Abstract
Background:
Prolongation of the inflammatory process in hyperglycemic interferes with bone formation, inhibits the healing process, and triggers bone resorption. A combination of spirulina and chitosan in the tooth socket of Rattus norvegicus is expected to promote the bone remodeling process. This study aimed to evaluate the effect of spirulina and chitosan on angiogenesis, osteoclast, and osteoblast cell in tooth socket models of type 1 diabetes.
Materials and Methods:
A laboratory-based experiment involving 36 R. norvegicus, divided into three groups (nondiabetes mellitus (DM), uncontrolled DM, and controlled DM) and further divided into six subgroups. The controlled groups (K1, K2, and K3) were induced with 3% carboxymethyl cellulose Na, while the treated groups were induced with 12% spirulina and 20% chitosan. On the 14th day, the mandibles of the rats were removed. The capillary lumen, osteoblasts, and osteoclast cells were counted by hypothalamic–pituitary–adrenal examination and the results analyzed by means of Shapiro–Wilk, Levene's, one-way ANOVA, and post hoc Tukey's honestly significant difference test.
Results:
There was a significant increment in the number of capillary lumen, osteoblast cells, and a decrease in osteoclasts in all three treated groups (P1, P2, and P3).
Conclusions:
A combination of spirulina and chitosan can effectively promote the healing process in postextraction sockets of type 1 DM R. norvegicus.
Keywords: Bone, chitosan, diabetes mellitus, spirulina, wound healing
Introduction
Diabetes mellitus (DM) is a syndrome characterized by high blood sugar levels (hyperglycemia).[1] The International Diabetes Federation stated in 2012 that the number of individuals afflicted by DM amounted to 371 million people. In a hyperglycemic state, the healing process takes longer due to the accumulation of advanced glycation end-products (AGEs) that increases pro-inflammatory cytokines, with a consequent more prolonged inflammatory process. Prolongation of the inflammatory process interferes with bone formation, inhibits the healing process, and triggers bone resorption.[2,3]
The replacing of missing teeth postextraction is necessary to prevent the adverse effects of tooth loss, such as the migration of a neighboring tooth into the vacant space, supraeruption of an antagonist tooth, loss of mastication or phonetic functions, and decreased esthetics of the oral cavity. Preparation of this prosthesis requires prominent bone for retention and stability of dental prosthesis.[4] Tooth extraction, both traumatic and atraumatic, will reduce both bone dimensions and volume, in contrast to prosthodontic treatments that require prominent bone.[5] In addition, the tendency of patients with systemic diseases, especially DM, to experience tooth loss will trigger a biological cascade that causes irreversible anatomical changes.[1]
Angiogenesis disorders in patients with hyperglycemia, in addition to impeding bone formation, affect the rate at which wounds heal. Angiogenesis is the formation of new blood vessels from existing ones and constitutes a marker for wound healing.[6] In order to overcome these problems, preservation sockets containing substances that suppress inflammation, resorption, and accelerate healing are required.[5] At present, natural ingredients are often used as a therapeutic option because they are regarded as safe. In addition, natural ingredients frequently represent the subject of further study and have been developed as an alternative therapy. Two natural ingredients known to potentially suppress inflammation, while accelerating wound healing, are spirulina and chitosan. Chitosan is a natural polysaccharide, derived from chitin through a deacetylation process,[6] which possesses strong osteoconductive properties and can improve bone regeneration in cases of dental bone loss.[7] In addition to chitosan, spirulina, which is a kaempferol-containing microalgae, also possesses antioxidant properties and is a pro-inflammatory cytokine inhibitor.[8]
The combination of spirulina and chitosan can accelerate the bone healing process because it produces an alkaline pH suitable for alkaline phosphatase activity. A combination of 12% spirulina and 20% chitosan in the tooth socket of Rattus norvegicus is also known to promote the bone remodeling process characterized by an increase in osteoblast cell counts and decreased osteoclast cells.[1]
In this study, histopathologic examination was performed on the 14th-day postextraction because, under diabetic conditions, the angiogenesis process experiences delay at that point while new bone cells begin to form.[9] The purpose of this study was to determine the effect of a combination of spirulina and chitosan on the number of lumen in the blood vessels and osteoblasts and osteoclasts in the sockets of diabetic mice.
