Abstract
Familial adenomatous polyposis (FAP), a common inherited form of colorectal cancer (CRC), causes the development of hundreds to thousands of colonic adenomas in the colorectum beginning in early adolescence. In absence of a prophylactic surgery, FAP patients almost inevitably develop CRC by the age of 40 to 50. The lack of valuable prognostic biomarkers for FAP patients makes it difficult to predict when the progression from adenoma to malignant carcinoma occurs. Decreased tryptophan (TRP) plasma levels and increased indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan hydroxylase 1 (TPH1) enzymatic activities have been associated to tumour progression in CRC. In the present study, we aimed at investigating whether an altered TRP metabolism might also exist in FAP patients. Our results highlighted that plasma levels of TRP and its main catabolites are comparable between FAP patients and healthy subject. On the contrary, FAP patients presented significantly higher TRP levels with respect to high-grade adenoma (ADE) subjects and CRC patients. Obtained data lead us to evaluate IDO1 and TPH1 enzymes activity in the study groups. For both enzymes, it was possible to discriminate correctly between FAP subject and ADE/CRC patients with high sensitivities and specificities. By receiver operating characteristic (ROC) curve analysis, the cut-off values of IDO1 and TPH1 enzymatic activities associated to the presence of an active malignant transformation have been calculated as >38 and >5.5, respectively. When these cut-off values are employed, the area under the curve (AUC) is > 0.8 for both, indicating that TRP metabolism in patients with FAP may be used to monitor and predict the tumorigenic evolution.
Keywords: colorectal cancer, familial adenomatous polyposis (FAP), biomarkers, tryptophan, IDO1, TPH1
Introduction
Colorectal cancer (CRC) is one of the most frequent malignancies in developed countries. Despite most of CRCs are sporadic, from 2% to 5% of colon cancers have a hereditary. A common inherited form of CRC is the Lynch Syndrome, in which the affected individuals develop colonic adenomas with greater frequency than the general population even in absence of polyposis. Another inherited form, the familial adenomatous polyposis (FAP) causes the development of hundreds to thousands of colonic adenomas and, in its attenuated form (aFAP), the polyps’ number drops from 10 to less than a hundred. Classic FAP disease presents a germline mutation in the adenomatous polyposis coli (APC) gene that accelerates the tumorigenesis initiation. FAP patients develop hundreds of adenomas in the colo-rectum beginning in early adolescence1 and almost inevitably develop CRC by the mean age of 40 to 50 years (~100% of subjects). Similar clinical features are present in the MutYH-Associated Polyposis (MAP), which is caused by the biallelic mutations in MUTYH gene.2 Periodic surveillance by sigmoidoscopy/colonoscopy and prophylactic surgery are the sole ways to prevent CRC in these subjects.3 However, several clinical features may influence surgery scheduling and operative management in FAP patients under active surveillance (Figure 1). As a consequence thereof, premature colectomies can cause an important loss of quality of life in absence of immediate risk.4
Figure 1.
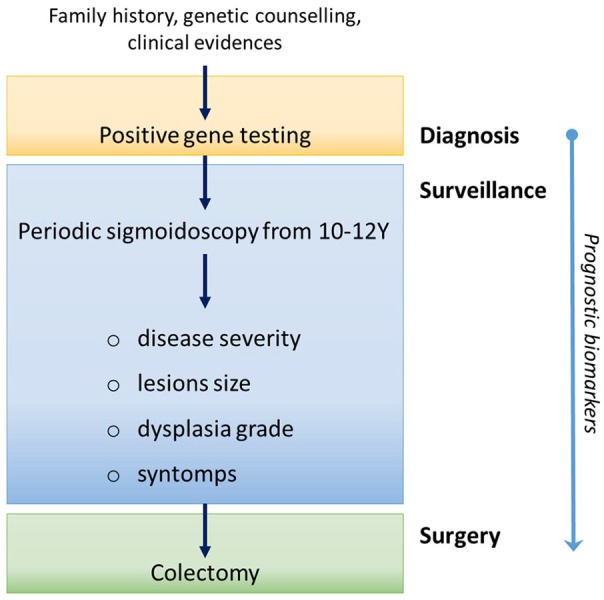
Schematic representation of FAP patients’ management after the genetic diagnosis.
