Abstract
The burden of hepatitis C infection is considerable among people who inject drugs (PWID), with an estimated prevalence of greater than 40%, representing an estimated 5.6 million people who have recently injected drugs living with hepatitis C infection. As such, PWID are a priority population for enhancing prevention, testing, linkage to care, treatment and follow-up care in order to meet World Health Organization (WHO) hepatitis C elimination goals by 2030. There are many barriers to enhancing hepatitis C prevention and care among PWID including; poor global coverage of harm reduction services, restrictive drug policies and criminalization of drug use, poor access to health services, low hepatitis C testing, linkage to care and treatment, restrictions for accessing DAA therapy, and the lack of national strategies and government investment to support WHO elimination goals. On 5 September 2017, the International Network of Hepatitis in Substance Users (INHSU) held a roundtable panel of international experts to discuss remaining challenges and future priorities for action from a health systems perspective. The WHO health systems framework comprises six core components; service delivery, health workforce, health information systems, medical procurement, health systems financing, and leadership and governance. Communication has been proposed as a seventh key element which promotes the central role of affected community engagement. This review paper presents recommended strategies for eliminating hepatitis C as a major public health threat among PWID and outlines future priorities for action within a health systems framework.
Keywords: elimination, health systems, people who inject drugs, viral hepatitis C
Introduction
Hepatitis B and hepatitis C account for approximately 1.34 million deaths globally, surpassing all chronic infectious diseases including HIV, malaria and tuberculosis4. It is estimated that 71 million people are living with chronic hepatitis C infection5. The burden of hepatitis C-related morbidity and mortality continues to rise4,6. However, broad access to direct-acting antiviral (DAA) hepatitis C regimens with cure rates of over 95%7, provides an opportunity to reverse the rising burden of liver disease attributable to hepatitis C infection.
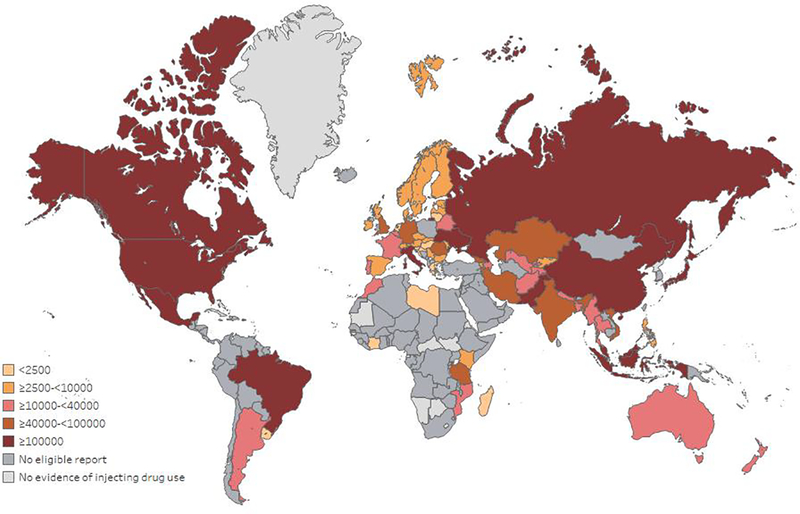
The burden of hepatitis C infection is considerable among people who inject drugs (PWID), with an estimated prevalence of greater than 40%, representing an estimated 6.1 million people who have recently injected drugs living with hepatitis C infection (9% of all infections globally)5,8,9. There is also considerable heterogeneity in the burden of hepatitis C infection among people who have recently injected drugs (Figure 1), with half of infections from just four countries: the Russian Federation, the United States, China, and Brazil9. PWID are a priority population for enhancing prevention, testing, linkage to care, treatment and follow-up care.
Figure 1. Estimated number of people with recent injecting drug use living with HCV viraemic infection, by country.
This figure has been reproduced with permission from9.
In 2016, the World Health Organization (WHO) set an ambitious goal to eliminate hepatitis C as a major public health threat by 2030. Specific targets include increasing sterile needles/syringes distributed from 20 to 200 per person per year for PWID, reducing new hepatitis C infections by 80%, and hepatitis C -related deaths by 65%, and increasing hepatitis C diagnoses from <20% to 90%, and the number of people receiving hepatitis C treatment from <10% to 80%10,11.
There are many barriers to enhancing hepatitis C prevention, diagnosis, linkage to care and treatment to achieve the WHO targets among PWID. Challenges include poor global coverage of harm reduction services, restrictive drug policies and criminalization of drug use, poor access to health services, low hepatitis C testing, linkage to care and treatment, restrictions for accessing DAA therapy, and the lack of national strategies and government investment to support WHO elimination goals7,12.
However, recent advances in the simplification of hepatitis C testing, diagnosis and treatment present an opportunity to enhance hepatitis C care among PWID. On 5 September 2017, prior to the 6th International Symposium on Hepatitis in Substance Users (INHSU 2017), the International Network of Hepatitis in Substance Users (INHSU) held a roundtable panel of international experts in drug and alcohol, infectious diseases, and hepatology to discuss remaining challenges and future priorities for action from a health systems perspective. Concepts and priorities were further developed through subsequent consultation. This paper presents recommended actions based on the expert input from the roundtable, follow-up consultation and evidence from the literature. It highlights the available scientific evidence regarding strategies to enhance hepatitis C prevention, testing, linkage to care, and treatment for PWID and outlines future priorities for action within a health systems framework.
Interventions to enhance hepatitis C prevention, testing and treatment to achieve hepatitis C elimination among people who inject drugs
Hepatitis C prevention
In 2015, there were an estimated 1.7 million new hepatitis C infections globally, with 23% occurring among people who inject drugs as a result of sharing of non-sterile injecting equipment11, highlighting the elevated hepatitis C incidence among PWID in many settings13–16, particularly in the initial years of injecting14,17.
There is evidence of the effectiveness of combined opioid substitution therapy (OST) and high-coverage needle and syringe programmes (NSP) on reducing the risk of hepatitis C acquisition in PWID14,15. OST is associated with a 50% reduction in hepatitis C acquisition risk, while combined OST and NSP are associated with a 74% reduction in hepatitis C transmissions18. NSP are also recognized as one of the most cost-effective public health interventions19. NSP and OST also have many other social, health and economic benefits beyond hepatitis C prevention, including prevention of HIV transmission and reducing death from overdose20–22.
