 (215.0, 640.0) | 360.00 (215.0, 665.0) |
| New York Heart Association classification, n (%) | 0.974 | |||||
| I | 4 ( 6.6) | 0 | 4 ( 2.7) | 76 ( 3.1) | 69 ( 2.8) | 145 ( 3.0) |
| II | 20 (32.8) | 44 (49.4) | 64 (42.7) | 1102 (45.1) | 1052 (43.4) | 2154 (44.2) |
| III | 33 (54.1) | 43 (48.3) | 76 (50.7) | 1175 (48.1) | 1211 (49.9) | 2386 (49.0) |
| IV | 4 ( 6.6) | 2 ( 2.2) | 6 ( 4.0) | 92 ( 3.8) | 94 ( 3.9) | 186 ( 3.8) |
| CHA2DS2-VASC Score, median (IQR) | 4 (3, 6) | 5 (4, 6) | 5 (4, 6) | 4 (3, 5) | 4 (3, 5) | 4 (3, 5) |
| Baseline therapies, n (%) | ||||||
| Aspirin | 53 (86.9) | 85 (95.5) | 138 (92.0) | 2276 (93.0) | 2261 (93.2) | 4537 (93.1) |
| Thienopyridine | 30 (49.2) | 29 (32.6) | 59 (39.3) | 1013 (41.4) | 943 (38.9) | 1956 (40.1) |
| Dual antiplatelet therapy | 24 (39.3) | 26 (29.2) | 50 (33.3) | 1696 (34.8) | 883 (36.1) | 813 (33.5) |
| ACEi or ARB | 55 (90.2) | 83 (93.3) | 138 (92.0) | 2291 (93.7) | 2231 (92.0) | 4522 (92.8) |
| ARNI | 0 | 0 | 0 | 18 ( 0.7) | 23 ( 0.9) | 41 ( 0.8) |
| β-Blocker | 54 (88.5) | 81 (91.0) | 135 (90.0) | 2246 (91.8) | 2261 (93.2) | 4507 (92.5) |
| MRA | 49 (80.3) | 66 (74.2) | 115 (76.7) | 1869 (76.4) | 1856 (76.5) | 3725 (76.5) |
Intent-to-Treat Analysis Set includes all randomized unique subjects who have a signed valid informed consent.
Percentages are calculated with the number of subjects in each category and treatment group as denominator.
Race and ethnicity are self-reported by the subject.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; NT-proBNP, N-terminal B-type natriuretic peptide; TIA, transient ischaemic attack.
Figure 2.
Distribution of COMMANDER HF participants and observed stroke or transient ischaemic attack rates by CHA2DS2-VASc score. * Given strong treatment effect of rivaroxaban vs. placebo on stroke/transient ischaemic attack, Cox proportional hazards models for risk prediction were performed in the placebo group alone.
Stroke severity and subsequent adverse events
Stroke severity as assessed using the mRS (score 0–6) among 133 patients included 31% fatal events (mRS 6), 16.5% with moderate-to-severe disability (mRS 3–5), while 51.1% were non-disabling events (mRS 0–2). Patients surviving after a stroke or TIA event faced risks of mortality of 26% (33 out of 126), recurrent stroke or TIA of 7% (9 out of 126), and rehospitalization for HF of 21% (26 out of 126) during study follow-up.
Predicting stroke or transient ischaemic attack after an episode of worsening chronic heart failure with reduced ejection
In a multivariate model among placebo-treated patients when clinically relevant variables were simultaneously tested, only prior history of stroke, low body mass index, and region were independently predictive of stroke/TIA after worsening HFrEF. Patients in Latin America, Western Europe, and South Africa independently carried the highest risks of stroke or TIA. Optimism-corrected C-statistic of this model with selected clinically relevant variables was 0.70 (percentile corrected interval 0.65–0.74) (Table 2). Per point, the CHA2DS2-VASc was significantly associated with risk of first stroke/TIA [hazard ratio (HR) 1.29; 95% CI 1.13–1.48; P < 0.001] among placebo-treated patients; the score displayed modest discrimination (C-statistic 0.62).
