Abstract
Aim:
This study evaluated in vitro the remineralization capacity of commercial toothpastes with different fluoride (F) concentrations and their effectiveness when they are acidified.
Materials and Methods:
One hundred and twelve caries-free teeth were used to prepare enamel specimens, and the specimens were divided into 16 groups (n = 7). Baseline surface Vickers microhardness was measured for all the specimens and all the tested groups were subjected to the pH-cycling regime involved five demineralization challenges each day for 10 days, and surface Vickers microhardness was then measured. Once daily, specimens were exposed for 30 min after last demineralization challenge of the day to the slurry of each toothpaste containing 1450 ppm F, 1000 ppm F, 450 ppm F, and 0 ppm F. The slurry was in original pH or acidulated on 6.5, 6.0, or 5.5 pH. The difference among tested group was assessed by analysis of variance and Newman–Keuls test (P < 0.05).
Results:
The highest increase in microhardness was detected after treatment with toothpaste containing 1450 ppm fluoride (percentage of increase in microhardness was 6.20%), and the biggest loss was detected after treatment with toothpaste containing no fluoride (percentage of decrease was 6.82%), but there was no significant difference between tested groups.
Conclusions:
The highest increase in microhardness was detected after treatment with toothpaste containing more fluorides (1450 ppm F) regardless of the acidity.
Keywords: Enamel demineralization, fluoride, pH cycling, prevention
Introduction
Toothpastes have been used since ancient times,[1] yet the fluorides, as the most active ingredients, have been added for the first time in 1914 in the United Kingdom. Toothpastes with fluoride did not enter into massive usage until 1956 in America. Today, the fluoride toothpastes make up >95% of all toothpaste sales in America and Europe.
During topical fluoridation, a calcium fluoride (CaF2)-like material is formed on the tooth enamel. It acts as an F reservoir and is a source of free ions during cariogenic challenges. Thus, it makes CaF2 the most effective compound in inhibiting enamel demineralization.[2]
The widespread use of fluoride has led to reducing the incidence of dental caries but has also increased the rate of dental fluorosis. The risk of fluorosis is especially high in preschool children because they tend to swallow the toothpaste during daily tooth brushing.[3,4] Pessan et al. showed that the main source of fluoride intake in preschool children was the dentifrices.[5] In many countries including Croatia, it is recommended that children should use toothpastes containing <600 ppm.[3] Santos et al. in their systematic review have proved that those low-fluoride toothpastes do not have the same anticariogenic effect as those containing higher F concentrations. They showed that children who brushed with low-fluoride toothpaste had an increased risk of caries.[6]
Whereas, it is known that the formation of CaF2 increases as the pH of a topical F agent decreases.[7,8] There are also studies that tested low-fluoride toothpastes with acidic level. Some authors evaluated the effectiveness of acidic toothpaste with reduced fluoride concentrations.[9,10,11,12] Brighenti et al. found in their study that the low-fluoride toothpaste was more effective when it was acidified than toothpaste with neutral pH. Furthermore, they found that the effectiveness of acidified low-fluoride toothpaste (550 ppm F) was similar to neutral higher fluoride (1100 ppm F) toothpaste.[9] Olympio et al. found that the acidulated dentifrice with 550 ppm F led to similar salivary concentrations of fluoride as the neutral dentifrice with 1100 ppm.[10] Furthermore, the results of Buzalaf et al. indicate that the reduction of dentifrice pH increases F uptake in dental plaque.[11]
The purpose of this study was to evaluate in vitro the remineralization capacity of commercial toothpastes with different F concentrations and their effectiveness when they are acidified.
The aim was to investigate how commercially available toothpastes with different fluoride levels perform on original and adjusted pH level and to study their abilities to prevent enamel demineralization.
