Abstract
Neuroregeneration following peripheral nerve injury is largely mediated by Schwann cells (SC), the principal glial cell that supports neurons in the peripheral nervous system. Axonal regeneration in vivo is limited by the extent of SC migration into the gap between the proximal and distal nerve, however, little is known regarding the principle driving forces for SC migration. Engineered microenvironments, such as molecular and protein gradients, play a role in the migration of many cell types, including cancer cells and fibroblasts. However, haptotactic strategies have not been applied widely to SC. Herein, a series of tethered laminin-derived peptides were analyzed for their influence on SC adhesion, proliferation, and alignment. Concentration gradient substrates were fabricated using a controlled vapor deposition method, followed by covalent peptide attachment via a thiol-ene reaction, and characterized by X-ray photoelectron spectroscopy (XPS) and MALDI-MS imaging. While tethered RGD peptides supported SC adhesion and proliferation, concentration gradients of RGD had little influence on biased SC directional migration. In contrast, YIGSR promoted less SC attachment than RGD, yet YIGSR peptide gradients directed migration with a strong bias to the concentration profile. With YIGSR peptide, overall speed increased with the steepness of the peptide concentration profile. YIGSR gradients had no haptotactic effect on rat dermal fibroblast migration, in contrast to fibroblast migration on RGD gradients. The response of SC to these tethered peptide gradients will guide the development of translationally relevant constructs designed to facilitate endogenous SC infiltration into defects for nerve regeneration.
Keywords: Cell migration, Schwann cells, laminin-derived peptides, concentration gradient, haptotaxis
Graphical Abstract

INTRODUCTION
Peripheral nerve injuries occur as a result of physical distress or compression during a traumatic injury [1]. Peripheral nerve injuries are reported in 3% of all trauma patients with more than 360,000 cases reported each year in the United States resulting in $150 billion in medical costs [2]. To address the limitations of current clinical approaches, an improved understanding of the neuroregenerative process is necessary to design therapeutic devices that lead to functional recovery [3, 4]. Following an injury, Schwann cells (SC) de-differentiate and migrate into the damaged site to assist fibroblasts and macrophages in phagocytosis and clearing of debris [5, 6]. SC also release growth factors, organize the basement matrix, and finally, myelinate and protect the regenerated nerve [7, 8]. Recognizing the importance to SC involvement in nerve regeneration, methods and substrates that are able to specifically guide the directional migration of Schwann cells into defect sites may facilitate improved recovery and function [9].
Random cell migration is a stochastic process that is driven by both soluble and bound factors. [10, 11]. Cell migration is integral to any regenerative process, and the study of immune [12–14], fibroblast [15–17], epithelial [18, 19], as well as many other cell types, has yielded a wealth of information regarding endogenous tissue regeneration. Chemotaxis, or directed migration due to soluble concentration gradients, has been investigated for over 130 years, and is a driving force for many processes in vivo [20–22]. While directed migration of SC due to adsorbed laminin was discovered more than 35 years ago [23], the focus of the neural migration literature has been the axon [24]. Therefore, little information exists on how to utilize extracellular matrix (ECM)-based gradients to direct SC response in nerve repair.
The ECM provides binding sites between cells and their microenvironments, helps to regulate proliferation, migration, growth factor sequestration, and provides cell signaling through surface receptors [25]. Laminin is one of the primary components of the basement membrane. Laminin-1, laminin-2, and fibronectin have been shown to increase Schwann cell migration in vitro [26]. In addition to its role in directed migration, laminin is crucial in facilitating nerve regeneration, myelination, and increasing axonal growth, which are essential for proper function of the nervous system [27]. Laminin-1 is a large and flexible glycoprotein that consists of three polypeptide chains designated as α1, β1 and γ1. Several fragments of the laminin sequence have been identified and tested for bioactivity, including RGD, YIGSR, IKVAV, RNIAEIIKDI, and PDSGR [28, 29]. For example RGD, which is known to be a versatile cell attachment site in fibronectin and in various other proteins [30], has been grafted to synthetic [31] and natural [32] materials to promote the neuroregenerative process. In combination, YIGSR and RGD have been found to support SC attachment and proliferation [33], stimulate neurite guidance in vitro and promote sciatic nerve regeneration in vivo [34]. Polypyrrole doped with IIKDI supported neurite outgrowth [35]. IKVAV selectively promoted neuronal differentiation [36, 37], and PDSGR was found to enhance attachment and survival of dissociated brain tissue [38]. Peptides have the advantage of higher stability and increased purity compared to the native protein, and are more economical to synthesize than the entire molecule by recombinant methods [39, 40].
We have shown previously the utility of tethered peptide concentration gradients as a rapid, efficient, and precise platform for the quantitative analysis of cellular phenomena [41–45]. In the present study, laminin-derived peptides were investigated for their capacity to influence primary SC responses. A confined channel vapor deposition method was used to generate well-defined linear concentration gradients, which were characterized by multiple methods. SC were seeded onto the substrates to quantify adhesion, proliferation, and morphology relative to peptide sequence, peptide concentration and slope of the gradient. Finally, migration experiments yielded mean squared displacement, bias direction and cell velocity in the direction of the concentration gradient, determined relative to both the peptide and steepness of the gradient concentration profile.
EXPERIMENTAL
Microscope glass coverslips (Fisher Scientific) were used as substrates for functionalization with concentration gradient of five different peptides: CRGDS (Cys-Arg-Gly-Asp-Ser), CYIGSR (Cys-Tyr-Ile-Gly-Ser-Arg), CQAAIKVAV (Cys-Gln-Ala-Ala-Ile-Lys-Val-Ala-Val), CRNIAEIIKDI (Cys-Arg-Asn-Ile-Ala-Glu-Ile-Ile-Lys-Asp-Ile) and CPDSGR (Cys-Pro-Asp-Ser-Gly-Arg). The RGD sequence used has been derived from fibronectin. Tethered self-assembled monolayers with uniform peptide concentrations were used as controls.
Materials
All materials were used as received unless otherwise noted. 5-hexanyldimethylchlorosilane was purchased from Gelest (Morrisville, PA). Fluorenylmethyloxycarbonyl (FMOC)-protected amino acids and resins for peptide synthesis were purchased from Aapptec (Louisville, KY). Gradient substrates were fabricated on glass coverslips (No 1; 25 × 25 mm, Fisherbrand™ borosilicate) and used for cell culture after sterilization by a 12 h ethylene oxide cycle using an Anprolene benchtop sterilizer (Anderson products, Inc, Haw River, NC), according to manufacturer’s protocol at room temperature and 35 % humidity, followed by a 48 h purge. All other chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Materials for cell study
Forskolin, N2 supplement, bovine pituitary extract, anti-S100b antibody (LOT 093M4784), poly-L-lysine hydrobromide, and primary anti-vinculin antibody (LOT 016M4857V) were purchased from Sigma Aldrich (St. Louis, MO). Alexa Fluor 546 anti-mouse secondary antibody (LOT 665662) was purchased from Invitrogen (Carlsbad, CA). Alexa Fluor 488 Phalloidin, fetal bovine serum, and Dulbecco’s Modification of Eagle’s Medium (DMEM) were purchased from ThermoFisher Scientific (Waltham, MA).
Solid phase peptide synthesis
Peptides were synthesized by standard solid phase FMOC chemistry [46]. Peptides were purified by equilibrium dialysis and freeze-dried by lyophilization. The chemical structures of the products were confirmed by electrospray ionization (ESI) and/or matrix-assisted laser desorption ionization (MALDI) mass spectrometry: Cys-Arg-Gly-Asp-Ser (CRGDS) [M + H]+ = 577.3 Da, Cys-Tyr-Ile-Gly-Ser-Arg (CYIGSR) [M + H]+ = 698.4 Da, Cys-Gln-Ala-Ala-Ile-Lys-Val-Ala-Val (CQAAIKVAV) [M + H]+ = 903.2 Da, Cys-Arg-Asn-Ile-Ala-Glu-Ile-Ile-Lys-Asp-Ile (CRNIAEIIKDI) [M + H]+ = 1288.5 Da and Cys-Pro-Asp-Ser-Gly-Arg (CPDSGR) [M + H]+ = 634.3 Da.