Materials and Methods
This study applied an experimental laboratory study design with a posttest-only control group. The sample comprised 36 male Wistar rats (R. norvegicus), weighing 130–150 grams and were divided into three groups (1, 2, and 3) and each group divided into two subgroups (K and P). All subject in group K2, P2, K3, and P3 were first induced with streptozotocin (STZ) at low doses of 50 mg/kg body weight to produce a hyperglycemic model. This condition could be achieved after 4–12 days of STZ induction.[10]
The K3 and P3 groups were induced intragastrical with daily doses of metformin at 250 mg/kg bodyweight, as oral antidiabetic therapy, with administration being initiated after the rats had been diagnosed with hyperglycemia.[11] Metformin was administered to obtain a controlled blood glucose level (BGL). In this study, uncontrolled hyperglycemia was characterized by random BGL >200 mg/dl, while controlled hyperglycemia was characterized by randomized BGL <200 mg/dl.
On the 6th day, after the subjects had been diagnosed with hyperglycemia, the left incisor of their mandibula was extracted. The sockets in the groups K1, K2, and K3 were induced with 3% CMCNa, while in the P1, P2, and P3 were induced with 12% spirulina and 20% chitosan. After induction, the socket was sutured using 3/03 silk thread to prevent contamination.
The subjects were sacrificed on day 14 postextraction with ketamine 22–44 mg/kg body weight and the left mandible was taken for hypothalamic–pituitary–adrenal (HPA) preparation. Calculation of lumen in blood vessels, osteoclasts, and osteoblasts was conducted by hematoxylin-eosin staining and observing HPA preparations under a light microscope, while observations were made of the 1/3 apical socket area.
The results obtained underwent analysis by means of a Shapiro–Wilk test to establish whether the data were normally distributed, followed by a Levene test to identify variance homogeneity. The data were subsequently tested with a one-way ANOVA and post hoc Tukey honestly significant difference (HSD) to identify any significant differences between groups.
Results
The results of the study regarding the number of lumen in blood vessels, osteoclast, and osteoblast are contained in Table 1. Table 1 shows the highest number of the blood vessels new formation have been found in P1 with the mean value 13.50 ± 1.31 and the lowest number has been found in K2 by the mean value 8.50 ± 0.93. Microscopically, the number of the blood vessels has been shown in Figure 1.
Table 1.
The mean value and standard deviation of osteoclast, osteoblast, and the new blood vessel formation in socket postextraction
| Group | Osteoclast | Osteoblast | Angiogenesis |
|---|---|---|---|
| K1 | 8.67±0.82a | 18.67±0.82a | 11.38±1.06a |
| K2 | 12.17±0.75b | 14.33±0.82b | 8.50±0.93b |
| K3 | 9.67±0.82a | 18.33±0.82a | 10.25±1.03a |
| P1 | 7.00±0.63c | 21.67±2.25c | 13.50±1.31c |
| P2 | 10.17±1.47d | 17.83±1.47d | 9.75±1.03d |
| P3 | 7.83±0.75c | 20.83±0.75c | 12.00±0.76c |
Different superscript letter indicates there was a significant difference between group according to Tukey HSD test. HSD: Honestly significant difference
Figure 1.