Table 2.
Risk predictors of stroke or transient ischaemic attack in final prediction model
| Wald statistics χ2 | Hazard ratio (HR) | P-value | |
|---|---|---|---|
| Region | 14.49 | 0.006 | |
| Asia Pacific vs. Eastern Europe | 1.45 (0.41–5.15) | ||
| Western Europe & South Africa vs. Eastern Europe | 2.97 (1.59–5.57) | ||
| North America vs. Eastern Europe | 1.74 (0.56–5.37) | ||
| Latin America vs. Eastern Europe | 2.54 (1.23–5.23) | ||
| History of prior stroke | 10.09 | 2.35 (1.39–3.98) | 0.002 |
| Body mass index (kg/m2) | 3.87 | 0.95 (0.91–1.00) | 0.049 |
| History of hypertension | 3.01 | 1.67 (0.94–2.99) | 0.083 |
| Age (per year) | 1.39 | 1.01 (0.99–1.04) | 0.239 |
| Time from index episode of worsening heart failure to randomization (per day) | 0.58 | 1.01 (0.99–1.03) | 0.448 |
| Left ventricular ejection fraction (per %) | 0.51 | 0.99 (0.96–1.02) | 0.473 |
| New York Heart Association class | 0.34 | 0.952 | |
| Class I vs. Class IV | |||
| Class II vs. Class IV | 1.38 (0.33–5.85) | ||
| Class III vs. Class IV | 1.49 (0.35–6.37) | ||
| History of diabetes mellitus | 0.23 | 1.11 (0.71–1.74) | 0.633 |
| White race | 0.10 | 1.19 (0.40–3.49) | 0.754 |
| Optimism-corrected C-statistic (percentile-correct interval) | 0.70 (0.65–0.74) | ||
No events occurred in the placebo arm of New York Heart Association Class I patients, so a hazard ratio was not estimable.
Treatment effects of rivaroxaban on occurrence of first and recurrent stroke/transient ischaemic attack and safety events
In this post hoc analysis, rivaroxaban significantly reduced the primary neurological endpoint of all-cause stroke or TIA compared with placebo by 32% (2.4% vs. 3.5%; 1.29 events vs. 1.90 events per 100 patient-years; HR 0.68; 95% CI 0.49–0.94; P = 0.02); Take homefigure. Known stroke subtype events (including haemorrhagic stroke) and TIA all directionally favoured rivaroxaban vs. placebo, however, only ischaemic stroke was significantly reduced by rivaroxaban vs. placebo by 36% (0.86 events vs. 1.34 events per 100 patient-years; HR 0.64; 95% CI 0.43–0.95; P = 0.028); Table 3. Consistent reductions were observed for all-cause stroke alone and the composite of ischaemic stroke or TIA. Fatal or moderate-severely disabling strokes, defined by mRS 3–6, were lower with rivaroxaban compared with placebo (39.6% vs. 52.5%). After adjusting for clinically relevant covariates (Table 2), rivaroxaban retained significant and independent risk reduction of stroke/TIA (HR 0.68; 95% CI 0.49–0.94). During follow-up, a total of nine recurrent stroke or TIA events occurred (two in the rivaroxaban arm and seven in the placebo arm).
Take home figure.
Time to first occurrence of stroke or transient ischaemic attack. Cox proportional hazards models were adjusted for all covariates presented in Table 2. Analyses were performed in the intention-to-treat cohort including all randomized unique subjects who have a signed valid informed consent. CI, confidence interval; HR, hazard ratio; TIA, transient ischaemic attack.
Table 3.