Functional concentration gradient substrate fabrication
Vinyl-terminated gradient fabrication
Peptide concentration gradients substrates were fabricated using a “vacuum away” confined channel vapor deposition method. The resulting substrates possess a linear chemical concentrations gradient profile. Vinyl terminated chemical gradient precursors were fabricated using a vapor deposition method previously reported [44, 45, 47, 48]. Substrates were washed with methanol and toluene and blown dry with nitrogen followed by a pretreatment with ultraviolet light generated ozone (UVO; Jelight Company Inc. Model No. 42A) for one hour to remove organic contaminants. A Teflon support, with a channel for a chlorosilane reservoir (1.5 × 2.5 × 1.1 cm) and the glass substrate (7.5 x 2.5 x 1.0 cm), was inserted into a rectangular glass tubing (30 × 2.5 × 1.3 cm) which was interned in a sealed metal chamber. A dynamic vacuum (4 mPa) was pulled from the side nearest the reservoir and away from the substrate. Methanol was injected through the opposite side to quench the diffusion process. A 10 % solution of 5-hexenyldimethylchlorosilane in toluene (150 μL, neat in the Teflon reservoir) was used as the chemical source for the fabrication of vinyl-terminated gradient. Vapor deposition was performed for 10 seconds, and substrates were washed successively with methanol and toluene, and blown dry under nitrogen. Samples were stored in a vacuum desiccator at room temperature until use.
One pot functionalization
The concentration profile can be varied using a combination of diffusion distance, stoichiometry and time the substrate is exposed to the vapor. Establishment of the concentration gradient was followed by a thiol-ene “click reaction”. The respective cysteine tagged peptides (5 mmol.mL−1) were dissolved in H2O/dimethylformamide (DMF) (9:1 by volume) and Irgacure 2959 was added as a photoinitiator (25 mmol.mL−1). Samples were immersed in the solution and left to react under UV (254 nm) for 2 h at room temperature [49]. Surface-adsorbed residues were removed by short sonication (2 min) in DMF, washed with methanol and blown dry with nitrogen gas. Samples were stored in a vacuum desiccator at room temperature until use.
Contact angle measurement
Advancing contact angles were measured using an Advanced Goniometer (Rame-Hart Instrument Co., Model 500) at room temperature and analyzed by a drop shape analysis method (ImageJ, http://imagej.net/Downloads). Seven different probe liquids were used to obtain the gradient’s position dependant surface tension using the Zisman method: ultrapure water (18 MΩ cm−1), propylene glycol (≥ 99.5 %, Sigma Aldrich), tetraethylene glycol (99 %, Alfa Aesar), diethylene glycol (99 %, Alfa Aesar), triethylene glycol (99 %, Alfa Aesar), ethylene glycol (99 %, Sigma Aldrich), and glycerol (99 %, Sigma Aldrich). Data for each probe liquid was collected from five equidistant points on the surface of each substrate at 3 mm intervals for 18 × 18 mm2 samples, 5 mm intervals for 18 × 30 mm2 samples, and 10 mm intervals for 18 × 50 mm2 samples. Standard uncertainty was determined by standard error calculations between measurements on three samples prepared under identical conditions with each measurement performed at a virgin site of the analyzed material and taken after a 5-15 sec equilibration period. Small droplet volumes of 2 μL were used to minimize the effect of gravity on the measurements.
X-ray Photoelectron Spectroscopy
The concentration profile and functionality of the gradient substrates were determined by XPS measurements, using silicon wafers as substrates. The XPS spectra were obtained using a VersaProbe II Scanning XPS Microprobe from Physical Electronics (PHI), under ultrahigh vacuum conditions (2×10−6 Pa). Automated dual beam charge neutralization was used during the analysis of the samples. The analyzer pass energy was 117.4 eV for the survey spectra, and 11.75 eV or 23.5 eV for the high-resolution scans in the C1s or N1s regions, respectively. The survey scans in the range 0-700 eV were used to evaluate the percentage of different atoms present on the surface of the samples. Atomic concentrations were calculated with PHI MultiPak software. The XPS high resolution spectra of C1s and N1s were decomposed into respective components by using the curve fitting routine in MultiPak. A quality of fit (χ) higher than 1.6 was achieved for each data set. Each spectrum was collected using a monochromatic (Al Kα) x-ray beam (E = 1486.6 eV) over a 100 μm diameter probing area with a beam power of 100 W. For each given position of the gradient, the fraction of total vinyl sites at which the functionalization and peptide linking conversion occurred were calculated by determining the local ratio of nitrogen to carbon. Based on the reaction chemistry and the expected ratios of nitrogen to carbon for each step, the extent of peptide tethering to the surface was converted to density (and concentration) based on the molecular mass of the peptide [45].
Surface MALDI-MS and mass spectroscopy imaging (MSI) data acquisition
MALDI-ToF-MS measurements were performed with a Bruker UltraFlex III ToF/ToF instrument, equipped with a Nd/YAG laser emitting at a wavelength of 355 nm. The polymer standard used to calibrate the m/z scale was 2000 g.mol−1 poly(methyl methacrylate) using trans-2-(3-(4-tert-butylphenyl)-2-methyl-2-propenylidene) malononitrile) (DCTB) matrix and sodium trifluoroacetate (NaTFA) to enable ionization. The MALDI-ToF-MS experiments were performed using the following parameters: Reflectron Ion Mode; detection mass range: 600-2000 Da; sample rate and digitizer settings: 1.00 GS/s; realtime smoothing: High; analog offset: 50.8 mV; ion suppression up to: 600 m/z; mode: Gating; gating strength: Medium; Voltages: Ion Source 1: 25.03 kV; ion source 2: 23.42 kV; lens: 6.00 kV; pulsed ion extraction: 100 ns; polarity: Positive; Laser: Nd/YAG 355 nm); global attenuator offset: 57 %; attenuator offset: 17 %; attenuator range: 19 %; focus offset: 0%; focus range: 100 %; focus position: 34 %; smartbeam parameter set: 100.0 Hz; digitizer sensitivity: 100 mV; analog offset linear: 50.8 mV; analog offset reflector: 51.2 mV; trigger level: 1000 mV; digital offset linear: 127 cnt; digital offset reflector: 127 cnt; Detector Voltages: detector gain: 34×1837 V; linear base: 1300.00 V; reflector base: 1400.00 V; linear boost: 0.00 V; reflector boost: 0.00 V. Data analyses were conducted using the Bruker Daltonics flexAnalysis software and flexImaging v2.1 software.
Cell experiments
Primary SC was isolated from adult female Sprague Dawley rat sciatic nerves after sacrifice under an approved IACUC protocol. The purification process was based on a selective culture medium according to previously published protocol, using forskolin (5 μM) and N2 supplement (1 % vol/vol) plus bovine pituitary extract (20 μg.mL−1) as SC growth stimulant, and D-valine as inhibitor for fibroblast overgrowth [50]. After 21 days, the SC culture had a 97 % purity average, confirmed by anti-S100b antibody immunocytochemistry. Cells were maintained using Dulbecco’s Modified Eagle’s Medium (DMEM) with 10 % fetal bovine serum (FBS), and maintenance flasks were coated with 1.5 μg.cm−2 poly-L-lysine. Cells were passed at confluence and used between passages 3 and 10. Rat dermal fibroblasts (ScienCell Research Laboratories, Carlsbad, CA) isolated from adult rat skin were cultured with recommended Fibroblast Medium-2 (ScienCell Research Laboratories) according to the protocol provided by the ScienCell, and used at passage 3.
Cell adhesion and growth
The concentration gradient samples (25 mm2) of peptides and respective uniform self-assembled monolayers controls were placed in 6-well plates. Cells were seeded at a density of 3 × 104 cells.cm−2 in a serum-free medium for 4 h to eliminate adsorbed proteins interfering with the initial cell attachment, rinsed and cultured in serum-containing media (10 % FBS + DMEM). After 24 h and 3 days of culture, the nuclei were labeled with DAPI (6 μL/10mL in PBS for 20 min) and samples were imaged in their entirety with an IX81 microscope (Olympus). Cell numbers at each concentration region were measured by nuclei counting.
Doubling time for cells cultured in each one of the peptide substrates was calculated using the standard equation:
Cell focal adhesion complexes and actin organization
Cells were seeded on samples and cultured as described above. After 24 h and 3 day incubation periods, the samples were fixed with 3.7 % paraformaldehyde in PBS, permeabilized with 0.5 % TritonX-100 for 10 minutes and quenched with 0.05 % fresh sodium borohydride to remove excess formaldehyde. Samples were then blocked from non-specific binding with 5 % donkey serum in PBS for 1 h. Incubation in the primary anti-vinculin antibody (mouse monoclonal IgG, diluted 1:100 in 1 % donkey serum in PBS) was conducted at 4 °C overnight. The following day, the primary antibody solution was aspirated and substrates were incubated with anti-mouse Alexa Fluor 546 (diluted 1:400 in PBS) and Alexa Fluor 488 Phalloidin (diluted 1:40 in PBS) to label the actin filaments. Nuclei were labeled with DAPI (6 μL/10mL in PBS). Cells were observed using fluorescence microscopy and five images were taken at each concentration on each substrate (n = 3). Cell morphology and focal adhesion were characterized by visual examination.
