Abstract
Background:
The relationship between pretreatment C-reactive protein to albumin ratio (CAR) and colorectal cancer (CRC) prognosis has been extensively studied in various tumors. However, little is known on CAR and its association with prognosis in CRC. This study aims to investigate the prognostic value of pretreatment CAR in CRC.
Methods:
We conducted a systematic search of MEDLINE, EMBASE, and Cochrane Library databases for eligible studies evaluating the associations of CAR with survival and/or clinicopathology of CRC. Overall survival (OS), disease-free survival (DFS), relapse-free survival (RFS), and clinicopathological features were synthesized and compared.
Results:
Nine studies including 3431 patients were analyzed in this meta-analysis. Pooled results showed that elevated pretreatment CAR was associated with poor OS (pooled hazards ratio [HR]: 2.18, 95% confidence interval [CI]: 1.70-2.78, P < .001) and DFS/RFS (pooled HR: 2.36, 95% CI: 1.40-3.98, P < .001). Moreover, elevated pretreatment CARs were correlated with male patients, large tumor diameter, late III-IV tumor node metastasis stage tumors, high serum carcinoembryonic antigen and carbohydrate antigen 19-9, and presence of lymphatic invasion and venous invasion.
Conclusion:
Elevated pretreatment CAR could be an adverse prognostic indicator in patients with CRC.
Keywords: C-reactive protein to albumin ratio, colorectal cancer, prognosis, meta-analysis
Introduction
Colorectal cancer (CRC) remains an important global health problem and may lead to 1.4 million new cancer cases every year and 700 000 cancer-related deaths worldwide. The incidence and death rate of CRC have been increasing in China.1 Radical surgical intervention remains the primary treatment for CRC.2 Nevertheless, approximately 25% of patients with CRC develop recurrence or distant metastasis.3 The overall prognosis of CRC remains poor, with 50% estimated 5-year survival.4 Therefore, better understanding of carcinogenic mechanisms and the use of ideal cancer biomarkers can promote the diagnosis and prognosis of CRC.
There is a well-documented correlation between inflammation and cancer, although the exact mechanism is still not fully understood. It has been reported that cancer-associated inflammation can increase the risk of tumor development and angiogenesis.5 Tumor-associated inflammatory response consists of inflammatory cells and a range of inflammatory mediators.6 Systemic inflammatory scoring systems, such as the albumin to globulin ratio, the neutrophil to lymphocyte ratio (NLR), the platelet to lymphocyte ratio, and the lymphocyte to monocyte ratio (LMR), have been reported in many solid tumors as prognostic markers.7-9 As a new inflammation-based scoring index, combination of C-reactive protein (CRP) and albumin, has been reported continuously in recent years. The C-reactive protein to albumin ratio (CAR) has been widely investigated for their value in predicting prognosis of patients with CRC.9,10 However, the prognostic value of CAR in CRC remains controversial.11,12 For example, Climent et al11 found that there was no significant difference in both overall survival (OS) and disease-free survival (DFS) between the high- and low-CAR groups (P = .12 and .33, respectively). Thus, the purpose of this meta-analysis is to assess the relationship of pretreatment CAR with the prognosis and clinicopathology of patients with CRC.
Materials and Methods
Search Strategy
Relevant studies were performed through MEDLINE, Embase, and Cochrane Library databases up to March 20, 2019. The following search terms were used: (“C-reactive protein to Albumin ratio” or “C-reactive protein-to-Albumin ratio” or “C-reactive protein Albumin ratio” or “C-reactive protein/Albumin ratio” or “CRP/Alb ratio”) and (“colorectal cancer” or “colorectal carcinoma” or “colorectal tumor” or “colorectal neoplasms” or “colon cancer” or “rectal cancer” or “CRC”). Detailed search strategies refer to supplemental materials.
Inclusion and Exclusion Criteria
Published articles were selected for study based on the following inclusion criteria: (1) studies reporting the association between pretreatment CAR and prognosis in CRC; (2) patients did not receive any treatment (such as surgery or chemotherapy) before obtaining samples; and (3) the study provided sufficient information for extraction or calculation of the individual hazard ratio (HR) or odds ratio (OR) and associated 95% confidence intervals (CIs). The criteria for exclusion of studies were as follows: (1) studies did not include any survival outcomes, (2) duplicate publications, and (3) reviews, meta-analysis, letters, and conference abstracts.
Data Extraction
Two reviewers independently reviewed articles for inclusion/exclusion qualifications. The following information was extracted: study details (first author, year and country of study, sample size, and number of patients involved and distribution of age and gender), pathological characteristics (tumor diameter, differentiation, tumor node metastasis [TNM] stage, and lymphatic invasion and venous invasion), and clinical features (type of treatment applied, CAR cutoff values, carcinoembryonic antigen [CEA] and carbohydrate antigen 19-9 [CA19-9] level, patient’s survival outcome, and duration of follow-up period).
