Summary
Background
Since the 1918 influenza pandemic, non-randomised studies and small clinical trials have suggested that convalescent plasma or anti-influenza hyperimmune intravenous immunoglobulin (hIVIG) might have clinical benefit for patients with influenza infection, but definitive data do not exist. We aimed to evaluate the safety and efficacy of hIVIG in a randomised controlled trial.
Methods
This randomised, double-blind, placebo-controlled trial was planned for 45 hospitals in Argentina, Australia, Denmark, Greece, Mexico, Spain, Thailand, UK, and the USA over five influenza seasons from 2013–14 to 2017–18. Adults (≥18 years of age) were admitted for hospital treatment with laboratory-confirmed influenza A or B infection and were randomly assigned (1:1) to receive standard care plus either a single 500-mL infusion of high-titre hIVIG (0·25 g/kg bodyweight, 24·75 g maximum; hIVIG group) or saline placebo (placebo group). Eligible patients had a National Early Warning score of 2 points or greater at the time of screening and their symptoms began no more than 7 days before randomisation. Pregnant and breastfeeding women were excluded, as well as any patients for whom the treatment would present a health risk. Separate randomisation schedules were generated for each participating clinical site using permuted block randomisation. Treatment assignments were obtained using a web-based application by the site pharmacist who then masked the solution for infusion. Patients and investigators were masked to study treatment. The primary endpoint was a six-category ordinal outcome of clinical status at day 7, ranging in severity from death to resumption of normal activities after discharge. The choice of day 7 was based on haemagglutination inhibition titres from a pilot study. It was analysed with a proportional odds model, using all six categories to estimate a common odds ratio (OR). An OR greater than 1 indicated that, for a given category, patients in the hIVIG group were more likely to be in a better category than those in the placebo group. Prespecified primary analyses for safety and efficacy were based on patients who received an infusion and for whom eligibility could be confirmed. This trial is registered with ClinicalTrials.gov, NCT02287467.
Findings
313 patients were enrolled in 34 sites between Dec 11, 2014, and May 28, 2018. We also used data from 16 patients enrolled at seven of the 34 sites during the pilot study between Jan 15, 2014, and April 10, 2014. 168 patients were randomly assigned to the hIVIG group and 161 to the placebo group. 21 patients were excluded (12 from the hIVIG group and 9 from the placebo group) because they did not receive an infusion or their eligibility could not be confirmed. Thus, 308 were included in the primary analysis. hIVIG treatment produced a robust rise in haemagglutination inhibition titres against influenza A and smaller rises in influenza B titres. Based on the proportional odds model, the OR on day 7 was 1·25 (95% CI 0·79–1·97; p=0·33). In subgroup analyses for the primary outcome, the OR in patients with influenza A was 0·94 (0·55–1·59) and was 3·19 (1·21–8·42) for those with influenza B (interaction p=0·023). Through 28 days of follow-up, 47 (30%) of 156 patients in the hIVIG group and in 45 (30%) of 152 patients in the placebo group had the composite safety outcome of death, a serious adverse event, or a grade 3 or 4 adverse event (hazard ratio [HR] 1·06, 95% CI 0·70–1·60; p=0·79). Six (4%) patients in the hIVIG group and five (3%) in the placebo group died, but these deaths were not necessarily related to treatment.
Interpretation
When administered alongside standard care (most commonly oseltamivir), hIVIG was not superior to placebo for adults hospitalised with influenza infection. By contrast with our prespecified subgroup hypothesis that hIVIG would result in more favourable responses in patients with influenza A than B, we found the opposite effect. The clinical benefit of hIVIG for patients with influenza B is supported by antibody affinity analyses, but confirmation is warranted.
Funding
NIAID and NIH. Partial support was provided by the Medical Research Council (MRC_UU_12023/23) and the Danish National Research Foundation.
Research in context.
Evidence before this study
We identified 9520 articles through searching PubMed with the terms “influenza”[All Fields]) AND (“immunotherapy”[All Fields]) AND “human”. The search was restricted to articles in English. We did not include any date restrictions; the earliest article we found was published in 1946. Although numerous case reports or small randomised or non-randomised studies of passive immunotherapy as either primary or adjunctive therapy have been published over the past century, to our knowledge, none have provided definitive evidence that there is a true clinical and virological benefit of passive immunotherapy for patients with severe influenza.
Added value of this study
In this international, randomised, double-blind, placebo-controlled trial we found that despite robust increases in haemagglutination inhibition titres for influenza A, and smaller magnitude increases in titres for influenza B, there was no clinical benefit observed in patients receiving a single infusion of weight-based anti-influenza hyperimmune intravenous immunoglobulin (hIVIG) either overall or for the predefined subgroup of interest with influenza A. Paradoxically, and contrary to our expectation, the addition of hIVIG to standard care for patients with influenza B had both a significant clinical benefit at day 7 and a significant virological benefit at day 3 compared with placebo. Anti-haemagglutinin antibody affinities were measured in the hIVIG lots administered, and much stronger antibody affinities were observed for B strains than for A strains.
Implications of all the available evidence
This trial provides strong evidence that passive immunotherapy as an adjunctive therapy for adults hospitalised with severe influenza A does not provide clinical benefit. Although the beneficial effect of hIVIG for patients with influenza B is supported by the antibody affinity analyses done on the lots of the study drug, confirmation of clinical and virological benefit is warranted in a further randomised controlled trial enrolling participants hospitalised with influenza B.
Introduction
About 650 000 people worldwide are estimated to die annually from respiratory complications of influenza,1 and all-cause excess mortality could be affecting twice as many people.2 The main treatment for both outpatients and inpatients with influenza is with neuraminidase inhibitors, although therapy with cap-dependent endonuclease inhibitors has also become available. However, considerable morbidity and mortality due to influenza still occur.3, 4, 5
During the 1918 influenza pandemic, which accounted for an estimated 50 million deaths,6 convalescent sera, plasma, or blood from individuals who recovered from influenza were given to patients with severe influenza-induced pneumonia. These studies were largely predicated on the assumption that rapidly increasing titres of anti-influenza antibodies in acutely ill patients to protective convalescent concentrations might prevent clinical deterioration while the host's immune system mounts its own endogenous response. A meta-analysis of these studies suggested that there was clinical benefit for those patients who were treated early in the course of disease.7 A subsequent systematic review of studies using convalescent plasma or serum also found evidence of clinical benefit, but the investigators reported that these studies, none of which were randomised trials, were of poor quality by contemporary standards and recommended the evaluation of convalescent plasma in new, well designed clinical trials.8 Since that systematic review, a phase 2 trial of immune plasma plus standard care versus standard care alone was done in 98 patients with influenza A and B infection.9 The study did not show a clear benefit for immune plasma on the basis of the primary endpoint of normalisation of respiratory status at day 28, but favourable trends in several outcomes led to the design of a phase 3 trial of high-titre versus low-titre anti-influenza plasma in patients with influenza A infection.10 However, in that trial, high-titre plasma did not confer significant clinical benefit over low-titre plasma.
A single randomised trial11 in severely ill patients with influenza A serotype H1N1 infection has also been done using anti-influenza hyperimmune intravenous immunoglobulin (hIVIG). Although not definitive, it suggested the need for further investigation on the basis of a subgroup analysis. In that trial, hIVIG was selected for its neutralising activity against the circulating strain A(H1N1)pdm09 and was compared with control intravenous immunoglobulin without neutralising activity. Overall, there was no evidence of a survival benefit of hIVIG in the 34 evaluable patients, who were in intensive care and on ventilator support. However, of the 22 patients who were treated within 5 days of symptom onset, there were no deaths in the hIVIG group (n=12), but there were four in the control group (n=10). Conversely, for those patients treated later than 5 days after symptom onset, all patients in the hIVIG group died (n=5), but none did in the control group (n=7). The subgroup results for patients who were treated early led the investigators to conclude that intravenous immunoglobulin should be considered in future pandemics.
Considering the limitations of the two meta-analyses and the results of the two clinical trials, the hypothesis that treating ill patients with convalescent products from immune patients might have clinical benefit remains unproven. To fill the gap in evidence on the efficacy of hIVIG and to potentially identify a treatment that could reduce morbidity and mortality in adults admitted to hospital with influenza infection, we did a double-blind randomised trial of clinical outcomes in patients treated with hIVIG plus standard care versus standard care alone.