Cell orientation
To study the influence of peptide gradient orientation and bias on cell alignment, images were analyzed using a previous described actin alignment method written in MATLAB, with a few modifications.[51] The method uses edge detection and statistical analysis to measure percent alignment ± 10° of each cell based on the concentration gradient direction.
Cell migration
A cell front migration assay was used to study cell migration behaviors on intermediary positions of the YIGSR and RGD gradients, and their respective uniform self-assemble monolayer controls and a uniform concentration of adsorbed laminin sample. Cells were seeded at a high concentration of 7.5 × 104 cells.cm−2 around confluency, in a 0.5 mm × 2 mm, 1.0 cm2 restrained space, using serum free media. The gap was created by silicone isolator pieces placed on the top of each sample and fixed with stereo grease. A diamond pen was used to create a visual mark on the substrates close to the edge of the isolators, identifying the initial position of the attached cells. After 8 h, the isolators were removed, samples were washed with 1X PBS and media were replaced to serum containing DMEM. Cell front migration behaviors were monitored in situ every 15 min for 24 h using a time-lapse phase-contrast microscope equipped with an incubator chamber (37 °C and 5 % CO2 humidified atmosphere). A manual tracking plugin in NIH ImageJ software was used to track the centroid of each individual cell. The original position of each cell was defined as the origin (0,0) and a sequence of (x,y) coordinators were recorded over time, allowing to reconstruct the individual cell trajectories over the observation time. At least 30 cells were chosen randomly from each sample for analysis, excluding any mitotic and spherical dead cell. The migration direction of cells in each sample was determined by plotting the total distance travelled over the 24 h period by the angle of displacement between each cell’s initial and final position. The average mean square displacement was calculated over each time interval (Eq. 1)
| (Eq. 1) |
where x and y are the positions at times t0 and t + t0 [15].
To study the effect of concentration gradient slope on SC behavior, a free cell migration assay was used. CYIGSR gradients with three different steepness. A 5-hexenyldimethylchlorosilane gradient and uniform SAM of the respective peptide were used as controls. Schwann cells were seeded uniformly along the entire sample at a low density of 1 × 104 cells.cm−2 to avoid cell-cell interactions, and let adhere and spread using a serum free medium. After 8 h, the samples were rinsed with PBS, the media was replaced to a serum containing DMEM. The cells at intermediary concentrations were tracked for 24 h as described above. A minimum of 50 cells were recorded for each study group.
For both studies, the analysis of cell motions were based on a dynamic model for the motion of individual cells. The concentration gradient is assumed to be directed along the x-axis. The overall velocity (x,y) is assumed to be a vector composed of x and y components, and a bias velocity (vx) is the average rate of motion along the direction of the chemical gradient.
Statistical analysis
Minitab was used for statistical evaluation and a normality test was performed for each experiment. Experiments for cell growth, focal adhesion, and actin alignment were conducted three times (n = 3), using 3 gradient substrates for each peptide and 3 uniform concentration SAM substrates as controls. Quantitative data for such experiments are presented as the average ± standard deviation. Two-way ANOVA (to assess the effect of time and peptide) with Tukey’s (cell growth and focal adhesion) or Dunnett’s (cell alignment) post hoc analysis was performed via General Linear Model and used to express statistical differences with 95% confidence interval. For migration studies, average ± standard deviation are presented for velocity values, and average ± standard error presented for MSD. A minimum of 30 cells were tracked for each study group. A significance level of p < 0.05 was considered to detect significant differences.
RESULTS AND DISCUSSION
Controlling SC migration in neural tissue engineering has the potential to direct and support axonal infiltration across injured nerve gaps [52]. Haptotaxis is the directional cell migration along gradient profiles of surface-bound cues. Several haptotatic cues for guiding axon growth have been identified, including the laminin molecule and its derivatives. Herein, we investigated how laminin peptides can directly influence SC migration.
Gradients fabrication and surface characterization
A sufficient external concentration difference is required to activate cell migration. Cells tend to respond to a gradient of surface-bound ligand density by directing migration towards the direction of the higher peptide concentration [53], in the present study represented by laminin-derived peptides. The guidance cue is dependent not only on the mean concentration of the peptide guidance molecule, but also on the relative slope of the concentration profile. A confined channel vapor deposition method was used to create several well-defined linear gradient chemical concentration profiles of vinyl functional groups on glass cover slides (Supporting Information – SI 1). The allyl-functionalized organosilane and substrate were placed in a sealed chamber and a dynamic vacuum pulled from the opposite direction to create the diffusion concentration gradient, from the most to the least concentrated position. This method, previously developed by Epps et al., and adapted by our group, affords control of the organosilane deposition profile by confining the vapor diffusion to a small gap (≈ 1.5 mm) on the substrate surface. The position of the reservoir, the concentration of the organosilane and the size of the substrate can be tuned to vary the concentration profile [42, 44, 45, 54]. The magnitude, or steepness of the gradient, was controlled by tuning the dimensions of the glass substrates, from 18 × 18 mm2 to 18 × 50 mm2. Organosilane-based self-assembled monolayers (SAM) present allyl groups for photoinitiated thiol-ene addition reactions of thiol-(cysteine) functionalized peptides (Fig. 1A) [55].
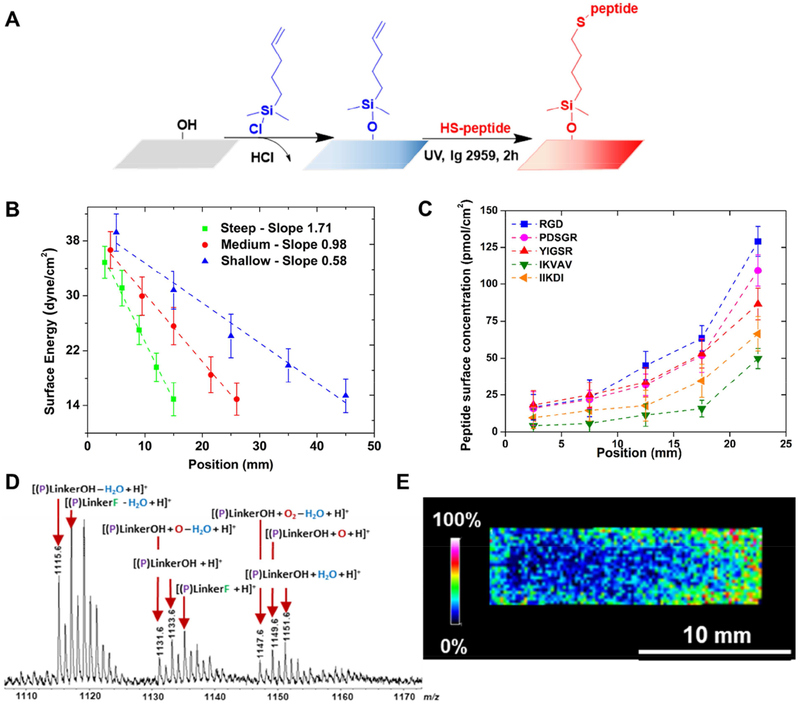
Figure 1.
Functional self-assembled peptide gradients fabrication and characterization. (A) Fabrication scheme. First, one-dimensional, vinyl-terminated concentration gradient substrates are fabricated on a confined chamber by vapor deposition using 5-hexenyldimethylchlorosilane and 25 mm2 glass slides as substrates. Cysteine-terminated peptide can then be attached to the surface using a thiol-ene “click” reaction. (B) The vinyl end group gradient profile was characterized by surface energy. Data was collected from five equidistant points on the surface at 5 mm intervals. (C) Average peptide concentration calculated based on XPS C1s and N1s high resolution peaks, based on three individually fabricated and characterized substrates for each peptide of interest. Peptides concentration increased monotonically along the x direction of the substrate. (D) The peptide gradient profile was confirmed by MALDI-MS analysis, here represented by a CQAASIKVAV concentration gradient sample. MS peaks corresponding to the tethered peptide mass and linker. (E) The choropleth intensity image corresponds to the m/z signal, which increases along the concentration gradient.
One of the most common parameters used to characterize the surface of materials is the surface free energy, which can be determined by measurement of the surface tension using the sessile drop method. To verify the linearity of the concentration gradients of the vinyl-functionalized substrates, static contact angle measurements of several testing liquids were performed.