FAP, Familial Adenomatous Polyposis.
A critical issue for improving management of FAP patients is to understand when the progression from adenoma to malignant carcinoma occurs. Indeed, in FAP individuals, CRC screening by flexible sigmoidoscopy or colonoscopy begin around age 10 to 12. During the screening, polyps number, size, distribution, and histologies are tracked annually. However, the high polyp number (>100) makes this endoscopic control difficult.
For these reasons, the identification of new prognostic biomarkers could represent a valuable possibility to replace colonoscopy in younger subjects and to postpone surgery without increasing the CRC risk, finally improving the FAP patients’ life quality. In the search for new valuable prognostic biomarkers, mass spectrometry–based proteomics has been employed to detect changes in tissue and plasma proteins5,6 or peptides7 correlated to disease progression. These studies seem promising for monitoring, and potentially predicting, the malignant transformation of polyps in FAP patients. In another study, the serum proteomic profiles of pre- and post-celecoxib-treated patients lead to the identification of several modulated proteins in a FAP-celecoxib prevention trial.8 Unfortunately, after the initial discovery step, in all these studies no putative protein biomarker has been validated for FAP disease progression monitoring.
Recently, we have showed that changes occurred in the tryptophan (TRP) metabolism in a cohort of CRC patients at different tumour stages and in subjects at risk of developing CRC (ie, high-grade adenoma (ADE) patients and ulcerative colitis patients).9 By monitoring TRP plasmatic levels and those of its metabolites, we highlighted the importance of TRP in CRC as a diagnostic marker of tumour progression. Indeed, decreased TRP concentration and increased indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan hydroxylase 1 (TPH1) enzymatic activities were detectable in plasma samples concomitant to pre-cancerous lesion and persisted in CRC patients at different stages (I-IV). Observed changes in the circulating TRP levels are mainly driven by the IDO1 overexpression at the tumour microenvironment9-11 and directly correlated with the raising of circulating pro-inflammatory cytokines and gamma-interferon.12 Under these conditions, IDO1 activity drives CRC progression and directly supports cancer cells growth by promoting the β-catenin activation and stimulating neoplastic growth.13,14 In addition, at stromal level, TRP deletion exerts an immune modulatory action against the infiltrate immune cells of both adaptive and innate tolerance, giving rise to the so-called immune escape.15
In the present study, we aimed at investigating whether an analogous pattern of TRP metabolism might exist in FAP patients, and if it could be employed as prognostic biomarkers. To do this, we analysed the plasma concentrations of TRP and its metabolites kynurenine (KYN), 5-hydroxy-tryptophan (5-HTP) and serotonin (5-hydroxytryptamine [5-HT]) in 25 FAP patients. Obtained results were compared to those of ADE and CRC described in our previous investigations (reference study)9 in order to determine if TRP metabolism in FAP patients might be used to monitor and predict the tumorigenic evolution.
Materials and Methods
Chemicals
l-tryptophan (TRP) was supplied by EGA-CHEMIE (Steinheim/Albuch W. Germany); 5-HTP, hydrochloride serotonin (5-HT), and l-kynurenine sulphate salt (KYN) were supplied by Sigma-Aldrich (Saint Louis, MO, USA). High performance liquid chromatography (HPLC) grade acetonitrile was provided by Avator Performance Materials BV (Deventer, Netherlands), analytical grade anhydrous potassium hydrogen phosphate (K2HPO4) and potassium dihydrogen phosphate (KH2PO4) were provided by Fluka Chemie (Buchs, Switzerland).
Patients’ samples
FAP plasma patients’ samples (n = 25) were from the First Surgical Clinic of Padua Hospital (Ethical Committee Approved Protocol Number: P448). FAP clinical parameters are as follows: median age 33 (11-61, min-max); sex: male = 7, female = 18; pathogenic gene mutation: in APC = 21, in MutYH = 4. A subset of healthy subjects (n = 5) was included and analysed as control samples to verify the data homogeneity with respect to the reference study.