Effects of rivaroxaban vs. placebo on stroke or transient ischaemic attack
| Rivaroxaban |
Placebo |
|||||
|---|---|---|---|---|---|---|
| n/N (%) | Incidence rate per 100 patient-years | n/N (%) | Incidence rate per 100 patient-years | HR (95% CI) | P-value | |
| Primary neurological endpoint: all-cause stroke or TIA | 61/2507 (2.43) | 1.29 | 89/2515 (3.54) | 1.9 | 0.68 (0.49, 0.94) | 0.02 |
| All-cause stroke | 51/2507 (2.03) | 1.08 | 76/2515 (3.02) | 1.62 | 0.67 (0.47, 0.95) | 0.025 |
| Ischaemic stroke | 41/2507 (1.64) | 0.86 | 63/2515 (2.50) | 1.34 | 0.64 (0.43, 0.95) | 0.028 |
| Haemorrhagic stroke | 6/2507 (0.24) | 0.13 | 8/2515 (0.32) | 0.17 | 0.74 (0.25, 2.13) | 0.572 |
| Subarachnoid haemorrhage | 1/2507 (0.04) | 0.02 | 3/2515 (0.12) | 0.06 | 0.33 (0.03, 3.16) | 0.334 |
| Uncertain type of stroke | 4/2507 (0.16) | 0.08 | 2/2515 (0.08) | 0.04 | 2.01 (0.37, 10.99) | 0.420 |
| TIA | 10/2507 (0.40) | 0.21 | 13/2515 (0.52) | 0.27 | 0.77 (0.34, 1.75) | 0.525 |
| Ischaemic stroke or TIA | 51/2507 (2.03) | 1.08 | 76/2515 (3.02) | 1.62 | 0.66 (0.46, 0.95) | 0.023 |
Cox proportional hazards models were used to determine hazard ratios (HR) and 95% confidence intervals, adjusted for time from index heart failure event to randomization and stratified by region.
TIA, transient ischaemic attack.
Overall, we estimate that 164 patients per year would need to be treated with rivaroxaban to prevent 1 stroke or TIA event. The efficacy and safety of rivaroxaban vs. placebo did not differ by background dual antiplatelet therapy or the CHA2DS2-VASc risk score with cut-off at the median integer (4); all interaction P-values >0.30. Among patients with CHA2DS2-VASc ≤4, rivaroxaban reduced stroke/TIA from 2.8% to 2.2% (HR 0.78; 95% CI 0.49–1.25) with an NNT of 316 patient-years. Among patients with CHA2DS2-VASc above 4, rivaroxaban reduced stroke/TIA from 4.5% to 2.7% (HR 0.59; 95% CI 0.37–0.92) with an NNT of 96 patient-years (Table 4).
Table 4:
Application of the CHA2DS2-VASc risk score with cut-off at the median score of 4 to the COMMANDER HF trial
| Rivaroxaban |
Placebo |
||||||
|---|---|---|---|---|---|---|---|
| n/N (%) | Incidence rate (per 100 patient-years) | n/N (%) | Incidence rate (per 100 patient-years) | NNT patient-years | HR (95% CI) | P-value | |
| Primary neurological endpoint: all-cause stroke or TIA | |||||||
| COMMANDER HF cohort | 61/2507 (2.4%) | 1.29 | 89/2515 (3.5%) | 1.90 | 164 | 0.68 (0.49–0.94) | 0.02 |
| CHA2DS2-VASc ≤ 4 | 31/1412 (2.2%) | 1.13 | 40/1424 (2.8%) | 1.44 | 316 | 0.79 (0.49–1.26) | 0.382b |
| CHA2DS2-VASc > 4 | 30/1095 (2.7%) | 1.52 | 49/1091 (4.5%) | 2.56 | 96 | 0.59 (0.37–0.93) | |
|
| |||||||
|
Rivaroxaban
|
Placebo
|
||||||
| n/N (%) | Incidence rate (per 100 patient-years) | n/N (%) | Incidence rate (per 100 patient-years) | NNH patient-yearsa | HR (95% CI) | P-value | |
|
| |||||||
| Principal safety endpoint: fatal bleeding or bleeding into a critical space | |||||||
| COMMANDER HF cohort | 18/2499 (0.7%) | 0.44 | 23/2509 (0.9%) | 0.55 | – | 0.81 (0.44–1.49) | 0.491 |
| CHA2DS2-VASc ≤ 4 | 8/1406 (0.6%) | 0.33 | 13/1422 (0.9%) | 0.53 | – | 0.65 (0.27–1.56) | 0.495b |
| CHA2DS2-VASc > 4 | 10/1093 (0.9%) | 0.60 | 10/1087 (0.9%) | 0.60 | – | 1.00 (0.42–2.40) | |
Cox proportional hazards models were used to determine hazard ratios (HR) and 95% confidence intervals (CI), adjusted for time from index heart failure event to randomization and stratified by region. Number needed to treat (NNT) or number needed to harm (NNH) was calculated based on the difference in incidence rates per 100 patient-year between the treatment groups.