Increasing hepatitis C treatment among PWID also has potential prevention benefits and is cost-effective23–25. As per international guidelines, given PWID are at a high risk of hepatitis C transmission, and hepatitis C treatment resulting in cure eliminates infectiousness which may yield transmission reduction benefits, PWID are a high priority for treatment26–29.
However, mathematical modelling studies suggest that whilst hepatitis C treatment for PWID can lead to substantial reductions in hepatitis C prevalence and reduce transmission30–34, prevention benefits are greatest when delivered in combination with OST and NSP31,35,36. Similarly, theoretical modelling indicates that whilst harm reduction has likely averted high HCV prevalence in some settings, scale-up of OST and NSP alone is unlikely to achieve WHO elimination incidence targets31,37. Therefore, a combination prevention strategy including hepatitis C treatment as prevention and increased coverage of harm reduction interventions is critical for achieving reductions in hepatitis C prevalence/incidence among PWID23.
Hepatitis C testing
Globally, hepatitis C testing and diagnosis remains inadequate, both in terms of numbers (<20% diagnosed) and completeness of tests offered (even fewer have been HCV RNA tested), in particular for PWID38–41. In a systematic review of the effectiveness of interventions to improve hepatitis C testing among PWID, on-site testing with pre-test discussion and education and dried blood spot (DBS) testing were demonstrated to be effective in increasing hepatitis C testing among PWID when compared to control interventions42. Other strategies that have been evaluated (without any comparator intervention) include physical and electronic medical chart reminders to prompt targeted risk-based assessment and testing43–47, peer-delivered outreach hepatitis C testing and hepatitis C education48, prison-based outreach testing49, patient referral contact tracing with monetary incentive for testing50, and point-of-care hepatitis C testing51–57. Decisions on what intervention(s) to implement to enhance hepatitis C testing will depend on the setting (and prevalence of hepatitis C infection), the model of care, the local context, and healthcare system. Decisions should also be based on engagement with the affected community to assess what testing interventions are most appropriate. Interventions should be implemented in a way that is respectful of individual choice and priorities. There is a lack of quality evidence on the most effective testing strategies, as such, strategies should be trialed and implemented in a way that is consultative and responsive rather than prescriptive.
Linkage to hepatitis C care and treatment
Linkage of PWID to hepatitis C care and treatment is insufficient internationally38–40. In a systematic review of studies to improve linkage to hepatitis C care for PWID in the interferon-era, facilitated referral (either a nurse, peer-support worker or patient navigator) for hepatitis C assessment and scheduling of specialist appointments was associated with improved linkage to hepatitis C care42. Integrated hepatitis C care within drug use and psychiatric services delivered by a multidisciplinary team with case management services, with or without non-invasive liver disease assessment, was associated with improved hepatitis C treatment uptake42. Other strategies evaluated and shown to enhance hepatitis C linkage to care and treatment include dried blood spot testing58, point-of-care hepatitis C testing54,55, non-invasive liver disease screening using transient elastography (FibroScan®) with facilitated referral to care59–61, integrated hepatitis C care43,62–66, patient navigation programs67,68, peer-based support69–81, financial incentive programs82,83, and telemedicine84–87. However, the majority of interventions that have been evaluated are specific for the interferon-era. Further research is needed to evaluate optimal interventions for linkage to hepatitis C care and treatment with interferon-free DAA therapy. Similar to efforts to increase hepatitis C testing, decisions on what intervention(s) to implement to enhance hepatitis C linkage to care and treatment will depend on the setting and prevalence of hepatitis C infection, the model of care, and the local context and healthcare system (which includes who can prescribe therapy and the reimbursement restrictions in place).
Models of hepatitis C care
There is evidence that different models of care are effective for linkage of PWID to hepatitis C care and treatment including in hospital-based specialist clinics, community health centers, drug treatment clinics, prisons, NSP, supervised consumption rooms, and primary care88. The common theme from this spectrum of hepatitis C care models is that “one size does not fit all”88. Models of care which provide on-site hepatitis C care in venues where PWID are already accessing services are important88. With the availability of simple DAA therapies, the expansion of hepatitis C care to primary care, prisons, and other non-hospital settings, as well as broadening the types of health care professionals providing care, will greatly enhance access to hepatitis C care and treatment for PWID.
Hepatitis C treatment
DAA therapy has improved the feasibility of hepatitis C treatment among PWID compared to interferon-based therapies, given DAA therapies have limited psychiatric side-effects, are simpler (oral, once-daily vs. weekly injections), and shorter in duration (8–12 weeks vs. 24–48 weeks). DAA therapy is effective among PWID receiving OST89–99, people with a history of injecting drug use82,100–105, and recent PWID99,106–108, including those with hepatitis C/HIV co-infection93–95,101,104,105,109. There is no impact of drug use prior to or during treatment on response to DAA therapy among people receiving OST98,99 or people with recent injecting drug use99,108. Concomitant alcohol use also has no impact on DAA treatment outcomes110. Hepatitis C reinfection incidence among PWID is 0.0–5.3/100 person-years111–118, with higher rates among those with ongoing injecting (4.9–6.4/100 person-year)112,114,115,117. Strategies to enhance hepatitis C prevention, such as access to high-coverage NSP and OST (>200 needle-syringes distributed per PWID and >40 OST recipients per 100 PWID) are crucial to minimize hepatitis C reinfection risk.
Remaining challenges and key recommendations for action from a health systems perspective to achieve hepatitis C elimination among people who inject drugs
Health Systems Building Blocks
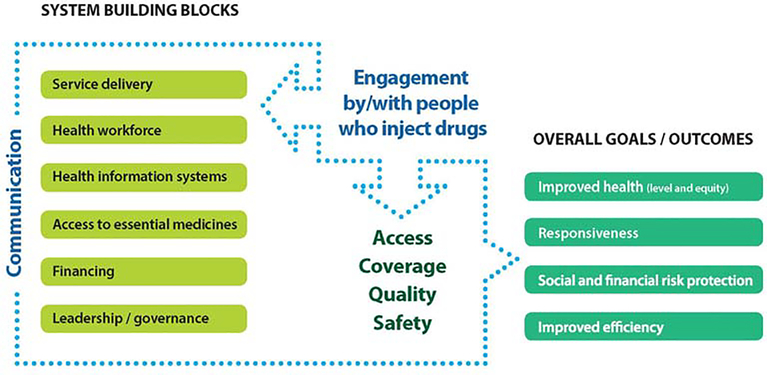
A health system, as defined by WHO is all the organizations, institutions, resources and people whose primary purpose is to improve health1,2. The WHO health systems framework comprises six core components, some of which underpin other components, such as leadership/governance and health information systems, others are input components to the health system (financing and the health workforce), or reflect the outputs of the health system (medical products and technologies and service delivery)1,2. Communication has been proposed as a seventh key element. This promotes the central role of communication in the context of affected community engagement and ensures dynamic interaction among the six traditional building blocks1,2.