Quality Assessment
In this meta-analysis, the quality assessment for the nonrandomized studies was evaluated by 2 reviewers independently based on the Newcastle-Ottawa quality assessment scale (NOS).13 In this scale, studies were awarded a maximum score of 9 points; studies with NOS values greater than 6 are considered high quality studies.
Statistical Analysis
We used Stata version 13.0 (StataCorp, College station, Texas) to pool HRs for OS, progression-free survival, and ORs for clinicopathological parameters. In this meta-analysis, the HRs and 95% CIs were directly extracted if a study reported the survival analysis, otherwise, they were computed from the Kaplan-Meier graph by using the software of Engauge Digitizer (version 4.1).14,15 The heterogeneity among the eligible studies was calculated by the Cochran Q-test and I2 statistic. If I2 ≤ 50% or P > .05 indicated low heterogeneity, we used fixed-effect models by using inverse variance method. Otherwise, we used random-effect models using the DerSimonian and Laird method, which considers both within-study and between-study variations.16 We did sensitivity analyses in order to validate the robustness of the pooled results by removing each study. Publication bias was evaluated using Begg and Egger tests and defined significantly at a P value <.05.17,18
Results
Study Selection
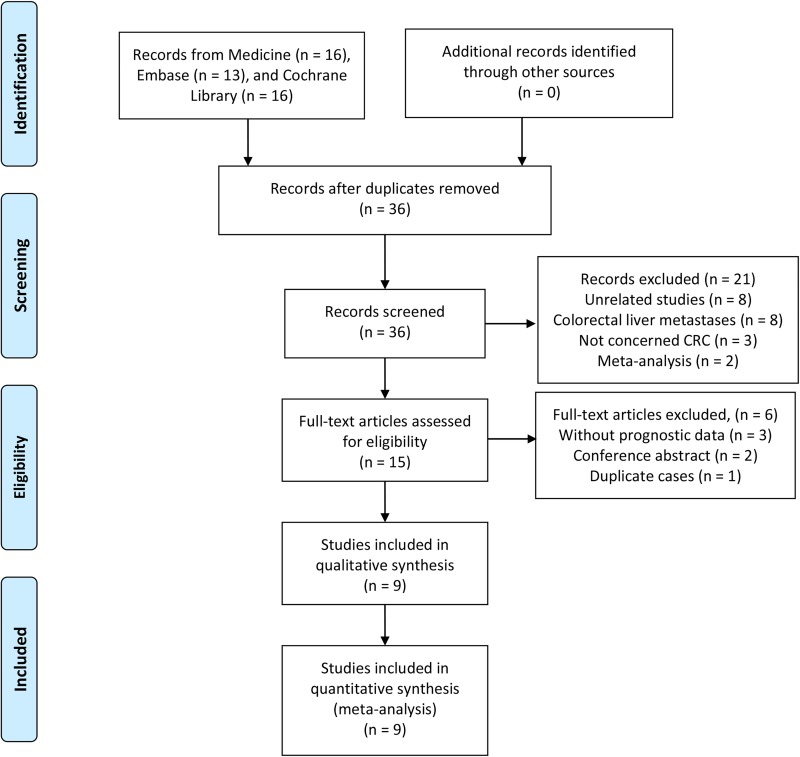
Literature research identified 45 records: 16 from Medline, 13 from Embase, and 16 from Cochrane Library. As shown in the flow diagram (Figure 1), 36 articles were left after removing duplications. After screening titles and abstracts, 15 full-text articles remained for further assessment, and 6 articles were excluded according to the inclusion criteria. In the final, a total of 9 articles involving 3431 patients were eligible for quantitative synthesis.9,11,12,19-24
Figure 1.
Flow diagram of study retrieval and selection processes.
Study Characteristics
All included studies were published from 2016 to 2019. Among them, 6 studies were from Japan and 1 each from China, the United Kingdom, and Ireland. There were 6 studies reported at mixed disease, and 3 studies reported in metastatic disease. The eligible articles consisted of the following: 9 on OS and 5 on DFS/RFS. In addition, prognostic data were directly retrieved from 6 studies on OS or DFS/RFS. Cutoff values of CAR ranged from 0.0271 to 0.65. The HR and 95% CI data were evaluated using univariate analysis in 4 studies and multivariate analysis in 5 studies. The NOS scores ranged from 6 to 9 stars and were all regarded as high quality. The detailed characteristics of the eligible articles are presented in Table 1.
Table 1.
