Abstract
Background:
Many people who inject drugs (PWID) are denied treatment for hepatitis C virus (HCV) infection, even if they are receiving opioid agonist therapy (OAT). Research suggests that HCV in PWID may be treated effectively, but optimal models of care for promoting adherence and sustained virologic response (SVR) have not been evaluated in the direct-acting antiviral (DAA) era.
Objective:
To determine whether directly observed therapy (DOT) and group treatment (GT) are more effective than self-administered individual treatment (SIT) in promoting adherence and achieving SVR among PWID receiving OAT.
Design:
Three-group, randomized controlled trial conducted from October 2013 to April 2017. (ClinicalTrials.gov: )
Setting:
Three OAT programs in Bronx, New York.
Participants:
Persons aged 18 years and older with genotype 1 HCV infection who were willing to receive HCV therapy on site in the OAT program. Of 190 persons screened, 158 were randomly assigned to a study group and 150 initiated treatment: DOT (n = 51), GT (n = 48), and SIT (n = 51).
Intervention:
2 intensive interventions (DOT and GT) and 1 control condition (SIT).
Measurements:
Primary: adherence, measured by using electronic blister packs. Secondary: HCV treatment completion and SVR 12 weeks after treatment completion.
Results:
Mean age was 51 years; 65% of participants had positive results on urine drug testing during the 6 months before treatment, and 75% reported ever injecting drugs. Overall adherence, estimated from mixed-effects models using the daily timeframe, was 78% (95% CI, 75% to 81%) and was greater among participants randomly assigned to DOT (86% [CI, 80% to 92%]) than those assigned to SIT (75% [CI, 70% to 81%]; difference, 11% [CI, 5% to 18%]; Bonferroni-corrected P = 0.001). No significant difference in adherence was observed between participants randomly assigned to GT (80% [CI, 74% to 86%]) and those assigned to SIT (difference, 4.7% [CI, −2% to 11%]; Bonferroni-corrected P = 0.29). The HCV treatment completion rate was 97%, with no differences among groups (P = 0.53). Overall SVR was 94% (CI, 89% to 97%); the SVR rate was 98% in the DOT group, 94% in the GT group, and 90% in the SIT group (P = 0.152).
Limitation:
These findings may not be generalizable to PWID not enrolled in OAT programs.
Conclusion:
All models of onsite HCV care delivered to PWID in OAT programs resulted in high SVR, despite ongoing drug use. Directly observed therapy was associated with greater adherence than SIT.
People who inject drugs (PWID) are at the heart of the hepatitis C virus (HCV) epidemic, and most HCV cases in the United States and other developed nations are related to illicit drug use (1). In the United States, cases of acute HCV infection increased annually from 2010 through 2015, rising more than 2.9-fold over this period, a growth driven predominantly by injection drug use (2). Hepatitis C virus results in up to 15 000 deaths annually and is the leading cause of cirrhosis and liver transplantation in the United States (3, 4). Outcomes of HCV treatment have improved substantially with the emergence of direct-acting antiviral (DAA) agents, which are associated with nearly 100% sustained virologic response (SVR) or HCV cure, defined as absence of detectable virus 12 weeks after therapy completion, convenient dosing, and few side effects (5). Sustained virologic response has been shown to improve quality of life (6–8) and to lengthen survival (9–11).
Despite the central role PWID occupy in the HCV epidemic, few are offered DAA treatment because of concerns regarding suboptimal adherence, cost, and medication resistance (12, 13). People who inject drugs face many adherence challenges, including mental illness, homelessness, lack of positive social support, poor adherence-related skills, low HCV-related knowledge, and poor access to and mistrust of the health care system (14–17). Yet, most PWID with HCV are willing to undergo treatment (18–21), and the simplicity of DAA therapy promises great advantages for achieving HCV cure. Despite these advances, optimal models of care that promote adherence and SVR for PWID have not been elucidated.
Opioid agonist therapy (OAT) programs are ideal settings in which to co-locate HCV therapy. Opioid agonist therapy is an effective strategy to reduce HCV risk behavior and active drug use (22, 23). Nationwide, more than 375 000 patients receive OAT in the form of methadone or buprenorphine from approximately 1500 opioid treatment programs (OTPs) (24), and conservative estimates suggest that more than 60% of PWID in OTPs have HCV infection (25). Several studies evaluating multidisciplinary models of care in drug-using and methadone-maintained patients in OTPs have demonstrated HCV treatment outcomes similar to those found in PWID who received interferon-based HCV therapy in large clinical trials (26, 27). However, limited DAA-era data on real-world outcomes among PWID in OAT programs suggest that SVR may be lower (85%) than has been observed in large clinical trials (28). Whether intensive models of care in the DAA era can improve outcomes among PWID in OAT programs is not yet known.
The goal of this randomized trial was to assess the effectiveness of 2 models of intensive onsite HCV care-directly observed therapy (DOT) and group treatment (GT)–compared with self-administered individual treatment (SIT) for promoting adherence, completing treatment, and achieving SVR. We hypothesized that rates of adherence, treatment completion, and SVR would be higher in the intensive intervention groups versus the control group. An additional goal was to examine the relationship between adherence and SVR, the relationship between drug use and adherence, and patient-level factors associated with both adherence and SVR.
Methods
Participants and Setting
Hepatitis C virus–infected PWID from 3 OAT programs in Bronx, New York, were enrolled beginning in October 2013, and participants were followed until April 2017. Potential participants were referred by clinicians if they were eligible for HCV treatment on the basis of guidelines from the American Association for the Study of Liver Diseases and Infectious Diseases Society of America (AASLD/IDSA) (29). Eligibility was assessed by an oral screener and a confirmatory chart review. Eligible participants were aged 18 years or older, spoke English or Spanish, had HCV genotype 1, were psychiatrically stable, were willing to receive HCV therapy on site in their OAT program, were HCV treatment naive (or treatment experienced with interferon-based regimens after December 2014, when combination DAA treatment was available and interferon exposure no longer predicted response to therapy), were receiving OAT in person at the OTP medication window at least 3 times per week (once per week after June 2015), and could provide informed consent. Exclusion criteria included decompensated cirrhosis, inability to provide informed consent, pregnancy or breastfeeding, and hypersensitivity to HCV medication.