Given the interdependent nature of health system components, barriers to hepatitis C care and treatment should be systematically addressed across all elements of the health systems framework to support sustainable improvement throughout the care cascade (Figure 2).
Figure 2. Proposed modified WHO Health Systems Framework for PWID.
This figure has been modified from_ENREF_3_ENREF_33.
Communication and Engagement
A people-centered approach to the health systems framework promotes health care that is respectful of, and responsive to, the preferences, needs and values of affected communities. If communication and engagement is established as essential to the health systems framework this component provides a central tenet on which health strategies can be structured. As people who are actively involved in their own health care tend to have better outcomes119, there is potential to move beyond inefficient and inequitable health systems by focusing on patient participation and community-led health interventions.
Key actions to enhance hepatitis C care for PWID through communication and patient engagement include:
Enhancing health care worker communication through education on stigmatizing language/terminology, attitudes, practices and policies;
Providing peer-led hepatitis C, health promotion and health literacy education through drug user organisations;
Facilitating consumer participation in hepatitis service design and delivery;
Facilitating patient engagement in hepatitis C communication strategies;
Ensuring patient representation on national hepatitis C strategy planning committees/reference groups.
Service Delivery
Service delivery is the provision of healthcare to people. All inputs to the health system, for example health workforce, medical procurement and health information systems are intended to enhance service delivery. WHO categorises good service delivery as possessing the following key characteristics: comprehensiveness, accessibility, coverage, continuity, quality, person-centeredness, coordination, and accountability and efficiency1.
As previously mentioned, there are many effective models of hepatitis C service delivery shown to successfully link PWID to care and treatment, all of which require contextual considerations such as individual diversity and culture. The same considerations need to be applied to pro-active testing outreach campaigns for those individuals not connected to any healthcare services.
New DAA therapies have simplified on-treatment monitoring and the resulting hepatitis C care pathway. This has increased the number of settings where hepatitis C services can be provided and has enabled a broader range of practitioners to be involved in prescribing (drug and alcohol specialists, general practitioners, pharmacists, nurses, and physician assistants) and supporting people through testing and care. This simplification has led to a wide spectrum of models of care that can improve hepatitis C service delivery.
Key actions to enhance hepatitis C service delivery include:
Establishing and supporting hepatitis C testing methodologies that do not require venepuncture (e.g. finger-stick and saliva) to enhance hepatitis C diagnosis, linkage to care, and treatment in a variety of settings;
Supporting the concept of task-shifting towards the continued expansion of available practitioners who can provide hepatitis C testing, linkage to care and treatment;
Delivering services to PWID in a non-judgmental and non-stigmatizing way.
Health Workforce
The health workforce is defined as ‘all people engaged in actions whose primary intent is to enhance health120. WHO identifies human resources as clinical staff, as well as management and support staff, i.e. those who do not deliver services directly but are essential to the performance of health systems1.
Given the ease and lower side-effect profile of DAA therapy, it is possible to increase hepatitis C treatment through simplified models of care across a range of settings88. Integrating hepatitis C care into new settings, for example drug and alcohol services, entails service delivery by a broader multidisciplinary health workforce not previously involved in hepatitis C management42.
Key actions to enhance hepatitis C care for PWID through strengthening the health workforce include:
Addressing health workforce limitations through increased hepatitis C education. Education must be contextually and culturally appropriate and provided through flexible, blended learning i.e. online and face-face. Education should focus on capacity strengthening within health systems through ‘train the trainer’ models and include a key focus on providing non-stigmatizing care;
Developing and expanding the peer workforce. Peer-based models of care receive a high level of patient acceptability and are an effective way of creating trust between services, healthcare providers and patients69,121. Health practitioner definitions should be expanded to include peer workers as valued members of the health workforce and peers should be supported through appropriate remuneration and professional support/supervision;
Encouraging and driving leadership within the workforce e.g. by reaching out to professional groups to create champions in various relevant disciplines.
Health Information Systems
Health information systems are the foundation of decision-making across the health system. They enable decision-makers to identify problems and needs, make evidence-based decisions on health policy, and allocate resources optimally122.
Despite epidemiological estimates relating to hepatitis C prevalence and burden of disease within PWID, there are still gaps in research and monitoring data. Addressing evidence gaps and improving methods for data collection is a priority for meeting global hepatitis C elimination goals12.
Key actions to enhance hepatitis C care for PWID through health information systems include:
Developing systems to enable electronic health medical record alerts to enhance hepatitis C testing in people at-risk who have not previously been tested or require ongoing risk-based testing;
Assisting clients to understand how their data will be used and how their privacy will be protected;
Collecting minimum program information at the outset of hepatitis C treatment scale-up that can monitor the uptake of hepatitis C case-finding among PWID, including the number and proportion that enter hepatitis C treatment programs;
Creating a hepatitis C treatment registry with linkage between laboratories and community hepatitis C treatment providers;
Developing more efficient/flexible digital means of capturing data on hepatitis C testing and treatment among PWID (particularly in settings where no registry exists or can be established);
Evaluating the impact of DAA treatment on hepatitis C-related morbidity and mortality, including hepatitis C prevalence and incidence, incidence of liver cancer and advanced liver disease (e.g. decompensated cirrhosis), and death among PWID.
It is noted that as less restrictive care pathways are enabled through point-of-care testing and treatment access in community settings, it may become more challenging to establish or maintain classical disease registries. This reinforces the need to create alternative, digital means of capturing data.
Medical Procurement
According to WHO, a well-functioning health system ensures equitable access to essential medical products and technologies of assured quality, safety, efficacy and cost-effectiveness123.
The availability of new hepatitis C diagnostics that are highly sensitive, quick and inexpensive, has facilitated the simplification of hepatitis C testing124–129. DAA therapies have also dramatically simplified on-treatment monitoring needs124.
Point-of-care and DBS testing have been shown to increase uptake of hepatitis C testing42,47,54–56,130 and linkage to hepatitis C care54,55,58. Both have the potential to reduce non-attendance to off-site phlebotomy and provide more immediate results to facilitate enhanced education and linkage to care. This is particularly useful for remote/rural and outreach settings.
