Abstract
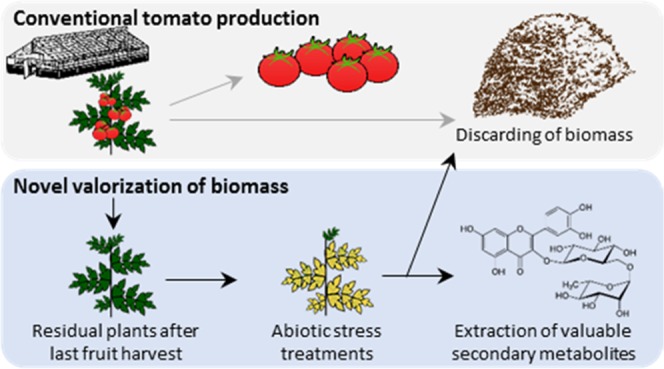
At the end of the annual horticultural production cycle of greenhouse-grown crops, large quantities of residual biomass are discarded. Here, we propose a new value chain to utilize horticultural leaf biomass for the extraction of secondary metabolites. To increase the secondary metabolite content of leaves, greenhouse-grown crop plants were exposed to low-cost abiotic stress treatments after the last fruit harvest. As proof of concept, we evaluated the production of the flavonoid rutin in tomato plants subjected to nitrogen deficiency. In an interdisciplinary approach, we observed the steady accumulation of rutin in young plants under nitrogen deficiency, tested the applicability of nitrogen deficiency in a commercial-like greenhouse, developed a high efficiency extraction for rutin, and evaluated the acceptance of the proposed value chain by its key actors economically. On the basis of the positive interdisciplinary evaluation, we identified opportunities and challenges for the successful establishment of horticultural leaf biomass as a novel source for secondary metabolites.
Introduction
As the world’s demand on food, feed, and fuel is increasing, novel approaches are needed to enhance the resource efficiency of food production systems.1 Bioeconomic approaches aim to develop novel processes that exploit currently underutilized by-products of agricultural and horticultural food production.2 In the case of tomato, the most widely grown vegetable in Europe and the U.S., about 33 kg of leaf and stem biomass per 100 kg of harvested tomatoes accrue during and at the end of the growing period.3 Although stem biomass contributes to about 70% of the residual green biomass after the last fruit harvest, each tomato plant yields about 0.75 kg of leaf biomass, resulting in about 15 t ha–1 (Mauricio Hunsche, personal communication). The large quantities of residual plant biomass generated by horticultural food production are currently either utilized for the production of biofuel or compost or are disposed with costs.4,5
Recently, cultivated solanaceous crop species including tomato have been shown to contain significant amounts of secondary metabolites.6,7 In plants, secondary metabolites are involved in the protection against abiotic and biotic stresses and, correspondingly, their biosynthesis is often enhanced under stress conditions.8,9 Due to their beneficial properties for human health, many plant secondary metabolites are also utilized as dietary food supplements and phytomedicines and in cosmetics.10 Chemically, secondary metabolites exhibit enormous diversity and complexity, which makes their industrial chemical synthesis difficult and expensive.11 To enhance resource efficiency, many agricultural, horticultural, and food processing by-products have been investigated as sources for valuable secondary metabolites.12−14
Tomato leaves contain high amounts of phenolic compounds such as flavonoids, which have antioxidant and health-promoting properties.15 The flavonoid rutin is of high interest for industrial applications, as it is used as a food supplement, in functional foods and cosmetics, and exhibits pharmacological activities.16 Dried tomato leaves contain about 0.8–3.1 0.6 g kg–1 rutin.17−19 Nitrogen deficiency, low temperatures, and increased light intensities have been shown to induce the accumulation of rutin and other phenolic compounds in leaves of tomato plants.18,20 Nitrogen deficiency is applicable in commercial production of greenhouses but has so far only been investigated to enhance the secondary metabolite content of fruits or medicinal plants.21 Further abiotic stresses that could be applied to selectively trigger plant secondary metabolism include, e.g., drought,8,22 osmotic stress by application of salt,23 spraying of growth regulators,24 and changes in growing temperature by passive cooling or warming due to increased/reduced ventilation.25 Depending on the available greenhouse technology, active regulation of growing temperature and increased irradiation by growth lights are possible but associated with costs.25 Abiotic stress treatments of greenhouse-grown crop plants like tomato after the last fruit harvest have the potential to induce the accumulation of secondary metabolites in leaves. By the proposed timing at the end of the annual production cycle, leaf biomass would be valorized for the extraction of secondary metabolites like rutin without interfering with fruit quality and yield. Typically, greenhouses in temperate climate zones are unused for a short time of a year, when costs for heating of the greenhouses are increasing and low light conditions further reduce the potential benefit of fruit production.26 The exposure of residual postharvest crop plants to low-cost stress treatments, which increase the secondary metabolite content of leaf biomass and make it suitable for the extraction of valuable secondary metabolites, could thus make use of the winter gap.
Establishment of tomato leaf biomass for the production of rutin requires targeting of industrial extraction procedures toward this novel source. Analytical extraction procedures for rutin employing modern extraction techniques like ultrasound- or microwave-assisted extraction have been established for several plant species.27 Industrial extraction methods based on conventional industrial extraction methods such as solid–liquid extraction by maceration, immersion, or percolation have been mostly developed for buckwheat biomass, which is the major source for rutin in food supplements.28 Typically, dried crushed plant material is used and extracts are purified by crystallization.29 To establish tomato leaves as a novel source, the extraction efficiency for rutin must be maximized while cost- and energy inputs as well as environmental impact are minimized.30 The key parameter for efficient extraction is the selection of a solvent with high solubility for rutin that pertains to the regulatory framework of the target market, which includes various organic solvents.16,27 Additionally, the extraction method, biomass pretreatment, and particle size must be optimized on the basis of the extraction kinetics of small-scale industrial-like extractions and an extraction time must be determined, which maximizes the space–time yield.31 To foster the sustainability of the extraction process, energy costs, extraction time, and solvent input should be minimized.
To establish a novel value chain that valorizes residual biomass for the extraction of secondary metabolites, the process must be practically feasible, economically viable, and accepted by actors of the value chain.12,32 As there is an increasing interest in secondary metabolites as food supplements, which is, e.g., indicated by a growing number of patented food supplements using rutin as an ingredient,33 the valorization of tomato leaf biomass could open up new market opportunities for companies in the food supplement sector. Nevertheless, the proposed process requires adjustments to the conventional agri-food value chain. Value chain actors need to face and overcome emerging challenges associated with the implementation of novel practices.34 Therefore, an explorative analysis of factors easing or hindering the feasibility and acceptance of rutin extraction from tomato leaf biomass along the whole emerging value chain from tomato farmers to the supplemental food industry and eventually consumers becomes pivotal.
This study proposes a novel value chain for the production of secondary metabolites from leaf biomass of solanaceous plants: residual plants after the last annual fruit harvest are subjected to low-cost abiotic stress treatments, which induce the accumulation of secondary metabolites in leaf biomass. As proof of concept, we assessed the feasibility of the proposed value chain for the extraction of rutin from leaf biomass of nitrogen-deficient tomato plants. We analyzed the plasticity of foliar rutin levels in young tomato plants, the applicability of nitrogen deficiency stress in commercial greenhouses, a noninvasive estimation of rutin content in leaf biomass, the extractability of rutin from tomato leaf biomass, and the acceptance by actors along the value chain. By this interdisciplinary approach, we identified the key factors for the successful implementation of the proposed value chain, which has the potential to valorize residual leaf biomass and thus enhance resource efficiency in horticultural food production.