Materials and Methods
Preparation of enamel slabs
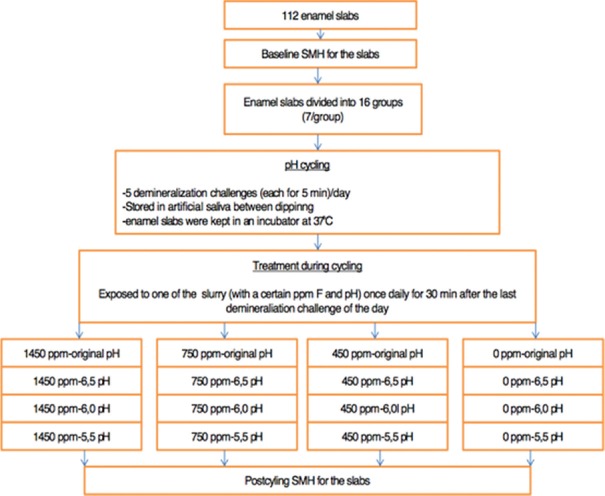
For this experiment, 112 caries-free teeth, which had been extracted for orthodontic reasons, were used and equally divided into 16 groups (n = 7). The teeth were stored immediately after extraction in 2% formaldehyde solution at 25°C. Enamel specimens were prepared from the buccal surface, using a water-cooled diamond saw, according to the relevant standard operating procedure (about 4 mm wide, 4 mm long, and 2 mm depth) [Figure 1]. In addition, each of the enamel slabs was embedded in acrylic resin and cured for 24 h. The surfaces of the resin blocks were progressively polished with an automatic polishing machine (RotoPol 11, Struers, Copenhagen, Denmark) under running water with carborundum discs (500 and 1200 grit; FEPA, Struers, Denmark). These procedures were implemented to achieve parallel planar surface for the Vickers microhardness tests.
Figure 1.

Enamel slabs embedded in acrylic resin (4 mm × 4 mm)
Microhardness measurements
A Vickers diamond indenter (HMV Shimadzu, Japan) was used in a standard microhardness tester for specimen indentation. A load of 980 mN applied for 10 s was used to make indentations. Each sample was subjected to six indentations before and after demineralization cycling.
pH-cycling regime
All the tested groups were subjected to the five demineralization challenges each day for 10 days. For the demineralization, the method proposed by Ten Cate was used.[13] The specimens were dipped in 50 ml of the demineralization solution once a day for 5 min (1.5 mM CaCl2, 0.9 mM KH2PO4, 50 mM acetic acid, and pH 4.8). Subsequently, the specimens were rinsed in distilled water for a minute and placed in 50 ml of artificial saliva for 90 min. Preparation of artificial saliva was made according to Al-Mullahi and Toumba.[14]
During the daytime, the formulation of artificial saliva was as follows: calcium carbonate 0.07 g/l, magnesium carbonate 0.019 g/l, potassium dehydrogenate phosphate 0.554 g/l, HEPES (Bioanalytic GmbH, Umkirch/Freiburg, Germany) buffer (acid form) 4.77 g/l, potassium chloride 2.24 g/l, and pH 6.8. After the last demineralization, the specimens were exposed to the slurry of toothpaste, once a day, and after that they were rinsed with distilled water and immersed in 50 ml of nighttime artificial saliva formulation (calcium carbonate 0.05 g/l, magnesium carbonate 0.019 g/l, potassium dehydrogenate phosphate 0.068 g/l, HEPES buffer [acid form] 4.77 g/l, potassium chloride 2.24 g/l, and pH 6.8).[14] Everyday, the pH values of demineralization and remineralization solutions were measured with a pH meter (HI 8014, HANNA instruments, Biobloch Scientific). After 10 days of the pH-cycling regime, all enamel specimens were rinsed with distilled water and stored in sealed containers with wet gauze until being analyzed.
Toothpaste treatment
The toothpastes used in this study were Vademecum Anticaries and Naturel (1450 ppm F, Henkel, Slovenia), Kalodont Pro-Care Herbal (1000 ppm F, Saponia Osijek, Croatia), Signal Kids (450 ppm F, Unilever, France), and Kalodont Wild Strawberry (0 ppm F, Saponia Osijek, Croatia).
Once a day, for 30 min, during 10 days, after the last demineralization, the enamel specimens were exposed to the toothpaste/deionized water slurries (1:3 w/w).[14] The solution was prepared daily before application by mixing toothpaste and deionized water in the ratio of 1:3, and the pH values were measured in each slurry with a pH meter (HI 8014, HANNA instruments, Biobloch Scientific). The original pH of the slurries and their standard deviations for 10 days during the experiment are listed in Table 1. Once the initial pH of the slurries was measured, it serves as a control group. The pH of the experimental slurries was adjusted to pH 6.5, 6.00, and 5.5 using the hydrochloric acid (0.1 mol/L) [Figure 2]. In each group, four different slurries were prepared, one with the original pH and three were acidified with hydrochloric acid.