Point-of-care hepatitis C testing can include oral fluid rapid diagnostic testing125–129, finger-stick whole-blood rapid diagnostic testing126–129,131,132, on-site venepuncture-based testing133,134, and finger-stick capillary whole blood testing57. Although DBS testing is not strictly point-of-care, the ability to collect a finger-stick sample at the point-of-care simplifies sample collection, transportation to the laboratory, and diagnosis130,135–137.
Key actions to enhance hepatitis C care for PWID through medical procurement include:
Simplifying, and disinvesting from, existing clinical algorithms for testing and treatment, ensuring a focus on improvement engagement with PWID;
Increasing certification of currently available diagnostics – particularly those that do not require a venous blood draw - e.g. oral tests, finger-stick blood tests – to increase access to testing for PWID;
Developing and certifying affordable diagnostics – particularly those that focus on community-based testing and reduce phlebotomy – to increase access to testing for PWID.
Health Systems Financing
Health financing is fundamental to the functionality of the health system. It involves both revenue generation/collection and purchasing/provision of services. Optimal health care financing allows access to needed services through efficient resource utilization.
The high cost of hepatitis C treatment continues to be a topic of concern; however, given economic and population prevention benefits, scaling up hepatitis C treatment and care in PWID has been shown to be cost-effective despite high drug costs and risk of reinfection23–25.
Globally, there is a lack of transparency in hepatitis c treatment financing mechanisms. Greater clarity and sharing of funding mechanisms would allow for a greater coordinated and effective global response.
Exploring new funding mechanisms and ensuring the financial sustainability of hepatitis C prevention and treatment programs should be an important focus for all health systems.
Key actions for enhancing hepatitis C care through financing include:
Identifying models of hepatitis C elimination success in settings with different economic health system structures and epidemic characteristics;
Advocating for transparent sharing of successes in drug procurement and pricing;
Developing investment cases including budgetary impact, epidemic impact (general and among PWID), cost-effectiveness and optimal resource allocation strategies ensuring equity.
Leadership and Governance
Effective health system leadership and governance enables strategic policy frameworks, effective service delivery oversight, coalition-building, regulation, attention to system design and accountability123. As a cross-cutting component of the health systems framework, leadership and governance is an integral part of improving health outcomes.
In the context of eliminating hepatitis C, although “early adopter” countries, and regions / sites within countries, many of whom have developed national strategies, action plans and clinical guidelines, are showing that rapid scale up of testing and treatment can be achieved through committed political leadership11, not all areas have such governance guidelines. To meet elimination targets by 2030 a comprehensive and global public health approach is needed.
Key actions for enhancing hepatitis C prevention and care through leadership and governance include:
Encouraging all countries to develop a national strategy with an action plan;
Ensuring engagement of key affected populations, preferably through leadership roles, in the development of national strategies and action plans;
Developing treatment guidelines specifically noting that PWID should not be excluded from treatment and addressing primary prevention to prevent reinfection;
Ensuring a financial commitment from national and regional/state governments;
Identifying what scale-up of harm reduction interventions are required to support hepatitis C treatment as prevention strategy;
Developing mechanisms for monitoring and evaluation to be able to provide data on whether progress is being made;
Identifying champions to drive change – from the community, clinicians, public health and government.
Conclusion
People who inject drugs, one of the populations most affected by hepatitis C, should be a priority population for interventions to prevent and treat the infection. If hepatitis C elimination is to be achieved a people-centered health systems approach is required, providing a framework for action in which PWID are engaged in all components of their care, from diagnosis to treatment and follow-up care. At present, this is seldom the case. This paper presents a series of recommendations, based on expert opinion and published evidence, for how to improve care for PWID in each of the WHO six health systems buildings blocks. The seventh central component - ensuring adequate communication among the different parts of the health system and the PWID population - is put forth as a core element of the hepatitis C elimination response.
Key Points.
PWID are a priority population in efforts to eliminate hepatitis C globally
There are many interventions effective for hepatitis C prevention, linkage to testing, care, and treatment in PWID
Future efforts to eliminate hepatitis C among PWID requires interventions across the six core components of a health systems framework including: service delivery, health workforce, health information systems, medical procurement, health systems financing, and leadership and governance
Communication is a key component of the hepatitis C elimination response, promoting the central role of community engagement and ensuring dynamic interaction among the six traditional building blocks
Acknowledgments
The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. JG is supported by a National Health and Medical Research Council Career Development Fellowship. NM is supported by the National Institute for Drug Abuse [grant number R01 DA037773] and the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214]. The authors wish to thank Tim France for preparing the original figure3 which has been adapted for Figure 2.
Declaration of interests
ED nothing to declare. JG is a consultant/advisor and has received research grants from AbbVie, Cepheid, Gilead Sciences and Merck/MSD outside of this work. JVL is a consultant/advisor or has received research grants from AbbVie, Cepheid, Gilead Sciences and Merck/MSD outside of this work. CT nothing to declare. NM has received unrestricted research grants and honoraria from Gilead Sciences and Merck outside of this work. OD has received research funding from Abbvie Gilead Sciences and MSD and is on advisory boards for Abbvie and MSD outside of this work. JD has received grant/research support from AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Roche, Genedrive and speaker honoraria from AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Roche outside of this work. JB has nothing to declare. AL is a consultant/advisor and has received research grants from Gilead Sciences and Merck/MSD outside of this work. MM has nothing to declare. PB is a consultant/advisor and has received research/travel grants from AbbVie, Gilead Sciences and Merck/MSD outside of this work. BN has nothing to declare. ST has received grant support from Gilead Sciences outside of this work.
Abbreviations
- DAA
direct-acting antiviral
- PWID
people who inject drugs
- WHO
World Health Organization
- INHSU
International Network on Hepatitis in Substance Users
- OST
opioid substitution therapy
- NSP
needle and syringe programmes
- DBS
dried blood spot
Footnotes
Financial Support
Nothing to report
REFERENCES
- 1.WHO. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva, Switzerland: 2010. [Google Scholar]
- 2.WHO. Everybody business : strengthening health systems to improve health outcomes : WHO’s framework for action. Geneva, Switzerland: 2007. [Google Scholar]
- 3.Lazarus JV, France T. A new era for the WHO health system building blocks? 2014; http://www.healthsystemsglobal.org/blog/9/A-new-era-for-the-WHO-health-system-building-blocks-.html. Accessed June 30, 2018, 2018.