Results and Discussion
Accumulation of Rutin in Young Tomato Plants as a Prerequisite To Valorize Leaf Biomass
The key idea of the proposed value chain is to enhance the value of the residual horticultural biomass by an inexpensive stress treatment that induces the accumulation of secondary metabolites. As a proof of concept, we selected the extraction of rutin from tomato biomass, due to its known plasticity in response to nitrogen deficiency.18,20,35 We suggest that only secondary metabolites should be considered for the proposed value chain, which fulfill the following criteria: (1) presence in relatively high amounts in leaf biomass, (2) strong induction by abiotic stresses, and (3) increasing accumulation in response to abiotic stresses, without degradation under extreme stress. To validate the suitability of rutin, we conducted an experiment with young tomato plants in response to nitrogen deficiency in combination with increased light intensities and/or chilling temperatures, which proved most efficient to induce foliar rutin content in an earlier study.36 We further extended the duration of the stress period to investigate if rutin steadily accumulates or degrades under extreme stress. One month old control plants showed stable rutin content of 0.6 g kg–1 per leaf dry weight (DW, Figure 1a). Induction of the foliar rutin content by nitrogen deficiency, especially in combination with increased light intensity, was observed starting 4 days after nitrogen depletion of the substrate and lead to a steady accumulation of 0.2–0.3 g kg–1 DW per day. Tomato plants exposed to nitrogen deficiency, increased light intensities, and chilling temperatures showed highest foliar rutin levels, with a maximum of 5.8 g kg–1 DW at the end of the stress treatment. The observed 10-fold induction exceeds the 2–5-fold increases observed in earlier studies.18,20
Figure 1.
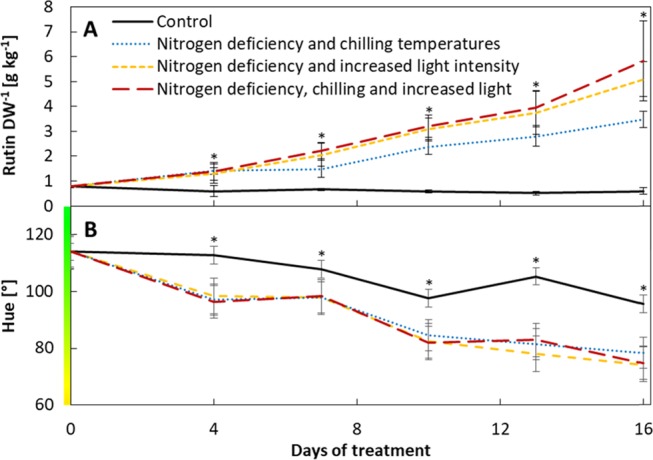
Effect of stress treatments in young tomato plants over 16 days of nitrogen deficiency in combination with increased light intensities and/or chilling temperatures compared to control plants. (a) Foliar rutin content per leaf dry weight (DW, g kg–1). (b) Leaf color assessed by smartphone image analyses (hue). Data represent the mean of N = 4 plants [±standard deviation (SD)]. Significant differences of stress to control plants [p < 0.05 using one-way-analysis of variance (ANOVA)] are indicated by asterisks.
The additional inducing effect of increased light intensities was previously observed20 and can be related to an increased demand for photoprotection from photooxidative damage, which is among others mediated by the accumulation of antioxidants such as rutin.37 In contrast, the effect of chilling temperatures was negligible, as it was likely only inducing the flavonoid biosynthesis during acclimation to lower temperatures, but was not cold enough to significantly affect the flavonoid metabolism.20 Most interesting with regard to the valorization of leaf biomass is the steady accumulation of rutin with increasing duration of the stress treatment and thus stress intensity. The antioxidant capacity of rutin is based on the physical quenching of reactive oxygen species (ROS). Therefore, rutin is not degraded under oxidative conditions.38 The value of leaf biomass thus increases with length and intensity of abiotic stress treatments, which should therefore be performed as long as the farmers’ procedures and schedules allow. The experiment with young tomato plants thus demonstrates the potential for the extraction of rutin from nitrogen-deficient tomato leaf biomass. Likely, other antioxidant secondary metabolites with physical ROS quenching activity will show a similar accumulation in response to increasing stress duration and intensity and are thus promising target metabolites for the valorization of leaf biomass.
Due to the increasing interest in smartphones as simple phenotyping tools for plant performance in agricultural applications,39 we assessed, if smartphone images reflect stress-related changes in leaf color. We observed a decrease of the leaf color index hue in stressed plants from 114° to a minimum of 74° (Figure 1b). This reflects decreasing chlorophyll content and yellowing of leaves,39,40 which accompanied the increasing rutin levels in response to nitrogen deficiency (data not shown). In contrast, control plants only showed a minor decrease of hue from 114 to 96°, which can be attributed to developmental variation.
Applicability of Abiotic Stress Conditions in a Production-Like Greenhouse
To evaluate the applicability of stress treatments in commercial production greenhouses, mature tomato plants in a commercial-like greenhouse were exposed to 3 week stress treatments, which would comply with farmer’s schedules at the end of the annual horticultural production cycle. In addition to nitrogen deficiency, a complete nutrient deficiency by watering with tap water was included, as premixed fertilizers in many production greenhouses hamper the application of nitrogen deficiency without changes in other nutrients.41
Young leaves showed a small but significant induction of rutin content in response to both stress treatments, while rutin levels of mature leaves were unaffected (Figure 2a). Young leaves of control plants had 3.4 g kg–1 rutin in the dry leaf biomass, while nitrogen and nutrient-deficient plants contained 5.3 and 4.8 g kg–1, respectively. The lower induction compared with the experiment with young tomato plants despite a longer stress treatment indicates that mature plants, which had a height of 5–6 m, were able to compensate the nitrogen/nutrient deficiency of the substrate by reallocation of nitrogen from stems and old leaves.42 Generally, lower foliar rutin content of 2.0 g kg–1 in mature leaves might be related to their lower light exposure compared with young leaves of the upper canopy. The rutin-inducing effect of increased light intensities was observed in the experiment with young tomato plants and previous experiments.20,38 In addition, young leaves may have a higher demand for antioxidant compounds, which has also been seen in young buckwheat leaves.37 The relatively higher importance of young leaves for plant growth may also contribute to the stronger induction of rutin in young compared with mature leaves, which were closer to senescence. To analyze the total secondary metabolite content of residual crop plants, future studies should investigate the secondary metabolite of the total leaf biomass or even within a gradient across plants, to assess the accumulation of secondary metabolites in leaves of all ages.
Figure 2.
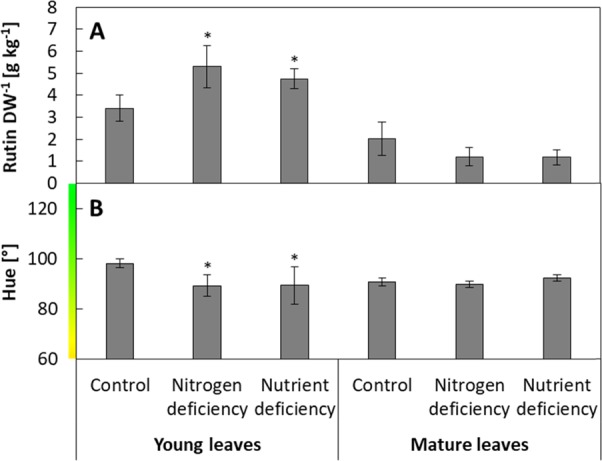
Effect of stress treatments in greenhouse-grown mature tomato plants after a 21 day stress period of nitrogen deficiency and nutrient deficiency compared with control plants. (a) Foliar rutin content per leaf dry weight (DW, g kg–1). (b) Leaf color assessed by smartphone image analyses (hue). Data represent mean of N = 5 plants (±SD). Significant differences of stressed to control plants (p < 0.05 using one-way-ANOVA) are indicated by asterisks.