The Zisman method was used to determine the critical surface free energy (γc) at each position of the vinyl terminated gradients [56, 57]. The relationship between wettability and surface free energy (SFE) can be given by Young’s equation:
| (Eq. 2) |
where, θ is the water contact angle, γs is the SFE of the solid, γsl the SFE of the solid-liquid interface, and γl the SFE of the liquid. According to Zisman method, an interpretation of the Young’s equation (Eq. 3) gives the critical surface concentration, γc which can be determined from an empirical investigation consisted of the contact angle measurement for the surface of the solid and a series of organic compound liquids of known γ1
| (Eq. 3) |
A plot of the cosine values of contact angle as a function of γl values for the corresponding liquids was traced, and the yc deduced by extrapolation at cosθ = 1. The water contact angle increased ~ 30° along the length of the gradients following the hydrophobic nature of the organosilane precursor (Supporting Information - SI 2A). Using this method, three different gradient concentration profiles of grafting were characterized. The gradual change of the surface energy was noted by the variation of concentrations, demonstrating that the gradients were successfully fabricated. Surface energies underwent a mean decrease along the length of all three different steepness substrates, ranging from 39.2 dyne.cm−2 to 15.4 dyne.cm−2 for shallow samples, 26.7 dyne.cm−2 to 14.9 dyne.cm−2 for medium steepness samples, and 34.9 to dyne.cm−2 to 14.2 dyne.cm−2 for steep samples with slopes equal to 0.58 dyne.cm−3, 0.98 dyne.cm−3 and 1.71 dyne.cm−3, respectively (Fig. 1B). It is important to notice that the surface energy decreases slightly after the addition of peptide compared to the precursor vinyl-organosilane treated substrate (as shown in Supporting Information – SI 2B for YIGSR peptide).
Our approach for fabrication of bioactive surface gradients can be extended to a variety of species, since different functionalities can be added to the substrate. Each of the peptides used in this study had a cysteine amino acid at the N-terminus allowing a sequential thiol-ene reaction between thiol groups and vinyl functional groups. The grafting was performed using UV irradiation (λ = 254 nm) at ambient temperature. The functionalization of each engineered (uniform and gradient) substrate was verified using a X-ray photoelectron spectroscopy (XPS) survey spectra (0-700 eV), which allows identification of the individual elements within the monolayer (Supporting Information – SI 3). In the survey spectra for each one of the five peptide functionalized substrates, it was observed the expected peaks from oxygen (O1s), silicon (Si2s and Si2p), carbon (C1s), and nitrogen (N1s), confirming that 5-hexenyldimethylchlorosilane was successfully grafted onto the surface and efficiently covalently linked to each peptide through the surface thiol-ene reaction.
The concentration profiles were further verified using high resolution XPS. The C1s and N1s curves were fitted based on the Gauss-Lorentz function with Multipak software, considering a linear background, and peak areas were calculated by integration as described previously [42] (Supporting Information – SI 4). The peak areas increased along the direction of the gradient for each peptide studied, which supports the concept of linear gradient fabrication. The nitrogen-to-carbon ratios were used to determine the fraction of alkyl-silane-peptide linkages per surface area, and the actual immobilized peptide concentrations were calculated from each peptide molecular mass (Fig. 1C). As expected, the peptide concentrations increased monotonically. Differences observed in absolute values varied for each peptide that can be attributed to the particular physico-chemical and molecular properties of each sequence of amino acids. Indeed, the different sizes, charges and water-solubilities influence the availability and accessibility of the reactive sites for each biomolecule during the thiol-ene reaction. However, all the peptide densities display a similar progression, ranging from 15 pmol.cm−2 to 130 pmol.cm−2 for RGD, 15 pmol.cm−2 to 110 pmol.cm−2 for PDSRG, 20 pmol.cm−2 to 90 pmol.cm−2 for YIGSR, 10 pmol.cm−2 to 70 pmol.cm−2 for IIKDI and 5 pmol.cm−2 to 50 pmol.cm−2 for IKVAV.
When chemical compounds are covalently tethered to a surface, their exact chemical identity is difficult to determine, especially for biomolecules such as peptides. Nevertheless, sensitive and accurate characterization of these molecules can be achieved by soft mass spectroscopy (MS) techniques, like electrospray ionization (ESI) or matrix-assisted laser desorption ionization – time-of-flight (MALDI-ToF). For this, a preparative approach was developed for the analysis of the peptides covalently bound on gradient substrates via siloxane (Si-O) connectivity, based on the known ability of fluoride ions to break Si-O bonds [58]. Ammonium fluoride (NH4F) solution was applied onto the functionalized substrate (prior to MALDI-ToF analysis) to selectively cleave in situ the Si-O bonds connecting the peptide to the surface. The liberated peptide retained the linker chemistry and was terminated with either OH or F end groups.
MALDI-ToF spectra obtained, using α-cyano-4-hydroxycinnamic acid (CHCA) matrix, illustrated this method’s ability to observe intact molecular ions containing peptide (P) and - S(CH2)5Si(CH3)2X linker, where X is either OH or F (as shown in Fig. 1D for IKVAV peptide). Ions representative of this chemistry included [(P)LinkerOH + H]+ (m/z 1133.6) and [(P)LinkerF + H]+ (m/z 1135.6). Additional oxidation (+16 Da), water addition (+18 Da), and water loss (−18 Da) resulted in the presence of a distribution of ions.
MALDI-MS imaging (MALDI-MSI) was performed and revealed the localization of the peptides on the substrates. To accomplish this analysis, NH4F was first applied to an IKVAV-grafted substrate by using a thin layer chromatography (TLC) sprayer to liberate the peptide-linker without effectively disturbing the positions of the tethered species on the surface. Next, a CHCA matrix solution (20 mg.mL−1) was sprayed uniformly on the sample surface and an image was acquired for a 5 × 15 mm region-of-interest (ROI) on the glass surface with a 150 μm MALDI-MSI resolution. The image displayed an intensity gradient of the ions at m/z 1110-1160, as represented by the choropleth image, showing gradually higher intensity signal from left to right of the ROI (Fig. 1E). This result provided strong evidence that the Si-O bond cleavage and matrix application steps preserved the spatial resolution of the peptide on the surface and visually revealed the tethered peptide gradient.
Effect of peptide gradient on Schwann cell response
Nerve repair following injury is largely mediated by SC, the principal glia that supports neurons in the peripheral nervous system. During neuroregeneration, axonal recovery only proceeds to the extent to which SC migrate within the damaged tissue, and therefore, enhancing SC migration through directional control could improve the endogenous repair [59]. SC use the basement membrane to support migration [60], and laminin is a key glycoprotein of the basement membrane associated with this process [29]. Specific fragments of laminin have been shown to mimic partial functions of the larger molecule [61]; such peptides can be easily synthesized, modified with additional functional groups, and immobilized on surfaces. As previously introduced, the five sequences of peptides selected for the present study have been described in several works for their potential in peripheral nerve regeneration. Herein, we characterized their haptotactic capacity to direct SC migration.
Cell adhesion and growth
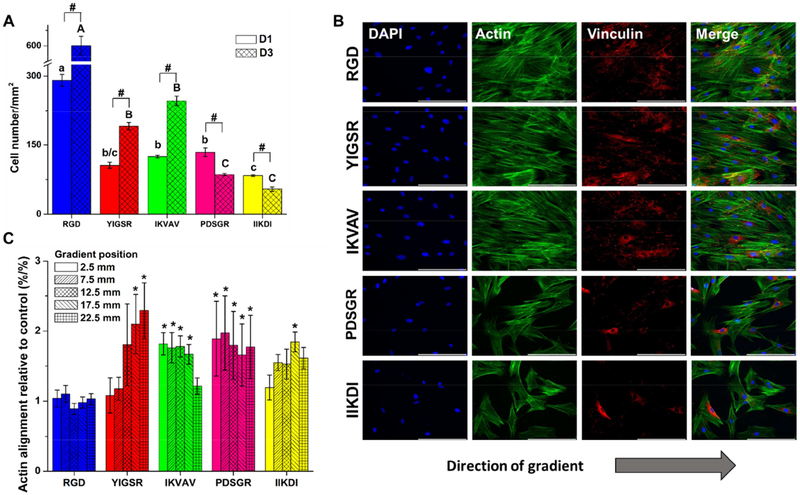
Laminin-derived peptides were first examined for their ability to support SC growth. The purity of the primary SC culture was confirmed by immunofluorescence for S100 (Supporting Information – SI 5). Cells were seeded in serum-free conditions to measure the impact of immobilized peptides on cell adhesion, and then cultured in serum-containing media to sustain the culture. Cell number at 24 h and 3 days was plotted for each concentration range of the gradients and for each SAM uniform controls (Supporting Information – SI 6). Overall, cell adhesion and expansion did not exhibit concentration dependent behavior, with no significant differences in the cell numbers versus position on the gradient. The one exception, IIKDI peptide, exhibited lower cell numbers at higher concentration. Thereafter, a second plot was created maintaining the SAM uniform controls to compare the influence of each peptide on cell adhesion and growth (Fig. 2A). At day 3 (D3 on the graph), evidence of proliferation activity was observed for RGD (142 pmol/cm2), YIGSR (95.1 pmol/cm2) and IKVAV (54.8 pmol/cm2), with significant cell number increase compared to day 1 (D1). RGD substrates had an average doubling time of 46 ± 2 h; 49 ± 3 h for IKVAV; and 56 ± 2 h YIGSR; the average doubling time of the primary SC in the typical growth culture was 56 ± 5 h. PDSGR (120.2 pmol/cm2) and IIKDI (73.0 pmol/cm2) did not support cell growth, and cell number decreased for both peptides over time compared to the previous three peptides. The results for RGD were in agreement with previously reported studies where RGD peptide was described as one of the major recognition systems for cell adhesion and proliferation [33, 62]. It is important to mention that there was a potential for protein deposition to the samples from the serum supplemented media used for the cell culture. Nevertheless, the results clearly show that the peptides were active and that they could be recognized and used by the cells to bind to the substrate.