- 4.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastro Hepatol. 2017;2(3):161–176. [DOI] [PubMed] [Google Scholar]
- 6.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nature Reviews in Gastroenterology & Hepatology. 2013;10(9):553–562. [DOI] [PubMed] [Google Scholar]
- 7.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Annals of internal medicine. 2017;166(9):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Global Health. 2017;5(12):e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Global health sector strategy on viral hepatitis 2016–2021. 2017; http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1. Accessed June 5, 2017.
- 11.WHO. Global Hepatitis Report 2017. . Geneva: World Health Organization;2017. [Google Scholar]
- 12.Grebely J, Bruneau J, Lazarus JV, et al. Research priorities to achieve universal access to hepatitis C prevention, management and direct-acting antiviral treatment among people who inject drugs. The International journal on drug policy. 2017;47:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiessing L, Ferri M, Grady B, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PloS one. 2014;9(7):e103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. American journal of epidemiology. 2008;168(10):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57 Suppl 2:S32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris MD, Shiboski S, Bruneau J, et al. Geographic Differences in Temporal Incidence Trends of Hepatitis C Virus Infection Among People Who Inject Drugs: The InC3 Collaboration. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(7):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy E, Boudreau JF, Boivin JF. Hepatitis C virus incidence among young street-involved IDUs in relation to injection experience. Drug Alcohol Depend. 2009;102(1–3):158–161. [DOI] [PubMed] [Google Scholar]
- 18.Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson DP, Donald B, Shattock AJ, Wilson D, Fraser-Hurt N. The cost-effectiveness of harm reduction. The International journal on drug policy. 2015;26 Suppl 1:S5–11. [DOI] [PubMed] [Google Scholar]
- 20.Lawrinson P, Ali R, Buavirat A, et al. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1484–1492. [DOI] [PubMed] [Google Scholar]
- 21.Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. The Cochrane database of systematic reviews. 2011(8):CD004145. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. The International journal on drug policy. 2014;25(1):34–52. [DOI] [PubMed] [Google Scholar]
- 23.Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384(9958):1953–1997. [DOI] [PubMed] [Google Scholar]
- 24.Martin NK, Vickerman P, Miners A, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55(1):49–57. [DOI] [PubMed] [Google Scholar]
- 25.Martin NK, Vickerman P, Dore GJ, et al. Prioritization of HCV treatment in the direct-acting antiviral era: An economic evaluation. Journal of hepatology. 2016;65(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AASLD/IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2017; https://www.hcvguidelines.org/. Accessed March 18, 2017.
- 27.EASL. EASL Recommendations on Treatment of Hepatitis C 2016. Journal of hepatology. 2017;66(1):153–194. [DOI] [PubMed] [Google Scholar]
- 28.Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. The International journal on drug policy. 2015;26(10):1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva, Switzerland: 2014. [PubMed] [Google Scholar]
- 30.Martin NK, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. Journal of hepatology. 2011;54(6):1137–1144. [DOI] [PubMed] [Google Scholar]
- 31.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57 Suppl 2:S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vos AS, Prins M, Kretzschmar ME. Hepatitis C Virus treatment as prevention among injecting drug users: who should we cure first? Addiction. 2015. [DOI] [PubMed] [Google Scholar]
- 34.Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–1870. [DOI] [PubMed] [Google Scholar]
- 35.Fraser H, Zibbell J, Hoerger T, et al. Scaling-up HCV prevention and treatment interventions in rural United States-model projections for tackling an increasing epidemic. Addiction. 2018;113(1):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. Journal of hepatology. 2018;68(3):402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107(11):1984–1995. [DOI] [PubMed] [Google Scholar]
- 38.Saraswat V, Norris S, de Knegt RJ, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 2. Journal of viral hepatitis. 2015;22 Suppl 1:6–25. [DOI] [PubMed] [Google Scholar]
- 39.Liakina V, Hamid S, Tanaka J, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 3. Journal of viral hepatitis. 2015;22 Suppl 4:4–20. [DOI] [PubMed] [Google Scholar]
- 40.Bruggmann P, Berg T, Ovrehus AL, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. Journal of viral hepatitis. 2014;21 Suppl 1:5–33. [DOI] [PubMed] [Google Scholar]
- 41.Lazarus JV, Sperle I, Spina A, Rockstroh JK. Are the testing needs of key European populations affected by hepatitis B and hepatitis C being addressed? A scoping review of testing studies in Europe. Croatian medical journal. 2016;57(5):442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. The International journal on drug policy.In Press. [DOI] [PubMed] [Google Scholar]
- 43.Zhou K, Fitzpatrick T, Walsh N, et al. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. The Lancet Infectious diseases. 2016. [DOI] [PubMed] [Google Scholar]
- 44.Krauskopf K, Kil N, Sofianou A, et al. Evaluation of an electronic health record prompt for hepatitis c antibody screening of baby boomers in primary care-a cluster randomized control trial. Journal of General Internal Medicine. 2014;29:S88–S89. [Google Scholar]
- 45.Litwin AH, Smith BD, Drainoni ML, et al. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2012;44(6):497–503. [DOI] [PubMed] [Google Scholar]
- 46.Drainoni ML, Litwin AH, Smith BD, et al. Effectiveness of a risk screener in identifying hepatitis C virus in a primary care setting. American journal of public health. 2012;102(11):e115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer JP, Moghimi Y, Marcus R, Lim JK, Litwin AH, Altice FL. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. The International journal on drug policy. 2015;26(10):922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aitken CK, Kerger M, Crofts N. Peer-delivered hepatitis C testing and counselling: a means of improving the health of injecting drug users. Drug and alcohol review. 2002;21(1):33–37. [DOI] [PubMed] [Google Scholar]