The observed stress response is reflected by changes in hue of leaves from 98 to 89° in response to both stress treatments only in young leaves, while the hue of mature leaves remained unchanged at about 91° (Figure 2b). The relatively lower change in leaf color compared with young plants fits the observed lower stress levels of the mature plants.
Altogether, the nitrogen deficiency treatment does not seem suitable to induce stress in mature tomato plants, as the induction of foliar rutin content is rather low. Therefore, the increased rutin content is likely insufficient to compensate for the costs of the stress treatment. Increased light intensities by supplemental lighting may induce a stronger accumulation of rutin, but the operation of light-emitting diode-panels in commercial greenhouses is costly and energy-consuming. Although the experiment with young tomato plants suggests that longer stress treatments would increase foliar rutin levels, stress treatments exceeding 3 weeks will not comply with farmers’ schedules. Instead, further inexpensive abiotic stresses, such as changes in growing temperature by passive cooling or warming due to increased/reduced ventilation, osmotic stress by application of salt23 or desiccation of plants, or spraying of elicitors, should be evaluated. Here, special focus should be put on a fast stress response, to maximize induction while minimizing the period, during which the greenhouse must be operated. Metabolite profiling of such stressed plants may identify further valuable secondary metabolites of industrial relevance.
Establishment of Leaf Color Indicators for Foliar Secondary Metabolite Content
To provide tomato farmers with a simple phenotyping tool, we tested the suitability of a smartphone camera to quantify leaf color as an indicator for plant stress. In both experiments, the increase of foliar rutin levels in response to nitrogen deficiency was reflected by a decrease of the color parameter hue (Figure 3). The rutin content of leaves and hue showed high correlations with R2 = 0.78 in the experiment with young tomato plants and R2 = 0.48 for young leaves of greenhouse-grown plants. A correlation for mature leaves cannot be derived due to the invariability of rutin content. Hue is a suitable indicator to estimate nitrogen deficiency stress levels, as it is sensitive to the decreasing chlorophyll content in response to nitrogen deficiency.39,40 Consequently, it also highly correlates with rutin levels, although the contribution of rutin to leaf color is likely very subtle. In addition, hue is largely insensitive to changes in irradiation, which contributes to a reliable assessment of leaf color even under variable light conditions.43 The development of a smartphone application provides an easy-to-use and inexpensive tool, which contributes to the establishment of abiotic stress treatments in new greenhouse facilities and thus the successful establishment of the proposed value chain.
Figure 3.
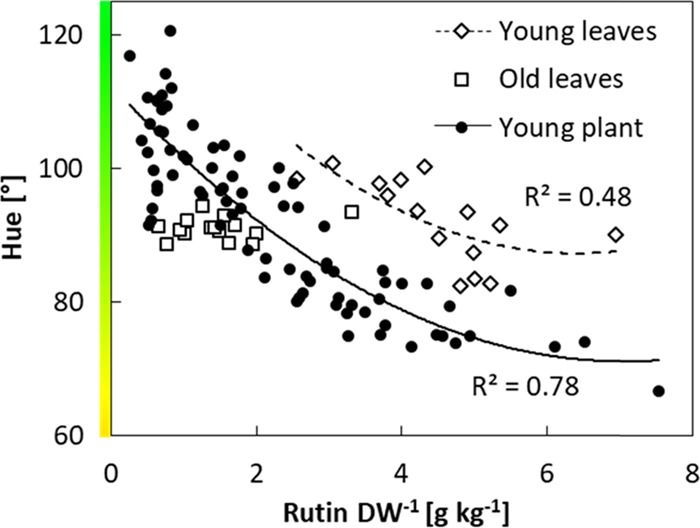
Correlation between foliar rutin content per leaf dry weight (DW, g kg–1) and leaf color assessed by smartphone image analyses (hue). Every data point represents measurements of an individual leaf sample. Trend lines represent polynomic regression fit.
Development of an Efficient Industrial-like Extraction of Rutin from Tomato Leaf Biomass
As a prerequisite for the establishment of the proposed value chain, secondary metabolites must be efficiently extracted from leaf biomass. We adapted the extraction of rutin by immersion, which previously proved efficient for the extraction of rutin from buckwheat16,29 to tomato leaf biomass, and evaluated its maximum efficiency. First, solubility for rutin in six possible solvents that pertain to the European food supplement market was analyzed. It ranked from 55.8 g kg–1 for ethanol, 51.6 g kg–1 for methanol, 17.8 g kg–1 for isopropyl alcohol, 3.7 g kg–1 for acetone, 0.6 g kg–1 for ethyl acetate, to a minimum of 0.04 g kg–1 for water (compare Zi et al.44). Ethanol additionally allows for a green and cost-efficient processing of leaf biomass, as it can be easily recycled by evaporation.45 The extraction of rutin from dried buckwheat biomass has been shown to be more efficient when using aqueous solvents,16 which lead to swelling of the dried leaf biomass and allowed faster diffusion of the solvent into the plant cells.46
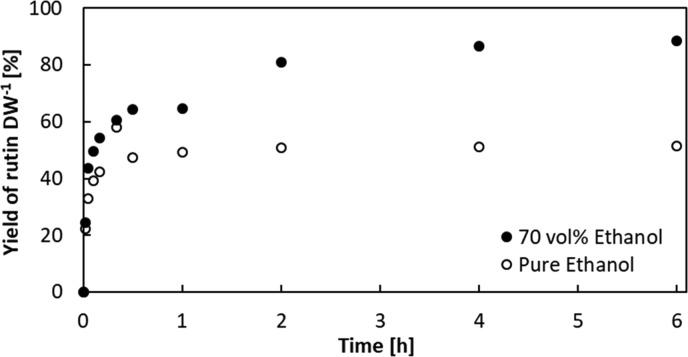
We therefore compared the extraction kinetics of rutin using pure ethanol and 70 vol % ethanol. Pure ethanol extracted only 51% of the total rutin content as determined by repeated analytical extractions, while 70% ethanol showed a maximum extraction yield of 89% and exhibited faster extraction kinetics (Figure 4). The yield of the rutin extraction with 70 vol % ethanol saturated after about 2 h, when yield was 81% and thus only 8% lower compared with an extraction time of 6 h. Therefore, the extraction time was empirically determined as 2 h to maximize the space–time yield.47 Subsequently, yield of the industrial-like extraction from dried tomato leaves with 70 vol % ethanol after 2 h extraction time was confirmed by the analysis of leaf biomass from four additional plants. The average yield of the industrial-like extraction from dried leaf material is 87 ± 19% of the repeated analytical extraction of frozen leaf aliquots. This demonstrates that drying of biomass at 40 °C had only a minor effect on the foliar rutin content, which facilitates transport and storage due to reduced biochemical degradation and risk of microbiological contamination.48
Figure 4.
Kinetics of the rutin extraction from dried tomato leaf material (DW, %). Extraction kinetics were determined with leaf particles sized 1–1.4 mm in batch extraction mode at room temperature and related to the total rutin content determined by repeated analytical extraction of leaf samples from the same plant frozen immediately after harvest.