Serum samples from FAP (n = 4), ADE (n = 6), and CRC (n = 8) were further obtained from the same institution for the quantification of pro-inflammatory cytokines and chemokines. Blood collection was performed using a DB Vacutainer® Blood Collection Tube (Becton Dickinson and Company, Franklin Lake, NJ, USA). Plasma was prepared by centrifuging 7 mL of peripheral blood at 3000 x rpm for 10 minutes at 4°C and stored at −80°C until analysis.
TRP and metabolites quantification
Quantitative analysis of TRP, 5-HTP, and 5-HT in plasma was performed according to the reference study,9 using a LC-10AD coupled to a RF-10AXL detector (Shimadzu Corporation, Kyoto, Japan). Excitation and respective emission wavelengths were set at λ = 285 and 345 nm, respectively. Chromatographic separation was carried out using a Platinum EPS C18 5 μm, 250 mm x 4.6 mm (Grace, Deerfield, IL, USA) column with an Alltech RP-8 (25-40 μm Lichroprep, Merck, Darmstadt, Germany) as guard column. Analytes were eluted in isocratic mode with acetonitrile and a 0.004 M phosphate buffer solution (15:85 v/v) at 1 mL/min flow rate.
Quantitative analysis of KYN was performed using the LC-10AD coupled to a UV-VIS Detector Model SPD-10A (Shimadzu Corporation, Kyoto, Japan). The absorbance wavelength was set at λ = 360 nm. Chromatographic separation was carried out at room temperature using a GraceSmart RP 18 column 5 μm 250 mm x 4.6 mm of (Grace, Deerfield, IL, USA). KYN elution was obtained using the same isocratic gradient previously described.
Human inflammatory cytokines detection
Circulating cytokines were finally evaluated in serum plasma samples by the MultiAnalyte ELISArray kit following the manufacturer’ instructions (Qiagen, MEH-004A). Cytokines evaluated were as follows: interleukin-1α (IL1α), IL1β, IL2, IL6, IL8, IL10, IL12, IL17A, interferon γ (IFNγ), tumour necrosis factor α (TNFα), and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Statistical analysis
Statistical analysis was performed with GraphPad Prism, version 5.00, 2007 (La Jolla, CA, USA). Normality of data was evaluated using the D’Agostino-Pearson omnibus normality test, and parametric (or non-parametric) statistical analyses were completed accordingly. To evaluate the sensitivity and specificity of IDO1 and TPH1 enzymes activity, univariate receiver operating characteristic (ROC) curves were employed, after the generation of the optimal cut-off points by the Youden’s index. Data in the text and in tables are presented as median and interquartile ranges (Q1, Q3) unless otherwise stated.
Results and Discussion
Data homogeneity with respect to the reference study9 was evaluated through the analysis of a new set of control samples (Table 1). Obtained results were consistent with the previous ones for all the analytes and the IDO1/TPH1 enzyme activities, as verified by Mann Whitney, confirming the absence of any analytical bias between the two studies.
Table 1.
Data homogeneity verification with respect to the reference study.
| Controls (n = 5) |
Controls (n = 30)a |
P value | |
|---|---|---|---|
| Median (Q1; Q3) | Median (Q1; Q3) | ||
| TRP µg/mL | 9.07 (7.08; 11.81) | 10.25 (8.99; 11.46) | .385 |
| KYN µg/mL | 0.43 (0.33; 0.45) | 0.38 (0.28; 0.46) | .587 |
| 5HTP µg/mL | 0.05 (0.04; 0.06) | 0.06 (0.04; 0.08) | .153 |
| 5-HT µg/mL | 0.04 (0.02; 0.07) | 0.02 (0.01; 0.03) | .057 |
| IDO1 activity | 40.35 (34.82; 57.18) | 38.27 (29.27; 48.52) | .436 |
| TPH1 activity | 5.09 (3.31; 6.16) | 5.68 (3.96; 6.928) | .564 |
Abbreviations: 5HTP, 5-hydroxy-tryptophan; IDO1, indoleamine 2,3-dioxygenase 1; KYN, kynurenine; TPH1, tryptophan hydroxylase 1; TRP, tryptophan; 5-HT, serotonin
New recruited control samples have been compared to those previously analysed.