Characteristics of the Studies Included in the Meta-Analysis.
| Author | Year | Country | Location | Sample Size | Age (years) | Treatment | Stage | Cutoff Value | Outcome | Analysis | Confounding Variables | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ide et al | 2017 | Japan | Rectum | 115 | 64 (33-83) | Mixed | Mixed | 0.049 | OS/DFS | MV | TNM stage, vascular invasion, CEA, mGPS | 7 |
| Ishizuka et al | 2016 | Japan | CRC | 627 | NA | Surgery | Mixed | 0.038 | OS | MV | Gender, tumor size, tumor type, Grade, WBC count, platelet count, CEA, CA19-9, NLR, GPS, stage | 9 |
| Tominaga et al | 2016 | Japan | CRC | 136 | NA | Mixed | Metastatic | 0.1 | OS/DFS | UV | 7 | |
| Chen et al | 2017 | China | CRC | 163 | 55 (15-81) | Mixed | Mixed | 0.132 | OS | UV | 8 | |
| Shibutani et al | 2019 | Japan | CRC | 40 | NA | Chemotherapy | Metastatic | 0.122 | OS/PFS | MV | Age, gender, PS, site, No. of prior regimens, RAS status, No. of metastasis, combined targeted therapy, LDH | 8 |
| Shibutani et al | 2016 | Japan | CRC | 705 | 68 (26-90) | Surgery | Mixed | 0.0271 | OS/RFS | MV | T stage, tumor size, lymphatic involvement, venous involvement, LNM | 7 |
| Dolan et al | 2018 | United Kingdom | Colon | 801 | NA | Mixed | Mixed | 0.22 | OS/CSS | MV | TNM stage, NLR, NLS, PLR, PLS, LMR, LMS, NPS, mGPS | 7 |
| Climent et al | 2019 | Ireland | CRC | 804 | 69.9 ± 12.3 | Mixed | Mixed | 0.464 | OS/DFS | UV | 7 | |
| Ikeguchi et al | 2017 | Japan | CRC | 40 | 68 (35-94) | Chemotherapy | Metastatic | 0.65 | OS | UV | 6 |
Abbreviations: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRC, colorectal cancer; CSS, cancer-specific survival; DFS, disease-free survival; GPS, Glasgow Prognostic Score; LDH, lactate dehydrogenase; LMR, lymphocyte–monocyte ratio; LMS, lymphocyte–monocyte score; LNM, lymph node metastasis; mGPS, modified Glasgow prognosis score; MV, multivariate; NA, not available; NLR neutrophil–lymphocyte ratio; NLS neutrophil–lymphocyte score; NPS, neutrophil–platelet score; NOS, Newcastle-Ottawa quality assessment scale; OS, overall survival; PFS, progression-free survival; PLR, platelet–lymphocyte ratio; PLS, platelet–lymphocyte score; PS, performance status; RAS, RAS type GTPase family status; RFS, relapse-free survival; TNM, tumor node metastasis; UV, univariate; WBC, white blood cell.
Meta-Analysis
C-reactive protein to albumin ratio and OS in CRC
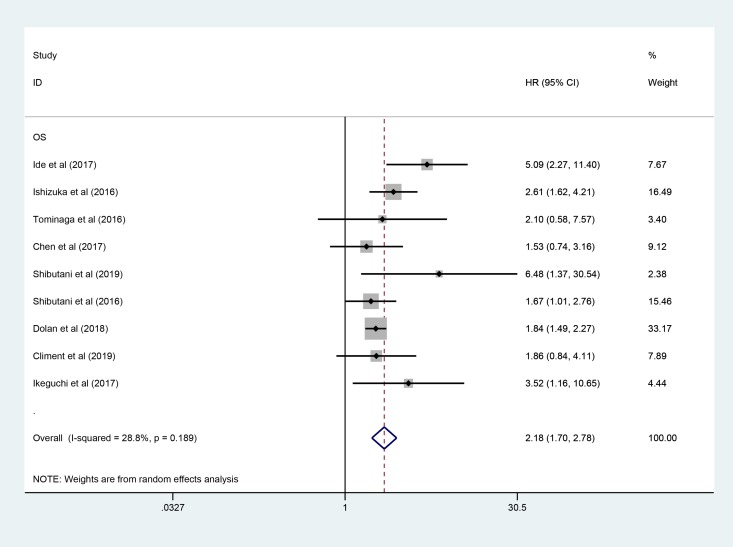
All included studies involving 3431 patients reported the predicting role of pretreatment CAR for OS. Figure 2 showed that elevated pretreatment CAR was predictive of a short OS (pooled HR: 2.18, 95% CI: 1.70-2.78, P < .001). Furthermore, subgroup analysis was performed to further explore the prognostic value of CAR in CRC (Table 2). The pooled HRs for most subgroups were not significantly altered by the study characteristics. Exploratory subgroup analysis, based on tumor stage, indicated that patients with mixed stage (pooled HR: 2.07; 95% CI = 1.59-2.69; P < .001) and metastatic stage (pooled HR: 3.41; 95% CI = 1.63-7.12; P < .001) were all significantly associated with worse OS. Similarly, stratified analysis by cutoff for CAR showed that significant relationship between elevated CAR and shorter OS was detected in patients with CAR ≥ 0.1 (pooled HR: 1.89; 95% CI = 1.57-2.28; P < .001) and patients with CAR < 0.1 (pooled HR: 2.61; 95% CI = 1.51-4.53; P = 0.001). Moreover, the region, sample size, treatment, and analysis method also did not affect the significant predictive value of CAR in patients with CRC.