Table 1.
List of toothpaste used in the study
| Toothpaste | Manufacturer | Ingrediens | F (ppm) | pH (slurry 1:3 w/w) |
|---|---|---|---|---|
| Vademecum Anticaries and Naturel | Henkel, Slovenia | Aqua, Sorbitol, Hydrated Silica, PEG-32, Sodium auryl sulfate, Aroma, Glycerin, Cellulose gum, Sodium fluoride, Panthenol, Magnesium sulfate, Disodium phosphate, Sodium saccharin, Chamomilla recutita flower extract, Calcium glycerophosphate, Trisodium phosphate, Zinc sulfate, Propylene glycol, Sodium sulfate, Retinyl palmitate, Lactic acid, Manganese sulfate, Melissa officinalis leaf extract, Salvia Officinalis leaf extract, Thymus vulgaris Extract, Sodium benzoate, Limonene, CI 42090, CI 47005, CI 77891 | 1450 | 6.83±0.04 |
| Kalodont Pro-care Herbal | Saponia Osijek, Croatia | Aqua, Hydrated Silica, Sorbitol, Glycerin, PEG-8, Sodium lauryl sulfate, Aroma, Xanthan gum, Sodium monofluorophosphate, Propylene glycol, Ethoxydiglycol, Matricaria extract, Sage extract, Balm Mint extract, Witch-hazel extract, Tormentil extract, Rosemary extract, Cocamidopropyl betaine, Tetrapotassium pyrophosphate, Sodium methylparaben, Sodium saccharin, Limonene, Citral, Linalool, Citronellol, Mica, CI 77891, CI 74160, CI 74260 | 1000 | 6.92±0.04 |
| Signal Kids | Unilever, France | Sorbitol, Aqua, Hydrated Silica, PEG-32, Sodium lauryl sulfate, Cellulose gum, Sodium fluoride, Sodium saccharin, Tocopheryl acetate, Calcium gluconate, Glycerin, CI 74160, CI 77891 | 450 | 7.09±0.05 |
| Kalodont Wild Strawberry | Saponia Osijek, Croatia | Aqua, Hydrated Silica, Sorbitol, Glycerin, Xylitol, PEG-8, Sodium lauryl sulfate, Aroma, Sodium saccharin, Panthenol, Xanthan gum, Cocamidopropyl betaine, Tetrapotassium pyrophosphate, Dmdm Hydantoin, Mica, Benzyl Alcohol, CI 77891, CI 16255 | 0 | 6.81±0.07 |
Figure 2.
Flowchart illustrating the study protocol
Statistical analysis
The data were subjected to analysis of variance (ANOVA) using a general linear model procedure of Statistica 7.0 (StatSoft, Inc., Tulsa, OK, USA) package. Statistica was set to P < 0.05. The difference among tested group was assessed by ANOVA and Newman–Keuls post hoc test (P < 0.05).
Results
The baseline and final microhardness as well as the difference in microhardness before and after treatment and the percentage of change in microhardness are presented in Table 2.
Table 2.