TIA, transient ischaemic attack.
As the principal safety endpoint occurred at a higher incidence rate in the placebo arm compared with rivaroxaban arm in the overall COMMANDER HF trial and by CHA2DS2-VASc subgroups, NNH was not calculated.
Interaction P-value.
The principal safety endpoint, fatal bleeding, or bleeding into a critical space with potential for permanent disability, occurred at a similar rate in rivaroxaban-treated patients compared with placebo-treated patients (0.44 events vs. 0.55 events per 100 patient-years). As bleeding events were directionally lower in the rivaroxaban arm with respect to the principal safety endpoint, there was no signal of net harm observed (Table 4).
Discussion
In this post hoc analysis of a large, global, randomized placebo-controlled clinical trial, we found that patients recently treated for an episode of worsening HF in sinus rhythm face a risk of stroke (1.6 per 100 patient-years) approaching rates observed among patients with chronic HF and AF (2.0 per 100 patient-years).4 Ischaemic strokes are the first such event in 82% of patients. This risk is noted to increase early immediately following the index episode of worsening HF, peaks by 6-months and persists throughout the period of observation. Nearly half of all first stroke events are either fatal or disabling and those individuals that survive these events continue to face risk of major adverse cardiovascular events, including death. The history of a prior stroke, low body mass index, and geographic region represent important independent predictors of such events. The addition of rivaroxaban 2.5 mg b.i.d. to background antiplatelet therapy markedly reduces risk of first stroke or TIA compared with placebo by 32%, when adjusted for clinically relevant covariates. The reduced risk of stroke among rivaroxaban-treated patients after worsening HFrEF in COMMANDER HF mirrors rates observed among studies of stable chronic HFrEF in sinus rhythm4 and translates into a NNT of 164 per year, a number that is considerably improved when applying the CHA2DS2-VASc score of >4, with a NNT of 96 per year. Rivaroxaban at a low dose is associated with a safe and acceptable bleeding profile; we did not observe between-arm differences in fatal or critical space bleeding (the principal safety endpoint), haemorrhagic stroke, or death. Rivaroxaban did increase bleeding when compared with placebo using secondary measures of safety endpoints as reported in the primary publication of the COMMANDER HF trial.13
Early clinical trials that tested the usefulness of vitamin K antagonists compared with antiplatelet therapy or no antithrombotic therapy in HFrEF were relatively small, underpowered, and did not demonstrate a clear net clinical benefit in stroke reduction.7–9 In the larger Warfarin vs. Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial of 2306 patients with chronic HFrEF in sinus rhythm, warfarin did not influence the primary endpoint of ischaemic stroke, intracerebral haemorrhage, or death compared with aspirin. However, warfarin did significantly reduce ischaemic stroke at the expense of increased major haemorrhage as compared with aspirin.10 COMMANDER HF evaluated rivaroxaban at a low-dose and safely reduced stroke or TIA, but did not modify the primary endpoint of the trial which was a composite of death, myocardial infraction, or stroke. This was largely because the lower incidence of a first stroke event was overwhelmed by a high proportional occurrence of HF deaths as the principal event. However, our findings on the stroke reduction signal in this unique population that suffered a recent episode of worsening HF are also supported by prospective trials evaluating extended-duration therapy with a factor Xa inhibitor, betrixaban, among patients hospitalized for medical illness.18,19 Low-dose rivaroxaban, which at this dose decreases thrombin generation,20 may attenuate residual thrombotic risk early and late following worsening HFrEF18 and among patients with stable atherosclerotic vascular disease21 or after acute coronary syndromes.22