Figure 2.
SC response to peptide concentration gradients. (A) SC number on peptide uniform SAMs of RGD, YIGSR, IKVAV, CPDSGR and IIKDI, respectively. Cell number was collected at each sample position after 1 and 3 days of culture. Values are represented as means ± standard deviation, with n = 3. # represents significant difference for cell number from D1 to D3, lowercase letters compare cell number for different peptides at D1, and capital letters compare different peptides at D3 (ANOVA with Tukey’s). (B) Immunofluorescent staining of nuclei (blue), vinculin (red), and actin (green) after 3 days of culture showing cell alignment to the gradient direction. Images were taken at 20x magnification. Scale bar: 100 μm. Images indicate that SC have high proliferative potential in RGD samples, reaching confluence after 3 days of culture, while YIGSR promoted cell alignment to the gradient direction, identified by the directionality of the actin filaments. (C) SC alignment as a function of the direction of concentration gradient. A Matlab code based on an edge detection method was used to quantify actin fiber alignment. Data is given as function of percent actin aligned relative to each respective uniform control (%/%) at day 3. Values are represented as means ± standard deviation, with and n = 3. * represents significant difference for actin alignment from a certain position compared to the respective control (ANOVA with Dunnett’s). Data show that RGD concentration gradient, for example, does not promote cell alignment, as opposed to YIGSR, where cell alignment increased with peptide concentration.
Cell binding
While the adhesion and growth data suggested specific interactions between the cells and the substrates, it was important to visualize the ability of the cells to specifically interact with the peptide substrates. Cell substrate interaction is controlled by the density of ligand-receptor pairs [53]. Integrin signaling play a major role on the cytoskeletal assembly leading to changes in cell shape, motility, and adhesion [63]. Milner et al. have shown that β1 integrins mediate SC migration on laminin-1 and laminin-2, while α5 integrins mediate migration on fibronectin. It has been stated that several variants of integrins allow cell binding to the RGD peptide, including α5β1, α5β3, α5β5 [64]. Both IKVAV and YIGSR are thought to be bound through a combination of α3β1, α4β1, and α5β1 [65]. While IKVAV also bounds to a 110 kDa receptor, YIGSR is recognized by a 32 kDa and a 67 kDa laminin receptors [33].
Herein, the focal adhesion sites of SC to peptide substrates were noted through vinculin staining (Fig. 2B and SI 7 for high resolution). All substrates showed clear regions of focal adhesions, with overlapping vinculin (in red) and actin (in green) labeling focal adhesion plaques (in yellow), but it was visible a higher predominance in RGD, YIGSR and IKVAV samples. Quantification of focal adhesion was done by processing of the immunofluorescence images using the Image J software using a protocol previously described by Horzum et. al [66]. As with cell adhesion, focal adhesions did not exhibit concentration dependent behavior, with no significant differences in the individual cell focal adhesion number versus position on the gradient. Therefore, the plot of focal adhesion number per cell represents the values obtained from uniform SAM samples for each peptide (Supporting Information – SI 8). RGD, YIGSR and IKVAV samples presented higher focal adhesion numbers per cell, and for RGD those numbers increased significantly comparing days 1 and 3.
Schwann Cell alignment
Peptide gradients have been previously found to influence cellular polarization [67–69]. Here, polarization was measured by actin alignment to the direction of the gradient in the range of ± 20° from 0°. The percentages of actin alignment for all concentration positions and peptides were normalized to their respective uniform SAM controls (Fig. 2C and SI 9). Surprisingly, RGD peptide gradient substrates produced no significant differences between SC actin alignment of the uniform samples and the gradient samples. On the contrary, in YIGSR samples, actin alignment increased as the peptide concentration increased, reaching a maximum of 2.2 times higher actin alignment at high peptide concentration positions relative to uniform controls. IKVAV, PDSGR and IIKDI gradients promoted SC alignment to the direction of the gradient, but no significant differences were observed in different positions of the sample, implying that the cells were affected by the chemical gradient independently of the peptide concentration.
Cell migration is a highly integrated multistep process, and both directional sensing and polarization are fundamental components. In general, when cells are exposed to an external chemical attractant gradient, they sense the gradient and polarize. Cell polarity is actually inherent to actin filaments polarization, and that is used to drive membrane protrusions. After sensing of an external gradient through membrane receptors, the signal is transmitted to the cell interior. The first step of chemotaxis and haptotaxis involves increasing in actin nucleation and branching, or polarization. In a second step, a combination of filaments elongation and an elastic-Brownian-motion unbending mechanism provides the driving force for protrusion and extension [70–72]. All peptides other than RGD promoted actin alignment of SC to the concentration gradient. In addition to promoting actin alignment overall, increasing the peptide concentration on YIGSR gradients increased the alignment. While it was surprising that RGD gradients did not instigate SC polarization, we decided to continue investigating RGD due to its rich literature base, significant influence on proliferation, and its increase in focal adhesion between days 1 and 3.
It is interesting to bring awareness for the fact that SC will be influenced by peptide-bound interactions and secrete ECM proteins that ultimately have their own role on the cell behavior in each one of the substrates. In our previous work we have shown, for example, that SC cultured on nanofibers decorated with YIGSR peptide secreted laminin and that the same phenomena was not observed on samples with RGD only peptide [33]. The deposition of ECM molecules, such as laminin and collagens, is essential for regulation of SC functions, such as proliferation, actin cytoskeleton dynamics, radial sorting and myelination [73]. For future work, it would be interesting to investigate the ECM molecules secreted by cells cultured in each one of the peptides, and relate to their function in an in vivo nerve injury environment.
Cell migration
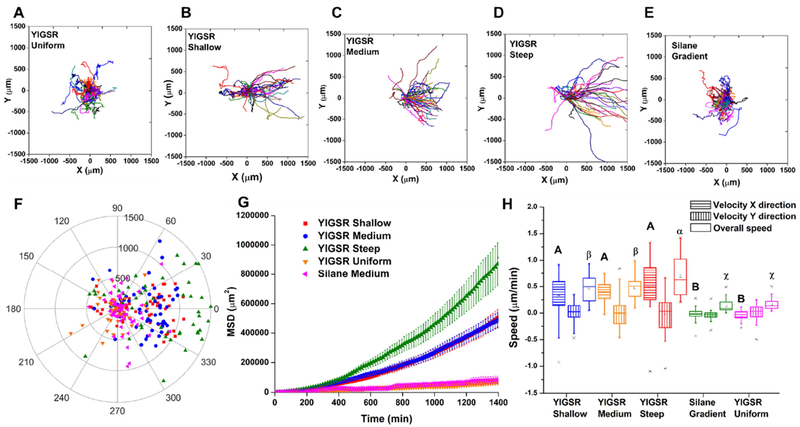
Previous studies of cell migration on gradients have shown that cells migrate preferentially towards regions of increasing adhesiveness [74]. Based on the cell adhesion, expansion, and polarization data, RGD and YIGSR peptide gradients were selected for migration studies. For the initial study of SC migration on the concentration gradient substrates, cells were seeded within an isolator at the center of the gradient and subsequently the isolator was removed (Supporting Information – SI 10A). Cells that migrated into the free space were tracked by time lapse microscopy, capturing the individual motility response of each cell. Directional components of cell migration were investigated by plotting individual migration traces in x and y directions (Fig. 3 A–E). In addition, the final migration distance of each cell versus the angle between the starting and ending positions, was plotted in order to compare the cell’s migration direction (Fig. 3F). From Figure 3A and blue plots on the Fig. 3F, it was clearly visible that cells tended to migrate in the x direction, displaying a directional preference for YIGSR concentration gradient samples, with angles of migration between ± 90° relative to the direction of the gradient. For all other samples, including RGD gradient, RGD uniform samples, YIGSR uniform samples, and laminin uniform samples, cells displayed a random walk, with no preferential direction to a negative or positive x position. Directed migration of Schwann cells along YIGSR gradients suggested that the cells responded haptotactically to these substrates.
Figure 3.