The mean (standard deviation) of enamel Vickers microhardness values (kg/mm2) before and after treatment with toothpastes with different pH level; difference in microhardness and percentage of changes
| Toothpaste | ppm | pH | Baseline microhardness | Final microhardness | Δ microhardness | Percentage changes |
|---|---|---|---|---|---|---|
| Vademecum Anticaries and Naturel | 1450 | 6.83 | 317.00 (11.89) | 336.9 (29.53) | 19.97 (22.08) | 6.20 (6.78) |
| 6.5 | 336.39 (29.41) | 354.28 (27.47) | 17.89 (21.20) | 5.55 (6.46) | ||
| 6.0 | 329.19 (17.13) | 338.21 (15.46) | 15.02 (14.09) | 4.76 (4.53) | ||
| 5.5 | 339.86 (35.02) | 340.36 (21.42) | 0.5. (22.75) | 0.64 (6.76) | ||
| Kalodont Pro-Care Herbal | 1000 | 6.92 | 346.62 (22.89) | 352.19 (20.79) | 5.57 (21.11) | 1.79 (6.18) |
| 6.5 | 345.11 (14.60) | 345.55 (27.56) | 1.00 (22.71) | 0.11 (6.66) | ||
| 6.0 | 369.43 (24.96) | 362.87 (24.87) | −6.56 (16.51) | −1.69 (4.43) | ||
| 5.5 | 330.78 (35.51) | 347.45 (34.72) | 16.67 (23,20) | 5.32 (7.26) | ||
| Signal Kids | 450 | 7.09 | 352.71 (18.11) | 360.95 (19.96) | 8.24 (18.28) | 2.44 (5.41) |
| 6.5 | 323.62 (33.55) | 332.81 (24.37) | 9.19 (21.43) | 3.23 (7.42) | ||
| 6.0 | 347.64 (20.00) | 353.55 (26.40) | 5.91 (14.61) | 1.66 (4.28) | ||
| 5.5 | 358.02 (33.65) | 339.00 (22.22) | −19.02 (20.71) | −4.95 (5.73) | ||
| Kalodont Wild Strawberry | 0 | 6.81 | 368.19 (45.58) | 342.76 (46.26) | −25.43 (24.61) | −6.82 (6.52) |
| 6.5 | 331.17 (36.01) | 330.17 (14.84) | −1.00 (31.00) | 0.60 (10.58) | ||
| 6.0 | 348.09 (30.04) | 331.10 (31.06) | −17.00 (38.27) | −4.45 (10.44) | ||
| 5.5 | 367.88 (29.98) | 361.98 (26.78) | −5.90 (10.02) | −1.53 (2.57) |
N=7 specimens per experimental condition. SDs are shown in parentheses. There is not statistically different at P<0.05 by Newman-Keuls test. SD: Standard deviation
The results of ANOVA test for tested toothpaste showed statistically significant difference between the initial and final microhardness (P < 0.05). According to the post hoc Newmann–Keuls tests (P < 0.05), there was no statistically significant difference among tested groups.
The highest increase in microhardness was detected after treatment with toothpaste containing 1450 ppm F (percentage of increase in microhardness was 6.2%), and the biggest loss was detected after treatment with toothpaste containing no fluoride (percentage of decrease was 6.82%), but there was no significant difference between the tested groups.
Discussion
Dental caries is the most common infectious disease, but it can be prevented. The use of fluoride along other preventive measures such as eliminating established M. streptococcus population from the oral cavity and control of the carbohydrate composition of the diet are the most successful healthy measures in dental care. Namely, fluorides could increase the acid resistance of the tooth.[15]
Fluoridated toothpastes are considered the most effective agents for preventing enamel demineralization, but their use increases the risk of dental fluorosis, especially in children who swallow large amounts of toothpaste during everyday brushing.[3] Therefore, the recommendations are to use a small amount of toothpaste[16] or a product with low-fluoride concentration.[9]
All the toothpastes used in this study had an original pH of the toothpaste/deionized water slurry (1:3 w/w) of above 6.80. The highest pH level was measured in the Signal Kids toothpaste (7.09), but all other toothpastes had pH level near the neutral value [Table 1].
The pH-cycling model used in this study was based on a study by Mulahi and Toumba,[14] and it was designed to stimulate the demineralizing and remineralizing episodes. This study was mainly focused on the prevention of demineralization, not remineralization of artificial preformed caries lesion,[17] and this protocol should have been able to stimulate the clinical situation.[18]
Microhardness measurements were used to provide evidence of enamel mineral loss or gain in connection with demineralization challenge and treatment with different toothpastes.[19,20]
Even though the findings obtained in this study are not statistically significant, they show a trend of increase in microhardness after treatment with toothpaste containing fluorides in all concentrations and decrease in microhardness when the specimens were treated with fluoride-free toothpaste. The absence of statistical significance could be due to small sample size in each group (n = 7).
When investigating how the original solutions of the toothpaste affected demineralization, it could be shown that the toothpaste with the highest level of fluoride (1450 ppm) prevented demineralization the most. Moreover, the microhardness values were higher at the end of the experiment.