Step-wise pharmacological and device developments over the last 3 decades have modified disease progression in HFrEF and led to longitudinal declines in sudden death.23 Despite this therapeutic success (primarily targeting neurohormonal pathways attenuating adverse myocardial remodelling), patients face residual thrombotic risks. Stroke, a most feared morbidity related to HF, remains a significant problem even among patients in sinus rhythm, across an ejection fraction spectrum.24 Although these events appear to occur at a relatively low frequency, we found that nearly half of index stroke events were fatal or disabling, highlighting the important lasting morbidity associated with this complication. Importantly, the analysis of this trial indicates that the early period after worsening HFrEF is ‘vulnerable’ with a large proportion of events occurring during that phase and accumulating thereafter with a peak within 6 months; however, there is no period when the risk is completely attenuated. Few data exist in this particular time period and even those that do evaluate such early post-discharge outcomes, do not provide long-term follow-up.18,19 A recent exploratory analysis of COMMANDER HF assessed the utility of rivaroxaban in modifying composite thromboembolic complications (inclusive of myocardial infarction, ischaemic stroke, sudden unwitnessed death, or symptomatic venous thromboembolism).25 Taken together with our study, these data highlight that patients after an episode of worsening HF face a broad range of residual thrombotic risks, of which stroke represents a critical modifiable event.
As patients presenting with worsening HFrEF have widely heterogeneous patient profiles, application of a clinical risk score may identify subpopulations that may particularly benefit from thromboprophylaxis. Few clinical risk scores have been validated to improve risk prediction to guide stroke prevention in HFrEF and sinus rhythm.26 The CHA2DS2-VASc score has been a validated and widely applied risk prediction tool among patients with AF, and in our analysis appears to be an important predictor of stroke outcomes among patients in sinus rhythm.4,27 This risk prediction score was significantly associated with first-time stroke or TIA, but its performance as a continuous variable was modest. This may reflect relatively low event rates, high observed competing risks of death and rehospitalization for worsening HF in this high-risk population,25 and lack of accounting of specific metrics of HF severity and status. In aggregate, we estimate that 164 patients per year would need to be treated with low-dose rivaroxaban to prevent 1 stroke or TIA event. If the CHA2DS2-VASc score is applied using a cut-point of 4 (the median score of our population), the NNT would reduce to 96 per year. Thus, a risk score targeted approach to cautious implementation of this preventive therapy may warrant further investigation in patients deemed at high risk for stroke in HF and without AF. Given the regional heterogeneity in event profiles consistent with prior observations across global HF programs,28,29 risk scores may need to be adapted accounting for local populations.
Study limitations
This is a post hoc analysis which used an endpoint that lacked formal independent adjudication by a clinical events committee and instead relied on site investigator-based event adjudication. However, as stroke was a component of the primary composite endpoint, data collection to support site adjudication was carefully performed. As imaging was not uniformly available to exclude cerebral infarction among patients presenting with transient neurological symptoms, we specifically focused on the composite of stroke or TIA as the primary neurological outcome. Patients with a history of stroke within 90 days of randomization were excluded which may lead to underestimation of stroke risk in this population. Given the exploratory nature of this analysis, treatment effects were adjusted for clinically relevant covariates (which did not modify the direction, magnitude, or significance of the results). These data should be considered exploratory, hypothesis-generating and require prospective validation, especially since rivaroxaban has not received regulatory approval for use for the indication of stroke prevention in patients with worsening HFrEF in the absence of other reasons for anticoagulation (such as AF).
Conclusions
In this exploratory analysis of a large, global, randomized clinical trial of patients with recently worsening HFrEF, CAD, and sinus rhythm, an ischaemic stroke was most often observed as the first stroke event and was frequently disabling or fatal. The addition of low-dose rivaroxaban appeared to safely attenuate risk of stroke or TIA in the vulnerable early and late phase after a recent episode of worsening HFrEF. Within the context of the relatively low absolute risk of stroke/TIA events, our data suggest that selected at-risk populations of patients with HFrEF and sinus rhythm may be identified using traditional risk scores and further investigation of such targeted approaches are warranted.