Figure 2.
Forest plot of the correlation between CAR and OS in patients with colorectal cancer. CAR indicates C-reactive protein to albumin ratio; OS, overall survival.
Table 2.
Pooled HRs for OS According to Subgroup Analyses.
| Subgroup | No. of Studies | No. of Patients | HR (95% CI) | P Value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I 2(%) | Ph | |||||
| Overall | 9 | 3431 | 2.18 (1.70-2.78) | <.001 | 28.8 | 0.189 |
| Region | ||||||
| Asia | 7 | 1826 | 2.49 (1.74-3.58) | <.001 | 33.4 | 0.173 |
| Europe | 2 | 1605 | 1.84 (1.51-2.25) | <.001 | 0 | 0.979 |
| Sample size | ||||||
| ≥300 | 4 | 2937 | 3.10 (1.70-5.32) | <.001 | 36.1 | 0.181 |
| <300 | 5 | 494 | 1.91 (1.60-2.27) | <.001 | 0 | 0.562 |
| Stage | ||||||
| Mixed | 6 | 3215 | 2.07 (1.59-2.69) | <.001 | 37.4 | 0.157 |
| Metastatic | 3 | 216 | 3.41 (1.63-7.12) | .001 | 0 | 0.546 |
| Cutoff for CAR | ||||||
| <0.1 | 3 | 1447 | 2.61 (1.51-4.53) | .001 | 63.3 | 0.066 |
| ≥0.1 | 6 | 1984 | 1.89 (1.57-2.28) | <.001 | 0 | 0.541 |
| Treatment | ||||||
| Surgery | 2 | 1332 | 2.11 (1.36-3.26) | .001 | 37.3 | 0.207 |
| Mixed | 5 | 2019 | 2.09 (1.46-2.97) | <.001 | 35.3 | 0.186 |
| Chemotherapy | 2 | 80 | 4.32 (1.76-10.64) | .001 | 0 | 0.530 |
| Analysis | ||||||
| Univariate | 4 | 1143 | 1.95 (1.24-3.05) | .004 | 0 | 0.670 |
| Multivariate | 5 | 288 | 2.39 (1.65-3.46) | <.001 | 58.5 | 0.047 |
Abbreviations: CAR, C-reactive protein to albumin ratio; CI, confidence interval; HR, hazard ratio.
C-reactive protein to albumin ratio and DFS/RFS in CRC
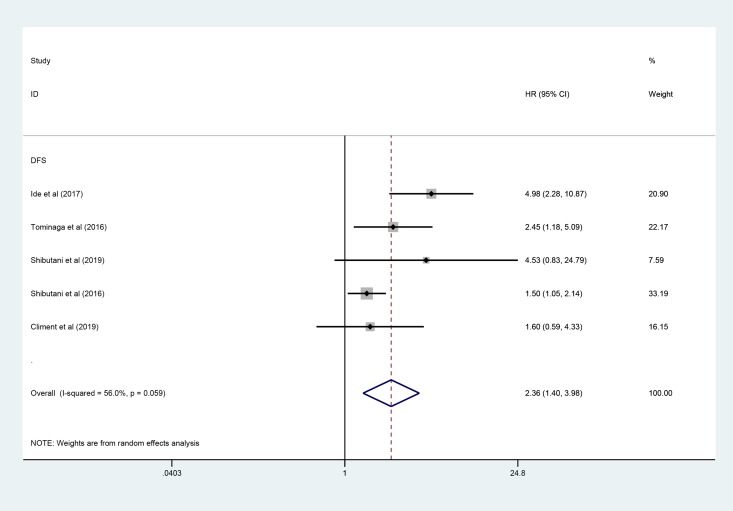
Five studies involving 1800 patients investigated the correlation between pretreatment CAR and DFS/RFS. Similar to the merge of HRs for OS, as shown in Figure 3, the pooled HR of CAR indicated that elevated CAR was associated with decreased DFS/RFS (pooled HR: 2.36, 95% CI: 1.40-3.98, P < .001).
Figure 3.
Forest plot of the correlation between CAR and DFS/RFS in patients with colorectal cancer. CAR indicates C-reactive protein to albumin ratio; DFS, disease-free survival; RFS, relapse-free survival.