In this study, toothpaste containing 450 ppm (Signal Kids) had a slightly better effect on the demineralization prevention than the toothpaste that contained 1000 ppm F (Kalodont Pro-care). The toothpaste Kalodont Pro-care contains 0.76% sodium monofluorophosphate (MFP) as opposed to Signal Kids, which contains sodium fluoride. In some studies, it has been shown that toothpastes containing MFP were slightly less effective than those containing sodium fluorides.[21,22,23] MFP needs to be hydrolyzed to release fluoride ions, and this ionization varies, depending on the activity of the enzyme phosphatase in the oral cavity.[24] Since this in vitro study was conducted with artificial saliva, this could partially explain the findings, attributed to the absence of enzymes required to break the MFP bond and release the fluoride ions.
The greatest decrease in microhardness occurred, as expected, in the specimens treated with the toothpaste not containing fluoride. However, this loss of minerals and microhardness in comparison with other toothpastes was not statistically significant. Again, this lack of statistical significance could be due to small sample size in each group (n = 7). Kalodont Wild Strawberry used in this study contained xylitol that other toothpastes did not. Xylitol acts as an inhibitor of demineralization, by preventing translocation of Ca++ and PO4 3− ions from lesions and also improves remineralization by acting as a Ca++ ions carrier from the remineralization solution.[25] Xylitol also has a buffering capacity.[26] When the original Kalodont Wild Strawberries toothpaste solution was acidified, much more acid was needed than in other pastes, and this could be a result of containing xylitol in its composition.
When the original solutions of toothpastes containing sodium fluoride were acidulated, the findings contradicted those obtained by Brighenti et al.[9] Namely, the microhardness was lower after treatment with acidulated toothpaste than original ones. After 10 days of treatment, the average values of microhardness were higher in all groups of toothpastes, except the toothpaste with 450 ppm acidulated at pH 5.5 [Table 2].
In the Brighenti et al. study, the tested toothpastes were experimental with the same ingredients except fluoride concentrations and pH levels.[9] In our study, we used the commercial toothpaste with different factory compositions [Table 1].
González-Cabezas et al. investigated the effect of low pH on surface rehardening efficacy of high-concentration fluoride treatments.[27] The results from their study suggested that high-concentration fluoride treatments with acidic pH were more effective. This is contradictory to the present findings, but their fluoride concentrations were much higher than what we used here.[27]
When we observed the changes in enamel microhardness after the treatments with acidulated toothpaste containing 1000 ppm F in the form of MFP, the greatest change occurred after treatment with solution acidulated at pH 5.5. The results were similar to those obtained after treatment with toothpaste with higher concentration of sodium fluoride (1450 ppm) and pH about 7.0. As mentioned earlier, MFP needs to be hydrolyzed. Furthermore, the dissociation of MFP in aqueous solution at neutral pH is only 6%, whereas sodium fluoride dissociates completely. The dissociation release of F from MPF can be accelerated in acidic medium.[28,29]
It could be concluded that for toothpaste containing MFP, it would have been more effective to be used in an acidic solution than neutral.
Today, they are many studies about the toxicity of fluoride exposure.[30,31,32] Choi et al. stated that fluoride exposure may produce developmental neurotoxicity.[33]
Therefore, because of fear, an increasing number of people refuse to use fluoride toothpaste. Toothpaste containing xylitol could be an alternative. According to the present findings, the enamel microhardness was not statistically different after treatment with fluoride-containing toothpaste versus fluoride-free (xylitol containing) toothpaste.