Methods
Study design and participants
FLU-IVIG was an international, double-blind, placebo-controlled trial designed and conducted by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT). We planned to include 45 major hospitals in Argentina, Australia, Denmark, Greece, Mexico, Spain, Thailand, UK, and the USA (appendix pp 3–4).
Adult patients (≥18 years of age) admitted for hospital treatment with influenza A (ie, A[H1N1]pdm09, H3N2) or B infection, locally determined by nucleic acid testing or by a rapid antigen test from a specimen obtained within 2 days before randomisation, were eligible if they had a National Early Warning (NEW) score12 of 2 points or greater at the time of screening, had onset of any symptoms no more than 7 days before randomisation, and were expected to be hospitalised for more than 24 h. If the NEW score for eligibility was obtained before the day of randomisation, an updated NEW score was obtained as baseline at the time of randomisation, which did not need to be 2 points or greater. Women of childbearing age were included if they were willing to abstain from sexual intercourse or to use at least one form of contraception until day 28 of the study. Pregnant or breastfeeding women were excluded, as were patients who had received any other investigational drug therapy within the 30 days before the screening, had a history of allergic reaction to blood or plasma products, had IgA deficiency, or were at risk of thrombosis for any reason. Additional exclusion criteria were suspicion of infection with another type of influenza, inability to comply with the protocol, increased risk due to treatment as judged by the site study investigator, and extracorporeal membrane oxygenation at screening. All patients provided written informed consent. The trial was approved by the institutional review board or ethics committee for each clinical site. The protocol is available online.
Randomisation and masking
Patients were randomly assigned (1:1) to receive 500 mL of either hIVIG solution (hIVIG group) or saline as placebo (placebo group), in addition to standard care. Randomisation was stratified by clinical site; a schedule for each site was prepared by the INSIGHT Statistical and Data Management Center at the University of Minnesota (Minneapolis, MN, USA) using permuted block randomisation. These schedules were maintained in secure files. After obtaining the treatment assignment using a web-based application, site pharmacists unmasked to treatment prepared the masked infusion bags of hIVIG or placebo. hIVIG solutions were slightly opaque. Thus, to ensure that the patient and clinical site staff were masked to the assigned treatment, the pharmacist placed a coloured sleeve over the 500 mL bag containing either the hIVIG or placebo.
Patients and investigators were masked to the study treatment administered. Apart from the site pharmacist, access to treatment assignment was restricted to the unmasked statisticians who prepared reports for the Data and Safety Monitoring Board. To assess the masking process, at the final follow-up visit on day 28, patients and a staff member primarily responsible for evaluating symptoms during follow-up were asked to guess which treatment was administered (appendix p 9).
Procedures
The hIVIG product was manufactured on an annual basis by Emergent BioSolutions (formerly Cangene Corporation; Winnipeg, MB, Canada) under contract to the National Institute of Allergy and Infectious Diseases, using high-titre anti-influenza plasma collected either from fractionated whole blood or by plasmapheresis from immune volunteers at sites in the USA and Canada. Donors and plasma units were selected on the basis of increased haemagglutination inhibition antibody titres against contemporary vaccine strains. Haemagglutination inhibition titres varied by hIVIG lot, and all lots were required to have substantial activity against contemporary circulating strains to be used (appendix p 12). The manufacturing methods and determination of neutralising antibody titres in the individual IVIG lots did not vary over the course of the trial. After unmasking of the primary study results, additional characterisation of the antibody composition of each hIVIG lot administered during the study was done. This included measurement of the total antibody binding and antibody affinity to the HA0 protein (the native functionally intact form of haemagglutinin) for each of the influenza vaccine strains (appendix p 7).13
Before randomisation, demographics information and medical history were obtained, NEW score was assessed, use of antiviral medication was recorded, and targeted symptoms were ascertained. Nasopharyngeal swabs from patients were obtained at baseline and on day 3 for central determination of influenza serotypes and viral loads by RT-PCR (appendix p 6). Blood was obtained before infusion of the assigned solution and on days 1, 3, and 7 for measurement of haemagglutination inhibition titres.
On the basis of the favourable pharmacokinetics and haemagglutination inhibition titres measured during a pilot trial,14 hIVIG was given at a dose of 0·25 g/kg (up to a maximum of 24·75 g, corresponding to approximately 100 kg actual bodyweight) dissolved in as much saline as needed to reach a total volume of 500 mL. A single infusion was given as soon as possible after randomisation over a period of approximately 2 h. 302 (98%) of patients were infused on the day of randomisation and six (2%) were infused on the day after randomisation.
Patients were followed up for 28 days after randomisation. Clinical data were collected on days 1–3, 7, 14, and 28. These data included NEW score on days 1–3 if the patient was hospitalised; targeted symptoms on days 3 and 7; nasopharyngeal swab on day 3; blood draws on days 1, 3, and 7; use of antiviral drugs; and adverse events throughout the entire follow-up period. Data were also collected on resolution of influenza symptoms. After infusion, the volume used and any subsequent adverse events were recorded.
Outcomes
An ordinal outcome with six mutually exclusive categories was used to describe the patient's clinical status during follow-up. The six categories were: (1) death; (2) in intensive care; (3) hospitalised but requiring supplemental oxygen; (4) hospitalised and not requiring supplemental oxygen; (5) discharged but unable to resume normal activities; or (6) discharged with full resumption of normal activities. Day 7 was the timepoint chosen for assesment of the primary ordinal outcome on the basis of the results of the pilot study,14 which showed that hIVIG rapidly increases haemagglutination inhibition titres against influenza A virus well above protective concentrations induced by seasonal vaccination15, 16, 17 and that differences in haemagglutination inhibition titres between hIVIG and placebo solutions were greatest in the first few days after infusion of hIVIG.14 We also considered that measures of clinical status at subsequent timepoints could probably reflect the effect of comorbidities instead of influenza infection and potentially obscure any treatment differences. The properties of the novel ordinal outcome we used for the primary endpoint have already been described.18
Prespecified secondary outcomes to assess the clinical status of patients are detailed in the panel and the rationale for any modifications is detailed in the appendix (pp 42–45).
Panel. Secondary outcomes.
Key outcomes
-
•
Five-category ordinal outcome on day 3 (death, in intensive care, hospitalised with NEW score ≥3, hospitalised with NEW score <3, or discharged from hospital)
-
•
The primary six-category ordinal outcome on day 3
-
•
Favourable outcome at day 7, accounting for location of enrolment (ie, in intensive care at enrolment to general ward or discharge before day 7, or in general ward at enrolment to discharge before day 7)
Other outcomes
-
•
The primary six-category outcome on days 14 and 28
-
•
The primary six-category outcome on days 1 through 7
-
•
Time to discharge
-
•
Time to death
-
•
Proportion of patients alive and discharged at day 28
-
•
Change in nasopharyngeal viral load from baseline to day 3
-
•
Change in haemagglutination inhibition titres from baseline to days 1, 3, and 7
-
•
Proportion of patients dead or re-admitted to hospital after discharge
-
•
Proportion of patients diagnosed on the day of randomisation or after with acute respiratory distress syndrome, acute renal failure, sepsis, pneumonia, enteritis, or bronchitis
-
•
Proportion of patients alive and discharged at day 14
-
•
Resumption of normal activities at day 14
NEW=National Early Warning.
Serious adverse events and grade 3 and 4 adverse events19 were categorised according to codes used in the Medical Dictionary for Regulatory Activities (MedDRA), version 21.0.
Statistical analysis
The trial was designed to enrol 320 patients. This sample size provides 80% power to detect an odds ratio (OR) of 1·77 for hIVIG versus placebo at the 0·05 (2-sided) level of significance (appendix p 7).20 ORs greater than 1·0 correspond to more favourable outcomes on hIVIG use compared with placebo.
An independent Data Safety Monitoring Board met five times during the trial to review interim safety and efficacy data (appendix p 13). The Board was asked to recommend early termination or modification of the trial only when there was clear and substantial evidence of a treatment difference. As a guideline, the Lan-DeMets spending function analogue of the O'Brien-Fleming boundaries was used to monitor the primary endpoint.21, 22 After approximately 70% of patients were enrolled, a futility analysis was provided to the Board by the unmasked statisticians. As a guideline, the Board was asked to consider futility if conditional power was less than 10%, given the observed data and the hypothesised treatment effect thereafter. These reviews by the Board did not lead to any modifications of the trial. No one except Board members and the unmasked statisticians had access to data summarising treatment comparisons.