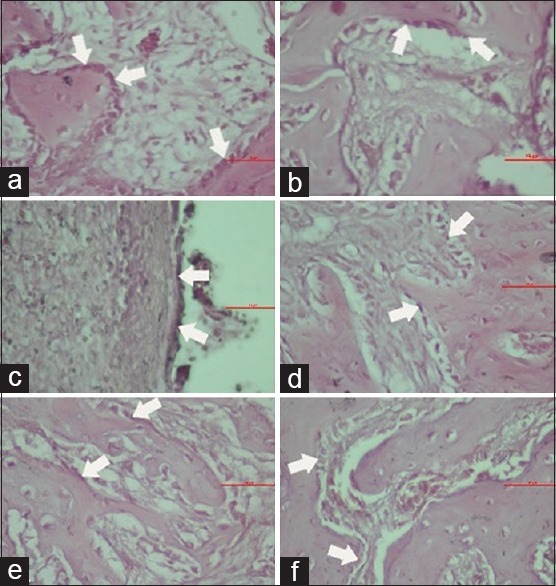
Microscopic view of osteoblast in groups K1 (a), P1 (b), K2 (c), P2 (d), K3 (e), and P3 (f)
The osteoclast in socket postextraction microscopically can be shown in Figure 2. The highest number of osteoclast showed in Group K2 by the mean value 12.17 ± 0.75 and the lowest number was found in Group P1 by the mean value 7.00 ± 0.63. The mean value of osteoclast number in Table 1 indicated a decrease in the number of osteoclast cells in the treatment group administered with gel consisting of 12% spirulina and 20% chitosan, compared to the control group which was only administered with 3% CMCNa gel base.
Figure 2.
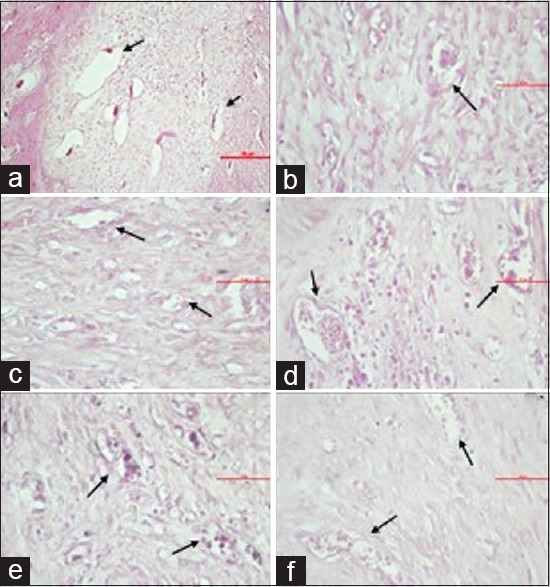
Microscopic view of the blood vessel lumen in groups K1 (a), P1 (b), K2 (c), P2 (d), K3 (e), and P3 (f)
Figure 3 shows that the average number of osteoblast cells in all group. The highest number of osteoblast showed in group P1 by the mean value 21.67 ± 2.25 and the lowest number was found in group K2 by the mean value 14.33 ± 0.82. This indicated an increase in the number of osteoblast cells in the treatment group, given a combination gel of spirulina and chitosan compared to the control group induced with 3% CMCNa gel.
Figure 3.
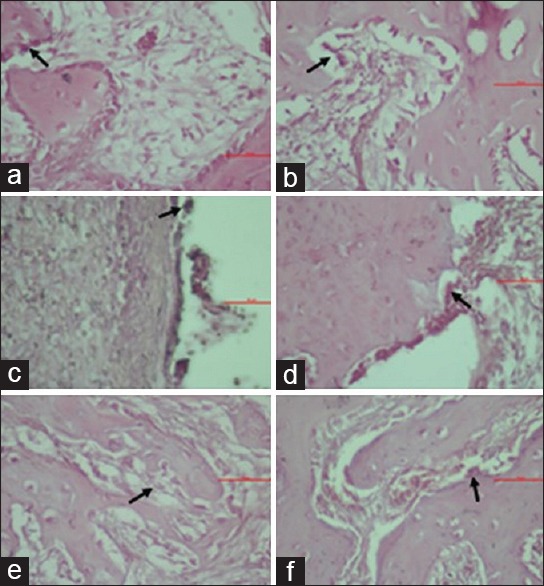
Microscopic view of osteoclasts in groups K1 (a), P1 (b), K2 (c), P2 (d), K3 (e), and P3 (f)
The data were then tested statistically by means of a Shapiro–Wilk test to determine the normality of distribution. From the analysis, it can be shown that the P > 0.05 which mean all the data have a normal distribution. Meanwhile, in order to establish the homogeneity of these three variables, the data were subjected to a Levene's statistic. This test resulting the P > 0.05 which confirmed a homogeneous data.