Migration behavior of SC in RGD and YIGSR concentration gradients. Time-lapse microscopy was used to track the position of individual cells. Images were taken every 10 min for a total of 24 h per experiment. (A, B, C, D, E) Centroid tracks from 30 representative cells in a typical experiment for YIGSR gradient, uniform YIGSR, RGD gradient, uniform RGD, and uniform laminin, respectively, with initial position of each track superimposed at 0,0 for clarity. (F) Polar plot showing the end point of each cell’s trajectory relative to its origin. Cells moving upward move toward the positive gradient of concentration of the respective peptide. (G) Mean square displacement curves for over 24 h period. (H) Overall velocity and bias velocity to the direction of the gradient μm/min (ANOVA with Tukey’s). Capital letter were used to correlate velocity in the x direction; lowercase letter were used to correlate velocity in the y direction; and Greek letters were used to correlate overall speed. Means that not share a letter were significant different. YIGSR concentration gradients had a positive influence on directing Schwann cell migration.
Mean square displacement (MSD) is a common metric for examining cell migration speed and overall distance traveled [75]. MSD values were averaged from the data for individual cells using overlapping intervals (Fig. 3G). The MSD average for each population showed that the gradient samples induced increased movement of Schwann cells compare to uniform SAM samples. In addition, the YIGSR gradient triggered an increased cell displacement than the RGD gradient and promoted faster overall migration than other study groups, with an overall speed of 0.48 ± 0.19 μm.min−1 (Fig. 3H). In addition to the overall cell velocity, the velocity of the cells in the direction of the gradient was also analyzed. The rate at which the cells migrated towards a higher peptide concentration was interpreted as the bias velocity, vx. The average bias velocity in the x direction stayed near zero on the RGD gradient, uniform RGD, uniform YIGSR, and laminin substrates because cells were just as likely to move to the left as they were to move to the right, resulting in no preferred direction of the cell motility. On the contrary, the cells responded to the YIGSR gradient with positive average x bias velocity values of 0.41 ± 0.18 μm.min−1, significantly higher than all other studied groups. The results indicated that SC exhibited haptotaxis towards higher concentrations of YIGSR peptide.
It is known that during peripheral nerve injury, fibroblasts from the surrounding connective tissue can quickly migrate to the wound site, impeding the progression of SCs and forming fibrous scars and painful neuroma [69]. It is important in neural tissue engineering to use strategies that facilitate the migration of SC over fibroblasts. Therefore, migration of fibroblasts in response to YIGSR and RGD gradients was also characterized using a free cell migration assay (Figure 4). While fibroblasts adhered and survived well on YIGSR samples, they showed a random migration on YIGSR gradient similar to the uniform samples for both RGD and YIGSR peptides. In contrast to SCs, fibroblasts migrated towards higher concentrations of the RGD gradient, in agreement with studies previously shown in literature [68, 76]. For fibroblasts, the x bias velocity (0.28 μm/min) and overall speed (0.29 μm/min) were higher on RGD gradient compared to the other three samples. The bias speed, vx, on YIGSR gradient was 0.02 μm/min, implying that the fibroblasts were as just as likely to move to up or down the YIGSR gradient sample, and that YIGSR did not have a haptotactic effect on fibroblasts.
Figure 4.
Migration behavior of rat dermal fibroblasts in RGD and YIGSR concentration gradients. Time-lapse microscopy was used to track the position of individual cells. Images were taken every 10 min for a total of 24 h per experiment. (A, B, C, D) Centroid tracks from 50 representative cells in a typical experiment for YIGSR gradient, uniform YIGSR, RGD gradient, and uniform RGD, respectively, with initial position of each track superimposed at 0,0 for clarity. (E) Polar plot showing the end point of each cell’s trajectory relative to its origin. Cells moving upward move toward the positive gradient of concentration of the respective peptide. (F) Mean square displacement curves for over 24 h period. (G) Overall velocity and bias velocity to the direction of the gradient μ:min (ANOVA with Tukey’s). Lowercase letter were used to correlate velocity in the x direction and capital letters were used to correlate overall speed. Means that not share a letter were significant different. RGD concentration gradients had a positive influence on directing fibroblast migration, but no influence was observed for YIGSR gradient.
While the SC alignment and migration studies on RGD gradients had excellent agreement, the lack of directional migration of SC on RGD gradients was still somewhat surprising. RGD gradients have been shown to induce alignment and increase migration of other cell types [68, 77, 78]. Additionally, fibronectin was found to increase migration and act as a chemoattractant in a Boyden chamber assay for SC [79], and has been found to generally support SC migration in vitro and in vivo [80]. However, specific data regarding the haptotactic impact of RGD peptide on SC migration is unavailable in the literature. SC proliferation increased with exogenously added fibronectin [79] and we have previously reported increased SC proliferation on RGD-tethered nanofiber substrates [33]. Both of these reports agree with the data reported herein, indicating that RGD-activation is a mediator of the enhanced proliferation. In contrast, the conflicting results with previously reported directional migration of SC with fibronectin are indicative that RGD-integrin activation is not likely to be the mediator of directional migration. In addition, the increased focal adhesions complexes found over time on RGD samples may also be associated with the inhibition of migration. High cell-substrate adhesion strength could act to decrease cell migration [76, 81, 82]. With that assumption, only YIGSR was selected for further investigation of gradient steepness influence on SC migration.
Spatial distribution of surface ligands can significantly affect cell motility. Cells preferentially move towards the direction of higher adhesiveness (ligand concentration) on substrates, and the velocity can be tuned by the slope of ligand (peptide) gradient [53]. In a pioneering study, McCarthy et al. showed evidence that adsorbed laminin promoted cell migration in vitro in a concentration-dependent manner, stimulating directed movement of a rat schwanomma cell line [23]. In the same study, they proposed that the magnitude of that response could be altered by changing the spatial density of the bound protein. Other cells have shown similar responses to gradients of whole proteins [79, 83]. Therefore, we sought to explore if the steepness of the gradient influenced the biased migration. SC were seeded over the entire length of shallow, medium, and steep YIGSR concentration gradients, as well as a YIGSR tethered surfaces of uniform concentration and an intermediate unfunctionalized organosilane gradient as controls (Supporting Information – SI 10B). Although YIGSR samples had three different concentration profiles, the range of surface peptide concentrations was similar for all samples, with the steepness being created using several different substrate lengths. SC tracked on each sample were present near the middle of the gradient at ~ 40 pmol.cm−2. The paths of individual cells were tracked over a 24 h migration (Fig. 5A–F). As expected, cells on controls, YIGSR grafted surface with uniform peptide density and the intermediate unfunctionalized organosilane gradient, displayed random migration behavior. For the steep YIGSR peptide gradient, MSD increased significantly over time compared to other YIGSR gradients and controls (Fig. 5G). While no significant differences among the x bias velocity were found for the three different steepness samples, cells on steep gradients had a significantly higher overall speed (Fig. 5H), 0 69 ± 0.36 μm.min−1. The results indicated that the steep YIGSR gradient could be used to promote directed and accelerated migration of Schwann cells.
Figure 5.
Influence of YIGSR gradient steepness on SC migration. Time-lapse microscopy was used to capture the motility responses on individual cells. Images were taken every 10 min for a total of 24 h per experiment. (A, B, C, D, E) Centroid tracks from a minimum of 50 representative cells in a typical experiment for YIGSR shallow, medium and steep gradients, uniform YIGSR SAM, and saline gradient, respectively, with initial position of each track superimposed at 0,0 for clarity. (F) Polar plot showing the end point of each cell’s trajectory relative to its origin. Cells moving upward move toward the positive gradient of concentration of the respective peptide. (G) Mean square displacement curves for over 24 h period. (H) Overall speed and bias velocity to the direction of the gradient μm/min (ANOVA with Tukey’s). Capital letter were used to correlate velocity in the x direction; lowercase letter were used to correlate velocity in the y direction; and Greek letters were used to correlate overall speed. Means that not share a letter were significant different. These data suggest that YIGSR steep gradient promoted faster directional cell migration.