From the outset of the FLU-IVIG trial, we planned to include the 16 patients who participated in the pilot study and met the eligibility criteria of FLU-IVIG in the primary analysis to reduce the cost of the trial (ie, enrolment costs and costs of preparing batches of hIVIG). The clinical outcomes of the 16 patients from the pilot study remained concealed until the results of the FLU-IVIG trial were unmasked. These patients were enrolled during the influenza season before the FLU-IVIG trial began.14 Data collected during the pilot study allowed all major outcomes to be determined.
All analyses were done in patients who received an infusion and for whom eligibility could be confirmed. A proportional odds model was used to analyse the primary ordinal outcome at day 7.23 The proportional odds model uses all six categories and estimates a common OR over all possible clinical states of the ordinal outcome. We defined an OR greater than 1 for a given category as the odds of patients in the hIVIG group being in a better category at day 7 than patients in the placebo group. The model included a treatment indicator and covariates: (1) whether the patient was enrolled from the intensive care unit or general ward and whether they required oxygen; (2) geographical region (USA, South America, and Mexico; Europe and Australia; and Thailand); and (3) participation in the previous pilot study. In addition to the adjusted OR specified for the primary analysis, an unadjusted OR is cited for the primary ordinal outcome. Proportional odds models with the same covariates are summarised for secondary ordinal outcomes at day 3, day 14, and day 28 (appendix p 9). Separate ORs were also estimated for each dichotomised definition of improvement from the ordered categorical scale on day 7. A test for the proportional odds assumption from a model that allowed different slopes for the baseline covariates (a partial proportional odds model) was done. Multiple imputation was used to estimate the day-7 clinical status of patients for whom data on the primary outcome were partially missing (appendix p 8). The ordinal outcome was also assessed on each of the first 7 days of follow-up. Generalised estimating equations were used to assess evidence of heterogeneity for these repeated assessments of the ordinal outcome. The interaction of study follow-up day and treatment was assessed and is referred to as an interaction p value. Sensitivity analyses are described in the appendix (p 8).
A major preplanned subgroup of interest were patients infected with influenza A serotypes A(H1N1) or A(H3N2) (appendix). We assumed that benefit would be greater for this subgroup, considering the substantial increase in haemagglutination inhibition titres that were seen after infusion with hIVIG in patients with A(H1N1) in the pilot study.14 Subgroups by influenza serotype and other measurements were defined at baseline by adding a cross-product term with treatment to the proportional odds model. Interaction p values are reported for this measure. Haemagglutination titres were analysed with geometric mean ratios and 95% CIs.
We used Kaplan-Meier curves and Cox regression to summarise safety outcomes, including a composite outcome of death, serious adverse events, or a grade 3 or 4 adverse event. Logistic regression was used to summarise binary outcomes such as the components of the ordinal outcome and the sliding dichotomy favourable outcome,24 defined as alive and out of intensive care for those enrolled via the intensive care unit, and discharged alive for those enrolled via the general ward. Analysis of variance was used to summarise log-transformed haemagglutination inhibition titres against different reference viruses for each influenza type at days 0, 1, 3, and 7, and log-transformed viral load changes from baseline to day 3. Baseline titres and viral load were included as covariates in these analyses.
Statistical analyses were done with the SAS software, version 9.4. All p values reported are 2-sided. There was no adjustment for type 1 errors for the number of secondary endpoints and subgroups examined. Thus, they should be interpreted with caution.
This study is registered with ClinicalTrials.gov, NCT02287467.
Role of the funding source
Staff from the funder, the US National Institutes of Health, collaborated with members of the writing group on the study design, data collection plan, interpretation of data analyses, and the writing of the report. Access to raw data was restricted to AGB, DW, and NE. The corresponding author had final responsibility for the decision to submit for publication.
Results
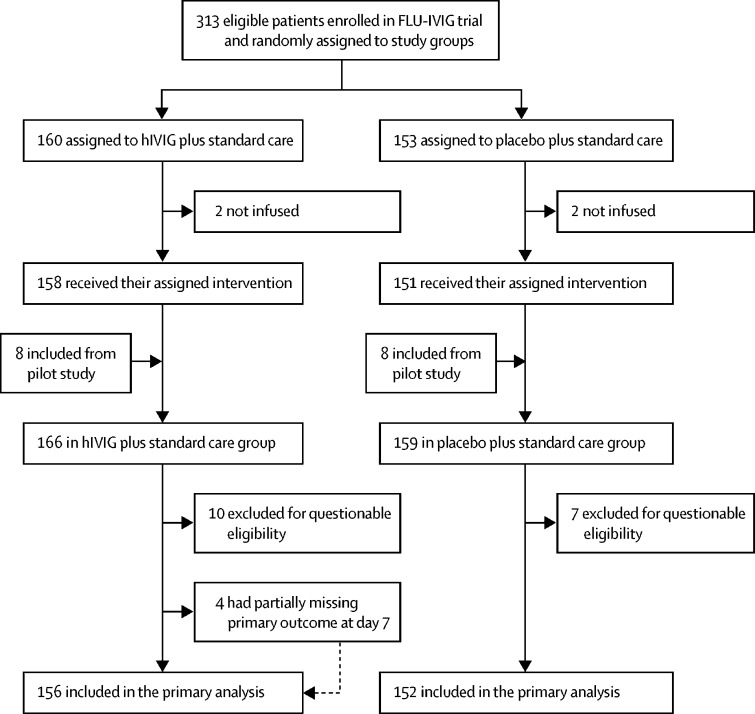
329 patients were enrolled by 34 clinical sites over five influenza seasons, from 2013–14 to 2017–18. The 329 patients included 313 enrolled directly in the FLU-IVIG trial between Dec 11, 2014, and May 28, 2018, and 16 patients from the pilot study enrolled between Jan 15, 2014, and April 10, 2014. 160 patients were assigned to the hIVIG group and 153 were assigned to the placebo group. Additionally, eight of the pilot-study patients received hIVIG and eight received placebo. Thus, 168 recruited patients were included in the hIVIG group and 161 in the placebo group (figure 1). 21 patients were excluded (12 from the hIVIG group and nine from the placebo group) because they did not receive an infusion or their eligibility could not be confirmed. Of the 308 remaining patients, the day-7 outcome was partially missing (discharge status was known) for four patients, but they were included in the analysis after data for them were imputed. Thus, the primary analysis included all 308 patients (156 in the hIVIG group and 152 in the placebo group). All four patients with missing data were discharged before day 7; one withdrew consent after discharge and three could not be contacted. On the last day of follow-up, survival status was unknown for two patients, one in each treatment group.
Figure 1.
FLU-IVIG trial profile
hIVIG=hyperimmune intravenous immunoglobulin.
Apart from the patients enrolled while in intensive care, more of whom were in the placebo group (23 [15%]) than the hIVIG group (12 [8%]), the two treatment groups were well balanced at baseline (table 1, appendix p 14). Median age was 57 years (IQR 45–68), and 168 (55%) of the 308 patients were women. 73 (24%) of all patients had an A(H1N1) infection, 137 (44%) had an A(H3N2) infection, and 84 (27%) had an influenza B infection. The proportion of patients with influenza A ranged from 69 (64%) of 107 patients recruited between October, 2016, and September, 2017, to 14 (87%) of 16 patients recruited between December, 2013, and September, 2014, over the five seasons of enrolment (appendix p 27). The median number of days since symptom onset at entry was 3 (2–5) and median NEW score at enrolment was 4 points (2–6). 256 (83%) of the 308 evaluable patients were randomly assigned to groups on the date of screening, and for these patients the baseline and eligibility NEW scores are identical. The remaining 52 (17%) patients were screened on the day before randomisation, and for these patients the baseline NEW score could have decreased below 2 points. Treatment with neuraminidase inhibitors (oseltamivir) was prescribed to 148 (95%) patients in the hIVIG group and to 144 (95%) patients in the placebo group (appendix p 14). For 155 (50%) patients, oseltamivir was administered no later than 2 days after symptom onset.