After the data were declared normal and homogeneous, one-way ANOVA and Tukey HSD tests were conducted. The overall results of the one-way ANOVA test for the group was a value of P < 0.05 which indicated significant differences between the groups. The contents of Table 1 confirm that the post hoc Tukey HSD test indicated no significant differences between the control group, the treatment groups (K1-P1 and K2-P3) and the treatment Group 1 (K1), or between the treatment Group 3 (P3) and treatment Group 1 (P1). In contrast, a significant difference did exist between control Group 1 (K1) and control 2 (K2), control Group 2 (K2) and control Group 3 (K3), treatment Group 1 (P1) and treatment Group 2 (P2), and treatment Group 2 (P2) and treatment Group 3 (P3).
Discussion
Data analysis using a Tukey HSD test confirmed that there was a significant difference between K1, K2, and P1, P2 in the total blood capillary, osteoclast, and osteoblast cells. This suggests that STZ induction in rats led to significant angiogenesis and postextraction wound healing. The induction of diabetes using STZ succeeded in destroying pancreatic beta cell DNA, resulting in the death of cells and reduction in the production of insulin.
The experimental rats suffered a progressive decrease in target cells for insulin secretion by pancreatic β-cells, leading to a chronic increase in blood sugar (hyperglycemia) due to STZ induction. This hyperglycemic state increases the interaction between glucose molecules and cellular molecules, such as those of proteins and fats. This interaction is termed a glycemic reaction that produces a modified product, AGEs, which subsequently plays a role in avoiding various complications in patients with DM.[4,5]
High AGEs levels can, in turn, increase those of reactive oxygen species (ROS) by bonding with receptors. The anti-angiogenic nuclear factor-kappa B pathway activated through increased plasminogen activator inhibitor-1 and endothelial cell apoptosis by AGEs and ROS. In hyperglycemia, AGEs bind to its receptors, RAGEs that occur in the neutrophil plasma membranes, induce neutrophil response disorders, and phagocytosis function. In addition, the AGEs–RAGEs bond will increase oxidative stress in the body by activating the NADPH oxidase enzyme, a pro-oxidative enzyme that produces ROS. The presence of ROS can trigger the phosphorylation and degradation of I-κB, thus causing activation of NF-κB, mRNA expression, cyclooxygenase-2 protein, PGE2 production, and secretion of pro-inflammatory cytokines (interleukin [IL]-1β, tumor necrosis factor-alpha [TNF-α], IL-6).[12] Excessive expression of these molecules can interfere with the inflammatory response, thereby inhibiting the post-extraction phase of wound healing in the next stage characterized by an increase in the number of osteoclasts.