Supplementary Material
Acknowledgements
We acknowledge the lasting contributions, memory, and legacy of the deceased Dr Mihai Gheorghiade, who was a member of the Steering Committee of COMMANDER HF. We thank all the patients, investigators, and site staff for participating in this trial; the entire Janssen Cross Functional Trial Team for their contributions to the statistical monitoring and analyses and the protocol development, safety monitoring, data management, and operational implementation of the trial.
Funding
COMMANDER HF was supported by Janssen Research & Development LLC. Employees of Janssen Research & Development LLC were involved with the design and conduct of the study (with the approval of the COMMANDER HF Steering Committee), collection, management, and analysis of data. However, data interpretation and manuscript drafting were carried out independently by study investigators.
Conflict of interest: M.R.M. reports personal fees from Janssen Research & Development LLC as a member of the steering committee of the COMMANDER HF trial and consulting fees during the conduct of the study and personal fees from Abbott, Medtronic, Portola Pharmaceuticals, Bayer, Mesoblast, Baim Institute for Clinical Research, Xogenex, NupulseCV and FineHeart outside the submitted work. M.V. is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Bayer AG, and Baxter Healthcare, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. M.F. reports other support from Janssen Research & Development LLC during the conduct of the study and is employed by and a shareholder of Johnson & Johnson. F.Z. and J.P.F. are supported by public grants overseen by the European Commission (EUFP7) and the French National Research Agency (ANR) as part of the second ‘Investissements d’Avenir’ programme FIGHT-HF (ANR-15-RHU-0004); programme HOMAGE under grant agreement N 305507; and project FIBROTARGETS grant HEALTH-2013- 602904. S.D.A. reports personal fees from Janssen Research & Development LLC as a member of the steering committee of the COMMANDER HF trial during the conduct of the study; personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Laboratoires Servier, Thermo Fisher Scientific, Vifor Pharma, V-Wave, CVRx, and Impulse Dynamics outside the submitted work; and grants from Vifor Pharma and Abbott Vascular outside the submitted work. J.G.F.C. reports personal fees from Janssen Research & Development LLC as a member of the steering committee of the COMMANDER HF trial and research grants and honoraria for speaking, committees, and advisory boards from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Medtronic, MyoKardia, Novartis, Philips Healthcare, Pharmacosmos, Pharma Nord, Sanofi, Laboratoires Servier, Stealth BioTherapeutics, Torrent Pharmaceuticals, and Vifor Pharma. C.S.P.L. reports personal fees from Janssen Research & Development LLC during the conduct of the study; research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; personal fees from serving on the advisory board/steering committee/executive committee/clinical end points committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia Medical, Stealth BioTherapeutics, Jana Care, Biofourmis, Applied Therapeutics Inc, WebMD Global LLC, Radcliffe Group Ltd, Takeda Pharmaceutical Company, and Darma Laboratories outside the submitted work; and support from a Clinician Scientist Award from the National Medical Research Council of Singapore. D.J.v.V. reports personal fees from Johnson & Johnson and Bayer during the conduct of the study and reports receiving board membership fees from Janssen Research & Development LLC as a member of the steering committee of the COMMANDER HF trial. W.M. B. reports other support from Janssen Research & Development LLC during the conduct of the study and is employed by and is a shareholder of Johnson & Johnson. T.S. is employed by and is a shareholder of Bayer. H.D. reports other support from Janssen Research & Development LLC during the conduct of the study and outside the submitted work and is employed by and a shareholder of Johnson & Johnson. F.Z. reports personal fees from Janssen Research & Development LLC and Bayer during the conduct of the study; personal fees from AstraZeneca, Boehringer Ingelheim, LivaNova, GE