Table 1.
Baseline characteristics of the patients
| hIVIG group (n=156) | Placebo group (n=152) | |
|---|---|---|
| Age (years) | ||
| <40 | 35 (22%) | 19 (13%) |
| 40–59 | 57 (37%) | 62 (41%) |
| ≥60 | 64 (41%) | 71 (47%) |
| Median (IQR) | 55 (41–68) | 57 (48–68) |
| Sex | ||
| Male | 76 (49%) | 64 (42%) |
| Female | 80 (51%) | 88 (58%) |
| Race | ||
| Asian | 33 (21%) | 36 (24%) |
| Black | 27 (17%) | 30 (20%) |
| White and other | 96 (62%) | 86 (57%) |
| Clinical status at enrolment | ||
| In intensive care | 12 (8%) | 23 (15%) |
| Hospitalised, requiring supplemental oxygen | 68 (44%) | 59 (39%) |
| Hospitalised, not requiring supplemental oxygen | 76 (49%) | 70 (46%) |
| Influenza serotype | ||
| A/H1N1 | 34 (22%) | 39 (26%) |
| A/H3N2 | 72 (46%) | 65 (43%) |
| A/subtype unknown | 8 (5%) | 6 (4%) |
| B/Victoria | 6 (4%) | 7 (5%) |
| B/Yamagata | 34 (22%) | 30 (20%) |
| B/lineage unknown | 2 (1%) | 5 (3%) |
| Days since symptom onset | ||
| ≤3 | 85 (54%) | 74 (49%) |
| 4 | 27 (17%) | 34 (22%) |
| ≥5 | 44 (28%) | 44 (29%) |
| Median (IQR) | 3 (2–5) | 4 (2–5) |
| NEW score | ||
| <2 | 19 (12%) | 21 (14%) |
| 2–3 | 55 (35%) | 46 (30%) |
| 4–5 | 38 (24%) | 40 (26%) |
| ≥6 | 44 (28%) | 45 (30%) |
| Median (IQR) | 4 (2–6) | 4 (2–6) |
Data are n (%) or median (IQR). Percentages might not add up because of rounding. NEW=National Early Warning.
The adjusted OR of hIVIG versus placebo for the primary endpoint was 1·25 (95% CI 0·79–1·97, p=0·33; table 2). The clinical status of patients on the basis of the primary ordinal outcome after data imputation is summarised in the appendix (p 29). The unadjusted OR was similar (1·26 [0·83–1·90], p=0·28), and there was no evidence that the proportional odds assumption was violated (p=0·79), as seen from the similarity of the five ORs (hIVIG vs placebo) estimated from the six categories of the ordinal outcome (table 2). Planned sensitivity analyses for the primary outcome showed consistent results, including the sensitivity analysis that excluded the pilot study participants (OR 1·31 [0·82–2·08], p=0·26; appendix p 17).
Table 2.
Outcomes summary
| hIVIG group (n=156) | Placebo group (n=152) | OR or HR (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Ordinal outcome on day 7 | .. | .. | 1·25 (0·79–1·97) | 0·33 |
| Binary components of the primary outcome | ||||
| Categories 1–5 (vs 6; ie, alive vs dead) | 153 (98%) | 150 (99%) | 0·66 (0·11–4·14) | 0·66 |
| Categories 1–4 (vs 5–6) | 147 (94%) | 139 (91%) | 1·20 (0·44–3·26) | 0·72 |
| Categories 1–3 (vs 4–6) | 132 (85%) | 123 (81%) | 1·17 (0·59–2·31) | 0·65 |
| Categories 1–2 (vs 3–6; ie, discharged vs hospitalised) | 124 (79%) | 111 (73%) | 1·33 (0·72–2·44) | 0·36 |
| Categories 1 (vs 2–6) | 68 (44%) | 60 (40%) | 1·29 (0·72–2·31) | 0·39 |
| Secondary outcomes | ||||
| Primary ordinal outcome on day 3 | .. | .. | 0·87 (0·57–1·33) | 0·52 |
| Primary ordinal outcome on day 14 | .. | .. | 1·17 (0·70–1·95) | 0·55 |
| Primary ordinal outcome on day 28 | .. | .. | 0·90 (0·50–1·62) | 0·73 |
| Ordinal five-category outcome on day 3 | .. | .. | 0·95 (0·61–1·48) | 0·84 |
| Favourable sliding dichotomy outcome at day 7 | 128 (82%) | 115 (76%) | 1·49 (0·81–2·74) | 0·20 |
| Alive and discharged on day 28 | 140/155 (90%) | 137/151 (91%) | 0·87 (0·38–1·98) | 0·74 |
| Time to discharge through day 7 | 119 (76%) | 110 (72%) | 1·11 (0·85–1·45) | 0·44 |
| Viral load below the lower level of detection at day 3* | 22/137 (16%) | 28/137 (20%) | 0·61 (0·31–1·20) | 0·15 |
Data are n (%) or n/N (%), unless otherwise indicated. Percentages might not add up because of rounding. ORs are adjusted for baseline clinical status, region, and participation in the pilot study. A HR is reported only for the time-to-discharge outcome, with worst rank for death and stratification by the same baseline characteristics. The categories of the primary outcome are: (1) discharged with full resumption of normal activities; (2) discharged but unable to resume normal activities; (3) hospitalised, not requiring supplemental oxygen; (4) hospitalised but requiring supplemental oxygen; (5) in intensive care; and (6) dead. Evaluable participants for the ordinal outcome on day 3 were 155 in the hIVIG group and 152 in the placebo group. For the ordinal outcome on day 14, the evaluable patients were 152 in the hIVIG troup and the 151 in the placebo group. For the ordinal outcome on day 28, the evaluable patients were 151 in the hIVIG group and 150 in the placebo group. For the five-category outcome, there were 155 evaluable patients in the hIVIG group and 152 in the placebo group. HR=hazard ratio. NEW=National Early Warning. OR=odds ratio.
Adjusted for baseline viral RNA, geographical region, and influenza subtype. Excludes participants with undetectable viral RNA at baseline and counts deaths before day 3 as detectable.
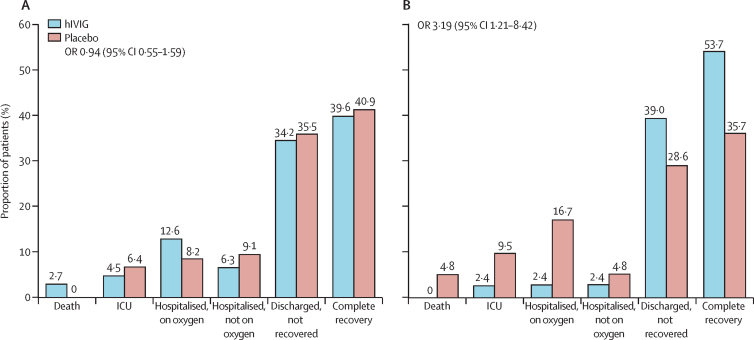
The OR for the primary ordinal outcome was determined for patients divided into subgroups according to characteristics at study entry (appendix pp 31–32). Findings for most subgroups, including duration of symptoms, ward of admission, use of oxygen at enrolment, and timing of antiviral use, were consistent with the overall findings. However, contrary to our preplanned hypothesis, a more favourable outcome for the hIVIG group than for the placebo group was seen in patients with influenza B infection (OR 3·19 [95% CI 1·21–8·42]) compared with those with influenza A infection (0·94 [0·55–1·59]; interaction p=0·023; figure 2). Other prespecified clinical outcomes followed a similar pattern to the primary endpoint in showing no benefit of hIVIG compared with placebo in patients with influenza A infection, but evidence of benefit of hIVIG for those with influenza B infection (appendix pp 21–22). Planned sensitivity analyses for the primary outcome gave consistent results for both the influenza A and B subgroups (appendix p 23). Furthermore, when the primary endpoint was assessed on each of the first 7 days of follow-up with recorded data, all but two of the ORs for patients with influenza A infection were less than 1·0, and those greater than 1·0 were both 1·06 on days 5 and 6. ORs on days 1 and 2 were significantly less than 1·0, favouring the placebo group (interaction p=0·99 for assessing the heterogeneity of the ORs over days 1–7; appendix p 24). For patients with influenza B infection, all ORs were greater than 1·0, particularly those on days 3–7, which were significant. The largest OR was on day 5 (OR 12·4 [4·1 to 37·6], interaction p=0·10; appendix p 25).
