Abstract
Background:
One of the important tumor markers having critically important applications in every aspect of treating men with prostatic illness is prostate-specific antigen (PSA), formed by prostate acini's epithelial cells. Where prostate is affected by inflammation or malignancy, the PSA levels rise to/and above 4 ng/ml. This study analyzes the interlink between different severity of periodontitis and prostatitis by assessment of PSA antigen levels and periodontal clinical parameters.
Materials and Methods:
In this study, 100 chronic prostatitis patients diagnosed to also have periodontal diseases were divided into four batches on the basis of the nature of prostatitis and levels of periodontal clinical attachment. The grouping was as: group 1A – clinical attachment level (CAL) <3 mm and mild prostatitis, Group 2A – CAL ≥3 mm and mild prostatitis, and Group 1B – CAL <3 mm and moderate-to-severe prostatitis, Group 2B – CAL ≥3 mm and moderate-to-severe prostatitis. Readings of CAL, probing pocket depth, bleeding on probing, plaque index, and gingival index (PI and GI) were recorded, followed by calculation and assessment of PSA values and correlation of periodontal parameters, respectively.
Results:
An important and affirmative correlation (r = 0.5549, P < 0.05) was seen between PSA and CAL scores at significance level of 5%, and also between PSA and probing depths (PD) scores at 5% (r = 0.5315, P < 0.05), indicating that PSA and CAL scores, as also PSA and PD scores are mutually dependent. The similar positive correlation was seen between PSA with PI (r = 0.3231, P < 0.05) and GI (r = 0.3567, P < 0.05) scores, respectively, at 5% level of significance, which shows PSA is also mutually dependent on PI and GI scores.
Conclusion:
Patients with of grades, moderate-to-severe prostatitis as well as periodontitis were found having higher PSA levels. The clinical readings of periodontal parameters were significantly higher in patients with moderate-to-severe prostatitis which shows a pathological link between the above two.
Keywords: Periodontitis, prostate carcinoma, prostate-specific antigen levels, prostatitis
Introduction
Periodontitis is an inflammatory condition of the tissues supporting the teeth caused by certain particular micro-organisms or clusters of such microorganisms, leading to the continuous destruction of the periodontal ligament and the alveolar bone with an increase in probing pocket depth, pocket formation, recession, or both. It may affect varying number of teeth with varying progression.[1] The emergence of host response paradigm to explain the pathogenesis of periodontal disease has expanded our knowledge to a better understanding of the disease. Research findings, of late, show potential links between periodontitis and systemic disorders, such as diabetes, coronary heart disease and lung disorders, stroke and pregnancy-related adverse conditions. These findings together with the surfacing of a new discipline of periodontal medicine, as a domain, has led to many developments in remedial and beneficial approaches about managing periodontitis.[2]
Of the various systemic disorders inflicting human beings, prostatitis is one of the disorders that has shown to have a relationship with periodontitis, the former being a common inflammatory condition below the age of 50. Prostatitis clinically presents with painful urination, difficulty in emptying the bladder, frequent tendency to urinate, pain in penis, testicles, even during or after ejaculation, with associated fever and chills.[3] Prostate-specific antigen (PSA), a glycoprotein produced mainly by the epithelial cells along the acini and ducts of the prostate gland, is a biological or tumor marker for conditions such as benign prostatic hyperplasia, prostate cancer, and the screening of which has proven to be beneficial in the confirmatory diagnosis of substantial numbers prostate cancer patients.[4] The serum PSA levels are normally very low; however, when the normal prostatic structural design is disrupted, like prostatic disease, inflammation or trauma, it permits entry of more PSA to the systemic circulation. PSA level below 4.0 ng/ml is normal and above 0.35 ng/ml/year has a relatively higher risk of death from prostate cancer, unlike those who had a PSA volume <0.35 ng/ml/year.[5]
Chronic prostatitis etiology depends on multiple host immuno inflammatory factors, likely to be channeled via pro-inflammatory cytokines, such as interleukin (IL-1 β) and tumor necrosis factor-α (TNF-α). Increased ratio of pro-inflammatory to anti-inflammatory cytokines imbalance has been concerned with the pathogenesis of periodontitis as well as prostatitis. Similarity in the etiopathogenesis of these denotes a possible relationship between the two which may be apparent with the elevated PSA values in circulation.[6] This study evaluates any link between periodontitis and PSA levels in chronic prostatitis patients, and compares the serum PSA levels with periodontal clinical parameters which include CALs, probing depths (PD), plaque index and gingival index (PI and GI), bleeding on probing between the study groups.
Materials and Methods
Patients who attended, Government General Hospital, Guntur presenting with signs and symptoms of lower urinary tract infections and diagnosed with prostatitis or elevated PSA levels PSA (4 ng/ml) together with chronic periodontitis were assigned in the study. Permissions as dictated by the ethics and protocols were obtained after sufficient explanations and clarifications to the patients about the treatment procedures and those who were willing to participate were selected for the study, as allowed by the Institute's Ethical Committee.