Healthcare, Amgen, Novartis, Quantum Genomics, Cardior Pharmaceuticals, CardioRenal, CVCT, Merck, CVRx, Vifor Fresenius Medical Care Renal Pharma, NovoNordisk, and MundiPharma outside the submitted work; fees for serving on a steering committee or a safety and data monitoring board from Actelion, Amgen, Bayer, Boehringer Ingelheim, Boston Scientific, CVRx, GE Healthcare, Janssen Research & Development LLC, Novartis, and ResMed; and consulting fees from AstraZeneca, Cardior Pharmaceuticals, CardioRenal, Quantum Genomics, and Vifor Fresenius Medical Care Renal Pharma. B.G. reports personal fees from Janssen Research & Development LLC as a member of the steering committee of the COMMANDER HF trial and consulting fees from Bayer during the conduct of the study; consulting fees from Janssen Research & Development LLC, Bayer, Novartis, Mesoblast, Ionis Pharmaceuticals, Zensun USA, and Cellular Dynamics outside the submitted work; speaker fees from Novartis and Otsuka Pharmaceutical outside the submitted work; and personal fees from AstraZeneca, Actelion, Amgen, EBR Systems, Impulse Dynamics, MyoKardia, Rocket Pharma, Sanofi, and Viking Therapeutics outside the submitted work.
See page 3602 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz523)
Meeting Presentation: Late-Breaking Clinical Trial Presentation at the European Society of Cardiology Heart Failure 2019 (6th World Congress on Acute Heart Failure) Meeting in Athens, Greece, 26 May 2019.
References
- 1. Stolker JM, Spertus JA, Cohen DJ, Jones PG, Jain KK, Bamberger E, Lonergan BB, Chan PS.. Rethinking composite end points in clinical trials: insights from patients and trialists. Circulation 2014;130:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaduganathan M, Patel RB, Yancy CW.. Stroke prevention in heart failure and sinus rhythm: where do we go from here? Eur J Heart Fail 2016;18:1267–1269. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS.. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 4. Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY.. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 2015;314:1030–1038. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira JP, Girerd N, Gregson J, Latar I, Sharma A, Pfeffer MA, McMurray JJV, Abdul-Rahim AH, Pitt B, Dickstein K, Rossignol P, Zannad F.. Stroke risk in patients with reduced ejection fraction after myocardial infarction without atrial fibrillation. J Am Coll Cardiol 2018;71:727–735. [DOI] [PubMed] [Google Scholar]
- 6. Gheorghiade M, Vaduganathan M, Fonarow GC, Greene SJ, Greenberg BH, Liu PP, Massie BM, Mehra MR, Metra M, Zannad F, Cleland JG, van Veldhuisen DJ, Shah AN, Butler J.. Anticoagulation in heart failure: current status and future direction. Heart Fail Rev 2013;18:797–813. [DOI] [PubMed] [Google Scholar]
- 7. Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole-Wilson PA.. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J 2004;148:157–164. [DOI] [PubMed] [Google Scholar]
- 8. Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK.. Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail 2006;8:428–432. [DOI] [PubMed] [Google Scholar]
- 9. Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, Jafri SM, Krol WF, O'Connor CM, Schulman KA, Teo K, Warren SR.. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation 2009;119:1616–1624. [DOI] [PubMed] [Google Scholar]
- 10. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R.. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012;366:1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lip GYH, Ponikowski P, Andreotti F, Anker SD, Filippatos G, Homma S, Morais J, Pullicino P, Rasmussen LH, Marin F, Lane DA, Lip GYH, Ponikowski P, Andreotti F, Anker SD, Filippatos G, Homma S, Morais J, Pullicino P, Rasmussen LH, Marin F, Lane DA, McMurray J, Hoes A, Ten Berg J, De Caterina R, Kristensen S, Zeymer U.. Thrombo-embolism and antithrombotic therapy for heart failure in sinus rhythm. A joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Eur J Heart Fail 2012;14:681–695. [DOI] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL.. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . Circulation 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 13. Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B.. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018;379:1332–1342. [DOI] [PubMed] [Google Scholar]