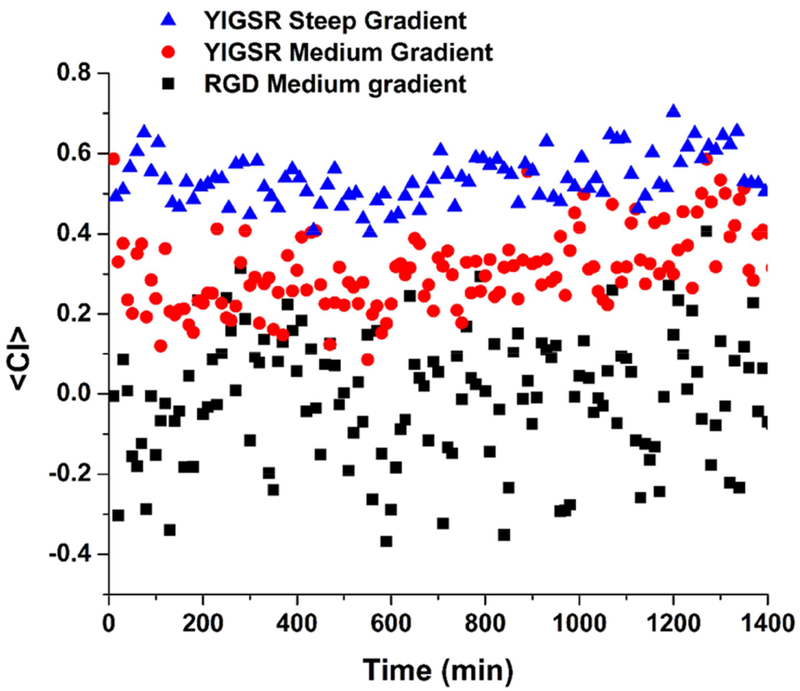
Another quantitative measurement of the cell population response to the concentration gradient can be determined by the chemotaxis index (CI), defined as the distance traveled by each cell in the direction of the gradient divided by the total path length [83, 84]. CI is an indicator of how directed the cell path is toward the gradient, ranging from 0 (random migration) to 1 (perfectly directed migration). Mean values of CI over time were calculated by averaging the CI of each tracked cell at each time point and plotted in Fig. 6. Overall, cells on steep YIGSR gradients had a significantly higher <CI> than medium gradient of same peptide, 0.54 ± 0.06 and 0.31 ± 0.09 respectively. Cells on YIGSR gradients travelled more consistently in the direction of the gradient, and this distance, compared to the total travelled distance, increased as the gradient steepness increased. <CI> for medium RGD gradient - 0.01 ± 0.15 was an indicative of random migration. Not shown in the plot, the CI for uniform SAM controls of peptides and laminin, and the unfunctionalized organosilane gradient, were as well average values around 0, similar to RGD medium gradient. The CI also indicated that the cells were continuously attracted by the YIGSR peptide concentration profile throughout the observation period. The CI values did not decrease with time, showing no saturation or degradation of the peptide.
Figure 6.
Mean chemotaxis index, <CI>, over time for SC migrating in response to the concentration of RGD and YIGSR medium gradients, and YIGSR steep gradient. CI was determined by averaging the chemotaxis index obtained from at least 30 cells at each time point. Overall, cells on YIGSR gradients travelled more consistently in the direction of the gradient represented by the higher <CI>, and this distance, compared to the total travelled distance, increased as the gradient steepness increased.
Insights gained from these in vitro studies have potential to be translated into an in vivo peripheral nerve injury model, verifying the relevance of the results in a more complex environment. Our ultimate goal is to identify and systematically optimize gradients that induce the directional migration of SC across a synthetic nerve guide material. Our research group has previously demonstrated aligned poly(ε-caprolactone) nanofibrous matrices as a potential scaffold for nerve regeneration. The post-electrospinning functionalization would enable to create gradients of concentration of peptides similar to the presented in this current work. We further aim to investigate the signaling pathways related to focal adhesion dynamics, regulation of actin alignment and the influence of SC migration behavior on axon generation and functional recovery.
CONCLUSION
In summary, a series of laminin-derived peptide concentration gradient substrates were fabricated. The peptides were analyzed for their effects on SC adhesion, proliferation and alignment with the concentration direction. Live cell migration studies on RGD and YIGSR gradients showed that the latter yields a more significant haptotactic response, with cell guidance migration in the direction of the concentration profile. Investigation of YIGSR gradient steepness on directional migration revealed that Schwann cells displayed a faster overall velocity and concentration dependent directional bias on steeper gradients. In contrast, did fibroblasts showed random migration on YIGSR gradient substrate, suggesting that YIGSR have a dominant and selective hypotactic effect on SC over fibroblasts. These investigations demonstrate show that the substrates with specific profiles of bioactive peptides may be useful for functionalizing scaffolds for nerve regeneration.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health (R15-GM113155) and the National Science Foundation (CBET BME 1603832). R.K.W. acknowledges the generous support of the Margaret F. Donovan Endowed Chair for Women in Engineering. M.L.B is grateful for support from the W. Gerald Austen Endowed Chair in Polymer Science and Polymer Engineering from the John S. and James L. Knight Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The raw and processed data required to reproduce these findings are available by request from the corresponding authors.
REFERENCES
- 1.Faweett J, Keynes RJ, Peripheral nerve regeneration. Annu Rev Neurosci 13(1):43–60 (1990). [DOI] [PubMed] [Google Scholar]
- 2.López-Cebral R, Silva-Correia J, Reis RL, Silva TH, Oliveira JM, Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater Sci Eng 3(12):3098–3122 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti J, Tissue engineering. Science 260(5110):920–926 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Shin H, Jo S, Mikos AG, Biomimetic materials for tissue engineering. Biomaterials 24(24):4353–4364 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Scheib J, Höke A, Advances in peripheral nerve regeneration. Nat Rev Neurol 9:668 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Fu SY, Gordon T, The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 14(1):67–116 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Thompson DM, Buettner HM, Oriented Schwann Cell Monolayers for Directed Neurite Outgrowth. Ann Biomed Eng 32(8): 1121–1131 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Xue J, et al. , Differentiation of Bone Marrow Stem Cells into Schwann Cells for the Promotion of Neurite Outgrowth on Electrospun Fibers. ACS Appl Mater Interfaces 9(14):12299–12310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt CE, Leach JB, Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu Rev Biomed Eng 5(1):293–347 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Ridley AJ, et al. , Cell migration: integrating signals from front to back. Science 302(5651): 1704–1709 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Lauffenburger DA, Horwitz AF, Cell migration: a physically integrated molecular process. Cell 84(3):359–369 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Ley K, Laudanna C, Cybulsky MI, Nourshargh S, Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7(9):678 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Baggiolini M, Dewald B, Moser B, Human chemokines: an update. Annu Rev Immunol 15(1):675–705 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z, Inflammation and cancer. Nature 420(6917):860 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder S, DeJulius C, Willits RK, Electrical Stimulation Increases Random Migration of Human Dermal Fibroblasts. Ann Biomed Eng 45(9):2049–2060 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Rhoads DS, Guan J-L, Analysis of directional cell migration on defined FN gradients: Role of intracellular signaling molecules. Exp Cell Res 313(18):3859–3867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen JH, et al. , Haptotaxis is cell type specific and limited by substrate adhesiveness. Cellular and molecular bioengineering 8(4):530–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LAPLANTE AF, Germain L, Auger FA, Moulin V, Mechanisms of wound reepithelialization: hints from a tissue-engineered reconstructed skin to long-standing questions. The FASEB Journal 15(13):2377–2389 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Vedula SRK, et al. , Epithelial bridges maintain tissue integrity during collective cell migration. Nat Mater 13(1):87 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Bagorda A, Parent CA, Eukaryotic chemotaxis at a glance. J Cell Sci 121(16):2621–2624 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Haastert PJ, Devreotes PN, Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol 5(8):626 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson PC (1998) Chemotaxis Encyclopedia of Immunology (Second Edition), ed Delves PJ (Elsevier, Oxford: ), pp 533–537. [Google Scholar]
- 23.McCarthy JB, Palm SL, Furcht LT, Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. The Journal of Cell Biology 97(3):772–777 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tessier-Lavigne M, Goodman CS, The molecular biology of axon guidance. Science 274(5290): 1123–1133 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Shuttleworth A (1998) Extracellular Matrix Encyclopedia of Immunology (Second Edition), ed Delves PJ (Elsevier, Oxford: ), pp 861–866. [Google Scholar]
- 26.Milner R, et al. , Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Developmental biology 185(2):215–228 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Chen Z-L, Strickland S, Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. Int J Biochem Cell Biol 163(4):889–899 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell SK, Kleinman HK, Neuronal laminins and their cellular receptors. The International Journal of Biochemistry & Cell Biology 29(3):401–414 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Nurcombe V, Laminin in neural development. Pharmacol Ther 56(2):247–264 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Tashiro K-I, et al. , The RGD containing site of the mouse laminin A chain is active for cell attachment, spreading, migration and neurite outgrowth. J Cell Physiol 146(3):451–459 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Sedaghati T, Jell G, Seifalian A, Investigation of Schwann cell behaviour on RGD-functionalised bioabsorbable nanocomposite for peripheral nerve regeneration. New Biotechnol 31(3):203–213 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Rafiuddin Ahmed M, Jayakumar R, Peripheral nerve regeneration in RGD peptide incorporated collagen tubes. Brain Res 993(1):208–216 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Zheng J, et al. , Enhanced Schwann Cell Attachment and Alignment Using One-Pot “Dual Click” GRGDS and YIGSR Derivatized Nanofibers. Biomacromolecules 16(1):357–363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, et al. , Noncovalent Bonding of RGD and YIGSR to an Electrospun Polyε-Caprolactone) Conduit through Peptide Self-Assembly to Synergistically Promote Sciatic Nerve Regeneration in Rats. Adv Healthcare Mater 6(8):1600860-n/a (2017). [DOI] [PubMed] [Google Scholar]