Study Design
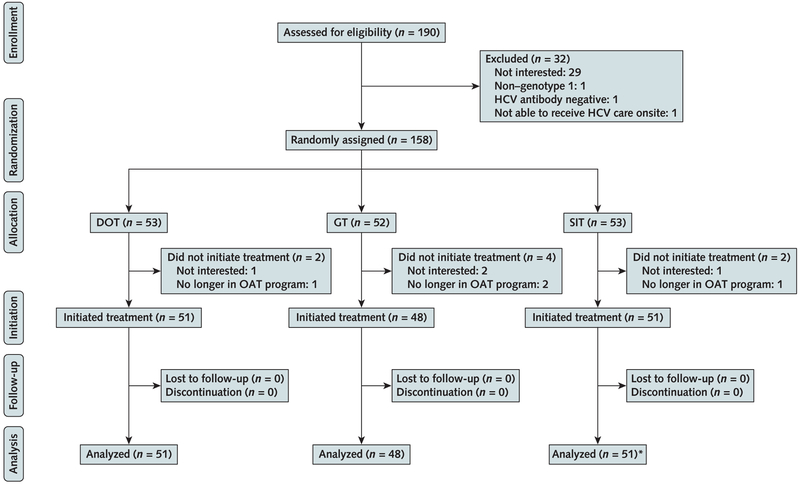
This 3-group, multisite, unblinded trial randomly assigned participants to 1 of 3 models of care (DOT, GT, or SIT) in a 1:1:1 ratio in varying block sizes (3 to 6 blocks) via central, computer-generated randomization (Figure 1). We stratified randomization by IL28B genotype (TC/TT vs. CC), HIV status, and stage of liver disease (cirrhosis vs. no cirrhosis, assessed by a combination of liver biopsy and noninvasive testing) (30).
Figure 1. Flow diagram of PREVAIL study participants.
DOT = directly observed therapy; GT = group treatment; HCV = hepatitis C virus; OAT = opioid agonist therapy; PREVAIL = Prevent Resistance Eliminate Virus and Improve Life; SIT = self-administered individual treatment.
* Three SIT participants with no available blister pack adherence data were not included in the analysis of the primary adherence outcome.
Research visits were conducted at baseline, then every 4 weeks during the first 12 treatment weeks (treatment weeks 4, 8, and 12; at the end of treatment if it was not at treatment week 12; and 4, 12, and 24 weeks after treatment). All 3 models of care included co-located, onsite care at the OAT program, which consisted of HCV care and substance use treatment. Participants received the following HCV treatments according to AASLD/IDSA guidelines: telaprevir, pegylated interferon, and ribavirin (TVR/IFN/RBV); sofosbuvir, pegylated interferon, and ribavirin (SOF/IFN/RBV); sofosbuvir and ribavirin (SOF/RBV); or a combination DAA regimen of sofosbuvir and simeprevir (SOF/SMV) or sofosbuvir/ledipasvir (SOF/LDV).
Study Assessments
Participants answered surveys using audio computer-assisted self-interview technology at each research visit (31). The surveys assessed factors hypothesized to be associated with adherence, including unstable housing, employment, relationship status, psychiatric illness (Patient Health Questionnaire-9), and substance use (Addiction Severity Index) (32, 33).
Treatment completion was determined on the basis of chart review. We obtained results of HCV RNA tests through medical chart review or from blood draws at baseline and treatment weeks 4, 8, and 12 (or end of treatment if it was not treatment week 12) and post-treatment weeks 4, 12, and 24. We defined SVR as an HCV RNA level below the limit of quantitation 12 weeks after treatment completion, using COBAS TaqMan real-time reverse transcriptase polymerase chain reaction assay (Roche Diagnostics), version 1.0 (<43 IU/mL), or version 2.0 (<15 IU/mL) after October 2014. At each research visit, participants provided urine specimens, which were tested for amphetamines, benzodiazepines, cocaine, methadone, opioids, and oxycodone with the enzyme multiplied immunoassay technique.
Study Interventions and Control Condition
DOT
Because DOT with HCV medications was linked to OTP methadone visits, the number of directly observed oral doses varied according to the number of days the participant attended the OTP to obtain methadone. We considered this intervention to be “modified” DOT, because not all oral medication doses were observed. The nonobserved doses were packaged in electronic blister packs as take-home doses for self-administration on non-OTP pick-up days. For participants receiving interferon-based therapy, providers administered interferon doses in the OTP. Nurses at the OTP clinics notified clinicians of declined doses, assessed side effects, and referred participants to onsite clinicians as necessary.
GT
The GT model, described in detail elsewhere (34), was adapted from models of HCV peer-based support (35). New participants were first oriented to the group and met other patients and the treatment team (physician and physician assistant). The treatment team then presented an overview of the HCV epidemic and its impact on PWID; HCV natural history; and the risks, benefits, and efficacy of HCV treatment. Weekly GT meetings had the following 5 components: a brief physical examination, psychosocial support from peers and providers, HCV education, side effect management, and a closing meditation on positive health. Six to 12 participants attended each group session, and group entry was rolling. Participants received interferon (for those receiving interferon-based therapy) and 7-day blister packs during GT.
SIT
Participants randomly assigned to SIT received all medications packaged in 7-day blister packs from an OTP clinic nurse and self-administered the medications at home. Patients receiving interferon were instructed on proper home administration. The provider administered the first interferon injection, and the participant administered the second injection under provider observation. Remaining doses were distributed in boxes containing 1 month’s supply for self-administration at home.
Study Outcomes
The primary outcome was adherence, measured in all 3 groups by using electronic Med-ic blister packs (Information Mediary), which have a 99.6% event accuracy (time of dose removal correctly recorded within ± 2 minutes) (36). Adherence was computed by using daily and window timeframes (Table 1) (37). Adherence was defined as a continuous outcome, calculated as the percentage of expected blister-pack medication dispensed during 2-week intervals. Secondary outcomes included HCV treatment completion, SVR, and cost-effectiveness (will be addressed elsewhere in a forthcoming article).
Table 1.
PREVAIL Study Primary and Secondary Outcomes
| Adherence |
| Daily adherence: Participants received credit if doses were taken on the specified day. |
| Window adherence: Participants received credit if doses were taken within a window based on 25% of the dosing interval. For example, a participant scheduled to take once-daily medication at 10:00 a.m. received credit if the dose was taken between 4:00 a.m. and 4:00 p.m. |
| HCV treatment completion |
| Completion of ≥80% of the planned treatment course. For example, ≥10 wk of a 12-wk course, or ≥20 wk of a 24-wk course. |
| SVR |
| Undetectable HCV RNA at posttreatment week 12. |
HCV = hepatitis C virus; PREVAIL = Prevent Resistance Eliminate Virus and Improve Life; SVR = sustained virologic response.
Sample Size Determination
Our previous pilot DOT study observed that mean adherence in the DOT group was 87% (SD, 12%), compared with 77% (SD, 20%) in the SIT group (standardized effect size [or Cohen d], 0.6) (38). On the basis of these estimates, we calculated a sample size of 150 participants (n = 50 per group) and anticipated a 20% attrition rate, resulting in 40 participants per group. The power of the mixed-effects linear model for the repeatedly measured continuous adherence outcome with 6 postbaseline time points would therefore be greater than 90%, even if anticipated intraclass correlation was as high as 0.5.
Statistical Analysis
Participant characteristics and outcomes were reported in percentages or frequencies and compared among the 3 groups. To compare both daily and window adherence rates among groups, we applied mixed-effects linear models (SAS Proc Mixed [SAS Institute]) to account for within-participant longitudinal correlations by using a first-order autoregressive covariance structure. The fixed effects were group, time, and group-by-time interactions, in addition to site and the 3 stratifying variables. We then repeated these analyses for the subgroup of participants who received a combination DAA regimen (SOF/LDV or SOF/SIM). We conducted 2 post hoc comparisons according to our study protocol (DOT vs. SIT and GT vs. SIT) of the outcomes of adherence with Bonferroni-corrected P values.