- 49.Skipper C, Guy JM, Parkes J, Roderick P, Rosenberg WM. Evaluation of a prison outreach clinic for the diagnosis and prevention of hepatitis C: Implications for the national strategy. Gut. 2003;52(10):1500–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brewer DD, Hagan H. Evaluation of a patient referral contact tracing programme for hepatitis B and C virus infection in drug injectors. Eurosurveillance. 2009;14(14). [PubMed] [Google Scholar]
- 51.Conway B, Vafadary S, Sharma S, et al. The community pop-up clinic as a tool of engagement for vulnerable populations with HCV and HIV infections. Journal of Hepatitis. 2015;2(1):1–4. [Google Scholar]
- 52.Cosmaro ML, Oldrini M, Rancilio L, et al. Facilitated access procedures for HIV and HCV testing in vulnerable groups. Infection. 2011;39:S33. [Google Scholar]
- 53.Remy AJ, Bouchkira H, Wenger H, Montabone S. News tools of screening viral hepatitis in real life: New french model of care. United European Gastroenterology Journal. 2015;1):A157. [Google Scholar]
- 54.Morano JP, Zelenev A, Lombard A, Marcus R, Gibson BA, Altice FL. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. Journal of community health. 2014;39(5):922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bottero J, Boyd A, Gozlan J, et al. Simultaneous Human Immunodeficiency Virus-Hepatitis B-Hepatitis C Point-of-Care Tests Improve Outcomes in Linkage-to-Care: Results of a Randomized Control Trial in Persons Without Healthcare Coverage. Open forum infectious diseases. 2015;2(4):ofv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beckwith CG, Kurth AE, Bazerman LB, et al. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. Journal of public health. 2016;38(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grebely J, Lamoury FMJ, Hajarizadeh B, et al. Evaluation of the Xpert HCV Viral Load point-of-care assay from venepuncture-collected and finger-stick capillary whole-blood samples: a cohort study. Lancet Gastroenterol Hepatol. 2017;2(7):514–520. [DOI] [PubMed] [Google Scholar]
- 58.McAllister G, Innes H, McLeod A, et al. Uptake of hepatitis C specialist services and treatment following diagnosis by dried blood spot in Scotland. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2014;61(3):359–364. [DOI] [PubMed] [Google Scholar]
- 59.Moessner BK, Jorgensen TR, Skamling M, et al. Outreach screening of drug users for cirrhosis with transient elastography. Addiction. 2011;106(5):970–976. [DOI] [PubMed] [Google Scholar]
- 60.Foucher J, Reiller B, Jullien V, et al. FibroScan used in street-based outreach for drug users is useful for hepatitis C virus screening and management: a prospective study. Journal of viral hepatitis. 2009;16(2):121–131. [DOI] [PubMed] [Google Scholar]
- 61.Marshall AD, Micallef M, Erratt A, et al. Liver disease knowledge and acceptability of non-invasive liver fibrosis assessment among people who inject drugs in the drug and alcohol setting: The LiveRLife Study. The International journal on drug policy. 2015;26(10):984–991. [DOI] [PubMed] [Google Scholar]
- 62.Cullen W, Stanley J, Langton D, Kelly Y, Staines A, Bury G. Hepatitis C infection among injecting drug users in general practice: a cluster randomised controlled trial of clinical guidelines’ implementation. The British journal of general practice : the journal of the Royal College of General Practitioners. 2006;56(532):848–856. [PMC free article] [PubMed] [Google Scholar]
- 63.Masson CL, Delucchi KL, McKnight C, et al. A Randomized Trial of a Hepatitis Care Coordination Model in Methadone Maintenance Treatment. American journal of public health. 2013;103(10):E81–E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. The American journal of gastroenterology. 2011;106(10):1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. The American journal of gastroenterology. 2006;101(10):2254–2262. [DOI] [PubMed] [Google Scholar]
- 66.Ho SB, Brau N, Cheung R, et al. Integrated Care Increases Treatment and Improves Outcomes of Patients With Chronic Hepatitis C Virus Infection and Psychiatric Illness or Substance Abuse. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13(11):2005–2014 e2001–2003. [DOI] [PubMed] [Google Scholar]
- 67.Trooskin SB, Poceta J, Towey CM, et al. Results from a Geographically Focused, Community-Based HCV Screening, Linkage-to-Care and Patient Navigation Program. J Gen Intern Med. 2015;30(7):950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falade-Nwulia O, Mehta SH, Lasola J, et al. Public health clinic-based hepatitis C testing and linkage to care in baltimore. Journal of viral hepatitis. 2016;23(5):366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crawford S, Bath N. Peer support models for people with a history of injecting drug use undertaking assessment and treatment for hepatitis C virus infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57 Suppl 2:S75–79. [DOI] [PubMed] [Google Scholar]
- 70.Grebely J, Knight E, Genoway KA, et al. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support. European journal of gastroenterology & hepatology. 2010;22(3):270–277. [DOI] [PubMed] [Google Scholar]
- 71.Norman J, Walsh NM, Mugavin J, et al. The acceptability and feasibility of peer worker support role in community based HCV treatment for injecting drug users. Harm Reduct J. 2008;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rance J, Treloar C. Integrating Treatment: Key findings from a qualitative evaluation of the Enhancing Treatment of Hepatitis C in Opiate Substitution Settings (ETHOS) study. Sydney: National Centre in HIV Social Research;2012. [Google Scholar]
- 73.Musgrove SM. NUAA’s ETHOS Projects: The Story of a Hep C Peer Support Worker & His Clinic. Paper presented at: 2nd International Symposium on Hepatitis in Substance Users2011; Brussels, Belgium. [Google Scholar]
- 74.Sylvestre DL, Zweben JE. Integrating HCV services for drug users: a model to improve engagement and outcomes. The International journal on drug policy. 2007;18(5):406–410. [DOI] [PubMed] [Google Scholar]
- 75.Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH. Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program. Journal of substance abuse treatment. 2012;43(4):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charlebois A, Lee L, Cooper E, Mason K, Powis J. Factors associated with HCV antiviral treatment uptake among participants of a community-based HCV programme for marginalized patients. J Viral Hepat. 2012;19(12):836–842. [DOI] [PubMed] [Google Scholar]
- 77.Roose RJ, Cockerham-Colas L, Soloway I, Batchelder A, Litwin AH. “It’s easier to do stuff that’s hard when you’ve got people to back you up:” description of a Hepatitis C Peer Education & Support Program in an opioid treatment program TBD. 2013;In Press. [Google Scholar]