Inclusion criteria were that patients aged 30–60, with ≥12 teeth, with elevated serum PSA levels ≥4 ng/ml, who did not undergo oral prophylaxis in the previous 6 months. Those with any history of systemically compromised conditions such as myocardial infarction, stroke, and organ transplant during the previous 6 months were not covered in the study.
One hundred prostatitis patients with periodontal problems were considered, of which 83 patients presented with the benign condition and 17 turned out to be malignant. They were further divided into four groups on the basis of prostatitis conditions and the level of periodontal clinical attachment. The groups were Group 1 A– clinical attachment level (CAL) <3 with mild prostatitis, Group 2 A-CAL ≥3 with mild prostatitis, Group 1B-CAL <3 with moderate or severe prostatitis, and Group 2 B-is CAL ≥3 mm with moderate or severe prostatitis. Five milliliters of intravenous blood was collected from the antecubital vein of each patient, and the samples were sent for determination of serum PSA levels. Prostate needle biopsy of prostate gland had been taken and sent for histopathological examination.
Estimation of prostate-specific antigen
The PSA chemiluminiscent immunoassay test, works on the principle of a solid phase enzyme-linked immunosorbent assay, using a rabbit anti-PSA antibody directed against intact PSA for solid phase immobilization (on the microtiter wells). The sample reacts with monoclonal-anti PSA antibody conjugate for 15 min at 37°C–40°C resulting in molecules getting sandwiched between the solid phase and enzyme-linked antibodies. The wells are then water-washed to remove unbound labeled antibodies and substrate reagent is added and incubated for 5 min.
Assay procedure
Serum is separated by adding ethylenediaminetetraacetic acid after collecting blood sample. Following which, 13 ml of serum is removed and the sample is placed in a well; later 50 ml of tracer reagent is added to that well. Swirl the plate for 60 s, and incubate the well for 45 min at 22°C–26°C. Decanting and washing are done five times with wash buffer, after which reagents are added in the ratio of 1:1 to the sample well, and incubated for 5 min following which the results of PSA will then be displayed on the screen.
Periodontal examination
After getting patients consent, periodontal examination was carried out using UNC-15 Probe (University of North Carolina-15), and assessment of periodontal parameters included measurement of probing depth from the gingival margin to the base of sulcus, measurement of CAL from the cement-enamel junction to the base of pocket, GI of Silness and Loe (1963), PI of Loe and Silness (1967), Bleeding index of Ainamo and Bay (1975).
Statistical analysis
Descriptive statistical analysis was carried out in the present study. For intragroup comparison, Kruskal–Wallis test was used. The Mann–Whitney test was used to help analyze the specific sample pairs for significant differences. Intergroup comparison was done with one-way analysis of variance. The relationship between PSA scores with all clinical parameters was done using Pearson's correlation coefficient technique. Difference in means as statistically significant was analyzed using Student's t-test.
Results
The age of the patients ranged between 40 and 75 years, with the mean age of 57.63 ± 13.65 in Group 1A and 60.50 ± 0.71 in Group 1B, 63.75 ± 8.03 in Group 2A, and in Group 2B, it is 63.97 ± 8.60 [Table 1]. The percentage of moderate/severe prostatitis patients with malignancy were 49% and with nonmalignancy are 51%. A significant difference was observed between mild, moderate, and severe types of prostatitis in relation to the status of malignancy (P < 0.05), as shown in Table 2. Table 3 shows the mean GI and PI scores in all the four groups. The mean bleeding index score in all the groups are depicted in Table 4 and mean CAL and probing depth scores of the groups are represented in Table 5. No difference was observed among four Groups 1A, 1B, 2A, and 2B with respect to PSA scores (P > 0.05), showing that the PSA scores are very similar in four groups [Table 6].
Table 1.
Mean and standard deviation age of study samples by four groups (1A, 1B, 2A, and 2B)
| Groups | Age (mean±SD) |
|---|---|
| Group 1A | 57.63±13.65 |
| Group 1B | 60.50±0.71 |
| Group 2A | 63.75±8.03 |
| Group 2B | 63.97±8.60 |
SD: Standard deviation
Table 2.
Distribution of samples by types of prostatitis and status of malignancy
| Status of malignant | Mild (%) | Moderate/severe (%) | Total |
|---|---|---|---|
| Malignant | 0 (0.00) | 15 (100.00) | 15 |
| Nonmalignant | 16 (50.00) | 16 (50.00) | 32 |
| Total | 16 (34.04) | 31 (65.96) | 47 |
| χ2 with Yates’s correction | 9.2533 | ||
| P | 0.0023* | ||
*P<0.05
Table 3.