To test the significance of differences in rates of treatment completion and SVR across the 3 study groups, we applied multivariable exact logistic regression models (SAS Proc Logistic), adjusting for site and the 3 stratifying variables. We repeated these analyses for the subgroup of participants who received a combination DAA regimen (SOF/LDV or SOF/SIM). We conducted 2 post hoc comparisons of SVR and treatment completion (DOT vs. SIT and GT vs. SIT) with Bonferroni-corrected P values, according to our study protocol (30). The 95% CIs for differences in proportions were computed on the basis of the Wilson method, with a continuity correction.
In addition, we determined treatment completion and SVR (and Clopper–Pearson exact 95% CI) among all randomly assigned participants (n = 158). We used Fisher exact tests to compare outcomes among study groups, considering participants who did not initiate treatment (n = 8) as having not completed treatment or not achieving SVR (intention-to-treat analysis). Finally, we examined differences among groups in another secondary outcome, HCV viral load over time, using a generalized mixed-effects linear model (SAS Proc Glimmix) with the logit link to determine effect of group on the repeatedly measured outcome of detectable (vs. undetectable) viral load, adjusting for time, site, and the stratifying variables. We then conducted post hoc logistic regressions on detectable viral load status at each time point with the same adjusting variables but without the time effect.
To test associations between adherence and SVR 12 weeks after treatment completion, we applied multivariable logistic regression models, adjusting for the following potential confounding variables: age, sex, race, psychiatric illness, unstable housing, use of alcohol to intoxication, DAA regimen, site, and study group. In these models, adherence was an independent variable and SVR was the outcome. We determined the proportion (and Clopper–Pearson exact 95% CI) of participants achieving SVR in each group among all those who initiated treatment (n = 150).
To identify participant characteristics, including the stratifying variables that might be associated with adherence, we applied a series of mixed-effects models using the characteristics of interest as main effects and adjusting for study group and site. To identify participant characteristics associated with SVR, we conducted a series of exact logistic regressions, adjusting for group and site.
We used SAS, version 9.4, for all statistical analyses, and statistical significance was determined at P < 0.050.
Informed Consent
All participants provided written informed consent, and the study was conducted in accordance with Good Clinical Practice and the ethical principles that originated in the Declaration of Helsinki. The study was approved by the institutional review board of Albert Einstein College of Medicine.
Results
Between October 2013 and May 2016, a total of 190 patients were screened for study inclusion and 32 were excluded. Of 158 patients randomly assigned to a study group, 150 received the intervention as assigned (Figure 1). Participants had a mean age of 51 years (SD, 10.6). Most were male, Latino, and unemployed and had genotype 1a HCV (Table 2). Most (65%) had used drugs in the previous 6 months, most commonly opioids (47%) or cocaine (47%), with 75% reporting ever injecting drugs. More than 75% of participants picked up methadone 4 to 6 times per week. Most participants (n = 115 [77%]) received combination DAA treatment (Table 2).
Table 2.
Baseline Characteristics of PREVAIL Study Participants*
| Characteristic | DOT (n = 51) | GT (n = 48) | SIT (n = 51) | Total (n = 150) |
|---|---|---|---|---|
| Mean age (SD),y | 51.4 (10) | 51.2 (11) | 51.0 (11) | 51.2 (11) |
| Male | 33 (65) | 32 (67) | 32 (63) | 97 (65) |
| Race/ethnicity | ||||
| Latino | 31 (61) | 24 (50) | 29 (57) | 84 (56) |
| African American | 13 (26) | 13 (27) | 14 (27) | 40 (27) |
| White | 4 (8) | 5 (10) | 3 (6) | 12 (8) |
| Other | 3 (6) | 6 (13) | 5 (10) | 14 (9) |
| Homeless | 9 (18) | 10 (21) | 15 (29) | 34 (23) |
| Unemployed | 43 (84) | 38 (79) | 41 (80) | 122 (81) |
| Married (living with partner) | 18 (35) | 21 (44) | 16 (31) | 55 (37) |
| Urine drug screen (6 mo before baseline)† | ||||
| Any drug | 34 (67) | 34 (71) | 30 (58) | 98 (65) |
| Opioids | 23 (45) | 26 (54) | 21 (41) | 70 (47) |
| Cocaine | 24 (47) | 23 (48) | 24 (47) | 71 (47) |
| Benzodiazepines | 15 (29) | 15 (31) | 13 (26) | 43 (29) |
| Urine drug screen (at baseline) | ||||
| Any drug | 26 (51) | 23 (48) | 25 (49) | 74 (49) |
| Opioids/oxycodone | 12 (24) | 14 (29) | 11 (22) | 37 (25) |
| Cocaine | 17 (33) | 11 (23) | 16 (31) | 44 (29) |
| Benzodiazepines | 9 (18) | 4 (8) | 10 (20) | 23 (15) |
| Amphetamines | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Self-reported drug use (30 d before baseline) | ||||
| Heroin | 9 (18) | 9 (19) | 10 (20) | 28 (19) |
| Other opioids/analgesics | 10 (20) | 8 (17) | 15 (29) | 33 (22) |
| Cocaine | 12 (24) | 12 (25) | 12 (24) | 36 (24) |
| Sedatives/hypnotics/tranquilizers | 9 (18) | 12 (25) | 12 (24) | 33 (22) |
| Amphetamines | 3 (6) | 0 (0) | 1 (2) | 4 (3) |
| Alcohol use to intoxication (30 d before baseline) | 13 (26) | 11 (23) | 12 (24) | 36 (24) |
| Injection drug use (ever) | 38 (75) | 40 (83) | 35 (69) | 113 (75) |
| Comorbid psychiatric conditions | ||||
| Any | 20 (39) | 22 (46) | 25 (49) | 67 (45) |
| Major depressive episode | 11 (22) | 15 (31) | 12 (24) | 38 (25) |
| Generalized anxiety disorder | 8 (16) | 10 (21) | 10 (20) | 28 (19) |
| Psychotic disorder | 12 (24) | 17 (35) | 20 (39) | 49 (33) |
| Current manic episode | 1 (2) | 6 (13) | 4 (8) | 11 (7) |
| Depression (PHQ-9) | ||||
| None or mild | 33 (65) | 33 (69) | 31 (60) | 97 (65) |
| Moderate or severe | 18 (35) | 15 (31) | 20 (39) | 53 (35) |
| HIV/HCV co-infection | 6 (12) | 6 (13) | 9 (18) | 21 (14) |
| HCV subtype | ||||
| 1a | 43 (84) | 41 (85) | 44 (86) | 128 (85) |
| 1b | 8 (16) | 7 (15) | 7 (14) | 22 (15) |
| IL28B CC | 9 (18) | 11 (23) | 13 (25) | 33 (22) |
| IL28B TC | 26 (50) | 26 (54) | 27 (53) | 79 (53) |
| IL28B TT | 16 (31) | 11 (23) | 11 (22) | 38 (25) |
| Cirrhosis | 15 (29) | 16 (33) | 10 (20) | 41 (27) |
| Treatment experienced | 4 (8) | 6 (13) | 6 (12) | 16 (11) |
| DAA regimen | ||||
| SOF/LDV | 31 (61) | 38 (79) | 35 (69) | 104 (69) |
| SOF/SMV | 5 (10) | 2 (4) | 4 (8) | 11 (7) |
| SOF/RBV | 9 (18) | 3 (6) | 5 (10) | 17 (11) |
| SOF/IFN/RBV | 5 (10) | 3 (6) | 7 (14) | 15 (10) |
| TVR/IFN/RBV | 1 (2) | 2 (4) | 0 (0) | 3 (2) |
| Opioid agonist therapy | ||||
| Methadone | 51 (100) | 47 (98) | 49 (96) | 147 (98) |
| Buprenorphine | 0 (0) | 1 (2) | 2 (4) | 3 (2) |
| Pick-up schedule | ||||
| 1–3 per week | 6 (12) | 12 (25) | 14 (28) | 32 (21) |
| 4–6 per week | 45 (88) | 36 (75) | 37 (73) | 118 (79) |
DAA = direct-acting antiviral; DOT = directly observed therapy; GT = group treatment; HCV = hepatitis C virus; IFN = pegylated interferon; LDV = ledipasvir; PHQ-9 = Patient Health Questionnaire-9; PREVAIL = Prevent Resistance Eliminate Virus and Improve Life; RBV = ribavirin; SIT = self-administered individual treatment; SMV = simeprevir; SOF = sofosbuvir; TVR = telaprevir.