TNF-α at normal concentrations is known to induce an inflammatory response by increasing vascular permeability and recruiting macrophages and neutrophils in the lesions. However, in a hyperglycemic state, the cytokines increase excessively, potentially producing a negative effect. Increased TNF-α and pro-inflammatory cytokines in hyperglycemia are known to stimulate bone resorption and inhibit bone formation.[13] Since pro-inflammatory cytokines can reduce gene expression, namely Runx2, that plays a role in the process of osteogenesis. Runx2 induces the differentiation of mesenchymal stem cells (MSC) into immature osteoblasts and directs the formation of immature bone.[14] If Runx2 decreases, a reduction in MSC differentiation into osteoblasts will also occur, in turn, causing the production of osteoblast cells to decrease.[15] In addition, in one study, it was stated that inhibited NF-κB can increase osteoblast cell differentiation or the number of mature osteoblast cells.[13]
A significant difference in the number of blood capillaries, osteoclasts, and osteoblasts was also found in K2 when compared to K3, as well as P2 in comparison to P3. This suggests that metformin medication in diabetic rats succeeded in lowering blood sugar levels, thus reducing AGE concentrations. The use of metformin in a controlled diabetic state may reduce the negative effects of AGE due to decreased blood glucose that occurs through metformin mechanisms, namely, decreased gluconeogenesis, increased insulin sensitivity, and reduced intestinal glucose absorption. Moreover, metformin is capable of enhancing angiogenesis through the enhancement of function and capacity of EPC and its protective effect against EPC. Minimal interaction between AGE and RAGE decreases the production of ROS and NF-κB which then reduces angiogenesis complications in these three pathways. It is known that metformin also has a positive effect on the regulation of osteoblast differentiation in bone marrow progenitor cells through the AMPK signaling pathway,[16] while metformin may stimulate osteoblast differentiation through the transactivation of Runx2.[17]
The application of combined gel of 12% spirulina and 20% chitosan in P1 increased angiogenesis and osteoblast cell counts and significantly decreased the osteoclast count when compared to K1. Similar results were also obtained when comparing K2 with P2 and K3 with P3. This showed the ability of 12% spirulina and 20% chitosan to decrease the number of osteoclast cells and increase angiogenesis as well as the number of osteoblast cells even when in a diabetic condition. This decrease occurred due to the therapeutic effects of both substances beyond the interference caused by high levels of ROS in the body. Decreased ROS and nuclear factor-kappa B are capable of increasing the rate of degradation of extracellular matrix, thus accelerating angiogenesis and preventing capcase-3 activation that stimulates endothelial cell apoptosis.
In addition, the substance contained in a combination of 12% spirulina gel and 20% chitosan is able to combat oxygen singlet influenced by lipid peroxidation and directly block the accumulation of ROS, thus inhibiting the expression of inflammation. Pro-inflammatory cytokines, for example, IL-1β, IL-6, and TNF-α, that play a role in bone resorption are also decreasing.[18,19] Decreased IL-6 will increase the number of osteoprotegerins that inhibit RANK interaction with RANKL. This condition results in the inhibition of osteoclastogenesis and decreases the number of osteoclasts. The properties of osteoinductivity and osteointegratability of chitosan will stimulate new bone growth and balance the number of osteoclasts and osteoblasts so that wound healing and efficient bone tissue repair can occur.[7]
Spirulina is known to contain phycocyanin and kaempferol which can decrease inflammation and chitosan which is capable of increasing bone mineralization by regulating the associated genes in the mineralization process and calcium-binding proteins during the osteoblast cell differentiation process in MSCs.[20,21] It is also known that chitosan may decrease pro-inflammatory cytokines TNF-α. Ma et al. mention that phycocyanin in spirulina contains phycocyanobilin which possesses a chemical structure similar to that of biliverdin enabling it to have antioxidant effects comparable to those of biliverdin and bilirubin.[8] The antioxidant effect is derived from its ability to inhibit the activity of NADPH oxidase which is the main source of ROS in the vascular tissue of diabetic patients. Azuma et al. stated that chitosan is able to prevent the activation of NF-κB and inhibit the production of TNF-α.[22] A study conducted by Tu et al. also mentions that chitosan is capable of decreasing degradation of NF-κB (I-κB) cytosolic inhibitors which can cause translocation of NF-κB in the nucleus.[23]
In both the controlled and uncontrolled DM groups, the results indicated a normal random BGL as in the nonhyperglycemic group. This suggests that the provision of metformin as a medication is capable of lowering BGLs, thereby minimizing barriers to effective healing. This is consistent with the theory that metformin in diabetic mice that are experimental subjects has been shown to decrease the levels of the phosphoenolpyruvate carboxykinase protein that affected the decrease in BGLs as opposed to glucagon.[24]
The study also provided evidence that a combination of 12% spirulina and 20% chitosan significantly increased angiogenesis and osteoblast cell count, while decreasing the postextraction osteoblast cell count in sockets of diabetic rats on the 14th day in a normal state, uncontrolled hyperglycemia, or controlled hyperglycemia.