Figure 2.
Proportional distribution of primary endpoint categories at day 7 of follow-up for patients infected with influenza A and B
(A) Proportions have been calculated from day-7 data for 111 patients in the hIVIG group and 110 in the placebo group. The OR estimate includes imputed data for three additional patients in the hIVIG group (n=114); these patients were discharged before day 7 and the imputation was on whether normal activities had been resumed. (B) Proportions have been calculated from day-7 data for 41 patients in the hIVIG and in the 42 placebo group. The OR estimate includes imputed data for one additional patient in the hIVIG group (n=42); this patient was discharged before day 7 and the imputation was on whether normal activities had been resumed. hIVIG=hyperimmune intravenous immunoglobulin. ICU=intensive care unit. OR=odds ratio.
The lack of efficacy of hIVIG measured by the primary endpoint for patients with influenza A was evident for both serotypes (A[H1N1] OR 0·61 [95% CI 0·21–1·78], A[H3N2] 0·79 [0·41–1·54]; appendix p 31). Conversely, treatment differences for the two influenza B lineages both favoured hIVIG. The OR for those patients with the major Yamagata lineage was 4·37 (1·3–14·7). For those with the minor Victoria lineage, the OR could not be determined, but all six patients randomly assigned to receive hIVIG were discharged at day 7, whereas only four of the 7 assigned to placebo had been discharged.
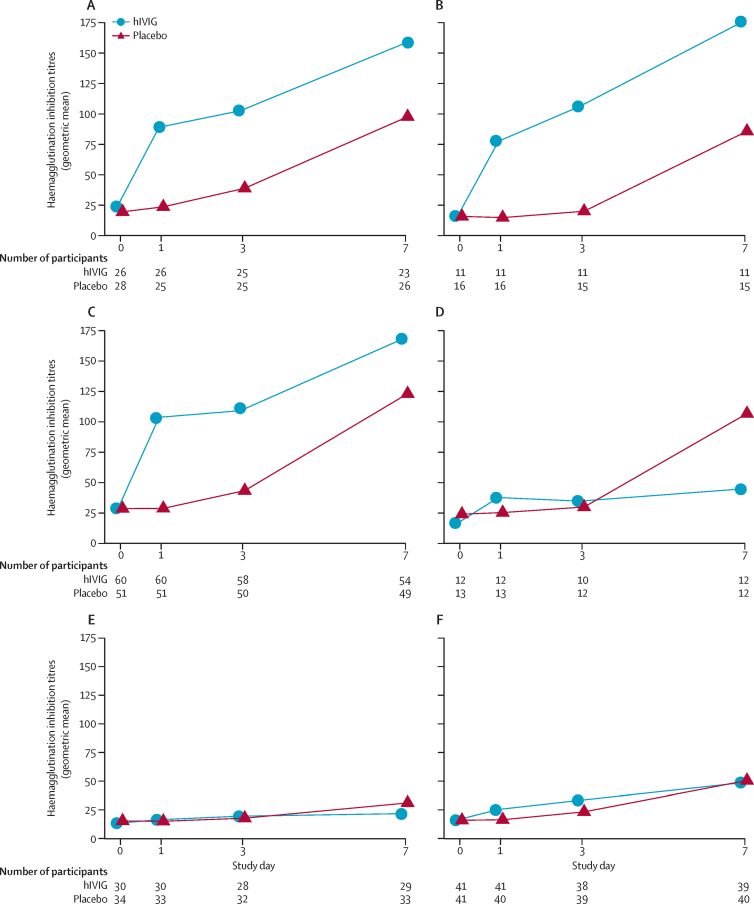
In patients with A(H1N1)pdm09 infection, haemagglutination inhibition titres for A/California/07/2009/A(H1N1) and A/Michigan/45/2015, the two reference viruses we considered, increased in the hIVIG group on day 1 (p<0·0001 for California, p<0·0001 for Michigan) and remained significantly higher than titres in the placebo group at day 3 (p=0·0001 for California, p<0·0001 for Michigan; figure 3, appendix p 15). By day 7, patients in the placebo group had developed natural immune responses and the treatment differences were no longer significant (p=0·18 for A/California/07/2009/A[H1N1], p=0·10 for A/Michigan/45/2015). A similar pattern of a smaller treatment difference in titres at day 7 (p=0·13) compared with day 1 (p<0·0001) and 3 (p<0·0001) was evident for patients infected with A(H3N2) for the reference virus A/Hong Kong/4801/2014. For those infected with A/Switzerland/2013/50/2012, titres in the hIVIG group differed from the placebo group at day 1 (p=0·0001) but not afterwards (p=0·65 for day 3, p=0·25 for day 7).
Figure 3.
Haemagglutination inhibition titres by reference virus and treatment group
Patients infected with influenza A(H1N1) with (A) A/California/07/2009 or (B) A/Michigan/45/2015 used as the reference virus for analysis. Patients infected with influenza A(H3N2) with (C) A/Hong Kong/4801/2014 or (D) A/Switzerland/2013/50/2012 used as the reference virus for analysis. Patients infected with influenza B with (E) B/Brisbane/60/2008 or (F) B/Phuket/3073/2013 used as the reference virus for analysis. hIVIG=hyperimmune intravenous immunoglobulin.
For patients with influenza B infection, haemagglutination inhibition titres were measured for both the B/Brisbane/60/2008 and B/Phuket/3073/2013 serotypes (figure 3). The increase in titres over time for both the hIVIG and placebo groups was lower than for patients with influenza A. Treatment differences were not significant at days 1, 3, or 7 for B/Brisbane/60/2008 (p values ranged from 0·13 to 0·33). For the B/Phuket/3073/2013 reference virus, titres in the hIVIG group were significantly higher than in the placebo group at days 1 (p=0·0004) and 3 (p=0·021), but not day 7 (p=0·78).
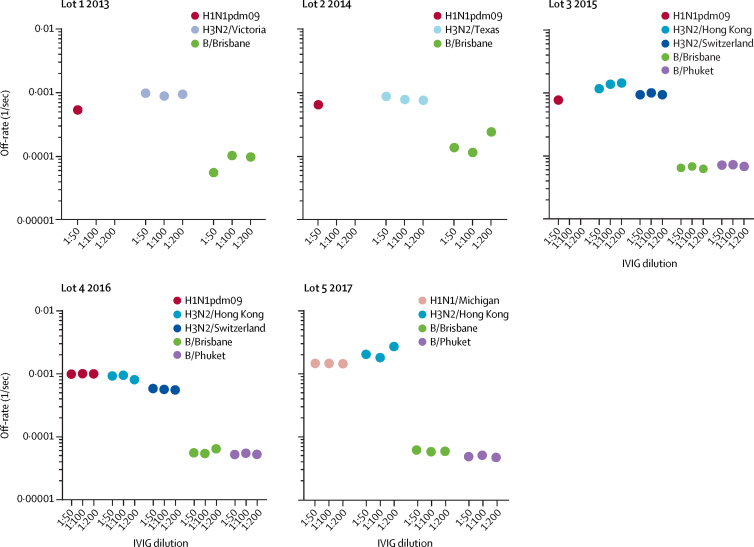
The greater increase in haemagglutination inhibition titres with hIVIG against influenza A compared with influenza B is consistent with the lot analysis (appendix p 12). However, in a post-trial exploratory laboratory analysis, the antibody kinetics showed an approximately tenfold (range 5·3–17·7) higher antibody affinity (slower antigen-antibody complex dissociation rates) against the haemagglutinin of influenza B strains than that of either the H1 or H3 vaccine strain for all hIVIG lots (figure 4). In most lots, anti-influenza B haemagglutinin-binding antibodies were higher than anti-H1 or anti-H3 antibodies, but not by much (appendix p 28).
Figure 4.