C-reactive protein to albumin ratio and clinicopathological characteristics
A total of 10 variables were investigated in the meta-analysis, including age, gender, tumor location, tumor diameter, differentiation, TNM stage, CEA level, CA19-9 level, lymphatic invasion, and venous invasion. The results demonstrated that elevated CAR was related to gender (male vs female; OR = 1.71, 95% CI: 1.30-2.24, P < .001), tumor diameter (≥ 50 mm vs < 50 mm; OR = 3.69, 95% CI: 2.61-5.21, P < .001), TNM stage (III-IV vs I-II; OR = 3.45, 95% CI: 1.76-6.77, P < .001), CEA (> 8.7 ng/mL vs <8.7 ng/mL; OR = 3.70, 95% CI: 2.51-5.47, P < .001), CA19-9 (>9.5 ng/mL vs <9.5 ng/mL; OR = 1.89, 95% CI: 1.37-2.60, P < .001), lymphatic invasion (yes vs no; OR = 1.29, 95% CI: 1.02-1.64, P = .03), and venous invasion (yes vs no; OR = 1.37, 95% CI: 1.04-1.81, P = .03). However, no obvious association was found between the CAR and age (>median vs <median; OR = 1.73, 95% CI: 0.84-3.56, P = .14), tumor location (left vs right; OR = 1.34, 95% CI: 0.33-5.42, P = .68), and differentiation (low vs moderate/high; OR = 1.51, 95% CI: 1.00-2.27, P = .05). The details of the relationship between CAR and clinicopathologic parameters are summarized in Table 3.
Table 3.
Meta-Analysis of the Association Between CAR and Clinicopathological Features of CRC.
| Characteristics | No. of Studies | No. of Patients | OR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|
| I 2 (%) | Ph | |||||
| Age (>median vs <median) | 3 | 830 | 1.73 (0.84-3.56) | .14 | 67 | 0.05 |
| Gender (male vs female) | 4 | 966 | 1.71 (1.30-2.24) | <.001 | 0 | 0.57 |
| Tumor location (left vs right) | 3 | 339 | 1.34 (0.33-5.42) | .68 | 83 | 0.003 |
| Tumor diameter (≥50 mm vs <50 mm) | 1 | 627 | 3.69 (2.61-5.21) | <.001 | - | - |
| Differentiation (low vs moderate/high) | 3 | 1468 | 1.51 (1.00-2.27) | .05 | 0 | 0.90 |
| TNM stage (III-IV vs I-II) | 1 | 163 | 3.45 (1.76-6.77) | <.001 | - | - |
| CEA (>8.7 ng/mL vs <8.7 ng/mL) | 1 | 627 | 3.70 (2.51-5.47) | <.001 | - | - |
| CA19-9 (>9.5 ng/mL vs <9.5 ng/mL) | 1 | 627 | 1.89 (1.37-2.60) | <.001 | - | - |
| Lymphatic invasion (yes vs no) | 3 | 1468 | 1.29 (1.02-1.64) | .03 | 43 | 0.17 |
| Venous invasion (yes vs no) | 3 | 1468 | 1.37 (1.04-1.81) | .03 | 0 | 0.43 |
Abbreviations: CAR, C-reactive protein to albumin ratio; CA19-9, carbohydrate antigen 19-9; CRC, colorectal cancer; CEA, carcinoembryonic antigen; CI, confidence interval; OR, odds ratio; TNM, tumor node metastasis.
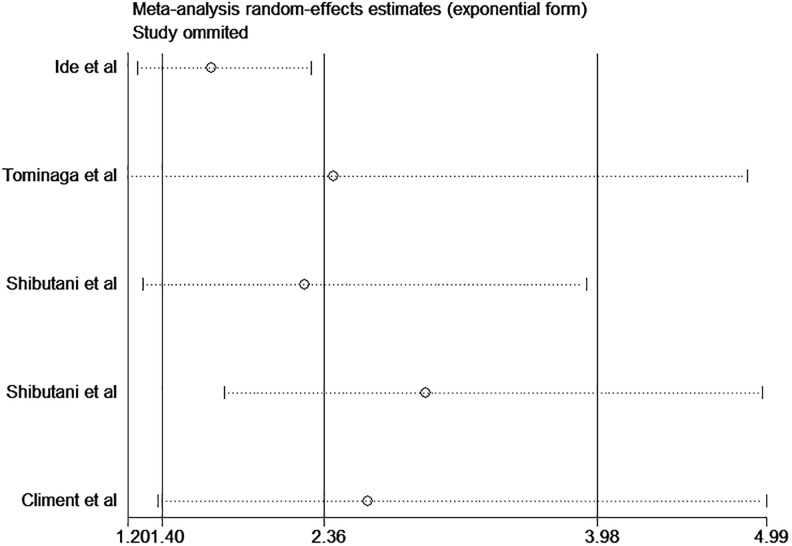
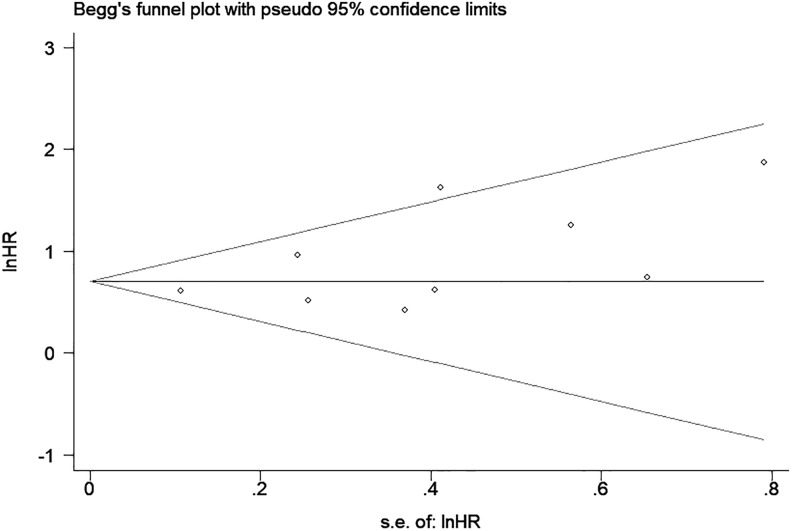
Sensitivity Analysis and Publication Bias
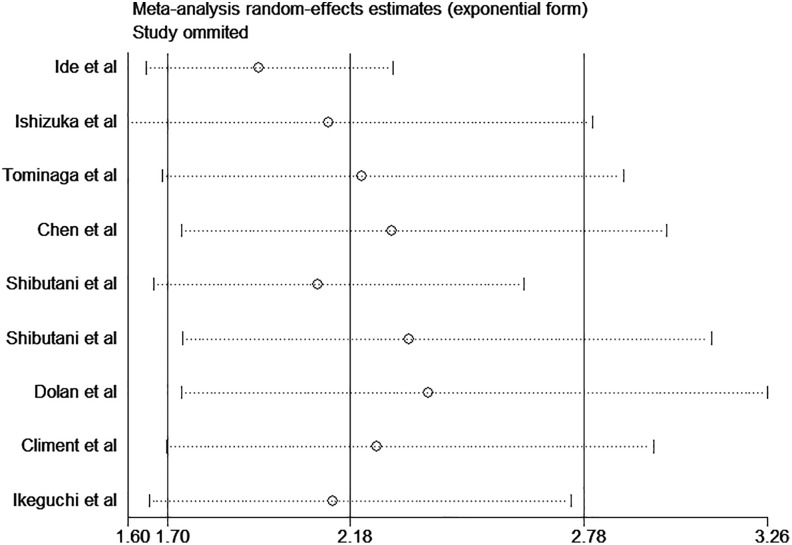
Sensitivity analyses were conducted to estimate the stability of CAR for OS and DFS/RFS. The results showed that no noteworthy influence was detected after removing any single study, which indicated that our conclusions were reliable (Figures 4 and 5). As shown in Figure 6, there was no significant publication bias in OS (P = .061 for Begg test and P = .115 for Egger test).
Figure 4.
Sensitivity analysis of CAR on OS in patients with CRC. CAR indicates C-reactive protein to albumin ratio; CRC, colorectal cancer; OS, overall survival.
Figure 5.