Altogether, the developed extraction process for rutin from tomato leaves is considered cost-efficient, as the one-step extraction showed high yield. The applied methods are widely available at low cost for industrial-scale extractions, which contributes to the feasibility and acceptance of the developed extraction method.49 While extraction processes must be targeted toward a specific source to maximize its yield, the purification of rutin from tomato extracts would be comparable to the buckwheat purification process, which is commonly performed by a crystallization step that recovers 92% of the rutin with a purity of more than 95%.50 When ethanol is evaporated from aqueous extracts, rutin crystallizes with high purity in water, due to its low solubility therein.29,51 The mass spectrometry (MS) analysis of rutin extract from tomato leaves revealed that most side components are more polar (i.e., early eluting compounds) than rutin (Figure 5a), which indicates a higher solubility in water and thus later crystallization. Rutin extracts may further contain low amounts of glycoalkaloids, mainly α-tomatine.51 Due to their insolubility in water, they would crystallize earlier than rutin or could be removed by additional purification processes.45 On the other hand, side components may also be beneficial or of commercial value.17 In our extracts, we identified chlorogenic acid and caffeic acid (Figure 5b), which are even enhanced under nitrogen deficiency (Figure 6 and 7). Therefore, the profitability of the proposed value chain may be enhanced by the simultaneous extraction of additional valuable metabolites.45
Figure 5.
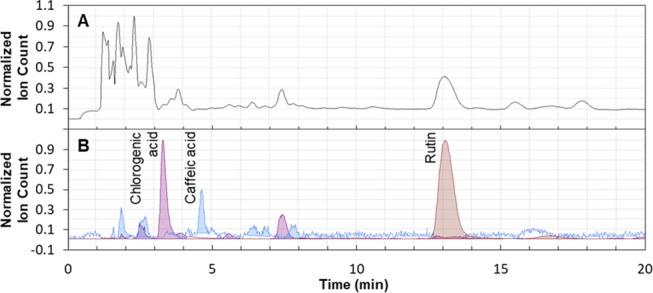
Liquid chromatography–mass spectrometry (LC–MS) chromatograms of a tomato leaf extract. The total ion count (a) reveals the presence of additional compounds besides rutin in extracts of tomato leaves, among which chlorogenic acid and caffeic acid were identified using the respective mass filters (b).
Figure 6.
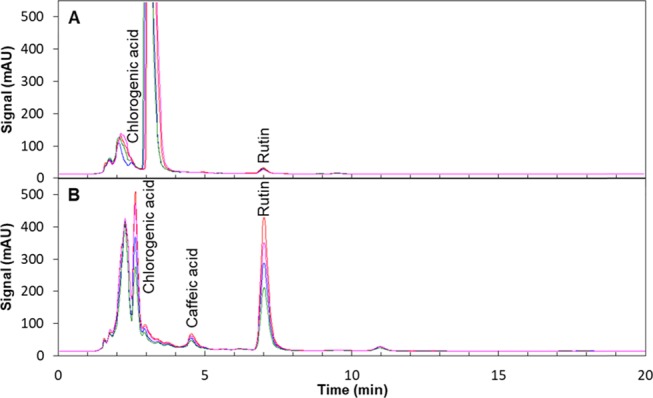
Representative high-performance liquid chromatography (HPLC) chromatograms recorded at 210 nm of rutin extracts from control plants (a) and plants stressed by nitrogen deficiency, high light, and cold (b) on day 16 extract revealed the induction of additional compounds besides rutin in extracts of tomato leaves. Blue, green, pink, and orange lines indicate chromatograms from four tomato plants.
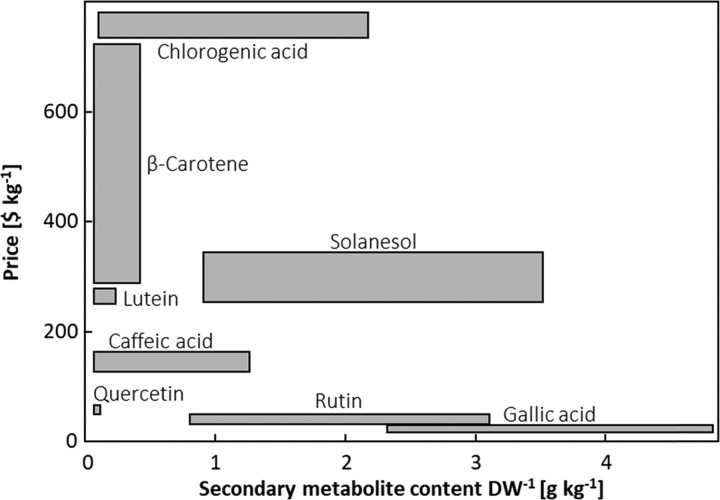
Figure 7.
Price ranges ($ kg–1) of secondary metabolites known to be present in relevant content ranges (DW, g kg–1) in tomato leaf biomass. Secondary metabolite content ranges are based on literature values.6,7,17,18,57,59 Market values were estimated based on requests on the trading platform alibaba.com in May 2016.
Analysis of Key Factors for the Establishment of the Proposed Value Chain
The establishment of horticultural leaf biomass as a novel source of secondary metabolites requires not only technical feasibility but also acceptance by its main actors, as additional steps need to be implemented in the existing horticultural practice, and acceptance by producers and consumers is pivotal for its successful establishment. Only few bioeconomic concepts have been incorporated in common practice, and the establishment of new resources and modification of existing value creation is challenging.2 We therefore complemented our study with an explorative analysis by expert interviews, to assess the value chain actors’ opinion on both opportunities and challenges of the proposed value chain and their provisional acceptance. By this interdisciplinary approach, we aim to identify all key factors necessary to consider for the establishment of the proposed value chain.
Tomato farmers confirmed the availability of high amounts of residual leaf biomass and their willingness to adopt novel practices to valorize these residuals, e.g., the application of stress treatments and drying of leaf biomass, when financial incentives are provided. Especially diversified and innovative farmers perceived the proposed value chain as a business opportunity to generate additional income. Therefore, subsidies for the establishment of such novel horticultural practices could further promote the implementation of the proposed value chain.52
Representatives of food supplement companies were generally skeptical about horticultural leaf biomass as a source of secondary metabolites. They were concerned by transportation costs for leaf biomass, supply security, purity of target components, regulation and labeling, and price competition with producers in Asia. Transportation costs of leaf biomass could be reduced by clustering of biomass suppliers, extraction companies, and producers of food supplements in the same geographical area.53 Short trade routes together with the sustainability of the raw materials might provide an advantage of rutin extracted from tomato leaf biomass compared with rutin from buckwheat that must be imported from Asia.54 Competitive prices and quality, i.e., high purity of the metabolites will have a major influence on its acceptance by the target markets and compliance with European legislation. To enhance the economic feasibility of the proposed value chain, multiple secondary metabolites should be purified from tomato plant extracts. Thereby, general production costs for transport, pretreatment, and extraction could be distributed across different products and prices are mainly determined by product specific costs. The increasing market demand for natural ingredients and marketing of bio-based products as healthy, ecologically sustainable, and regionally produced were perceived advantageous for the establishment of the novel value chain. Nevertheless, industry representatives emphasized that a steady supply of biomass is preferred. Therefore, leaf biomass gathered during regular pruning of horticultural plants throughout the growing season, e.g., from tomato and cucumber, should be further investigated for their secondary metabolite content. This especially applies to valuable secondary metabolites, which are abundant in leaf biomass without being induced by stress treatments.