- 78.Roose RJ, Cockerham-Colas L, Soloway I, Batchelder A, Litwin AH. Reducing barriers to hepatitis C treatment among drug users: an integrated hepatitis C peer education and support program. Journal of health care for the poor and underserved. 2014;25(2):652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Treloar C, Rance J, Bath N, et al. Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis C virus infection in opioid substitution treatment clinics: The ETHOS study, Australia. Int J Drug Policy. 2015(In Press). [DOI] [PubMed] [Google Scholar]
- 80.Alavi M, Grebely J, Micallef M, et al. Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opiate substitution setting: the ETHOS study. Clin Infect Dis. 2013;In Press. [DOI] [PubMed] [Google Scholar]
- 81.Keats J, Micallef M, Grebely J, et al. Assessment and delivery of treatment for hepatitis C virus infection in an opioid substitution treatment clinic with integrated peer-based support in Newcastle, Australia. The International journal on drug policy. 2015;26(10):999–1006. [DOI] [PubMed] [Google Scholar]
- 82.Sulkowski M, Ward K, Falade-Nwulia O, et al. Randomized controlled trial of cash incentives or peer mentors to improve HCV linkage and treatment among HIV/HCV coinfected persons who inject drugs: the CHAMPS Study. Journal of hepatology. 2017;66:S719. [Google Scholar]
- 83.Norton BL, Singh R, Agyemang L, Litwin AH. Contingency Management Improves HCV Linkage and Treatment Outcomes in Persons Who Inject Drugs: A Pilot Study. Paper presented at: 5th International Symposum on Hepatitis Care in Substance Users (INHSU 2016)2016; Oslo, Norway. [Google Scholar]
- 84.Mashru J, Kirlew M, Saginur R, Schreiber YS. Management of infectious diseases in remote northwestern Ontario with telemedicine videoconference consultations. Journal of telemedicine and telecare. 2017;23(1):83–87. [DOI] [PubMed] [Google Scholar]
- 85.Tahan V, Almashhrawi A, Kahveci AM, Mutrux R, Ibdah JA. Extension for Community Health Outcomes-hepatitis C: Small steps carve big footprints in the allocation of scarce resources for hepatitis C virus treatment to remote developing areas. World journal of hepatology. 2016;8(11):509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. The New England journal of medicine. 2011;364(23):2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lloyd AR, Clegg J, Lange J, et al. Safety and effectiveness of a nurse-led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(8):1078–1084. [DOI] [PubMed] [Google Scholar]
- 88.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57 Suppl 2:S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grebely J, Puoti M, Wedemeyer H, et al. Safety and Efficacy of Ombitasvir, Paritaprevir/Ritonavir and Dasabuvir With or Without Ribavirin in Chronic Hepatitis C Patients Receiving Opioid Substitution Therapy: A Pooled Analysis Across 12 Clinical Trials. Journal of hepatology. 2017;66:S514. [Google Scholar]
- 90.Grebely J, Dore GJ, Zeuzem S, et al. Efficacy and Safety of Sofosbuvir/Velpatasvir in Patients With Chronic Hepatitis C Virus Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ASTRAL Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grebely J, Mauss S, Brown A, et al. Efficacy and Safety of Ledipasvir/Sofosbuvir With and Without Ribavirin in Patients With Chronic HCV Genotype 1 Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ION Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016. [DOI] [PubMed] [Google Scholar]
- 92.Grebely J, Jacobson IM, Kayali Z, et al. SOF/VEL/VOX for 8 or 12 Weeks Is Well Tolerated and Results in High SVR12 Rates in Patients Receiving Opioid Substitution Therapy. Journal of hepatology. 2017;66:S513. [Google Scholar]
- 93.Christensen S, Schober A, Mauss S, et al. DAA-Treatment of HCV-infected patients on Opioid Substitution Therapy (OST): does the clinical setting matter? Data from the German Hepatitis C-Registry (DHC-R). Hepatology. 2016;64(S1):982A–983A. [Google Scholar]
- 94.Schutz A, Moser S, Marchart K, Haltmayer H, Gschwantler M. Direct Observed Therapy of Chronic Hepatitis C With Interferon-Free All-Oral Regimens at a Low-Threshold Drug Treatment Facility-a New Concept for Treatment of Patients With Borderline Compliance Receiving Opioid Substitution Therapy. The American journal of gastroenterology. 2016;111(6):903–905. [DOI] [PubMed] [Google Scholar]
- 95.Scherz N, Brunner N, Bruggmann P. Direct-acting antivirals for hepatitis C in patient in opioid substitution treatment and heroin assisted treatment: real-life data. Journal of hepatology. 2017;66:S726. [Google Scholar]
- 96.Dillon J, Mauss S, Nalpas C, et al. Efficacyand safety of Simeprevir-containing hepatitis C therapy in patients on opiate substitution therapy. Journal of hepatology. 2017;66:S520. [Google Scholar]
- 97.Boyle A, Marra F, Fox R, et al. Partial directly observed therapy with ombitasvir/paritaprevir based regimens allows for successful treatment of patients on daily supervised methadone. Journal of hepatology. 2017;66. [Google Scholar]
- 98.Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Annals of internal medicine. 2016;165(9):625–634. [DOI] [PubMed] [Google Scholar]
- 99.Conway B, Grebely J, Fraser C, et al. Paritaprevir/ritonavir/ombitasvir, dasabuvir + ribavirin in people with HCV genotype 1 and recent injecting drug use or receiving OST: The D3FEAT study. Paper presented at: 6th international Symposium on Hepatitis Care in Substance Users2017; New Jersey, United States. [Google Scholar]
- 100.Norton BL, Fleming J, Steinman M, et al. High HCV Cure Rates for Drug Users Treated with DAAs at an Urban Primary Care Clinic. Paper presented at: Conference on Retroviruses and Opportunistic Infections; Feb 22–24 2016; Boston, United States. [Google Scholar]
- 101.Conway B, Raycraft T, Bhutani Y, et al. Efficacy of All-Oral HCV Therapy in People Who Inject Drugs. Hepatology. 2016;64(S1):990A.26705089 [Google Scholar]
- 102.Morris L, Smirnov A, Kvassay A, et al. Initial outcomes of integrated community-based hepatitis C treatment for people who inject drugs: findings from the Queensland Injectors’ Health Network. The International journal on drug policy. 2017;In Press. [DOI] [PubMed] [Google Scholar]
- 103.Mason K, Dodd Z, Guyton M, et al. Understanding Real-World Adherence in the Directly Acting Antiviral Era: a prospective evaluation of adherence amongst people with a history of drug use at a community-based program in Toronto, Canada. The International journal on drug policy. 2017;In Press. [DOI] [PubMed] [Google Scholar]
- 104.Read P, Lothian R, Chronister K, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. The International journal on drug policy. 2017;In Press. [DOI] [PubMed] [Google Scholar]