Sensitivity analysis of CAR on DFS/RFS in patients with CRC. CAR indicates C-reactive protein to albumin ratio; CRC, colorectal cancer; DFS, disease-free survival; RFS, relapse-free survival.
Figure 6.
Begg funnel plot of publication bias test for OS in patients with CRC. CRC indicates colorectal cancer; OS, overall survival.
Discussion
Current systematic review and meta-analysis including 3134 patients with CRC give solid evidence of an association between elevated pretreatment CAR and worse prognosis. When stratified by region, sample size, tumor stage, cutoff value for CAR, treatment, and analysis method, the results remained constant. Moreover, elevated pretreatment CAR was correlated with advanced clinicopathological characteristics, such as large tumor diameter, advanced tumor stage, high CEA and CA19-9 levels, and positive lymphatic and venous invasion. Therefore, CAR could serve as a biomarker for the prognosis of patients with CRC.
It is well recognized that inflammation is an important factor in the development of tumor. Inflammatory response can promote tumorigenesis and progression by affecting the tumor microenvironment.6 Tumor-associated inflammatory responses lead to the release of a variety of mediators, such as acute phase proteins, chemokines, and cytokines, which stimulate tumor cell growth, promote angiogenesis, resist cell death and apoptosis, and enhance invasion ability of tumor cells.5,25 There is increasing evidence that high levels of systemic inflammatory cells have the potential to serve as diagnostic or prognostic markers in patients with CRC.9,26-28 Patel et al29 found an correlation between high systemic inflammatory response and right-sided CRC, assessed using modified Glasgow prognosis score, NLR, and LMR. Yang et al30 have shown that elevated NLR is indicative of a poor prognosis for patients with CRC receiving neoadjuvant chemoradiotherapy.
As an inflammatory marker, the CAR was calculated from the serum CRP and albumin levels; CAR was originally studied as a prognostic marker for patients with sepsis31 and was later used as a marker for patients with tumors.32
Recently, CAR has been developed to predict oncological outcomes in patients with CRC. However, the exact mechanism regarding its prognostic ability have not been clearly elaborated. C-reactive protein is an acute-phase protein that is synthesized in the liver, together with cytokines such as interleukin (IL) 1, IL-6, and tumor necrosis factor α.33,34 Research has discovered that CRP can produce inflammatory cytokines and chemokines, which leading to tumor progression.35 Several studies have shown that elevated CRP level was associated with poor prognosis of patients withCRC.36,37 Albumin is the most abundant plasma protein, accounting for about 50% of the total protein content. It is the most commonly used indicator of the nutritional status and is also involved in inflammatory response which acts as an acute-phase protein.38 Hypoalbuminemia caused by a condition under suppressed production of albumin results in activation of cytokines such as IL-1, IL-6, and tumor necrotic factor α.39,40 Thus, CAR may represent a balance between the inflammation and nutritional status, and an increased CAR indicates a poor prognosis.