Antibody affinity of hIVIG to properly folded haemagglutinin of seasonal influenza vaccine strains in SPR analysis
Sequential SPR analysis of human hIVIG was done against properly folded homologous haemagglutinin from the H1N1pdm09, H3N2, and B influenza vaccine strains for each lot of hIVIG used. 50-fold, 100-fold, and 200-fold dilutions of individual hIVIG lots were evaluated. Antibody off-rate constants that describe the fraction of antibody-antigen complexes decaying per second were determined directly from the hIVIG interaction with each of the three haemagglutinin proteins using SPR in the dissociation phase (appendix p 7). Slower dissociation kinetics (off-rate) of antigen-antibody complex means higher antibody affinity. hIVIG=hyperimmune intravenous immunoglobulin. SPR=surface plasmon resonance.
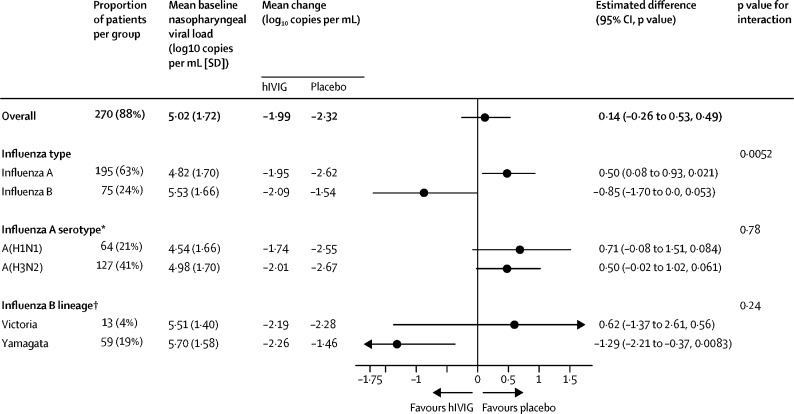
Shed viral load decreased by 1·99 log10 copies per mL in the hIVIG group and 2·32 log10 copies per mL in the placebo group during the first 3 days (p=0·49; figure 5, appendix p 16). At day 3, 22 (16%) of the 137 assessed patients in the hIVIG group and 28 (20%) of 137 assessed patients in the placebo group had no detectable virus (p=0·15; table 2). For the subgroup of patients who received antiviral drugs within 2 days of symptom onset, viral load decreased by 2·75 log10 copies per mL in the hIVIG group and 3·08 log10 copies per mL in the placebo group (p=0·74).
Figure 5.
Change in overall and serotype-specific nasopharyngeal viral load
Analyses only include participants with detectable viral load at baseline and are adjusted for baseline viral load and geographical region. The overall analysis was also adjusted for influenza subtype. *Excludes five participants with unknown serotype. †Excludes three participants with unknown lineage.
ORs of the ordinal outcome on each of the first 7 days of follow-up (data as observed with no imputation; appendix p 18) showed that outcomes on days 1–3 in the placebo group were more favourable, but not significant, than in the hIVIG group. The OR on day 5 was 1·75 (95% CI 1·13–2·70, p=0·013). The interaction p value for the test for heterogeneity of the OR over days 1 to 7 was 0·39.
Similar to the primary outcome, differences between the hIVIG and placebo group in other clinical outcomes were not significant (table 2).
Through 28 days of follow-up, a similar number of patients administered hIVIG and placebo died (six [4%] in the hIVIG group and five [3%] in the placebo group), had serious adverse events (25 [16%] in the hIVIG group, 26 [17%] in the placebo group), and had grade 3 or 4 adverse events (45 [29%] in the hIVIG group and 40 [26%] in the placebo group; table 3, appendix pp 19–20). The composite outcome of death, serious adverse events, or grade 3 or 4 events was seen in 47 (30%) patients in the hIVIG group and in 45 (30%) patients in the placebo group (hazard ratio [HR] 1·06 [95% CI 0·70–1·60], p=0·79; table 3, appendix p 30). Viral load and safety outcome treatment differences followed a pattern similar to the primary endpoint for patients with influenza A and B. In patients with influenza A, the decline in viral load from baseline to day 3 was actually greater for the placebo group than the hIVIG group (difference 0·50; p=0·021), whereas for those with influenza B the decline in viral load was greater for the hIVIG group than for the placebo group (difference −0·85, p=0·053, p=0·0052 for interaction between treatment group and influenza serotype; figure 5, appendix p 26).
Table 3.
Death and adverse events
| hIVIG group (n=156) | Placebo group (n=152) | |
|---|---|---|
| Event | ||
| Death | 6 (4%) | 5 (3%) |
| Any serious adverse event | 25 (16%) | 26 (17%) |
| Any grade 4 adverse event | 22 (14%) | 17 (11%) |
| Any grade 3 adverse event | 31 (20%) | 34 (22%) |
| Composite outcomes ranked by severity | ||
| Death | 6 (4%) | 5 (3%) |
| Death or serious adverse event | 25 (16%) | 26 (17%) |
| Death, serious adverse event, or grade 4 adverse event | 29 (19%) | 29 (19%) |
| Death, serious adverse event, or grade 3 or 4 adverse event* | 47 (30%) | 45 (30%) |
Adverse events are graded according to the DAIDS toxicity table. DAIDS=Division of Acquired Immunodeficiency Syndrome. HR=hazard ratio.
HR 1·06, 95% CI 0·70–1·60, p=0·79.
In patients with influenza A, the composite of death, serious adverse events, or grade 3 or 4 events occurred for 40 (35%) patients administered hIVIG and 29 (26%) patients administered placebo (HR 1·49 [95% CI 0·91–2·42], p=0·11; appendix p 33). For those with influenza B infection, this composite occurred for seven (17%) patients in the hIVIG group and 16 (38%) patients in the placebo group (0·38 [0·15–0·97], p=0·043 interaction p=0·010; appendix p 34).
Discussion
In this randomised controlled trial assessing immunotherapy as a potential therapeutic adjunct in patients with severe influenza, a significant treatment benefit of hIVIG plus standard care (oseltamivir) compared with placebo plus standard care was not found either overall or for the predefined subgroup of interest with influenza A infection (224 [73%] of 308 trial participants). The findings for the primary clinical outcome and several secondary clinical outcomes were matched by the inability of hIVIG treatment to induce a larger reduction in influenza A viral load in the upper respiratory tract than placebo. These findings for patients with influenza A are consistent with the results of a contemporaneous randomised trial (IRC005) of high-titre anti-influenza plasma done in 140 patients with influenza A infection.10 In that trial, which enrolled a moderately sicker cohort than ours, the same primary endpoint was used and the OR (high-titre vs low-titre plasma) for a favourable outcome on day 7 was 1·22 (95% CI 0·65–2·29). Taken together, the findings from the subgroup of patients with influenza A in our trial of hIVIG and the IRC005 trial provide no support for the use of immunotherapy to treat patients with severe influenza A.
Unlike the trial of high-titre anti-influenza plasma that only enrolled patients with influenza A, in our trial of hIVIG 84 (27%) patients had influenza B infection. Contrary to what was hypothesised on the basis of conventional haemagglutination inhibition titres, the addition of hIVIG to standard care did result in both a demonstrable clinical and a virological benefit in patients with influenza B. The OR in favour of a clinical benefit on the primary outcome for hIVIG use in patients with influenza B was 3·19 (95% CI 1·21–8·42). Secondary clinical and viral load outcomes were consistent with the primary outcome results for this subgroup. Particularly, the effect of hIVIG treatment on deaths and adverse events, which might reflect the underlying medical condition of patients better than adverse effects of treatment, was also greater in patients with influenza B than those with influenza A infection.
The rationale for choosing hIVIG as a potential therapeutic was based on the known course of the development of haemagglutination inhibition antibodies during natural infection that appears to correlate temporally with clinical recovery. Infusion of an anti-influenza IVIG product produces a substantial rise in haemagglutination inhibition titres against A(H1N1) and A(H3N2) at least 3–4 days sooner than those induced by natural infection itself.14 By contrast, acute infection with influenza B (or after seasonal vaccination) produces a haemagglutination inhibition response against B virus that typically is much more muted when tested by conventional means, and the corresponding increase in anti-influenza B titres after hIVIG infusion is generally smaller. For this reason, we launched the hIVIG trial expecting to observe a greater treatment effect against the two major influenza A subtypes.