No missing values wsere observed for any characteristics. Values are numbers (percentages) unless otherwise indicated.
Oxycodone and amphetamine data were not obtained via chart review.
Adherence
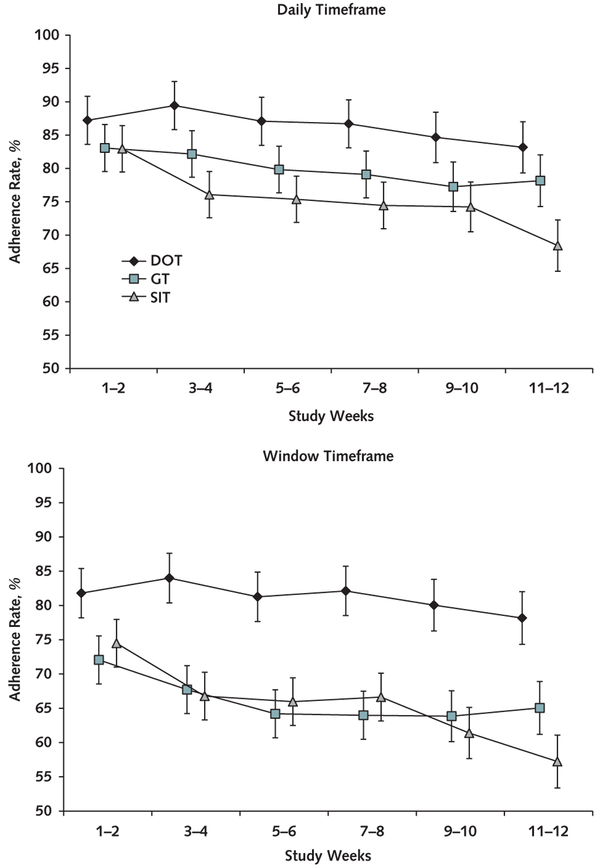
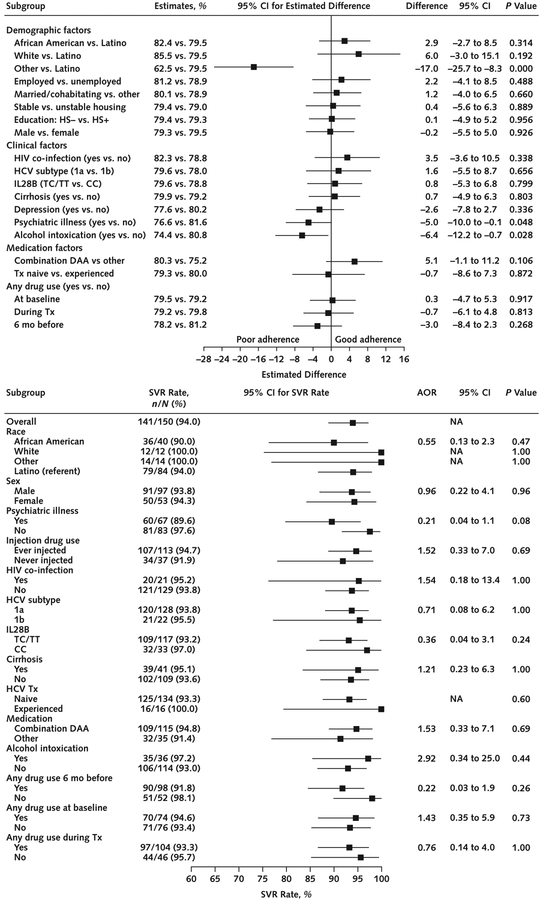
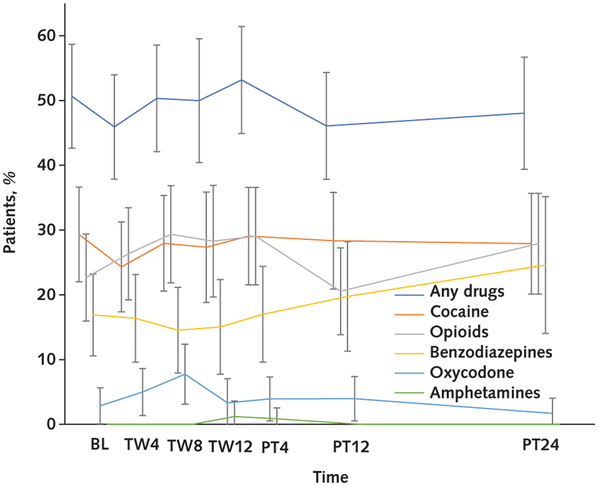
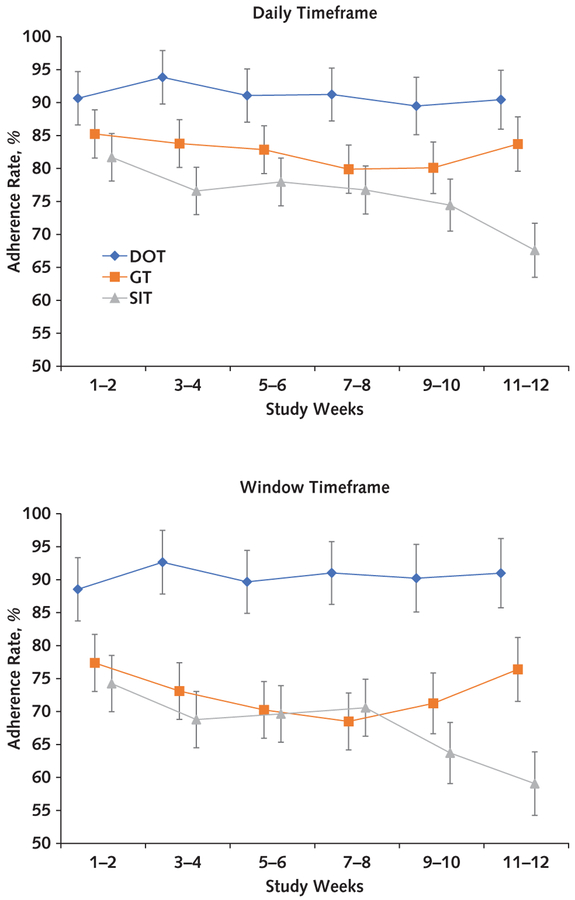
Overall adherence estimated from mixed-effects models using the daily timeframe and considering all treated participants (n = 150) was 78% (95% CI, 75% to 81%). The daily timeframe adherence was significantly different across the 3 groups (P = 0.003) and was greater in the DOT (86% [CI, 80% to 92%]) than the SIT group (75% [CI, 70% to 81%]; difference, 11% [CI, 5% to 18%]; Bonferroni-corrected P = 0.001). However, no significant difference was observed between the GT (80% [CI, 74% to 86%]) and the SIT group (difference, 4.7% [CI, −2% to 11%]; Bonferroni-corrected P = 0.29). Estimated overall window timeframe adherence among all treated participants was 67% (CI, 64% to 70%). The window timeframe adherence was significantly different among the groups (P < 0.001) and greater in the DOT (81% [CI, 74% to 88%]) than the SIT group (65% [CI, 59% to 72%]; difference, 16% [CI, 8% to 23%]; Bonferroni-corrected P < 0.001); however, no significant difference was observed between the GT (66% [CI, 59% to 73%]) and the SIT group (difference, 1% [CI, −7% to 8%]; Bonferroni-corrected P = 1.00) (Figure 2). All these results were virtually unchanged in sensitivity analyses of the handling of missing adherence data, including application of a fully conditional specification imputation model (data not shown). In the subgroup of participants receiving combination DAA therapy, daily adherence was significantly different among the groups (P < 0.001) and greater in the DOT (91% [CI, 84% to 98%]) than the SIT group (76% [CI, 70% to 82%]; difference, 15% [CI, 8% to 22%]; Bonferroni-corrected P < 0.001); however, no significant difference was observed between the GT (83% [CI, 77% to 89%]) and the SIT group (difference, 7% [CI, 0.3% to 13%]; Bonferroni-corrected P = 0.080). In the window timeframe, adherence was significantly different among the groups (P < 0.001) and was greater in the DOT (91% [CI, 82% to 99%]) than the SIT group (68% [CI, 61% to 75%]; difference, 23% [CI, 15% to 31%]; Bonferroni-corrected P <0.001); however, no significant difference was observed between the GT (73% [CI, 66% to 80%]) and the SIT group (difference, 5% [CI, −3% to 13%]; Bonferroni-corrected P = 0.39) (Appendix Figure 1, available at Annals.org). Factors significantly associated with poor daily adherence were psychiatric illness at baseline (P = 0.048) and drinking alcohol to intoxication in the 30 days before baseline (P = 0.028). Drug use was not significantly associated with poor adherence (Figure 3, top) and was noted to be consistent throughout the study period (Figure 4).
Figure 2. Adherence rates over time.