Data retrieved from the reference study.
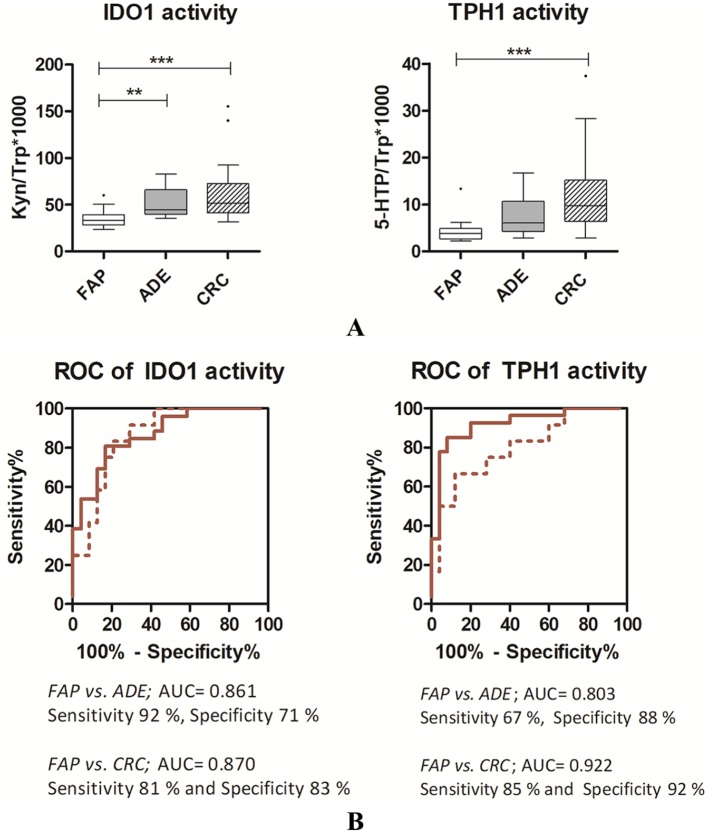
In Table 2, obtained results for FAP plasma samples are compared to those previously obtained for adenomas (n = 12) and for CRC patients with the malignancy specifically located in the colon (n = 41). Indeed, we demonstrated that the anatomical site of lesion significantly affects the TRP levels.9 As compared with colon cancer patients, FAP presented statistically significant differences in TRP (P = 0.001, Kruskal-Wallis) and 5-HTP (P < 0.001, Kruskal-Wallis) plasma concentrations. Indeed, increased TRP concentrations were present in FAP patients with respect to both ADE subjects and CRC patients, whilst lower plasma 5-HTP concentrations were detected (Table 2). Conversely, for both KYN and 5-HT, no changes were observable in the FAP-ADE-CRC comparison. These data are consistent with the typical CRC-associated inflammation that actively modulates the circulating levels of TRP, and its metabolites, along the adenoma–carcinoma sequence by means of an increased tissue IDO1 expression.9,16 Indeed, at the tumour microenvironment, TRP metabolites are produced in presence of an active inflammatory state and subsequently, they are shed into the blood. Consequently, tumour progression produces detectable changes in plasma concentrations of TRP and IDO1/TPH1 tissue activities are easily calculated based on them. In our samples, the KYN/TRP*1000 ratio was calculated as an estimate of IDO1 activity; similarly, 5-HTP/TRP*1000 ratio was calculated as an estimate of TPH1 activity. The activity of IDO1 and TPH1 enzymes for FAP patients have been then compared to those of subject presenting high-risk adenomas (ADE) and CRC patients (Figure 2, panel A). Our results revealed a lower IDO1 activity in FAP patients with respect to both ADE and CRC (P < 0.0001, Kruskal-Wallis) patients. Similarly, the results we reported in Figure 2 (panel A) also highlight a decreased TPH1 activity in FAP patients with respect to ADE and CRC patients (FAP vs. CRC: P < 0.0001, Kruskal-Wallis).