In preclinical studies of A(H5N1) infection in ferrets, increasing IVIG dose was associated with improved efficacy.25 Measurement of post-infusion haemagglutination inhibition titres against contemporaneously circulating influenza A strains in our trial did indeed suggest that the expected boost in titres was achieved in recipients of hIVIG. Accordingly, we anticipated that this measurably greater increase in haemagglutination inhibition titres against influenza A than against B might yield better efficacy in patients with influenza A infection. Yet this strategy to jumpstart the humoral response to acute infection by several days translated into more rapid clinical recovery and more rapid virological decline only in patients with influenza B, whose post-infusion titres increased little beyond baseline. As such, the conventional haemagglutination inhibition titres resulting from administration of hIVIG appear to have been poor predictors of both clinical and virological efficacy in our trial.
These results raise at least two obvious questions: (1) whether conventional haemagglutination inhibition antibody titres, despite their longstanding use as predictors of post-vaccination efficacy against both influenza A and B,15, 26 are truly an appropriate surrogate for an effective humoral response in therapeutic studies against influenza A, and hence whether better markers of antibody-mediated activity might exist that should be substituted, and (2) whether the overall host immune response to infection with influenza B might differ in some fundamental way from that against influenza A. It is known, for example, that acute influenza infection is also associated with pronounced impairment of the cellular immune response, including defective T-cell responses and a sometimes lethal unbridling of the proinflammatory cytokine cascade.27, 28 It is possible that the two major types of influenza virus interact differentially with the immune system and that, for example, the neutralising activity of virus-specific antibodies is more important in the host response to B virus infection than to infection with virus A. Alternatively, the immunomodulatory effects29 of the Fc portion of IVIG itself might behave differently in patients with either of these two types of influenza. It might also be conjectured that the generally greater antiviral potency of oseltamivir against influenza A subtypes than against influenza B might somehow have overshadowed the potential additive beneficial effects of the immunotherapy in the subset of patients with influenza A infection. However, arguing strongly against this possibility are the observations that (1) the improvement in viral titres over time in the influenza A subgroup was, in fact, worse than in the influenza B subgroup, and (2) the absence of benefit in the influenza A subgroup was no different in those for whom antiviral treatment was started early (ie, within 2 days of symptom onset) than in those in whom treatment was delayed.
Rather, perhaps a more probable explanation for this difference might be found in the antibody kinetic analyses of hIVIG lots after unmasking of the primary data. Striking differences between the affinities of anti-A and anti-B antibodies within the hIVIG lots were found. Unlike conventional haemagglutination inhibition testing, these analyses showed that the antibody avidity against influenza B was often up to tenfold higher than it was against either subtype of influenza A. A study30 showed that higher anti-haemagglutinin antibody affinity provided better control of viral load in nasal washes and weight loss in vaccinated ferrets after challenge with wild-type H7N7 or H7N9 influenza virus.30 Although the mechanism for these avidity differences in human plasma is unknown, it is clear that the role of antibody affinity and other antibody effector functions need to be carefully evaluated to assess their importance in providing protection against severe disease due to influenza A and influenza B.
A limitation of our results is that the subgroup with influenza B included only 84 patients (27% of all participants in the trial). Thus, our estimates of efficacy have wide CIs. The consistent findings for this subgroup across other clinical outcomes and viral load argue for further exploration of this finding. Another limitation is the fact that 17 patients from one site had to be excluded because their eligibility could not be confirmed. In the sensitivity analyses, their exclusion had little impact on estimated ORs for the primary endpoint, but did reduce our power slightly. Finally, we note that our target population of patients hospitalised with influenza included only 35 (11%) of patients in intensive care at the time of enrolment and about half had NEW scores less than or equal to 3 points. Thus, we are not able to generalise our findings to severely ill patients. However, subgroup analyses according to NEW score, location of enrolment, use of oxygen at entry, and duration of symptoms at the time of enrolment did not identify consistent trends. Taken together with the lack of overall benefit in severely ill patients in the two plasma trials9, 10 and the Hong Kong trial of hIVIG,11 the absence of benefit of immunotherapy for patients with influenza A might apply to a broader population than we studied.
Of note, the trial's ability to assess the effect of hIVIG on mortality would have required a much larger sample size than we were able to enrol.18 Instead, a novel ordinal primary outcome was defined for a trial with a feasible sample size, and several secondary clinical outcomes were defined to further characterise the possible benefit of hIVIG compared with placebo. An advantage of the primary outcome used compared with mortality, including improved power, is that it assesses both improvement and deterioration in health status.18
In summary, on the basis of our results and the two plasma trials,9, 10 hIVIG and plasma are not recommended for patients hospitalised with influenza A. By contrast, the beneficial clinical and viral load results of hIVIG consistently seen in patients with influenza B warrant further investigation at both the laboratory and clinical levels.
Data sharing
The research protocol, statistical analysis plan, and informed consent documents are available immediately upon publication of this Article. The protocol and informed consent can be found on the INSIGHT website, www.insight-trials.org. Primary and secondary study outcomes from this trial will be entered into ClinicalTrials.gov within 1 year following publication of the primary study results. Requests for access to more detailed study data and deidentified patient data can be submitted to the INSIGHT Scientific Steering Committee using the Research Proposal Form on the INSIGHT website at any time for review and approval on the basis of scientific merit of submitted proposals. Accepted proposals will be issued a letter of endorsement by the Committee.
Acknowledgments
Acknowledgments
The INSIGHT FLU-IVIG trial was primarily funded by the NIAID Intramural Research Program and the NIAID Division of Clinical Research, NIH, and the legal sponsor for the trial was the University of Minnesota. This project has been funded in whole or in part with US federal funds from the National Cancer Institute, National Institutes of Health, under contracts HHSN261200800001E and HHSN2612015000031. IND15771 is held by Regulatory Compliance and Human Subjects Protection Branch, NIAID. Support for the London and Copenhagen International Coordinating Centres was also provided by the UK Medical Research Council (MRC_UU_12023/23) and the Danish National Research Foundation. The content of this Article does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organisations imply endorsement by the US Government. There were no agreements concerning confidentiality of the data between the sponsor and the authors or the institutions named in the credit line. The authors wish to thank the patients who participated in this study.
Contributors
RTD, EF-C, NM, SP, AGB, DW, FG, VK, JL, JHB, HCL, and JDN designed the study. RTD, EF-C, NM, SP, NE, MKJ, VK, MNP, PR, KR, ZT, JL, JHB, HCL, and JDN collected the data. RTD, EF-C, NM, SP, AGB, DW, SK, NE, MKJ, VK, MNP, PR, KR, ZT, JL, JHB, HCL, JDN contributed to data analysis, data interpretation, writing of the manuscript, and figure preparation.
Declaration of interests
EF-C reports personal fees from the US National Institutes of Health (NIH), during the conduct of the study, and personal fees from OCTAPHARMA and grants from Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (Madrid, Spain), outside the submitted work. AGB reports grants from the University of Minnesota, (Minneapolis, MN, USA) and the UK Medical Research Council, during the conduct of the study. MKJ reports grants from National Institute of Allergy and Infectious Diseases (NIAID) Influenza Research Collaboration, during the conduct of the study. PR reports grants from NIAID/NIH, during the conduct of the study, and grants from Janssen Pharmaceutica and Emergent Biosolutions, outside the submitted work. KR reports grants from NIH, during the conduct of the study. JDN reports grants from NIAID, during the conduct of the study. The remaining authors declare no competing interests.
Contributor Information
Richard T Davey, Jr, Email: rdavey@niaid.nih.gov.