Adherence rates and their error bars are model-based estimates; SEs were obtained from the mixed-effects linear models adjusting for site and the 3 stratifying variables. Three SIT participants with no available blister pack adherence data were not included in this analysis. Error bars represent ± SEs. DOT = directly observed therapy; GT = group treatment; SIT = self-administered individual treatment.
Figure 3. Forest plots comparing participant characteristics with daily adherence and SVR.
AOR = adjusted odds ratio; DAA = direct-acting antiviral; HCV = hepatitis C virus; HS = high school; NA = not available; SIT = self-administered individual treatment; SVR = sustained virologic response; Tx = treatment. Top. Participant characteristics and daily adherence. All statistics, including P values, are estimated from a mixed-effects model adjusting for site and the study groups. Three SIT participants with no available blister pack adherence data were not included in this analysis. Bottom. Participant characteristics and SVR. AORs and their 95% CIs and P values were obtained from exact multivariable logistic regressions adjusting for site and the study groups. No missing data were observed for this analysis.
Figure 4. Urine toxicology testing over time.
Comparisons among substances at each time point were not conducted. Error bars represent 95% CIs. BL = baseline; PT = posttreatment week; TW = treatment week.
Treatment Completion and SVR
Among all participants who initiated treatment (n = 150), overall completion was 97% (CI, 92% to 99%), with no significant difference among groups (P = 0.53): DOT, 98% (CI, 90% to 100%); GT, 98% (CI, 89% to 100%); SIT, 94% (CI, 84% to 99%); DOT versus SIT difference, 3.9% (CI, −7% to 15% [Bonferroni-corrected P = 1.00]); and GT versus SIT difference, 3.8% (CI, −8% to 15% [Bonferroni-corrected P = 1.00]). Overall SVR was 94% (CI, 89% to 97%), with no significant difference among groups (P = 0.152): DOT, 98% (CI, 90% to 100%); GT, 94% (CI, 83% to 99%); SIT, 90% (CI, 79% to 97%); DOT versus SIT difference, 7.8% (CI, −4% to 20% [Bonferroni-corrected P = 0.40]); and GT versus SIT difference, 3.6% (CI, −10% to 17% [Bonferroni-corrected P = 1.00]). No participant characteristics were significantly associated with SVR (Figure 3, bottom). Among participants receiving combination DAA therapy, overall SVR was 95% (CI, 89% to 98%), with no significant difference among groups (P = 0.056): DOT, 100% (CI, 90% to 100%); GT, 95% (CI, 83% to 99%); and SIT, 90% (CI, 76% to 97%) (Appendix Table 1, available at Annals.org). With regard to the intention-to-treat analysis using all randomly assigned participants (n = 158), treatment completion was 92% (CI, 86% to 96%) overall, with no significant difference among groups (P = 0.77): DOT, 94% (CI, 84% to 99%); GT, 90% (CI, 79% to 97%); and SIT, 91% (CI, 79% to 97%). Overall SVR was 89% (CI, 83% to 94%), with no significant difference among groups (P = 0.36): DOT, 94% (CI, 84% to 99%); GT, 87% (CI, 74% to 94%); and SIT, 87% (CI, 75% to 95%).