However, on the consumer side, our interviews revealed a general lack of knowledge about environmental benefits of bio-based ingredients. This hampers the demand of bio-based products and represents a challenge for their promotion. On the opposite, health-conscious customers were interested in purchasing bio-based food supplements due to their natural and health-promoting ingredients. This is in accordance with earlier findings, that positive attitudes toward bio-based food supplements are primarily driven by consumers’ health consciousness and not by environmental concerns.55 Appropriate labeling of bio-based ingredients to communicate the beneficial health effects and/or environmental benefits would help to reach this important target group and increase consumer awareness.2 However, the current Nutrition and Health Claims Regulation requires high R&D expenditures from companies to apply health claims associated with food ingredients.56
Altogether, secondary metabolites extracted from horticultural biomass have a high market potential, which is further considered to rise with the twist of the European Union toward a bio-based economy.2 Rutin was selected as target metabolite for our study, as it is relatively abundant and highly inducible in tomato biomass. Nevertheless, with the long treatment duration needed to induce strong induction of rutin in greenhouse-grown tomato plants together with a rather low price for rutin extracted from buckwheat, the valorization of leaf biomass for the extraction of rutin does not seem economically feasible. To identify potential target metabolites abundant in tomato leaf biomass and of high commercial value, we combined a literature analysis of secondary metabolite contents in tomato leaf biomass with an estimation of their market value (Figure 5). Secondary metabolites abundant in tomato leaf biomass and of high commercial value, such as solanesol and chlorogenic acid, are more promising for the establishment of the proposed value chain compared with rutin. Therefore, further studies should elucidate the potential for the induction of these metabolites in residual postharvest crop plants. Nevertheless, the valorization of leaf biomass will be only profitable when secondary metabolites are strongly induced by a low-cost stress treatment. Therefore, the potential of inexpensive abiotic stress treatments or those that trigger a fast stress response in plants should be further explored. For example, a 12-fold induction of solanesol was observed in young tomato plants exposed for 1 week to day temperatures of 28 °C instead of 22 °C.36 Given the low number of studies on the secondary metabolite content of residual green biomass of horticultural crop plants, systematic metabolite profiling may reveal further valuable secondary metabolites.6,57
In conclusion, we identified key aspects for the utilization of residual leaf biomass for the extraction of secondary metabolites and the potential to valorize leaf biomass by abiotic stress treatments. Our interdisciplinary approach to investigate tomato leaf biomass as a source for the flavonoid rutin serves as a proof of concept for the potential of the proposed value chain. We showed strong induction and steady accumulation of rutin in leaves of young tomato plants but point out challenges in the application of nitrogen deficiency stress treatments in greenhouses. This limits the feasibility of the proposed value chain, despite a high efficiency of industrial-like extraction processes and general market potential for secondary metabolites derived from horticultural by-products. Future research should screen for further valuable secondary metabolites in the leaf biomass of different horticultural crops and if low-cost abiotic stress treatments can be applied in commercial greenhouses to substantially induce their content in residual leaf biomass. Thereby, the potential of horticultural biomass as novel source of secondary metabolites will be exploited, which contributes to increased sustainability of the horticultural food production.
Material and Methods
Plants and Materials Used for Experiments
For both experiments, tomato plants (Solanum lycopersicum L.) from a commercially commonly used line, the truss tomato F1 hybrid Lyterno (Rijk Zwaan Welver GmbH, Welver, Germany), were grown from seed in the Grodan rockwool substrate (Grodan, Roermond, The Netherlands).
Inducibility of Rutin Accumulation in Young Tomato Plants under Controlled Nitrogen Deficiency
The experiment was conducted in two controlled walk-in environmental chambers with 7.1 m2 growing area and a total volume of 22 m3 each (Hühren Kälte-Klima-Elektrotechnik, Erkelenz, Germany) at the Forschungszentrum Jülich GmbH, Jülich, Germany. Plants were randomly distributed among and within both chambers and grown at 22/18 °C day/night temperature with 10 h day length, 50% relative humidity, and 200 μmol m–2 s–1 photon light intensity using metal halide lamps placed at a height of 1.95 m. Plants took about 10 days to germinate in rockwool plugs watered with deionized water. One week after germination, plants were transferred to 7.5 × 7.5 cm2 rockwool cubes and fertilized with half-strength Hoagland solution. Two weeks later, plants were fertilized with full-strength Hoagland solution [5 mM KNO3, 5 mM Ca(NO3)2, 2 mM MgSO4, 1 mM KH2PO4, 90 μM FeEDTA, plus micronutrients]. One month after germination, plants were randomly assigned to four treatment groups (N = 4, in total 96 plants): control, nitrogen deficiency in combination with increased light intensity, nitrogen deficiency in combination with chilling temperatures, nitrogen deficiency in combination with increased light intensity and chilling temperatures. This stress combination is not transferrable to commercial greenhouse conditions but allowed for monitoring of rutin contents under severe stress conditions. Nitrogen deficiency was achieved by washing the rockwool substrate three times with 0.5 L of modified nitrogen-free Hoagland solution [KNO3 and Ca(NO3)2 were replaced by 2.5 mM K2SO4 and 5 mM CaCl2] and subsequent watering with nitrogen-free Hoagland solution. For the chilling temperature treatment, plants were transferred to an identical growth chamber with the same environmental conditions but set to 20/12 °C day/night temperature. For the increased light intensity treatment, plants were positioned closer to the lamps, where they received the doubled light intensity. At the start of the experiment, plants were about 30 cm high and had a fresh weight of 11.7 ± 1.8 g and a leaf area of 307 ± 45 cm2. Throughout the experiment, control plants were about 60 cm high, with a fresh weight of 11.7 ± 1.8 g and a leaf area of 2340 ± 141 cm2. Stress-treated plants showed reduced growth, with a fresh weight of 36.4 ± 2.9 g and a leaf area of 596 ± 64 cm2 at the end of the treatment (Figure S1). The fourth oldest leaf was sampled for analyses of the rutin content and immediately frozen in liquid nitrogen 0, 4, 7, 10, 13, and 16 days after onset of the stress treatment. Additionally, the third and fifth leaves were sampled and dried for 3 days at 80 °C to determine the fresh/dry weight ratio.
Nitrogen and Nutrient Deficiency Experiment with Greenhouse-Grown Tomato Plants
The applicability of a nitrogen deficiency treatment in commercial-like greenhouses was tested with mature tomato plants grown in a commercial-like ridge and furrow type greenhouse at the Research Campus Klein-Altendorf, Rheinbach, Germany, in a separated compartment sized 4.6 × 45.0 m2. Seeds were sown in January 2017 in rockwool cubes and cultivated under supplemental lighting. Four weeks after sowing, two plants were transferred onto one 1 m long rockwool slab in an open hydroponic gutter growing system. A total number of 420 plants was grown in six 35 m long rows, equaling a density of about 20 000 plants ha–1. The greenhouse was covered with a single layer of safety glass, and plants were cultivated under natural light conditions. The temperature regulation system maintained 20.7 ± 1.9 °C during the day and 18.1 ± 0.4 °C at night, with minimum and maximum temperatures of 17.5 and 25.4 °C, respectively. Computer-controlled drip irrigation with a standard nutrient solution (17.2 mM N, 5.4 mM Ca, 4.7 mM K, 0.4 mM P, 5.4 mM S, 2.4 mM Mg, 10 μM Fe plus micronutrients) ensured generous water and nutrient supply. Nitrogen and nutrient deficiency treatments were started on April 13, when plants were about 2 m tall. Stress treatments were conducted on five rockwool slabs (with a total number of 10 plants) randomly selected among the inner four rows excluding slabs at the beginning and end of rows, to minimize variation in microclimate and irradiation. Each slab was flushed with 60 L of tap water and subsequently watered with a modified nitrogen-free nutrient solution or tap water, respectively, using a Comfort 9000 watering system (GARDENA GmbH, Ulm, Germany). Equally, control plants were selected. On May 4, after 3 weeks of deficiency treatments, stress-treated plants showed comparable stress responses. Therefore, one plant per slab was sampled, equaling N = 5 plants from the control, nitrogen deficiency, or nutrient deficiency treatment group. Intermediate leaflets of mature leaves from the lower plant canopy and of fully developed young leaves from the upper canopy were immediately frozen in liquid nitrogen. Comparable leaflets were taken to analyze the fresh/dry weight ratio.