INSIGHT FLU-IVIG Study Group:
Richard T Davey, Eduardo Fernández-Cruz, Norman Markowitz, Sarah Pett, Abdel G Babiker, Deborah Wentworth, Surender Khurana, Nicole Engen, Fred Gordin, Mamta K Jain, Virginia Kan, Mark N Polizzotto, Paul Riska, Kiat Ruxrungtham, Zelalem Temesgen, Jens Lundgren, John H Beigel, H Clifford Lane, James D Neaton, Jessica Butts, Eileen Denning, Alain DuChene, Eric Krum, Merrie Harrison, Sue Meger, Ross Peterson, Kien Quan, Megan Shaughnessy, Greg Thompson, David Vock, Julia Metcalf, Robin Dewar, Tauseef Rehman, Ven Natarajan, Rose McConnell, Emily Flowers, Kenny Smith, Marie Hoover, Elizabeth M Coyle, David Munroe, Bitten Aagaard, Mary Pearson, Adam Cursley, Helen Webb, Fleur Hudson, Charlotte Russell, Aminata Sy, Cara Purvis, Brooke Jackson, Yolanda Collaco-Moraes, Dianne Carey, Rosemary Robson, Adriana Sánchez, Elizabeth Finley, Donna Conwell, Marcelo H Losso, Luciana Gambardella, Cecilia Abela, Paco Lopez, Helena Alonso, Giota Touloumi, Vicky Gioukari, Olga Anagnostou, Anchalee Avihingsanon, Kanitta Pussadee, Sasiwimol Ubolyam, Bola Omotosho, Clemencia Solórzano, Tianna Petersen, Kranthi Vysyaraju, Stacey A Rizza, Jennifer A Whitaker, Raquel Nahra, John Baxter, Patricia Coburn, Edward M Gardner, James A Scott, Leslie Faber, Erica Pastor, Linda Makohon, Rodger A MacArthur, L Monique Hillman, Marti J Farrough, Hari M Polenakovik, Linda A Clark, Roberto J Colon, Ken M Kunisaki, Miranda DeConcini, Susan A Johnson, Cameron R Wolfe, Laura Mkumba, June Y Carbonneau, Alison Morris, Meghan E Fitzpatrick, Cathy J Kessinger, Robert A Salata, Karen A Arters, Catherine M Tasi, Ralph J Panos, Laura A Lach, Marshall J Glesby, Kirsis A Ham, Valery G Hughes, Robert T Schooley, Daniel Crouch, Leticia Muttera, Richard M Novak, Susan C Bleasdale, Ariel E Zuckerman, Weerawat Manosuthi, Supeda Thaonyen, Thaniya Chiewcharn, Gompol Suwanpimolkul, Sivaporn Gatechumpol, Sirikunya Bunpasang, Brian J Angus, Monique Anderson, Marcus Morgan, Jane Minton, Maria N Gkamaletsou, Joe Hambleton, David A Price, Martin J Llewelyn, Jonathan Sweetman, Javier Carbone, Jose R Arribas, Rocio Montejano, Jose L Lobo Beristain, Iñaki Z Martinez, Jose Barberan, Paola Hernandez, Dominic E Dwyer, Jen Kok, Alvaro Borges, Christian T Brandt, Lene S Knudsen, Nikolaos Sypsas, Costas Constantinou, Antonios Markogiannakis, Spyros Zakynthinos, Paraskevi Katsaounou, Ioannis Kalomenidis, Analia Mykietiuk, Maria F Alzogaray, Mora Obed, Laura M Macias, Juan Ebensrtejin, Patricia Burgoa, Esteban Nannini, Matias Lahitte, Santiago Perez-Patrigeon, José Arturo Martínez-Orozco, and Juan Pablo Ramírez-Hinojosa
Supplementary Material
References
- 1.Iuliano AD, Roguski K, Chang HH. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cilek L, Chowell G, Farinas R. Age-specific excess mortality patterns during the 1918–1920 influenza pandemic in Madrid, Spain. Am J Epidemiol. 2018;187:2511–2523. doi: 10.1093/aje/kwy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paules C, Subbarao K. Influenza. Lancet. 2017;390:697–708. doi: 10.1016/S0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- 4.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomized controlled trials. Lancet. 2015;385:1729–1737. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez J, Peyrani P, Wiemken T. A randomized study evaluating the effectiveness of oseltamivir initiated at the time of hospital admission in adults hospitalized with influenza-associated lower respiratory tract infections. Clin Infect Dis. 2018;67:736–742. doi: 10.1093/cid/ciy163. [DOI] [PubMed] [Google Scholar]
- 6.Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 7.Luke TR, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 8.Mair-Jenkins J, Saavedra-Campos M, Baillie JK. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigel JH, Tebas P, Elie-Turenne MC. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med. 2017;5:500–511. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel JH, Aga E, Elie-Turenne M-C. Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2019 doi: 10.1016/S2213-2600(19)30199-7. published online Sept 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung Ivan FN, To Kelvin KW, Lee Cheuk-Kwong. Hyperimmune IV immunoglobulin treatment: a multicentre double-blind randomized controlled trial for patients with severe A(H1N1)pdm09 infection. Chest. 2013;144:464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Physicians of London National early warning score (NEWS): standardising the assessment of acute-illness severity in the NHS. 2012. www.rcplondon.ac.uk/resources/national-early-warning-score-news
- 13.Khurana S, Verma N, Yewdell JW. MF59 adjuvant enhances diversity and affinity of antibody mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.INSIGHT FLU005 IVIG Pilot Study Group INSIGHT FLU005: an anti-influenza virus hyperimmune intravenous immunoglobulin pilot study. J Infect Dis. 2016;213:574–578. doi: 10.1093/infdis/jiv453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohmit SE, Petrie JG, Cross RT. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 2011;204:1879–1885. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 16.Hobson D, Curry RL, Beare AS. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza viruses. 2007. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Guidance-for-Industry–Clinical-Data-Needed-to-Support-the-Licensure-of-Pandemic-Influenza-Vaccines.pdf
- 18.Peterson RL, Vock DM, Powers JM. Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials. 2017;14:264–276. doi: 10.1177/1740774517697919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute of Allergy and Infectious Diseases Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0, December 2004, clarification August 2009. 2004. https://rsc.niaid.nih.gov/sites/default/files/table-for-grading-severity-of-adult-pediatric-adverse-events.pdf
- 20.Whitehead J. Sample size calculations for ordered categorical data. Stat Med. 1993;12:2257–2271. doi: 10.1002/sim.4780122404. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 22.Lan KG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 23.Agresti A, Kateri M. Categorical data analysis. In: Lovric M, editor. International encyclopedia of statistical science. Springer; Berlin, Heidelberg: 2011. [Google Scholar]
- 24.Murray GD, Barer D, Choi S. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma. 2005;22:511–517. doi: 10.1089/neu.2005.22.511. [DOI] [PubMed] [Google Scholar]
- 25.Rockman S, Lowther S, Camuglia S. Intravenous immunoglobulin protects against severe pandemic influenza infection. EBioMedicine. 2017;19:119–127. doi: 10.1016/j.ebiom.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowling BJ, Lim WW, Perera RAPM. Influenza hemagluttination-inhibition antibody titer as a mediator of vaccine-induced protection for influenza B. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy759. published online Sept 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao R, Bhatnagar J, Blau DM. Cytokine and chemokine profiles in lung tissues from fatal cases of 2009 pandemic influenza A (H1N1): role of the host immune response in pathogenesis. Am J Pathol. 2013;183:1258–1268. doi: 10.1016/j.ajpath.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altenburg AF, Rimmelzwaan GF, de Vries RD. Virus-specific T cells as correlate of (cross-) protective immunity against influenza. Vaccine. 2015;33:500–506. doi: 10.1016/j.vaccine.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 29.Sicca F, Neppelenbroek S, Huckriede A. Effector mechanisms of influenza-specific antibodies: neutralization and beyond. Expert Rev Vaccines. 2018;17:785–795. doi: 10.1080/14760584.2018.1516553. [DOI] [PubMed] [Google Scholar]
- 30.Khurana S, Coyle EM, Verma S. H5 N-terminal β sheet promotes oligomerization of H7-HA1 that induces better antibody affinity maturation and enhanced protection against H7N7 and H7N9 viruses compared to inactivated influenza vaccine. Vaccine. 2014;32:6421–6432. doi: 10.1016/j.vaccine.2014.09.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research protocol, statistical analysis plan, and informed consent documents are available immediately upon publication of this Article. The protocol and informed consent can be found on the INSIGHT website, www.insight-trials.org. Primary and secondary study outcomes from this trial will be entered into ClinicalTrials.gov within 1 year following publication of the primary study results. Requests for access to more detailed study data and deidentified patient data can be submitted to the INSIGHT Scientific Steering Committee using the Research Proposal Form on the INSIGHT website at any time for review and approval on the basis of scientific merit of submitted proposals. Accepted proposals will be issued a letter of endorsement by the Committee.