- 105.Litwin AH, Agyemang L, Akiyama M, et al. The PREVAIL Study: Intensive Models of HCV Care for People Who Inject Drugs Journal of hepatology. 2017;66:S72. [Google Scholar]
- 106.Bouscaillou J, Kikvidze T, Butsashvili M, et al. Effectiveness of DAA-based treatment of HCV in active people who inject drugs living in middle income countries (MIC): the results of a prospective cohort study in Tbilisi, Georgia. Journal of hepatology. 2017;66:S409. [Google Scholar]
- 107.Boglione L, Mornese Pinna S, De Nicolo A, et al. Treatment with direct-acting antiviral agents of hepatitis C virus infection in injecting drug users: A prospective study. Journal of viral hepatitis. 2017. [DOI] [PubMed] [Google Scholar]
- 108.Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastro Hepatol. 2018;In Press. [DOI] [PubMed] [Google Scholar]
- 109.Dore GJ, Altice F, Litwin AH, et al. Elbasvir/Grazoprevir to Treat HCV Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Controlled Trial (C-EDGE CO-STAR). Annals of internal medicine. 2016;In Press. [DOI] [PubMed] [Google Scholar]
- 110.Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug and alcohol dependence. 2016;169:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cunningham EB, Applegate TL, Lloyd AR, Dore GJ, Grebely J. Mixed HCV infection and reinfection in people who inject drugs--impact on therapy. Nature reviews Gastroenterology & hepatology. 2015;12(4):218–230. [DOI] [PubMed] [Google Scholar]
- 112.Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57 Suppl 2:S80–89. [DOI] [PubMed] [Google Scholar]
- 113.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(6):683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Midgard H, Bjoro B, Maeland A, et al. Hepatitis C reinfection after sustained virological response. Journal of hepatology. 2016;64(5):1020–1026. [DOI] [PubMed] [Google Scholar]
- 115.Weir A, McLeod A, Innes H, et al. Hepatitis C reinfection following treatment induced viral clearance among people who have injected drugs. Drug and alcohol dependence. 2016;165:53–60. [DOI] [PubMed] [Google Scholar]
- 116.Pineda JA, Nunez-Torres R, Tellez F, et al. Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. The Journal of infection. 2015;71(5):571–577. [DOI] [PubMed] [Google Scholar]
- 117.Young J, Rossi C, Gill J, et al. Risk factors for hepatitis C virus reinfection after sustained virologic response in patients co-infected with HIV. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dore GJ, Grebely J, Altice F, et al. HCV reinfection and injecting risk behavior following elbasvir/grazoprevir treatment in patients on opioid agonist therapy: Co-STAR Three Year Follow-up Study. Hepatology. 2016;64(S1):431A. [Google Scholar]
- 119.Rifkin SB. Examining the links between community participation and health outcomes: a review of the literature. Health Policy Plan. 2014;29 Suppl 2:ii98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.WHO. The world health report 2006: working together for health. 2018. [DOI] [PubMed]
- 121.Treloar C, Rance J, Bath N, et al. Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis C virus infection in opioid substitution treatment clinics: The ETHOS study, Australia. The International journal on drug policy. 2015;26(10):992–998. [DOI] [PubMed] [Google Scholar]
- 122.WHO. Health Metrics Network. Framework and standards for country health information systems. . 2008.
- 123.WHO. The world health report 2006 – working together for health. Geneva, Switzerland: 2006. [Google Scholar]
- 124.Grebely J, Applegate TL, Cunningham P, Feld JJ. Hepatitis C point-of-care diagnostics: in search of a single visit diagnosis. Expert Rev Mol Diagn. 2017;17(12):1109–1115. [DOI] [PubMed] [Google Scholar]
- 125.Drobnik A, Judd C, Banach D, Egger J, Konty K, Rude E. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am J Public Health. 2011;101(11):2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jewett A, Smith BD, Garfein RS, Cuevas-Mota J, Teshale EH, Weinbaum CM. Field-based performance of three pre-market rapid hepatitis C virus antibody assays in STAHR (Study to Assess Hepatitis C Risk) among young adults who inject drugs in San Diego, CA. J Clin Virol. 2012;54(3):213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith BD, Teshale E, Jewett A, et al. Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin Infect Dis. 2011;53(8):780–786. [DOI] [PubMed] [Google Scholar]
- 128.Smith BD, Drobeniuc J, Jewett A, et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011;204(6):825–831. [DOI] [PubMed] [Google Scholar]
- 129.Shivkumar S, Peeling R, Jafari Y, Joseph L, Pant Pai N. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Annals of internal medicine. 2012;157(8):558–566. [DOI] [PubMed] [Google Scholar]
- 130.Coats JT, Dillon JF. The effect of introducing point-of-care or dried blood spot analysis on the uptake of hepatitis C virus testing in high-risk populations: A systematic review of the literature. The International journal on drug policy. 2015;26(11):1050–1055. [DOI] [PubMed] [Google Scholar]
- 131.Wong VW, Wong GL, Chim AM, et al. Targeted hepatitis C screening among ex-injection drug users in the community. J Gastroenterol Hepatol. 2013. [DOI] [PubMed] [Google Scholar]
- 132.Poiteau L, Soulier A, Rosa I, et al. Performance of rapid diagnostic tests for the detection of antibodies to hepatitis C virus in whole blood collected on dried blood spots. Journal of viral hepatitis. 2016;23(5):399–401. [DOI] [PubMed] [Google Scholar]
- 133.McHugh MP, Wu AHB, Chevaliez S, Pawlotsky JM, Hallin M, Templeton KE. Multicenter Evaluation of the Cepheid Xpert Hepatitis C Virus Viral Load Assay. J Clin Microbiol. 2017;55(5):1550–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gupta E, Agarwala P, Kumar G, Maiwall R, Sarin SK. Point -of -care testing (POCT) in molecular diagnostics: Performance evaluation of GeneXpert HCV RNA test in diagnosing and monitoring of HCV infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2017;88:46–51. [DOI] [PubMed] [Google Scholar]
- 135.Chevaliez S, Poiteau L, Rosa I, et al. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin Microbiol Infect. 2016;22(5):459 e451–456. [DOI] [PubMed] [Google Scholar]
- 136.Soulier A, Poiteau L, Rosa I, et al. Dried Blood Spots: A Tool to Ensure Broad Access to Hepatitis C Screening, Diagnosis, and Treatment Monitoring. The Journal of infectious diseases. 2016;213(7):1087–1095. [DOI] [PubMed] [Google Scholar]
- 137.Greenman J, Roberts T, Cohn J, Messac L. Dried blood spot in the genotyping, quantification and storage of HCV RNA: a systematic literature review. Journal of viral hepatitis. 2015;22(4):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]