Table 2.
Plasma concentration of TRP and its metabolites in FAP, high-risk adenoma and colon cancer patients.
| FAP (n = 25) |
Adenomaa (n = 12) |
Colon cancera (n = 41) |
|||||
|---|---|---|---|---|---|---|---|
| Median | Q1; Q3 | Median | Q1; Q3 | Median | Q1; Q3 | P values | |
| TRP µg/mL | 8.23 | 7.19; 10.18 | 7.34 | 5.44; 9.52 | 5.77 | 4.71; 7.17 | <.01 b |
| KYN µg/mL | 0.30 | 0.25; 0.34 | 0.35 | 0.28; 0.43 | 0.32 | 0.24; 0.40 | |
| 5HTP µg/mL | 0.03 | 0.03; 0.04 | 0.04 | 0.04; 0.06 | 0.05 | 0.04; 0.08 | <.05c; <.001b |
| 5-HT µg/mL | 0.01 | 0.01; 0.03 | 0.01 | 0.009; 0.01 | 0.01 | 0.01; 0.02 | |
Abbreviations: FAP, familial adenomatous polyposis, 5HTP, 5-hydroxy-tryptophan; KYN, kynurenine; TRP, tryptophan; 5-HT, serotonin
High-grade adenoma (ADE) and colorectal cancer (CRC) data retrieved from the reference study.
FAP vs ADE p value after Dunn’s Multiple Comparison Test
FAP vs CRC p value after Dunn’s Multiple Comparison Test.
Figure 2.
(Panel A) Box plots of IDO1 and TPH1 activities. Dunn’s Multiple Comparison Test, **: P < 0.01; ***; P < 0.001. (Panel B) ROC curves for IDO1 and TPH1 activities in FAP patients with respect to ADE (dashed line) and CRC (solid line) patients.
ADE, Adenoma; CRC, colorectal cancer; FAP, Familial Adenomatous Polyposis; IDO1, indoleamine 2,3-dioxygenase 1; ROC, receiver operating characteristic; TPH1, tryptophan hydroxylase 1.
TRP catabolism is a physiological mechanism controlling excessive acute innate immune response at tissue level. Beside this natural role, TRP catabolism is an important driving factor in chronic inflammation and neoplastic progression.17-19 Indeed, inflammatory mediators (eg, IFN-γ) induce the IDO1-mediated TRP catabolism in tumours with a chronic inflammatory component, including CRC.20 Although FAP patients develop precursor lesions in the form of adenomatous polyps prior to malignant transformation to cancer, this disease is generally not associated with inflammatory syndromes.18 Recent data in support of this view have been published, highlighting that decrements in plasma levels of the very-long-chain dicarboxylic acid (28:4), a potent anti-inflammatory molecule, are detectable in CRC patients, and not in FAP patients.21
In order to verify whether inflammatory cytokines may be associated to sporadic CRC and not to FAP, we performed the simultaneous detection of pro-inflammatory cytokines (or chemokines) in a new cohort of patients. To do this, IL1α, IL1β, IL2, IL6, IL8, IL10, IL12, IL17A, IFNγ, TNFα, and GM-CSF have been quantified in 50 µL of serum samples of ADE (n = 6), CRC (n = 8), and FAP (n = 4). Five cytokines (IL1α, IL8, IFNγ, TNFα, and GM-CSF) were detectable in the samples analysed, whereas the others were below the limit of detection for ⩾75% of the analysed samples. The acute phase cytokine tumour necrosis factor (TNFα) was present in a comparable amount in the three groups under study (P = 0.654 Kruskal-Wallis test). On the contrary, the GM-CSF was higher in ADE/CRC compared to FAP patients (P < 0.05, Kruskal-Wallis test; ADE vs. FAP P < 0.05, Dunn’s post test). In addition, the three cytokines mediating the systemic inflammation IL1α, IL8, and IFNγ were consistently detectable (ie, in at least 50% of samples) in the serum of ADE and CRC subjects, but not in those of FAP patients. These results seem to indicate that FAP patients are not characterized by an acute or chronic inflammatory state, whereas ADE and CRC patients present a certain degree of inflammation. Cytokines analysis, even if performed in a small cohort of samples, is in line with other studies in literature reporting increased levels of some pro-inflammatory ILs, including IL1 and IL8, in CRC patients.22-24 Present results indicate the absence of an active TRP metabolism in FAP patients under active surveillance before the onset of adenoma–carcinoma sequence. However, the APC mutation in FAP inevitability accelerates the tumorigenesis initiation, with the consequent establishment of the inflammation and cancer cross-talk typical of sporadic CRC. Similar clinical features are characteristic of the MutYH-Associated Polyposis (MAP), which share also similar levels of TRP and its metabolites. Indeed, we performed FAP patients’ stratification according to the germline mutation in APC or MUTYH genes. Statistical differences have not been found in metabolites levels nor in the IDO1/TPH1 enzymes activity (P > 0.2 for all).