This study has certain limitations. This was an in vitro study and was unable to stimulate complex intraoral conditions that lead to caries development. Thus, the findings can be used to plan further clinical studies with larger sample sizes investigating the benefits of reducing pH of low-fluoride toothpastes, especially those containing MFP, to decrease caries development. The pH in individuals’ oral environment varies, and it is dependent on the buffer capacity of their saliva. It would be interesting to investigate how the alterations in the pH of saliva could change the efficacy of toothpaste.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lippert F. An introduction to toothpaste-its purpose, history and ingredients. Monogr Oral Sci. 2013;23:1–14. doi: 10.1159/000350456. [DOI] [PubMed] [Google Scholar]
- 2.Arnold WH, Haase A, Hacklaender J, Gintner Z, Bánóczy J, Gaengler P. Effect of pH of amine fluoride containing toothpastes on enamel remineralization in vitro . BMC Oral Health. 2007;7:14. doi: 10.1186/1472-6831-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley EM, Ellwood RP, Davies RM. Fluoride ingestion from toothpaste by young children. Br Dent J. 1999;186:460–2. doi: 10.1038/sj.bdj.4800140. [DOI] [PubMed] [Google Scholar]
- 4.Moraes SM, Pessan JP, Ramires I, Buzalaf MA. Fluoride intake from regular and low fluoride dentifrices by 2-3-year-old children: influence of the dentifrice flavor. Braz Oral Res. 2007;21:234–40. doi: 10.1590/s1806-83242007000300008. [DOI] [PubMed] [Google Scholar]
- 5.Pessan JP, Silva SM, Buzalaf MA. Evaluation of the total fluoride intake of 4-7-year-old children from diet and dentifrice. J Appl Oral Sci. 2003;11:150–6. doi: 10.1590/s1678-77572003000200012. [DOI] [PubMed] [Google Scholar]
- 6.Santos AP, Oliveira BH, Nadanovsky P. Effects of low and standard fluoride toothpastes on caries and fluorosis: systematic review and meta-analysis. Caries Res. 2013;47:382–90. doi: 10.1159/000348492. [DOI] [PubMed] [Google Scholar]
- 7.Ogaard B. CaF(2) formation: cariostatic properties and factors of enhancing the effect. Caries Res. 2001;35(Suppl 1):40–4. doi: 10.1159/000049109. [DOI] [PubMed] [Google Scholar]
- 8.Tenuta LM, Cerezetti RV, Del Bel Cury AA, Tabchoury CP, Cury JA. Fluoride release from CaF2 and enamel demineralization. J Dent Res. 2008;87:1032–6. doi: 10.1177/154405910808701105. [DOI] [PubMed] [Google Scholar]
- 9.Brighenti FL, Delbem AC, Buzalaf MA, Oliveira FA, Ribeiro DB, Sassaki KT. In vitro evaluation of acidified toothpastes with low fluoride content. Caries Res. 2006;40:239–44. doi: 10.1159/000092232. [DOI] [PubMed] [Google Scholar]
- 10.Olympio KP, Bardal PA, Cardoso VE, Oliveira RC, Bastos JR, Buzalaf MA. Low-fluoride dentifrices with reduced pH: fluoride concentration in whole saliva and bioavailability. Caries Res. 2007;41:365–70. doi: 10.1159/000104794. [DOI] [PubMed] [Google Scholar]
- 11.Buzalaf MA, Vilhena FV, Iano FG, Grizzo L, Pessan JP, Sampaio FC, et al. The effect of different fluoride concentrations and pH of dentifrices on plaque and nail fluoride levels in young children. Caries Res. 2009;43:142–6. doi: 10.1159/000211717. [DOI] [PubMed] [Google Scholar]
- 12.Alves KM, Pessan JP, Brighenti FL, Franco KS, Oliveira FA, Buzalaf MA, et al. In vitro evaluation of the effectiveness of acidic fluoride dentifrices. Caries Res. 2007;41:263–7. doi: 10.1159/000101915. [DOI] [PubMed] [Google Scholar]
- 13.ten Cate JM, Exterkate RA, Buijs MJ. The relative efficacy of fluoride toothpastes assessed with pH cycling. Caries Res. 2006;40:136–41. doi: 10.1159/000091060. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mullahi AM, Toumba KJ. Effect of slow-release fluoride devices and casein phosphopeptide/amorphous calcium phosphate nanocomplexes on enamel remineralization in vitro . Caries Res. 2010;44:364–71. doi: 10.1159/000316090. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan M, Simmonds RS, Tagg JR. Dental caries is a preventable infectious disease. Aust Dent J. 2000;45:235–45. doi: 10.1111/j.1834-7819.2000.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida BS, da Silva Cardoso VE, Buzalaf MA. Fluoride ingestion from toothpaste and diet in 1- to 3-year-old Brazilian children. Community Dent Oral Epidemiol. 2007;35:53–63. doi: 10.1111/j.1600-0528.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 17.Comar LP, Souza BM, Gracindo LF, Buzalaf MA, Magalhães AC. Impact of experimental nano-HAP pastes on bovine enamel and dentin submitted to a pH cycling model. Braz Dent J. 2013;24:273–8. doi: 10.1590/0103-6440201302175. [DOI] [PubMed] [Google Scholar]