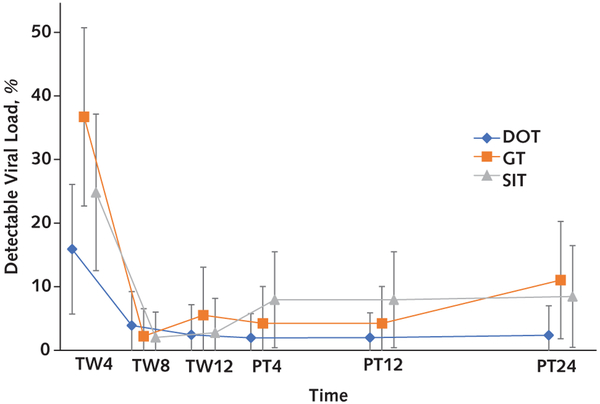
The proportions of participants with detectable HCV viral loads longitudinally over the treatment and posttreatment periods were significantly different across the 3 groups (P = 0.021): DOT versus GT adjusted odds ratio (AOR), 0.15 (CI, 0.04 to 0.58 [P = 0.006]), and DOT versus SIT AOR, 0.24 (CI, 0.06 to 0.93 [P = 0.039]). At week 4 in particular, on the basis of the post hoc analysis, 14% of DOT participants had week 4 detectable HCV viral load, compared with 37% of GT participants (AOR, 0.32 [CI, 0.12 to 0.86]; P = 0.025) and 27% of SIT participants (AOR, 0.54 [CI, 0.19 to 1.52]; P = 0.24) (Appendix Figure 2, available at Annals.org).
Sustained virologic response, by treatment regimen and characteristics of the 9 treatment failures, including 2 deaths, is shown in Appendix Tables 2 and 3 (available at Annals.org).
Adherence, SVR, and Drug Use
Greater adherence was significantly associated with SVR, with the odds of SVR 1.81 times higher for each 10% increase in daily adherence (CI, 1.06 to 3.11 [P = 0.030]) and 1.71 times higher for each 10% increase in window adherence (CI, 1.04 to 2.82 [P = 0.035]).
Discussion
To our knowledge, the PREVAIL (Prevent Resistance Eliminate Virus and Improve Life) study is the first randomized trial to test intensive models for providing HCV care with DAAs to PWID receiving OAT. Although other studies have used self-reported medication adherence questionnaires and electronic diaries (39–41), the PREVAIL trial used electronic blister packs to monitor adherence to DAAs. Using electronic blister pack technology, we found that adherence was greater in the DOT than the SIT group. Using both daily and window timeframes, we demonstrated that increases in adherence were associated with an increased likelihood of SVR. Drinking alcohol to intoxication and psychiatric illness were associated with poor adherence, which suggests that additional adherence support may be warranted for PWID who use alcohol or have a psychiatric disorder and are receiving HCV treatment.
All 3 models had a high proportion of patients receiving OAT who completed treatment and achieved SVR, including those actively using drugs. The overall SVR rate was 94% (CI, 89% to 97%), which is similar to that of a large registration trial in patients with genotype 1 HCV treated with SOF/LDV, in which the SVR rate was 99% (CI, 96% to 100%) (42). This result is notable, because approximately one quarter of study participants received interferon- or ribavirin-based HCV therapies that are associated with lower SVR rates, particularly when combined with certain host and viral factors, including African American race, advanced liver fibrosis, IL28B genotype, and prior treatment experience (43–45). Moreover, registration trials of DAA therapies generally have excluded or minimized entry of patients who are either active PWID or receiving OAT. Our study provides evidence that similar SVR rates may be achieved in PWID receiving OAT.
Although a greater percentage of patients were cured (achieved SVR) in the more intensive models of care (DOT and GT), no statistically significant differences in SVR were found among groups, despite a significant difference in adherence among groups and adherence being predictive of SVR. The threshold for optimal adherence that predicts SVR is not known in the DAA era. We postulate that a larger trial is needed to determine definitively whether the models differ with respect to SVR. We did, however, see significant differences in rates of decline of HCV viral load, driven primarily by rates of viral clearance in the first 4 weeks of treatment. Rapid virologic response, or undetectable viral load at treatment week 4, was identified as a predictor of SVR in the interferon era (46), but the importance of this outcome may be limited in the DAA era (47). However, with therapy being shortened to 8 weeks or less (48) and emerging discussions about the reintroduction of response-guided therapy (49), adherence throughout the treatment period may become more influential, with the benefits of adherence associated with DOT becoming more pronounced.
Our data demonstrate that active drug use has no substantial effect on virologic outcomes in HCV treatment. This finding supports treating PWID receiving OAT, regardless of current drug use. Two recent clinical trials (phase 2 and phase 3) examining DAA therapy among PWID receiving and those not receiving OAT demonstrated similar findings. In a study by Dore and colleagues (41), 96% of patients reported greater than 95% adherence using electronic diaries, with an SVR of 92%. Grebely and colleagues (50) observed 94% daily adherence among patients using electronic blister packs, with an SVR of 94%. In our trial, daily adherence was 78%, with an SVR of 94%, suggesting that lower adherence may be tolerated without affecting SVR. Further studies are needed to assess the effect of adherence on SVR to determine the threshold at which SVR is affected.
This study has several limitations. First, it occurred during the transition from interferon- and ribavirin-based treatments to state-of-the-art combination DAA therapy. Because of the emergence of combination DAA regimens, all participants did not receive the same therapy; however, we observed no differences in HCV treatment among groups. Second, our results may not be generalizable to HCV-infected PWID not enrolled in OAT programs or to rural populations. However, the intensive models of care studied may be even more important in other settings where PWID are served, including syringe service programs, in which attention to adherence and social support may have an even greater impact. Another limitation is that psychiatric stability is no longer a criterion for treatment eligibility, as it was in the interferon era. However, in this study, treatment eligibility was determined by providers, and nearly half of our participants were found to have psychiatric diagnoses; therefore, we do not believe this limits generalizability.
In conclusion, DOT in OAT settings was associated with greater adherence than self-administered treatment, and improved adherence was associated with SVR. All models of onsite HCV care resulted in high treatment completion and SVR rates despite ongoing drug use, thereby supporting treatment of PWID in the OAT setting.
Financial Support:
By grants R01DA034086 and K99DA043011 from the National Institute on Drug Abuse. Gilead Sciences also provided support (grant IN-337-1779) for the research and supplied study medication, SOF/LDV.
Disclosures: Dr. Akiyama has served on an advisory board for Gilead Sciences outside the submitted work. Dr. Norton reports grants from Merck and Co. outside the submitted work. Dr. Litwin reports grants from Gilead Sciences during the conduct of the study, and grants and personal fees from Gilead Sciences and Merck Pharmaceuticals outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-1715.
Primary Funding Source:
National Institute on Drug Abuse and Gilead Sciences.
Role of the Funding Source
This research was supported by the National Institute on Drug Abuse and Gilead Sciences. Gilead Sciences also provided study medication, SOF/LDV.
Appendix Figure 1. Adherence rates among PREVAIL participants receiving combination DAA therapy.