Finally, the potential of IDO1 and TPH1 activities as prognostic biomarker for FAP in terms of specificity and sensibility was then evaluated by ROC curves (Figure 2, panel B). In order to obtain the binary comparison that illustrates the ability of IDO1 or TPH1 activities in the classification of FAP people with respect to ADE and CRC, the optimal cut-off values were calculated by the Youden’s index. For IDO1 enzymatic activity, the calculated area under the curve (AUC) was, respectively, equal to 0.861 (versus ADE, cut-off value of 38.1) and 0.870 (versus CRC, cut-off value of 40.6). Thus, the evaluation of IDO1 activity allows the correct classification of FAP and ADE patients with sensitivity and specificity of 92% and 71%, respectively. In a similar way, FAP and CRC patients were correctly classified with sensitivity and specificity of 81% and 83%, respectively. Comparable performances were obtained by the TPH1 activity evaluation, with an AUC of 0.803 (versus ADE, cut-off value of 5.46) and of 0.922 (versus CRC, cut-off value of 5.83). These performances ensure sensitivities and specificities of 67% and 88% (versus ADE) and 85% and 92% (versus CRC), respectively.
Conclusion
The identification of prognostic biomarkers in FAP patients might permit to replace–or at least postpone–colonoscopy in younger subjects with less invasive assays and eventually delay the surgery in absence of immediate risks. Nowadays, colonoscopy still represent the gold standard technique for detecting CRC and eventually, the introduction of biomarker capable to highlight the onset of adenoma-carcinoma sequence, could improve the FAP clinical surveillance. Indeed, these subjects present a very high polyp number (>100) that makes an accurate endoscopic control extremely difficult.
In the present study, we evaluated the TRP metabolism as a possible source of prognostic marker for FAP patients. We demonstrated the possibility to distinguish FAP, ADE, and CRC patients based on IDO1 and TPH1 activity with high sensitivities and specificities (up to 92%). Increased levels of IL1α, IL8, IFNγ, and GM-CSF suggest that these differences in IDO1 and TPH1 activity may be related to the different inflammatory state characterizing sporadic adenomas and CRC, but not FAP subjects. Present study limitation is the small population of FAP patients analysed with respect to the CRC. However, this is inevitably due to the low disease prevalence (1-9/100’000) and the necessity to collect FAP samples before the prophylactic surgery. Further efforts are then necessary to collect new samples in order to verify whether an analogous TRP metabolism ultimately occurs in also those FAP patients who present CRC before the prophylactic surgery.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), project IG2016-n.19104.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SC Conception and design; writing the original draft.
CB Data curation; revising the article.
AB Acquisition of data; revising the article.
MD Acquisition of data.
MZ and EDLU samples collection and clinical database management.
MA Project supervisor and founding acquisition.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics committee of institution approved the study (Comitato Etico del Centro Oncologico Regionale, Protocol Number: P448).