- 18.Buzalaf MA, Hannas AR, Magalhães AC, Rios D, Honório HM, Delbem AC. pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J Appl Oral Sci. 2010 Aug 18;:316–34. doi: 10.1590/S1678-77572010000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seow WK, Thong KM. Erosive effects of common beverages on extracted premolar teeth. Aust Dent J. 2005;50:173–8. doi: 10.1111/j.1834-7819.2005.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 20.Chunmuang S, Jitpukdeebodintra S, Chuenarrom C, Benjakul P. Effect of xylitol and fluoride on enamel erosion in vitro . J Oral Sci. 2007;49:293–7. doi: 10.2334/josnusd.49.293. [DOI] [PubMed] [Google Scholar]
- 21.Itthagarun A, Wei SH, Wefel JS. The effect of different commercial dentifrices on enamel lesion progression: an in vitro pH-cycling study. Int Dent J. 2000;50:21–8. doi: 10.1111/j.1875-595x.2000.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 22.Marks RG, Conti AJ, Moorhead JE, Cancro L, D’Agostino RB. Results from a three-year caries clinical trial comparing NaF and SMFP fluoride formulations. Int Dent J. 1994;44(3 Suppl 1):275–85. [PubMed] [Google Scholar]
- 23.Duckworth RM, Jones Y, Nicholson J, Jacobson AP, Chestnutt IG. Studies on plaque fluoride after use of F-containing dentifrices. Adv Dent Res. 1994;8:202–7. doi: 10.1177/08959374940080021101. [DOI] [PubMed] [Google Scholar]
- 24.Casals E, Boukpessi T, McQueen CM, Eversole SL, Faller RV. Anticaries potential of commercial dentifrices as determined by fluoridation and remineralization efficiency. J Contemp Dent Pract. 2007;8:1–0. [PubMed] [Google Scholar]
- 25.Miake Y, Saeki Y, Takahashi M, Yanagisawa T. Remineralization effects of xylitol on demineralized enamel. J Electron Microsc (Tokyo) 2003;52:471–6. doi: 10.1093/jmicro/52.5.471. [DOI] [PubMed] [Google Scholar]
- 26.Ribelles Llop M, Guinot Jimeno F, Mayné Acién R, Bellet Dalmau LJ. Effects of xylitol chewing gum on salivary flow rate, pH, buffering capacity and presence of streptococcus mutans in saliva. Eur J Paediatr Dent. 2010;11:9–14. [PubMed] [Google Scholar]
- 27.González-Cabezas C, Jiang H, Fontana M, Eckert G. Effect of low pH on surface rehardening efficacy of high concentration fluoride treatments on non-cavitated lesions. J Dent. 2012;40:522–6. doi: 10.1016/j.jdent.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Guimarães IC, Rezende CC, Fracassi da Silva JA, Pereira de Jesus D. Simultaneous determination of free fluoride and monofluorophosphate in toothpaste by capillary electrophoresis with capacitively coupled contactless conductivity detection. Talanta. 2009;78:1436–9. doi: 10.1016/j.talanta.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 29.Gleisner H, Einax JW, Morés S, Welz B, Carasek E. A fast and accurate method for the determination of total and soluble fluorine in toothpaste using high-resolution graphite furnace molecular absorption spectrometry and its comparison with established techniques. J Pharm Biomed Anal. 2011;54:1040–6. doi: 10.1016/j.jpba.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Pérez N, Torres-Mendoza N, Borges-Yáñez A, Irigoyen-Camacho ME. Dental fluorosis: Concentration of fluoride in drinking water and consumption of bottled beverages in school children. J Clin Pediatr Dent. 2014;38:338–44. doi: 10.17796/jcpd.38.4.e77h557k0005077n. [DOI] [PubMed] [Google Scholar]
- 31.Denbesten P, Li W. Chronic fluoride toxicity: Dental fluorosis. Monogr Oral Sci. 2011;22:81–96. doi: 10.1159/000327028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DenBesten PK. Biological mechanisms of dental fluorosis relevant to the use of fluoride supplements. Community Dent Oral Epidemiol. 1999;27:41–7. doi: 10.1111/j.1600-0528.1999.tb01990.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi AL, Zhang Y, Sun G, Bellinger DC, Wang K, Yang XJ, et al. Association of lifetime exposure to fluoride and cognitive functions in Chinese children: A pilot study. Neurotoxicol Teratol. 2015;47:96–101. doi: 10.1016/j.ntt.2014.11.001. [DOI] [PubMed] [Google Scholar]