Adherence rates and their error bars are model-based estimates; SEs were obtained from the mixed-effects linear models adjusting for site and the 3 stratifying variables. No missing data were observed for this analysis. Error bars represent ± SEs. DAA = direct-acting antiviral; DOT = directly observed therapy; GT = group treatment; PREVAIL = Prevent Resistance Eliminate Virus and Improve Life; SIT = self-administered individual treatment.
Appendix Figure 2. Percentage of detectable HCV viral loads over time.
The overall percentages of longitudinal detectable HCV viral loads during and after treatment were significantly different across the 3 groups (P = 0.021). Error bars represent 95% CIs. DOT = directly observed therapy; GT = group treatment; HCV = hepatitis C virus; PT = posttreatment week; SIT = self-administered individual treatment; TW = treatment week.
Appendix Table 1.
SVR, by Group, for Study Participants Overall and for Those Receiving a Combination DAA Regimen*
| Group | Overall | Combination DAA Regimen | ||||
|---|---|---|---|---|---|---|
| Patients, n | SVR, n (%) | SVR, 95% CI, % | Patients, n | SVR, n (%) | SVR, 95% CI, % | |
| Overall | ||||||
| DOT | 51 | 50 (98) | 90 to 100 | 36 | 36 (100) | 90 to 100 |
| GT | 48 | 45 (94) | 83 to 99 | 40 | 38 (95) | 83 to 99 |
| SIT | 51 | 46 (90) | 79 to 97 | 39 | 35 (90) | 76 to 97 |
| Total | 150 | 141 (94) | 89 to 97 | 115 | 109 (95) | 89 to 98 |
| Difference in SVR (95% CI), percentage points | Difference in SVR (95% CI), percentage points | |||||
| Comparison | ||||||
| DOT vs. GT | – | 4 (–7 to 16) | – | 5 (–8 to 18) | ||
| DOT vs. SIT | – | 8 (–4 to 20) | – | 10 (–4 to 25) | ||
| GT vs. SIT | – | 4 (–10 to 17) | – | 5 (–10 to 21) | ||
DAA = direct-acting antiviral; DOT = directly observed therapy; GT = group treatment; SIT = self-administered individual treatment; SVR = sustained virologic response.
No significant differences in SVR were found across the 3 groups (P = 0.152) among all participants or among those receiving combination DAA treatment (P = 0.056), on the basis of multivariable exact logistic regression adjusting for site and the 3 stratifying variables. No missing data were observed for this analysis.
Appendix Table 2.
SVR, by Treatment Regimen*
| Regimen | Patients, n | SVR, n (%) | SVR, 95% CI, % |
|---|---|---|---|
| SOF/LDV | 104 | 98 (94) | 88–98 |
| SOF/SMV | 11 | 11 (100) | 72–100 |
| SOF/IFN/RBV | 15 | 14 (93) | 68–100 |
| SOF/RBV | 17 | 15 (88) | 64–99 |
| TVR/IFN/RBV | 3 | 3 (100) | 29–100 |
| Total | 150 | 141 (94) | 89–97 |
IFN = pegylated interferon; LDV = ledipasvir; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir; SVR = sustained virologic response; TVR = telaprevir.
No significant differences in SVR were found among treatment regimens (P = 0.69). No missing data were observed for this analysis.
Appendix Table 3.
Characteristics of Virologic Failure Cases
| Case Number | Group | Psychiatric Diagnosis/HIV | HCVVL, IU/mL | Genotype 1a or 1b | Cirrhosis | Tx Naive | Tx Regimen | Weeks of Treatment Regimen Completed (n/N) | Drug 6 Months Before Tx | Drug During Tx | Daily Adherence, % | Viral and Clinical Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DOT | Bipolar disorder | 213 832 | 1a | No | Yes | SOF/RBV | 4/24 | C/O/B | O | 79 | Week 4 VL: 233 110 IU/mL DOT 3×/wk, Tx discontinued at week 4 |
| 2 | SIT | Depression | 1 114 267 | 1b | Yes | Yes | SOF/RBV/IFN | 8/12 | O/B | None | No data | Week 4 VL: 93 692 IU/mL Week 8 VL: 3516IU/mL Tx discontinued because of side effects |
| 3 | SIT | Depression | 188 936 | 1a | No | Yes | SOF/LDV | 8/8 | No | B | 43 | ETR, no SVR4 or SVR12 |
| 4 | SIT | Depression | 2 471 964 | 1a | No | Yes | SOF/LDV | 8/8 | C/O | C/O | 31 | ETR, no SVR4 or SVR12 |
| 5 | GT | Depression | 7 300 001 | 1a | No | Yes | SOF/LDV | 12/12 | C/O | C/O | 38 | No ETR UD at weeks 4 and 8 |
| 6 | GT | None | 12 143 424 | 1a | No | Yes | SOF/RBV | 12/24 | C/O | O | 45 | Week 4 VL: 43 IU/mL Week 8 VL: 585 602 IU/mL Incarcerated |
| 7 | SIT | None | 15 961 170 | 1a | Yes | Yes | SOF/LDV | 12/12 | C/O | O | 82 | ETR, no SVR |
| 8 | GT | Depression HIV | 19 508 733 | 1a | No | Yes | SOF/LDV | 7/12 | C/B | C/O | 91 | UD at week 4 Deceased: cardiac |
| 9 | SIT | Depression | 621 760 | 1a | No | Yes | SOF/LDV | 8/12 | O/B | None | 86 | UD at week 8 Deceased: MVA |
B = benzodiazepines; C = cocaine; DOT = directly observed therapy; ETR = end-of-treatment response; GT = group treatment; HCV = hepatitis C virus; IFN = pegylated interferon; LDV = ledipasvir; MVA = motor vehicle accident; O = opioids; RBV = ribavirin; SIT = self-administered individual treatment; SOF = sofosbuvir; SVR = sustained virologic response; SVR4 = SVR at week 4; SVR12 = SVR at week 12; Tx = treatment; UD = undetectable; VL = viral load.
Footnotes
Note: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the U.S. government.
Reproducible Research Statement: Study protocol: See the Supplement (available at Annals.org). Statistical code: Not available. Data set: Available from Dr. Litwin (e-mail, alain.litwin@prismahealth.org).
References
- 1.Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. HCV Surveillance Report 2015. Accessed at http://www.cdc.gov/hepatitis/statistics/2015surveillance/on 30 January 2018.