In addition, five untreated tomato plants were sampled on July 20 for the development of the industrial-like extraction procedure. All leaves of one plant were pooled and dried for 4 days at 40 °C. Dried leaf samples were crushed by hand and sieved to retain different particle sizes. Prior to sampling of the leaves, small parts of intermediate leaflets of every second leaf were taken and immediately frozen in liquid nitrogen. Rutin content of these aliquots was determined by repeated analytical extraction as reference for the yield of the industrial-like extractions.
Smartphone Phenotyping To Quantify Leaf Color as an Indicator for Rutin Content
To investigate the potential of smartphones as simple phenotyping tools to estimate plant stress levels and secondary metabolite content, every sampled leaf was photographed using a smartphone (FP 1, Fairphone B.V., Amsterdam, The Netherlands) equipped with a Sony IMX179 camera with an 8 megapixel sensor and autofocus. Automatic flash was disabled, while automatic adjustments of white balance and ISO was kept. Images of individual leaves together with a gray reference were taken under room light conditions (mix of natural sunlight and low-pressure mercury-vapor gas-discharge lamps) from a 30 cm distance in the nadir position. The software ImageJ was used to select a circular region of interest, either in the top leaflet of young tomato plants or in the sampled leaflet of greenhouse-grown tomato plants,40 and extract the mean RGB color as digital numbers (R, G, B; 0–255) with the function histogram. As color analyses of the gray reference revealed little variation in illumination, RGB analysis was performed without further calibration. Hue was calculated as follows
| 1 |
Analytical Extraction of Rutin
To determine the rutin content of the frozen samples by analytical extractions, a methanol-based protocol was adapted.58 Prior to the analyses, frozen leaf samples were ground by hand in liquid nitrogen and stored at −80 °C. One hundred milligrams of ground leaf material was extracted with 0.7 mL of pure methanol for 4 h at room temperature on a shaker (1 × 4 h MeOH). This analytical method proved very efficient and extracted 95 ± 1% of the total rutin content, which was determined by repeated extraction with four subsequent extraction steps with 0.7 mL of methanol for 1 h each and pooling of supernatants (4 × 1 h MeOH).
Development of an Industrial-like Rutin Extraction Procedure Targeting Tomato Leaves
In the first step, a suitable solvent was selected based on the solubilization strength for rutin. Ethanol, methanol, isopropyl alcohol, acetone, ethyl acetate, and water were tested, as they comply with the requirements of the food supplement market. Pure rutin (0.1 g, TCI Deutschland GmbH, Eschborn, Germany) was added to 1 mL of each solvent at 25 °C, with three technical replicates. After shaking flasks for 24 h, they were centrifuged for 30 min at 13 500g, and the solubilized rutin in the supernatant was analyzed by HPLC.
Second, kinetics for the extraction of rutin from dried tomato leaf material in stirred batch mode were analyzed to empirically determine an extraction time that maximizes the space–time yield. In addition to pure ethanol, which had the highest solubility for rutin, we also investigated the extraction kinetics of 70 vol % ethanol, as the addition of water has been shown to improve the extraction kinetics for rutin from plant material.16 To determine the extraction kinetics shown in Figure 4, 3 g of dried leaf biomass with a particle size between 1.0 and 1.4 mm, which is typical for industrial-scale extractions from plant biomass,31 was extracted in 0.2 L of each solvent, respectively. Offline HPLC samples (0.8 mL of each) were taken over 6 h at room temperature in stirred batch extraction mode. On the basis of the superior efficiency of 70 vol % ethanol and saturation of the extraction after 2 h, these parameters were chosen for the industrial-like extraction.
Last, the yield of the industrial-like extraction was determined. One gram of dried leaf biomass was extracted in 15 mL of 70 vol % ethanol for 2 h for four plants with three technical replicates and related to the repeated analytical extraction of aliquots of the same leaves.
Quantification of Rutin Content in Extracts by High-Performance Liquid Chromatography
Rutin concentration of analytical and industrial-like extracts was quantified using the 1200 Series HPLC (Agilent Technologies Deutschland GmbH & Co. KG, Waldbronn, Germany) equipped with a NUCLEODUR 100-3 C18ec reversed phase column (125 mm × 3 mm; Macherey-Nagel GmbH & Co. KG, Düren, Germany) temperature-controlled at 25 °C. Extracts were filtered using syringe filters [0.2 μm poly(tetrafluoroethylene), VWR International GmbH, Germany] and diluted with water to match the isocratic eluent, 40 wt % methanol, and 60 wt % water (pH 2.7 adjusted with phosphoric acid). At a flow rate of 0.3 mL min–1, rutin eluted 7 min after injection of 10 μL of the extract using an autosampler and was detected at 210 nm by a UV detector and quantified according to a standard (TCI Deutschland GmbH, Eschborn, Germany).
The same method was applied on a LC-MS-QTOF System (Agilent Technologies Deutschland GmbH & Co. KG, Waldbronn, Germany) in electrospray ionization mode with positive ionization, equipped with a NUCLEODUR 100-5 C18ec column (250 mm × 3 mm; Macherey-Nagel GmbH & Co. KG, Düren, Germany) to investigate side components in an additional rutin extract from a mature leaf of a 10 month old tomato plant.
Value Chain Analysis Based on Semistructured Expert Interviews with Key Actors
To economically evaluate the feasibility and competitiveness of the proposed value chain, the acceptance and opinions of the potential key actors were assessed in a qualitative research approach. Semistructured interviews with four tomato farmers, three representatives of the food supplement industry, and with five consumers addressed (1) the professional background of the value chain actors; (2) their acceptance concerning novel farm practices (tomato farmers), using residual leaf biomass as raw materials (food supplement industry) and bio-based ingredients in products (consumers); and (3) their perceived opportunities and challenges of the proposed value chain. The interview length ranged from 20 to 60 min and participants were predominantly male. The interviews were transcribed, hand-coded by categories, and analyzed. On the basis of these interviews, we identified opportunities and challenges of the proposed value chain.
Market Value Estimation of Secondary Metabolites
To give an outlook about potential secondary metabolites of interest in tomato leaf biomass, we combined a literature analysis relevant to secondary metabolites known to be present in tomato leaves with a market-based analysis of their value. Values of secondary metabolite contents in tomato leaves were found in the literature.6,7,17,18,57,59 If literature values were estimated per fresh weight, we assumed a dry weight-to-fresh weight ratio of 1:9, which we experimentally determined for mature leaves of control plants grown in the commercial-like greenhouse. Market values of secondary metabolites in tomato leaves were estimated by requesting the price for a quantity of 1000 kg of each substance from international suppliers on the online business-to-business trading platform alibaba.com in May 2016. Price ranges for each metabolite were determined based on two to five answers from different retailers.