Comparison of four groups (1A, 1B, 2A, 2B) with respect to gingival index and plaque index scores by Kruskal–Wallis analysis of variance test and Pairwise comparison by Mann–Whitney Utest
| Groups | GI scores | PI scores | ||
|---|---|---|---|---|
| Mean±SD | Sum of ranks | Mean±SD | Sum of ranks | |
| Group 1A | 1.63±1.27 | 121.00 | 1.59±1.23 | 122.00 |
| Group 1B | 1.40±0.50 | 34.00 | 1.56±0.69 | 40.00 |
| Group 2A | 1.37±0.47 | 121.50 | 1.35±0.42 | 122.50 |
| Group 2B | 1.89±0.53 | 851.50 | 1.83±0.49 | 843.50 |
| H | 11.6201 | 10.6321 | ||
| P | 0.0088* | 0.0139* | ||
| Pair-wise comparison by Mann–Whitney U-test | ||||
| 1A versus 1B |
0.7940 | 0.6015 | ||
| 1A versus 2A |
0.7527 | 0.9164 | ||
| 1A versus 2B |
0.0134* | 0.0134* | ||
| 1B versus 2A |
1.0000 | 0.7940 | ||
| 1B versus 2B |
0.2278 | 0.3764 | ||
| 1A versus 2B |
0.0067* | 0.0103* | ||
*P<0.05. SD: Standard deviation; GI: Gingival index; PI: Plaque index
Table 4.
Comparison of four groups (1A, 1B, 2A, 2B) with respect to bleeding index scores by oneway analysis of variance test and Pairwise comparison by Tukeys multiple post hoc procedures
| Groups | BI (mean±SD) |
|---|---|
| Group 1A | 0.80±0.31 |
| Group 1B | 1.00±0.00 |
| Group 2A | 0.97±0.06 |
| Group 2B | 1.00±0.00 |
| F-test | 5.3210 |
| P | 0.0033* |
| Pair-wise comparison by Tukeys multiple post hoc procedures | |
| 1A versus 1B | 0.2040 |
| 1A versus 2A | 0.0458* |
| 1A versus 2B | 0.0017* |
| 1B versus 2A | 0.9918 |
| 1B versus 2B | 1.0000 |
| 1A versus 2B | 0.9417 |
*P<0.05. SD: Standard deviation; BI: Bleeding index
Table 5.
Comparison of four groups (1A, 1B, 2A, 2B) with respect to clinical attachment level and probing depth scores by oneway analysis of variance test and Pairwise comparison by Tukey’s multiple post hoc procedures
| Groups | Mean±SD | |
|---|---|---|
| CAL scores | PD scores | |
| Group 1A | 1.90±0.61 | 3.26±0.55 |
| Group 1B | 2.28±0.03 | 3.72±0.01 |
| Group 2A | 3.61±0.87 | 3.49±0.71 |
| Group 2B | 5.69±1.34 | 3.76±0.96 |
| F | 27.5763 | 0.8025 |
| P | 0.0000* | 0.4994 |
| Pair-wise comparison by Tukey’s multiple post hoc procedures | ||
| 1A versus 1B | 0.9759 | 0.9016 |
| 1A versus 2A | 0.0251* | 0.9516 |
| 1A versus 2B | 0.0002* | 0.4693 |
| 1B versus 2A | 0.4722 | 0.9849 |
| 1B versus 2B | 0.0014* | 0.9999 |
| 1A versus 2B | 0.0004* | 0.8547 |
*P<0.05. SD: Standard deviation; CAL: Clinical attachment level; PD: Probing depth
Table 6.
Comparison of four groups (1A, 1B, 2A, 2B) with respect to prostate-specific antigen scores by one-way analysis of variance test
| Groups | PSA (mean±SD) |
|---|---|
| Group 1A | 1.75±1.24 |
| Group 1B | 4.23±0.32 |
| Group 2A | 4.73±1.53 |
| Group 2B | 19.46±28.60 |
| F-test | 1.8265 |
| P | 0.1566 |
PSA: Prostate-specific antigen; SD: Standard deviation
The mean PSA in mild prostatitis patients is 3.23 ± 2.04, whereas in moderate-to-severe prostatitis is 18.47 ± 27.887. A significant difference was observed between mild and moderate/severe types of prostatitis with respect to PSA scores (P < 0.05), indicating that PSA scores are significantly higher in moderate-to-severe prostatitis as compared to mild prostatitis [Table 7].
Table 7.