- 3.Verna EC, Brown RS Jr. Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10:919–40. [DOI] [PubMed] [Google Scholar]
- 4.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004 [DOI] [PubMed] [Google Scholar]
- 5.Lok AS, Chung RT, Vargas HE, Kim AY, Naggie S, Powderly WG. Benefits of direct-acting antivirals for hepatitis C. Ann Intern Med. 2017;167:812–813. doi: 10.7326/M17-1876 [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Stepanova M, Henry L, Han KH, Ahn SH, Lim YS, et al. The effect of interferon-free regimens on health-related quality of life in East Asian patients with chronic hepatitis C. Liver Int. 2018;38:1179–1187. doi: 10.1111/liv.13650 [DOI] [PubMed] [Google Scholar]
- 7.Ohashi K, Ishikawa T, Suzuki M, Abe H, Koyama F, Nakano T, et al. Health-related quality of life on the clinical course of patients with chronic hepatitis C receiving daclatasvir/asunaprevir therapy: a prospective observational study comparing younger (<70) and elderly (= 70) patients. Exp Ther Med. 2018;15:970–976. doi: 10.3892/etm.2017.5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Feld J, Zeuzem S, Sulkowski M, Foster GR, et al. Sofosbuvir and velpatasvir combination improves patient-reported outcomes for patients with HCV infection, without or with compensated or decompensated cirrhosis. Clin Gastroenterol Hepatol. 2017;15:421–430. doi: 10.1016/j.cgh.2016.10.037 [DOI] [PubMed] [Google Scholar]
- 9.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–7. doi: 10.1093/cid/cir306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 11.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology. 2018;68:827–838. doi: 10.1002/hep.29811 [DOI] [PubMed] [Google Scholar]
- 12.Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grebely J, Raffa JD, Lai C, Krajden M, Kerr T, Fischer B, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16:352–8. doi: 10.1111/j.1365-2893.2009.01080.x [DOI] [PubMed] [Google Scholar]
- 14.Calvo M, MacFarlane J, Zaccaro H, Curtis M, Cabán M, Favaro J, et al. Young people who use drugs engaged in harm reduction programs in New York City: overdose and other risks. Drug Alcohol Depend. 2017;178:106–114. doi: 10.1016/j.drugalcdep.2017.04.032 [DOI] [PubMed] [Google Scholar]
- 15.Boyd J, Fast D, Hobbins M, McNeil R, Small W. Social-structural factors influencing periods of injection cessation among marginalized youth who inject drugs in Vancouver, Canada: an ethno-epidemiological study. Harm Reduct J. 2017;14:31. doi: 10.1186/s12954-017-0159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebo KA, Keruly J, Moore RD. Association of social stress, illicit drug use, and health beliefs with nonadherence to antiretroviral therapy. J Gen Intern Med. 2003;18:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–80. [DOI] [PubMed] [Google Scholar]
- 18.Strathdee SA, Latka M, Campbell J, O’Driscoll PT, Golub ET, Kapadia F, et al. ; Study to Reduce Intravenous Exposures Project. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40 Suppl 5:S304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001;61:211–5. [DOI] [PubMed] [Google Scholar]
- 20.Walley AY, White MC, Kushel MB, Song YS, Tulsky JP. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat. 2005;28:181–7. [DOI] [PubMed] [Google Scholar]
- 21.Zeremski M, Dimova RB, Zavala R, Kritz S, Lin M, Smith BD, et al. Hepatitis C virus-related knowledge and willingness to receive treatment among patients on methadone maintenance. J Addict Med. 2014;8:249–57. doi: 10.1097/ADM.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med. 2014;174:1974–81. doi: 10.1001/jamainternmed.2014.5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolan S, Dias Lima V, Fairbairn N, Kerr T, Montaner J, Grebely J, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction. 2014;109:2053–9. doi: 10.1111/add.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderks C Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (Update) 2017. Accessed at www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.html on 15 March 2018 [PubMed]
- 25.Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction. 2008;103:905–18. doi: 10.1111/j.1360-0443.2008.02188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris KA Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4:20–6. doi: 10.1097/ADM.0b013e3181add3de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litwin AH, Soloway IJ, Cockerham-Colas L, Reynoso S, Heo M, Tenore C, et al. Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program. Int J Drug Policy. 2015;26:1014–9. doi: 10.1016/j.drugpo.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butner JL, Gupta N, Fabian C, Henry S, Shi JM, Tetrault JM. Onsite treatment of HCV infection with direct acting antivirals within an opioid treatment program. J Subst Abuse Treat. 2017;75:49–53. doi: 10.1016/j.jsat.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 29.American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C 2017. Accessed at www.HCVguidelines.org on 15 January 2018.
- 30.Akiyama MJ, Agyemang L, Arnsten JH, Heo M, Norton BL, Schackman BR, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis. 2018;18:74. doi: 10.1186/s12879-018-2964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004;99:885–96. [DOI] [PubMed] [Google Scholar]
- 32.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH. Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program. J Subst Abuse Treat. 2012;43:424–32. doi: 10.1016/j.jsat.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sylvestre DL, Zweben JE. Integrating HCV services for drug users: a model to improve engagement and outcomes. Int J Drug Policy. 2007;18:406–10. [DOI] [PubMed] [Google Scholar]
- 36.Information Mediary Corp. Med-ic Smart Label. Accessed at www.informationmediary.com/med-ic on 1 February 2018.
- 37.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litwin AH, Li X, Heo M, Hidalgo J, Arnsten JH. Directly observed HCV treatment in methadone clinics—preliminary results [Abstract] Presented at International Conference on Viral Hepatitis 2011, Baltimore, MD, 11–12 April, 2011. Abstract no. 70762. [Google Scholar]
- 39.Mason K, Dodd Z, Guyton M, Tookey P, Lettner B, Matelski J, et al. Understanding real-world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int J Drug Policy. 2017;47:202–208. doi: 10.1016/j.drugpo.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 40.Grebely J, Mauss S, Brown A, Bronowicki JP, Puoti M, Wyles D, et al. Efficacy and safety of Ledipasvir/Sofosbuvir with and without ribavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: analysis of phase 3 ION trials. Clin Infect Dis. 2016;63:1405–1411. [DOI] [PubMed] [Google Scholar]
- 41.Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, et al. ; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165:625–634. doi: 10.7326/M16-0816 [DOI] [PubMed] [Google Scholar]
- 42.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. ; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 43.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. ; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912 [DOI] [PubMed] [Google Scholar]
- 44.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853 [DOI] [PubMed] [Google Scholar]
- 45.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804–11. doi: 10.1001/jama.2013.109309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55:69–75. doi: 10.1016/j.jhep.2010.10.032 [DOI] [PubMed] [Google Scholar]
- 47.Patel M, Rab S, Kalapila AG, Kyle A, Okosun IS, Miller L. Highly successful hepatitis C virus (HCV) treatment outcomes in human immunodeficiency virus/HCV-coinfected patients at a large, urban, Ryan White clinic. Open Forum Infect Dis. 2017;4:ofx062. doi: 10.1093/ofid/ofx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354–369. doi: 10.1056/NEJMoa1702417 [DOI] [PubMed] [Google Scholar]
- 49.Aghemo A, Colombo M. Response-guided duration of direct acting antiviral therapy for chronic hepatitis C: back to the future? Gastroenterology. 2017;152:1238–1239. doi: 10.1053/j.gastro.2017.02.022 [DOI] [PubMed] [Google Scholar]
- 50.Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, et al. ; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153–161. doi: 10.1016/S2468-1253(17)30404-1 [DOI] [PubMed] [Google Scholar]