Several limitations of this study should be considered. First, most of included studies included are carried out in Asian countries, more cohort studies from other regions are necessary. Second, the cutoff value of CAR applied in the enrolled studies was not uniform. Third, part of the HRs and 95% CIs could not be directly obtained and were estimated by software, which may reduce the overall accuracy of the combined results. Finally, this meta-analysis included the predominance of retrospective studies and lacked random control test studies, and the retrospective studies may bring confounding variables.
Conclusions
Our study suggested that elevated pretreatment CAR may serve as a promising indicator for prognostic evaluation of patients with CRC.
Supplemental Material
Supplemental Material, Search_strategies for C-Reactive Protein to Albumin Ratio in Colorectal Cancer: A Meta-Analysis of Prognostic Value by Qiang-ping Zhou and Xiu-jiang Li in Dose-Response
Footnotes
Authors’ Note: QPZ and XJL collected, analyzed, interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xiu-jiang Li  https://orcid.org/0000-0002-6582-8680
https://orcid.org/0000-0002-6582-8680
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338 PubMed PMID: 26808342. [DOI] [PubMed] [Google Scholar]
- 2. Benson AB, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(3):370–398. PubMed PMID: 28275037. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer (Oxford, England: 1990). 2006;42(14):2212–2221. doi:10.1016/j.ejca.2006.04.012 PubMed PMID: 16904315. [DOI] [PubMed] [Google Scholar]
- 4. Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38(5):451–466. doi:10.1016/j.ctrv.2011.09.001 PubMed PMID: 21945823. [DOI] [PubMed] [Google Scholar]
- 5. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025 PubMed PMID: 20303878; PubMed Central PMCID: PMCPmc2866629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013 PubMed PMID: 21376230. [DOI] [PubMed] [Google Scholar]
- 7. Niwa N, Matsumoto K, Ide H, Nagata H, Oya M. Prognostic value of pretreatment albumin-to-globulin ratio in patients with non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(3): e655–e661. doi:10.1177/2050313x17751838 10.1016/j.clgc.2017.12.013 PubMed PMID: 29366633. [DOI] [PubMed] [Google Scholar]
- 8. Akdogan B, Ozen H, Ding L, et al. Retrospective study of the prognostic significance of neutrophil-to-lymphocyte ratio for postsurgical outcomes of patients with endometrial carcinoma. Int J Clin Oncol. 2017;138(3):311–319. doi:10.1007/s10147-017-1150-x. 10.1002/ijgo.12230 PubMed PMID: 28599056. [DOI] [PubMed] [Google Scholar]
- 9. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23(3):900–907. doi:10.1245/s10434-015-4948-7 PubMed PMID: 26530445. [DOI] [PubMed] [Google Scholar]
- 10. Shibutani M, Maeda K, Nagahara H, Iseki Y, Hirakawa K, Ohira M. The significance of the C-reactive protein to albumin ratio as a marker for predicting survival and monitoring chemotherapeutic effectiveness in patients with unresectable metastatic colorectal cancer. Springerplus. 2016;5(1):1798 doi:10.18632/oncotarget.13053 PubMed PMID: 27812440; PubMed Central PMCID: PMCPmc5069226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Climent M, Ryan EJ, Stakelum A, et al. Systemic inflammatory response predicts oncological outcomes in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer. Int J Colorectal Dis. 2019. doi:10.1007/s00384-019-03274-6 PubMed PMID: 30993458. [DOI] [PubMed] [Google Scholar]
- 12. Tominaga T, Nonaka T, Sumida Y, Hidaka S, Sawai T, Nagayasu T. The C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in Stage III colorectal cancer patients. PLOS One. 2016;11(12): e0167967 doi:10.1371/journal.pone.0167967 PubMed PMID: 27930703; PubMed Central PMCID: PMCPmc5145220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z PubMed PMID: 20652370. [DOI] [PubMed] [Google Scholar]
- 14. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. PubMed PMID: 9921604. [DOI] [PubMed] [Google Scholar]
- 15. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 doi:10.1186/1745-6215-8-16 PubMed PMID: 17555582; PubMed Central PMCID: PMCPMC1920534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. PubMed PMID: 3802833. [DOI] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. PubMed PMID: 7786990. [PubMed] [Google Scholar]
- 19. Ide S, Toiyama Y, Okugawa Y, et al. Clinical significance of C-reactive protein-to-albumin ratio with rectal cancer patient undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2017;37(10):5797–5804. doi:10.21873/anticanres.12022 PubMed PMID: 28982904. [DOI] [PubMed] [Google Scholar]