Conclusions
The combination of spirulina and chitosan increased angiogenesis and osteoblast cells while decreasing the osteoclast cells in sockets, after tooth extraction of hyperglycemic R. norvegicus on day 14. The combination of spirulina and chitosan was able to accelerate the postextraction wound healing process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J Dent Res. 2014;93:950–8. doi: 10.1177/0022034514541127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widowati W. Potensi antioksidan sebagai antidiabetes. JKM. 2008;7:1–11. [Google Scholar]
- 3.Sanguineti R, Puddu A, Mach F, Montecucco F, Viviani GL. Advanced glycation end products play adverse proinflammatory activities in osteoporosis. Mediators Inflamm. 2014;2014:975872. doi: 10.1155/2014/975872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rostiny, Kuntjoro M, Sitalaksmi RM, Salim S. Spirulina chitosan gel induction on healing process of Cavia cobaya post extraction socket. Dent J Majalah Kedokteran Gigi. 2014;47:19–24. [Google Scholar]
- 5.Askary A. Victoria, Australia: Blackwell Munksgaard Publishing Company; 2007. Fundamentals of Esthetic Implant Dentistry; pp. 209–13. [Google Scholar]
- 6.Dwivedi S, Mahor A, Chanchal D. Chitosan nanoparticles: A review. World J Pharm Pharm Sci. 2015;5:433–9. [Google Scholar]
- 7.Ezoddini-Ardakani F, Navab-Azam A, Yassaei S, Fatehi F, Rouhi G. Effects of chitosan on dental bone repair. Health. 2011;3:200–5. [Google Scholar]
- 8.Ma QY, Fang M, Zheng JH, Ren DF, Lu J. Optimised extraction of β-carotene from spirulina platensis and hypoglycaemic effect in streptozotocin-induced diabetic mice. J Sci Food Agric. 2016;96:1783–9. doi: 10.1002/jsfa.7286. [DOI] [PubMed] [Google Scholar]
- 9.Steiner GG, Francis W, Burrell R, Kallet MP, Steiner DM, Macias R, et al. The healing socket and socket regeneration. Compend Contin Educ Dent. 2008;29:114–6. 118, 120-4. [PubMed] [Google Scholar]
- 10.Purwanto B, Liben P. Surabaya, Indonesia: PT Revka Petra Media; 2014. Model Hewan Coba untuk Penelitian Diabetes; pp. 6–29. [Google Scholar]
- 11.Onkowo P, Okoye Z. Comparative effects of antidiabetic drug, metformin and deferoxamine on serum lipids, serum ferritin and endocrine indicators of diabetes mellitus complications in streptozotocin diabetic rats. Int J Biochem Res Rev. 2014;4:538–40. [Google Scholar]
- 12.Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 13.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–9. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komori T. Regulation of osteoblast differentiation by runx2. Adv Exp Med Biol. 2010;658:43–9. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 15.Finastika C, Sutrisna E, Handayani F. The effect of atorvastatin per oral to the number of capillary blood vessel and osteoblast in the alveolar bone healing. BIMKGI. 2016;4:7–16. [Google Scholar]
- 16.Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of runx2. Bone. 2011;48:885–93. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone – Osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K, et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of spirulina: An overview. Arch Toxicol. 2016;90:1817–40. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 19.Friedman AJ, Phan J, Schairer DO, Champer J, Qin M, Pirouz A, et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: A targeted therapy for cutaneous pathogens. J Invest Dermatol. 2013;133:1231–9. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syarina PN, Karthivashan G, Abas F, Arulselvan P, Fakurazi S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015;14:385–93. doi: 10.17179/excli2014-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews S, Gupta PK, Bhonde R, Totey S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011;44:537–49. doi: 10.1111/j.1365-2184.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015;6:33–49. doi: 10.3390/jfb6010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu J, Xu Y, Xu J, Ling Y, Cai Y. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in caco-2 cells. Int J Biol Macromol. 2016;86:848–56. doi: 10.1016/j.ijbiomac.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Pernicova I, Korbonits M. Metformin – Mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]