Statistics
The effects of stress treatments on rutin content and leaf color were compared by one-way-ANOVA (function aov) performed with R 3.0.3 followed by Tukey’s multiple comparison test (function TukeyHSD, 95% family-wise confidence level and p < 0.05 level of significance). Homogeneity of variance was validated using the Levene’s test (function levene from the library car), and normality of distribution was validated using the Shapiro–Wilk-test (function shapiro.test). Correlations between leaf color in hue and foliar rutin content were calculated in Excel, and the best fitting regression function was chosen among linear, exponential, logarithmic and polynomial fit.
Acknowledgments
The authors thank Veronique Hellmund for help with conducting the experiment with young tomato plants, Moritz Doeker for help with extractions and analyses, and technical staff at all contributing research institutions for their support. We acknowledge funding by the Ministry of Innovation, Science and Research of the German State of North Rhine-Westphalia in the framework of the Bioeconomy Science Center (BioSC, NRW Strategieprojekt No. 313/323-400-002 13) and support from the Deutsche Forschungsgemeinschaft (INST 222/1077-1 FUGG).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01462.
Biomass and leaf area of young tomato plants under control and stress conditions (nitrogen deficiency in combination with increased light intensities and/or chilling temperatures compared with control plants) (Figure S1) (PDF)
Author Present Address
∇ Agroscope, Taenikon 1, 8356 Ettenhausen, Switzerland (T.G.).
The authors declare no competing financial interest.
Supplementary Material
References
- Lin C. S. K.; Pfaltzgraff L. A.; Herrero-Davila L.; Mubofu E. B.; Abderrahim S.; Clark J. H.; Koutinas A. A.; Kopsahelis N.; Stamatelatou K.; Dickson F.; Thankappan S.; Mohamed Z.; Brocklesby R.; Luque R. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. 10.1039/c2ee23440h. [DOI] [Google Scholar]
- Golembiewski B.; Sick N.; Bröring S. The emerging research landscape on bioeconomy: What has been done so far and what is essential from a technology and innovation management perspective?. Innovative Food Sci. Emerging Technol. 2015, 29, 308–317. 10.1016/j.ifset.2015.03.006. [DOI] [Google Scholar]
- Boulard T.; Raeppel C.; Brun R.; Lecompte F.; Hayer F.; Carmassi G.; Gaillard G. Environmental impact of greenhouse tomato production in France. Agron. Sustainable Dev. 2011, 31, 757–777. 10.1007/s13593-011-0031-3. [DOI] [Google Scholar]
- Antón M. A.; Muñoz P.; Castells F.; Montero J. I.; Soliva M. Improving waste management in protected horticulture. Agron. Sustainable Dev. 2005, 25, 447–453. 10.1051/agro:2005045. [DOI] [Google Scholar]
- Gómez L. D.; Amalfitano C.; Andolfi A.; Simister R.; Somma S.; Ercolano M. R.; Borrelli C.; McQueen-Mason S. J.; Frusciante L.; Cuciniello A.; Caruso G. Valorising faba bean residual biomass: Effect of farming system and planting time on the potential for biofuel production. Biomass Bioenergy 2017, 107, 227–232. 10.1016/j.biombioe.2017.10.019. [DOI] [Google Scholar]
- Taylor M. A.; Fraser P. D. Solanesol: Added value from solanaceous waste. Phytochemistry 2011, 72, 1323–1327. 10.1016/j.phytochem.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Campbell R.; Freitag S.; Bryan G. J.; Stewart D.; Taylor M. A. Environmental and genetic factors associated with solanesol accumulation in potato leaves. Front. Plant Sci. 2016, 7, 1263 10.3389/fpls.2016.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D.; Kleinwächter M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. 10.1016/j.indcrop.2012.06.020. [DOI] [Google Scholar]
- Dixon R. A. Natural products and plant disease resistance. Nature 2001, 411, 843. 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Wu S.; Chappell J. Metabolic engineering of natural products in plants; tools of the trade and challenges for the future. Curr. Opin. Biotechnol. 2008, 19, 145–152. 10.1016/j.copbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Oksman-Caldentey K.-M.; Inzé D. Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. 10.1016/j.tplants.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Galanakis C. M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. 10.1016/j.tifs.2012.03.003. [DOI] [Google Scholar]
- Fontana A. R.; Antoniolli A.; Bottini R. Grape pomace as a sustainable source of bioactive compounds: extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food. Chem. 2013, 61, 8987–9003. 10.1021/jf402586f. [DOI] [PubMed] [Google Scholar]
- Fromm M.; Loos H. M.; Bayha S.; Carle R.; Kammerer D. R. Recovery and characterisation of coloured phenolic preparations from apple seeds. Food Chem. 2013, 136, 1277–1287. 10.1016/j.foodchem.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Balasundram N.; Sundram K.; Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Gullón B.; Lú-Chau T. A.; Moreira M. T.; Lema J. M.; Eibes G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. 10.1016/j.tifs.2017.07.008. [DOI] [Google Scholar]
- Silva-Beltrán N. P.; Ruiz-Cruz S.; Chaidez C.; de Jesús Ornelas-Paz J.; López-Mata M. A.; Márquez-Ríos E.; Estrada M. I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. 10.1080/09603123.2014.938030. [DOI] [PubMed] [Google Scholar]
- Larbat R.; Paris C.; Le Bot J.; Adamowicz S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci. 2014, 224, 62–73. 10.1016/j.plantsci.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Bénard C.; Bourgaud F.; Gautier H. Impact of Temporary Nitrogen Deprivation on Tomato Leaf Phenolics. Int. J. Mol. Sci. 2011, 12, 7971. 10.3390/ijms12117971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvdal T.; Olsen K. M.; Slimestad R.; Verheul M.; Lillo C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613. 10.1016/j.phytochem.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Bénard C.; Gautier H.; Bourgaud F.; Grasselly D.; Navez B.; Caris-Veyrat C.; Weiss M.; Génard M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food. Chem. 2009, 57, 4112–4123. 10.1021/jf8036374. [DOI] [PubMed] [Google Scholar]
- Kleinwächter M.; Selmar D. New insights explain that drought stress enhances the quality of spice and medicinal plants: potential applications. Agron. Sustainable Dev. 2015, 35, 121–131. 10.1007/s13593-014-0260-3. [DOI] [Google Scholar]
- Amalfitano C.; Del Vacchio L.; Somma S.; Cuciniello A.; Caruso G. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of ‘Friariello’ pepper grown in hydroponics. Hortic. Sci. 2017, 44, 91–98. 10.17221/172/2015-HORTSCI. [DOI] [Google Scholar]
- Bloem E.; Haneklaus S.; Kleinwächter M.; Paulsen J.; Schnug E.; Selmar D. Stress-induced changes of bioactive compounds in Tropaeolum majus L. Ind. Crops Prod. 2014, 60, 349–359. 10.1016/j.indcrop.2014.06.040. [DOI] [Google Scholar]
- Kittas C.; Katsoulas N.; Bartzanas T.; Bakker J. C.. Greenhouse Climate Control and Energy Use. In Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; Baudoin W., Nono-Womdim R., Lutaladio N., Hodder A., Castilla N., Leonardi C., De Pascale S., Qaryouti M., Eds.; Food and Agriculture Organization of the United Nations: Rome, 2013; Vol. 127, pp 63–95. [Google Scholar]
- Heuvelink E.; Bakker M. J.; Hogendonk L.; Janse J.; Kaarsemaker R.; Maaswinkel R.. Horticultural Lighting in the Netherlands: New Developments; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2006; pp 25–34. [Google Scholar]
- Ameer K.; Shahbaz H. M.; Kwon J.-H. Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- Chua L. S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Wanping S.; Yingchun X.. A Method for the Extraction and Separation of Rutin from Buckwheat. 2014-06-04, 2016.