ORCID iD: Sara Crotti  https://orcid.org/0000-0001-6602-5153
https://orcid.org/0000-0001-6602-5153
References
- 1. Preston SL, Leedham SJ, Oukrif D, et al. The development of duodenal microadenomas in FAP patients: the human correlate of the Min mouse. J Pathol. 2008;214:294-301. [DOI] [PubMed] [Google Scholar]
- 2. Croner RS, Brueckl WM, Reingruber B, Hohenberger W, Guenther K. Age and manifestation related symptoms in familial adenomatous polyposis. BMC Cancer. 2005;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [DOI] [PubMed] [Google Scholar]
- 4. Campos FG. Surgical treatment of familial adenomatous polyposis: dilemmas and current recommendations. World J Gastroenterol. 2014;20:16620-16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung AT, Patel BB, Li XM, et al. One-hit effects in cancer: altered proteome of morphologically normal colon crypts in familial adenomatous polyposis. Cancer Res. 2008;68:7579-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quaresima B, Crugliano T, Gaspari M, et al. A proteomics approach to identify changes in protein profiles in serum of familial adenomatous polyposis patients. Cancer Lett. 2008;272:40-52. [DOI] [PubMed] [Google Scholar]
- 7. Agatea L, Crotti S, Ragazzi E, et al. Peptide patterns as discriminating biomarkers in plasma of patients with familial adenomatous polyposis. Clin Colorectal Cancer. 2016;15:e75-e92. [DOI] [PubMed] [Google Scholar]
- 8. Fatima N, Chelius D, Luke BT, et al. Label-free global serum proteomic profiling reveals novel celecoxib-modulated proteins in familial adenomatous polyposis patients. Cancer Genomics Proteomics. 2009;6:41-49. [PubMed] [Google Scholar]
- 9. Crotti S, D’Angelo E, Bedin C, et al. Tryptophan metabolism along the kynurenine and serotonin pathways reveals substantial differences in colon and rectal cancer. Metabolomics. 2017;13:148. [Google Scholar]
- 10. Meireson A, Chevolet I, Hulstaert E, et al. Peritumoral endothelial indoleamine 2, 3-dioxygenase expression is an early independent marker of disease relapse in colorectal cancer and is influenced by DNA mismatch repair profile. Oncotarget. 2018;9:25216-25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lob S, Konigsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown RR, Lee CM, Kohler PC, Hank JA, Storer BE, Sondel PM. Altered tryptophan and neopterin metabolism in cancer patients treated with recombinant interleukin 2. Cancer Res. 1989;49:4941-4944. [PubMed] [Google Scholar]
- 13. Thaker AI, Rao MS, Bishnupuri KS, et al. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416-425.e1-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bishnupuri KS, Alvarado DM, Khouri AN, et al. IDO1 and kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Cancer Res. 2019;79:1138-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435-5440. [DOI] [PubMed] [Google Scholar]
- 16. Chen IC, Lee KH, Hsu YH, Wang WR, Chen CM, Cheng YW. Expression pattern and clinicopathological relevance of the indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase protein in colorectal cancer. Dis Markers. 2016;2016:8169724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105:17073-17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230-240. [DOI] [PubMed] [Google Scholar]
- 19. Ferdinande L, Decaestecker C, Verset L, et al. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem. 2011;18:2257-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wood PL, Donohue MM, Cebak JE, et al. Reduced plasma levels of very-long-chain dicarboxylic acid 28:4 in Italian and Brazilian colorectal cancer patient cohorts. Metabolites. 2018;8:E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voronov E, Apte RN. IL-1 in colon inflammation, colon carcinogenesis and invasiveness of colon cancer. Cancer Microenviron. 2015;8:187-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kantola T, Klintrup K, Vayrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cui G, Yuan A, Goll R, Vonen B, Florholmen J. Dynamic changes of interleukin-8 network along the colorectal adenoma-carcinoma sequence. Cancer Immunol Immunother. 2009;58:1897-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]