- 14. Zannad F, Greenberg B, Cleland JG, Gheorghiade M, van Veldhuisen DJ, Mehra MR, Anker SD, Byra WM, Fu M, Mills RM.. Rationale and design of a randomized, double-blind, event-driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: the COMMANDER HF trial. Eur J Heart Fail 2015;17:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banks JL, Marotta CA.. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091–1096. [DOI] [PubMed] [Google Scholar]
- 16. Abdul-Rahim AH, Perez AC, Fulton RL, Jhund PS, Latini R, Tognoni G, Wikstrand J, Kjekshus J, Lip GY, Maggioni AP, Tavazzi L, Lees KR, McMurray JJ.. Risk of stroke in chronic heart failure patients without atrial fibrillation: analysis of the Controlled Rosuvastatin in Multinational Trial Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca-Heart Failure (GISSI-HF) Trials. Circulation 2015;131:1486–1494; discussion 1494. [DOI] [PubMed] [Google Scholar]
- 17. Austin PC, Lee DS, Fine JP.. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bajaj NS, Vaduganathan M, Qamar A, Gupta K, Gupta A, Golwala H, Butler J, Goldhaber SZ, Mehra MR.. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: a trial sequential and cumulative meta-analysis. PLoS Med 2019;16:e1002797.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibson CM, Chi G, Halaby R, Korjian S, Daaboul Y, Jain P, Arbetter D, Goldhaber SZ, Hull R, Hernandez AF, Gold A, Bandman O, Harrington RA, Cohen AT.. Extended-duration betrixaban reduces the risk of stroke versus standard-dose enoxaparin among hospitalized medically ill patients: an APEX Trial Substudy (Acute Medically Ill Venous Thromboembolism Prevention With Extended Duration Betrixaban). Circulation 2017;135:648–655. [DOI] [PubMed] [Google Scholar]
- 20. Gerotziafas GT, Elalamy I, Depasse F, Perzborn E, Samama MM.. In vitro inhibition of thrombin generation, after tissue factor pathway activation, by the oral, direct factor Xa inhibitor rivaroxaban. J Thromb Haemost 2007;5:886–888. [DOI] [PubMed] [Google Scholar]
- 21. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Stork S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S.. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 22. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM.. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 23. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray J.. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 24. Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA.. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–3744. [DOI] [PubMed] [Google Scholar]
- 25. Greenberg B, Neaton JD, Anker SD, Byra WM, Cleland JGF, Deng H, Fu M, La Police DA, Lam CSP, Mehra MR, Nessel CC, Spiro TE, van Veldhuisen DJ, Vanden Boom CM, Zannad F.. Association of rivaroxaban with thromboembolic events in patients with heart failure, coronary disease, and sinus rhythm: a post hoc analysis of the COMMANDER HF trial. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira JP, Girerd N, Alshalash S, Konstam MA, Zannad F.. Antithrombotic therapy in heart failure patients with and without atrial fibrillation: update and future challenges. Eur Heart J 2016;37:2455–2464. [DOI] [PubMed] [Google Scholar]
- 27. Ye S QM, Zhao B, Buchsbaum R, Sacco RL, Levin B, Tullio Mr D, Mann DL, Pullicino PM, Freudenberger RS, Teerlink JR, Mohr JP, Graham S, Labovitz AJ, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Lip GY, Thompson JL, Homma S, for the WARCEF Investigators. CHA2DS2-VASc score and adverse outcomes in patients with heart failure with reduced ejection fraction and sinus rhythm. Eur J Heart Fail 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferreira JP, Girerd N, Rossignol P, Zannad F.. Geographic differences in heart failure trials. Eur J Heart Fail 2015;17:893–905. [DOI] [PubMed] [Google Scholar]
- 29. Kristensen SL, Martinez F, Jhund PS, Arango JL, Bĕlohlávek J, Boytsov S, Cabrera W, Gomez E, Hagège AA, Huang J, Kiatchoosakun S, Kim K-S, Mendoza I, Senni M, Squire IB, Vinereanu D, Wong RC-C, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV.. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J 2016;37:3167–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.