- Azmir J.; Zaidul I. S. M.; Rahman M. M.; Sharif K. M.; Mohamed A.; Sahena F.; Jahurul M. H. A.; Ghafoor K.; Norulaini N. A. N.; Omar A. K. M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Pfennig A.; Delinski D.; Johannisbauer W.; Josten H.. Extraction Technology. Industrial Scale Natural Products Extraction; Wiley-VCH Verlag GmbH & Co. KGaA, 2011; pp 181–220. [Google Scholar]
- Mirabella N.; Castellani V.; Sala S. Current options for the valorization of food manufacturing waste: A review. J. Cleaner Prod. 2014, 65, 28–41. 10.1016/j.jclepro.2013.10.051. [DOI] [Google Scholar]
- Wensing J.; Bröring S. Functional Ingredients: Market research. Kirk-Othmer Encycl. Chem. Technol. 2017, 1–26. 10.1002/0471238961.koe00033. [DOI] [Google Scholar]
- Paula L.; Birrer F. Including public perspectives in industrial biotechnology and the biobased economy. J. Agric. Environ. Ethic 2006, 19, 253–267. 10.1007/s10806-005-6170-2. [DOI] [PubMed] [Google Scholar]
- Ercolano M. R.; Gomez L. D.; Andolfi A.; Simister R.; Troise C.; Angelino G.; Borrelli C.; McQueen-Mason S. J.; Evidente A.; Frusciante L.; Caruso G. Residual biomass saccharification in processing tomato is affected by cultivar and nitrogen fertilization. Biomass Bioenergy 2015, 72, 242–250. 10.1016/j.biombioe.2014.10.030. [DOI] [Google Scholar]
- Junker L. V.; Thiele B.; Schurr U.; Wiese-Klinkenberg A. In Nutzung von Tomatenpflanzen nach Tomatenproduktion zur Gewinnung von Sekundärmetaboliten für die weiterverarbeitende Industrie, DGG & BHGL Jahrestagung, 2017; pp 1–5.
- Suzuki T.; Honda Y.; Mukasa Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005, 168, 1303–1307. 10.1016/j.plantsci.2005.01.007. [DOI] [Google Scholar]
- Montaña M. P.; Massad W. A.; Criado S.; Biasutti A.; García N. A. Stability of flavonoids in the presence of riboflavin-photogenerated reactive oxygen species: A kinetic and mechanistic study on quercetin, morin and rutin. Photochem. Photobiol. 2010, 86, 827–834. 10.1111/j.1751-1097.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- Rigon J. P. G.; Capuani S.; Fernandes D. M.; Guimarães T. M. A novel method for the estimation of soybean chlorophyll content using a smartphone and image analysis. Photosynthetica 2016, 54, 559–566. 10.1007/s11099-016-0214-x. [DOI] [Google Scholar]
- Junker L. V.; Ensminger I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. 10.1093/treephys/tpv148. [DOI] [PubMed] [Google Scholar]
- Sonneveld C.; Voogt W.. Nutrient Solutions for Soilless Cultures; Springer Netherlands: Dordrecht, 2009; pp 257–275. [Google Scholar]
- Masclaux-Daubresse C.; Daniel-Vedele F.; Dechorgnat J.; Chardon F.; Gaufichon L.; Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. 10.1093/aob/mcq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. D.; Jiang X. H.; Sun Y.; Wang J. Color image segmentation: advances and prospects. Pattern Recognit. 2001, 34, 2259–2281. 10.1016/S0031-3203(00)00149-7. [DOI] [Google Scholar]
- Zi J.; Peng B.; Yan W. Solubilities of rutin in eight solvents at T=283.15, 298.15, 313.15, 323.15, and 333.15K. Fluid Phase Equilib. 2007, 261, 111–114. 10.1016/j.fluid.2007.07.030. [DOI] [Google Scholar]
- Chemat F.; Vian M. A.; Cravotto G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615. 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.-J.; Gao Y.; Liu Y.; Zheng X.-Q.; Ye J.-H.; Liang Y.-R.; Lu J.-L. Studies on the mechanism of efficient extraction of tea components by aqueous ethanol. Food Chem. 2016, 194, 312–318. 10.1016/j.foodchem.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Both S.; Koudous I.; Jenelten U.; Strube J. Model-based equipment-design for plant-based extraction processes – considering botanic and thermodynamic aspects. C. R. Chim. 2014, 17, 187–196. 10.1016/j.crci.2013.11.004. [DOI] [Google Scholar]
- Giovanelli G.; Paradiso A. Stability of dried and intermediate moisture tomato pulp during storage. J. Agric. Food. Chem. 2002, 50, 7277–7281. 10.1021/jf025595r. [DOI] [PubMed] [Google Scholar]
- Wang L.; Weller C. L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- Kim K. H.; Lee K. W.; Kim D. Y.; Park H. H.; Kwon I. B.; Lee H. J. Optimal recovery of high-purity rutin crystals from the whole plant of Fagopyrum esculentum Moench (buckwheat) by extraction, fractionation, and recrystallization. Bioresour. Technol. 2005, 96, 1709–1712. 10.1016/j.biortech.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Friedman M. Tomato Glycoalkaloids: Role in the Plant and in the Diet. J. Agric. Food Chem. 2002, 50, 5751–5780. 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- Becker D. R.; Moseley C.; Lee C. A supply chain analysis framework for assessing state-level forest biomass utilization policies in the United States. Biomass Bioenergy 2011, 35, 1429–1439. 10.1016/j.biombioe.2010.07.030. [DOI] [Google Scholar]
- Witjes S.; Lozano R. Towards a more Circular Economy: Proposing a framework linking sustainable public procurement and sustainable business models. Resour., Conserv. Recycl. 2016, 112, 37–44. 10.1016/j.resconrec.2016.04.015. [DOI] [Google Scholar]
- Porter M. E.Competitive Strategy: Techniques for Analyzing Industries and Competitors; 1st Free Press export edition ed.; Free Press: New York, 2004; p 396. [Google Scholar]
- Bornkessel S.; Bröring S.; Omta S. W. F.; van Trijp H. What determines ingredient awareness of consumers?: A study on ten functional food ingredients. Food Qual. Preference 2014, 32, 330–339. 10.1016/j.foodqual.2013.09.007. [DOI] [Google Scholar]
- Khedkar S.; Bröring S.; Ciliberti S. Exploring the nutrition and health claims regulation (EC) No. 1924/2006: What is the impact on innovation in the EU food sector?. Int. J. Food Sci. Nutr. 2017, 68, 10–17. 10.1080/09637486.2016.1212818. [DOI] [PubMed] [Google Scholar]
- Kim D.; Na H.; Kwack Y.; Chun C. Secondary metabolite profiling in various parts of tomato plants. Korean J. Hortic. Sci. Technol. 2014, 32, 252–260. 10.7235/hort.2014.13165. [DOI] [Google Scholar]
- Olsen K. M.; Slimestad R.; Lea U. S.; Brede C.; LØVdal T.; Ruoff P.; Verheul M.; Lillo C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant, Cell Environ. 2009, 32, 286–299. 10.1111/j.1365-3040.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- Gupta P.; Sreelakshmi Y.; Sharma R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5 10.1186/s13007-015-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.