- 20. Chen YY, Zhang JH, Zhang W, Yang ZK, Luo RC, Kang SJ. C-reactive protein/albumin ratio as a novel inflammation-based prognostic index for predicting outcomes of patients with colorectal cancer. J South Med Univ. 2017;37(5):622–627. doi:10.1038/s41598-017-03153-6 PubMed PMID: 28539284; PubMed Central PMCID: PMCPmc5462766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeguchi M, Ashida K. Prognostic significance of C-reactive protein/albumin ratio in patients with locally advanced unresectable colorectal cancer. Indian J Surg Oncol. 2017;8(3):263–266. PubMed PMID: 617960187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shibutani M, Nagahara H, Fukuoka T, et al. Prognostic significance of the C-reactive protein-to-albumin ratio in patients with metastatic colorectal cancer treated with trifluridine/thymidine phosphorylase inhibitor as later-line chemotherapy. Anticancer Res. 2019;39(2):1051–1057. doi:10.1111/1759-7714.12990. 10.21873/anticanres.13212 PubMed PMID: 30711994. [DOI] [PubMed] [Google Scholar]
- 23. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36(3):995–1001. PubMed PMID: 26976989. [PubMed] [Google Scholar]
- 24. Dolan RD, McSorley ST. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51. doi:10.1038/s41416-018-0095-9 PubMed PMID: 29789606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11): e493–e503. doi:10.1016/s1470-2045(14)70263-3 PubMed PMID: 25281468. [DOI] [PubMed] [Google Scholar]
- 26. Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep. 2017;7(1):1494 doi:10.1038/s41598-017-01652-0 PubMed PMID: 28473700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncology. 2017;3(12): e172319 doi:10.1001/jamaoncol.2017.2319 PubMed PMID: 28796857; PubMed Central PMCID: PMCPmc5824285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261 PubMed PMID: 28974892; PubMed Central PMCID: PMCPmc5603492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel M, McSorley ST, Park JH, et al. The relationship between right-sided tumour location, tumour microenvironment, systemic inflammation, adjuvant therapy and survival in patients undergoing surgery for colon and rectal cancer. Br J Cancer. 2018;118(5):705–712. doi:10.1038/bjc.2017.441 PubMed PMID: 29337962; PubMed Central PMCID: PMCPmc5846060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang J, Xu H, Guo X, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. 2018;8(1):3044 doi:10.1038/s41598-018-21093-7 PubMed PMID: 29445100; PubMed Central PMCID: PMCPmc5813153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLOS One. 2013;8(3): e59321 doi:10.1371/journal.pone.0059321 PubMed PMID: 23555017; PubMed Central PMCID: PMCPmc3595283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. doi:10.1245/s10434-014-4048-4050 PubMed PMID: 25190127. [DOI] [PubMed] [Google Scholar]
- 33. Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70(3):709–713. PubMed PMID: 1320454. [DOI] [PubMed] [Google Scholar]
- 34. Ruscetti FW. Hematologic effects of interleukin-1 and interleukin-6. Curr Opin Hematol. 1994;1(3):210–215. PubMed PMID: 9371284. [PubMed] [Google Scholar]
- 35. Asegaonkar SB, Asegaonkar BN, Takalkar UV, Advani S, Thorat AP. C-reactive protein and breast cancer: new insights from old molecule. Int J Breast Cancer. 2015;2015:145647 doi:10.1155/2015/145647 PubMed PMID: 26693355; PubMed Central PMCID: PMCPmc4674617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim WR, Han YD, Min BS. C-reactive protein level predicts survival Outcomes in rectal cancer patients undergoing total mesorectal excision after preoperative chemoradiation therapy. Ann Surgi Oncol. 2018;25(13):3898–3905. doi:10.1245/s10434-018-6828-4 PubMed PMID: 30288654. [DOI] [PubMed] [Google Scholar]
- 37. Egenvall M, Morner M, Martling A, Gunnarsson U. Prediction of outcome after curative surgery for colorectal cancer: preoperative haemoglobin, C-reactive protein and albumin. Colorectal Dis. 2018;20(1):26–34. doi:10.1111/codi.13807 PubMed PMID: 28685921. [DOI] [PubMed] [Google Scholar]
- 38. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus. 2013;11(suppl 4):18–25. doi:10.2450/2013.005s PubMed PMID: 24333308; PubMed Central PMCID: PMCPmc3853979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005; 39(4 suppl 2): S143–S146. PubMed PMID: 15758650. [DOI] [PubMed] [Google Scholar]
- 40. Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–389. doi:10.1245/s10434-006-9093-x PubMed PMID: 17160496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Search_strategies for C-Reactive Protein to Albumin Ratio in Colorectal Cancer: A Meta-Analysis of Prognostic Value by Qiang-ping Zhou and Xiu-jiang Li in Dose-Response