- 35.Stauffer WR, Cui XT, Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials 27(11):2405–2413 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Motoyoshi N, et al. , Structure-activity study of a laminin α1 chain active peptide segment Ile-Lys-Val-Ala-Val (IKVAV). FEBS Lett 365(2-3):227–231 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Alcmène C, M. TV, C KM, P RT, D. GM, The α1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J Neurobiol 33(2): 118–138 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Huber M, et al. , Modification of glassy carbon surfaces with synthetic laminin-derived peptides for nerve cell attachment and neurite growth. J Biomed Mater Res 41(2):278–288 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Huber M, et al. , Modification of glassy carbon surfaces with synthetic laminin-derived peptides for nerve cell attachment and neurite growth. J Biomed Mater Res 41(2):278–288 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Maeda M, et al. , Amino Acids and Peptides: XXXIII. A Bifunctional Poly(ethylene Glycol) Hybrid of Laminin-Related Peptides. Biochem Biophys Res Commun 248(3):485–489 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Smith Callahan LA, Ma Y, Stafford CM, Becker ML, Concentration dependent neural differentiation and neurite extension of mouse ESC on primary amine-derivatized surfaces. Biomater Sci 1(5):537–544 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, et al. , Concentration-Dependent hMSC Differentiation on Orthogonal Concentration Gradients of GRGDS and BMP-2 Peptides. Biomacromolecules 17(4):1486–1495 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Smith Callahan LA, Childers EP, Bernard SL, Weiner SD, Becker ML, Maximizing phenotype constraint and extracellular matrix production in primary human chondrocytes using arginine–glycine–aspartate concentration gradient hydrogels. Acta Biomater 9(7):7420–7428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, Zheng J, Amond EF, Stafford CM, Becker ML, Facile Fabrication of “Dual Click” One- and Two-Dimensional Orthogonal Peptide Concentration Gradients. Biomacromolecules 14(3):665–671 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallant ND, Lavery KA, Amis EJ, Becker ML, Universal Gradient Substrates for “Click” Biofunctionalization. Adv Mater 19:965–969 (2007). [Google Scholar]
- 46.Barlos K, Gatos D, 9-Fluorenylmethyloxycarbonyl/ tbutyl-based convergent protein synthesis. Peptide Science 51(4):266–278 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Moore NM, Lin NJ, Gallant ND, Becker ML, Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater 7(5):2091–2100 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Moore NM, Lin NJ, Gallant ND, Becker ML, The use of immobilized osteogenic growth peptide on gradient substrates synthesized via click chemistry to enhance MC3T3-E1 osteoblast proliferation. Biomaterials 31(7): 1604–1611 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Zheng J, et al. , Post-Electrospinning “Triclick” Functionalization of Degradable Polymer Nanofibers. ACS Macro Letters 4(2):207–213 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Kaewkhaw R, Scutt AM, Haycock JW, Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat Protocols 7(11):1996–2004 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Kemeny SF, Clyne AM, A Simplified Implementation of Edge Detection in MATLAB is Faster and More Sensitive than Fast Fourier Transform for Actin Fiber Alignment Quantification. Microsc Microanal 17(2):156–166 (2011). [DOI] [PubMed] [Google Scholar]
- 52.R. WM, G. SH, Directed Migration in Neural Tissue Engineering. Tissue Eng, Part B 20(2):93–105 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Sarvestani AS, Jabbari E, Analysis of cell locomotion on ligand gradient substrates. Biotechnol Bioeng 103(2):424–429 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Albert JNL, Baney MJ, Stafford CM, Kelly JY, Epps TH, Generation of Monolayer Gradients in Surface Energy and Surface Chemistry for Block Copolymer Thin Film Studies. ACS Nano 3(12):3977–3986 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Miles J, et al. , Design and Fabrication of Wettability Gradients with Tunable Profiles through Degrafting Organosilane Layers from Silica Surfaces by Tetrabutylammonium Fluoride. Langmuir 33(51):14556–14564 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Hejda F, Solar P, Kousal J (Surface free energy determination by contact angle measurements–a comparison of various approaches.
- 57.Zisman WA (1964) Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution Contact Angle, Wettability, and Adhesion, Advances in Chemistry, (AMERICAN CHEMICAL SOCIETY; ), Vol 43, pp 1–51. [Google Scholar]
- 58.Hartmeyer G, Marichal C, Lebeau B, Caullet P, Hernandez J, Fluorination of Silica Nanoparticles by Aqueous NH4F Solutions. The Journal of Physical Chemistry C 111(18):6634–6644 (2007). [Google Scholar]
- 59.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A, A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc, Interface 9(67):202–221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yurchenco PD, Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harbor Perspect Biol 3(2):a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masanori Y, et al. , IleχLysχValχAlaχVal (IKVAV) χ containing laminin α1 chain peptides form amyloidχlike fibrils. FEBS Letters 530(1-3):48–52 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Ruoslahti E, RGD AND OTHER RECOGNITION SEQUENCES FOR INTEGRINS. Annu Rev Cell Dev Biol 12(1):697–715 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Mitra SK, Hanson DA, Schlaepfer DD, Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6(1):56 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW, Ligand Binding to Integrins. J Biol Chem 275(29):21785–21788 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Widhe M, Johansson U, Hillerdahl C-O, Hedhammar M, Recombinant spider silk with cell binding motifs for specific adherence of cells. Biomaterials 34(33):8223–8234 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Horzum U, Ozdil B, Pesen-Okvur D, Step-by-step quantitative analysis of focal adhesions. MethodsX 1:56–59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang CE, Gemeinhart EJ, Gemeinhart RA, Cellular alignment by grafted adhesion peptide surface density gradients. J Biomed Mater Res, Part A 71A(3):403–411 (2004). [DOI] [PubMed] [Google Scholar]
- 68.DeLong SA, Gobin AS, West JL, Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J Controlled Release 109(1): 139–148 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Ren T, Yu S, Mao Z, Gao C, A complementary density gradient of zwitterionic polymer brushes and NCAM peptides for selectively controlling directional migration of Schwann cells. Biomaterials 56:58–67 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Weiner OD, Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol 14(2):196–202 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, et al. , Two phases of actin polymerization display different dependencies on PI (3, 4, 5) P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell 14(12):5028–5037 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devreotes P, Janetopoulos C, Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem 278(23):20445–20448 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S, Regulation of Schwann cell function by the extracellular matrix. Glia 56(14):1498–1507 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Park J, Kim D-H, Levchenko A, Topotaxis: A New Mechanism of Directed Cell Migration in Topographic ECM Gradients. Biophys J 114(6): 1257–1263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loosley AJ, O’Brien XM, Reichner JS, Tang JX, Describing Directional Cell Migration with a Characteristic Directionality Time. PLOS ONE 10(5):e0127425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG, Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci 113(10): 1677–1686 (2000). [DOI] [PubMed] [Google Scholar]
- 77.Brandley BK, Schnaar RL, Tumor cell haptotaxis on covalently immobilized linear and exponential gradients of a cell adhesion peptide. Developmental Biology 135(1):74–86 (1989). [DOI] [PubMed] [Google Scholar]
- 78.Guarnieri D, et al. , Covalently immobilized RGD gradient on PEG hydrogel scaffold influences cell migration parameters. Acta Biomater 6(7):2532–2539 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Baron-Van Evercooren A, Kleinman HK, Seppä HE, Rentier B, Dubois-Dalcq M, Fibronectin promotes rat Schwann cell growth and motility. Int J Biochem Cell Biol 93(1):211–216 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukhatyar VJ, et al. , Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials 32(16):3958–3968 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnhart E, Lee K-C, Allen GM, Theriot JA, Mogilner A, Balance between cell-substrate adhesion and myosin contraction determines the frequency of motility initiation in fish keratocytes. Proc Natl Acad Sci U S A 112(16):5045–5050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y-J, et al. , Confinement and Low Adhesion Induce Fast Amoeboid Migration of Slow Mesenchymal Cells. Cell 160(4):659–672 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Zhao X, Jain S, Benjamin Larman H, Gonzalez S, Irvine DJ, Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials 26(24):5048–5063 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Moghe PV, Nelson RD, Tranquillo RT, Cytokine-stimulated chemotaxis of human neutrophils in a 3-D conjoined fibrin gel assay. J Immunol Methods 180(2): 193–211 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.