Comparison of types of prostatitis with respect to prostate-specific antigen scores by t-test
| Types of prostatitis | Mean±SD | t | P |
|---|---|---|---|
| Mild | 3.2375±2.0422 | −2.1715 | 0.0352* |
| Moderate/severe | 18.4787±27.8877 |
*P<0.05. SD: Standard deviation
The mean CAL in mild prostatitis patients is 2.75 ± 1.14 whereas in moderate or severe prostatitis is 5.47 ± 1.54. A significant difference was observed between mild and moderate/severe types of prostatitis with respect to CAL scores (P < 0.05) [Table 8]. There is no significant difference while comparing the CAL <3 and ≥3 groups, with respect to PSA scores (P > 0.05) indicating that the PSA scores are similar in <3 CAL and ≥3 CAL groups [Table 9]. Table 10 shows the distribution of samples by type of prostatitis, malignancy, and CAL. The correlation coefficient values obtained among the four groups, i.e., (1A, 1B, 2A, and 2B) were 0.532, 1.00, 0.684, and 0.558 with values of P = 0.175, 0.01, 0.06, and 0.002, respectively. From Table 11, it can be inferred that statistically significant correlation between prostate specific antigen levels and clinical attachment level scores was seen in Group 1B, i.e. CAL <3 with moderate or severe prostatitis, and Group 2 B, i.e. CAL ≥3 mm with moderate or severe prostatitis, when examined among all the four groups.
Table 8.
Comparison of types of prostatitis with respect to clinical attachment level scores by t-test
| Types of prostatitis | Mean±SD | t | P |
|---|---|---|---|
| Mild | 2.7571±1.1439 | −6.1870 | 0.05* |
| Moderate/severe | 5.4705±1.5461 |
*P<0.05. SD: Standard deviation
Table 9.
Comparison of <3 clinical attachment level and ≥3 clinical attachment level groups with respect to prostate-specific antigen scores by t-test
| CAL | Mean±SD | t | P |
|---|---|---|---|
| <3 | 3.2450±1.867 | −1.6942 | 0.0971 |
| ≥3 | 18.2754±27.9676 |
SD: Standard deviation; CAL: Clinical attachment level
Table 10.
Distribution of samples by prostaitis, malignancy, clinical attachment level
| Attribute | Category | n (%) |
|---|---|---|
| Prostatitis | Mild | 16 (34.05) |
| Severe | 31 (65.95) | |
| Malignancy | M | 15 (31.91) |
| NM | 32 (68.09) | |
| CAL | <3 | 10 (21.28) |
| >3 | 37 (78.72) |
CAL: Clinical attachment level
Table 11.
Correlation between prostate-specific antigen levels and clinical attachment level scores across four groups (1A, 1B, 2A, 2B)
| Group | Pearson correlation coefficient value | Significance (P) |
|---|---|---|
| 1A | 0.532 | 0.175 |
| 1B | 1.000 | 0.01* |
| 2A | 0.684 | 0.06 |
| 2B | 0.558 | 0.002* |
*Correlation is statistically significant
A significant and positive correlation (r = 0.5549, P < 0.05) was observed between PSA and CAL scores at 5% level and also between PSA and PD scores at 5% (r = 0.5315, P < 0.05), showing that the PSA and CAL scores, as well as PSA and PD scores, are dependent on each other. Furthermore, a significant and positive correlation was observed between PSA and PI (r = 0.3231, P < 0.05) and GI (r = 0.3567, P < 0.05) scores at 5% level showing that the PSA is also dependent on PI and GI scores. A nonsignificant and positive correlation (r = 0.1331, P > 0.05) was observed between PSA and BI scores at 5% level showing that the PSA and BI scores are not dependent on each other [Table 12].
Table 12.
Relationship between prostate-specific antigen scores with all clinical parameters by Karl Pearson’s correlation coefficient technique
| Parameters | Relationship between PSA scores with | ||
|---|---|---|---|
| Correlation coefficient r (X, Y) | t | P | |
| CAL | 0.5549 | 4.4742 | 0.0001* |
| PD | 0.5315 | 4.2086 | 0.0001* |
| GI | 0.3567 | 2.5612 | 0.0139* |
| PI | 0.3231 | 2.2901 | 0.0268* |
| BI | 0.1331 | 0.9011 | 0.3723 |
*P<0.05. PSA: Prostate-specific antigen; GI: Gingival index; BI: Bleeding index; PI: Plaque index; PD: Probing depth; CAL: Clinical attachment level
Histological examination reveals that mild prostatitis usually appears as a localized process involving a small number of ducts or acini. The lumina are distended and filled with secretion mixed with inflammatory cells among which neutrophils were predominant [Figure 1]. Whereas in severe prostatitis, a localized process involving a large number of ducts or acini are present with the lumina distended and filled with secretion, mixed with large areas of inflammatory cells composed of lymphocytes, plasma cells and giant cells [Figure 2]. Microscopically, prostatic carcinoma composed of small-to-medium-sized glands with irregular outline and inner smooth surface. Individual cells show large, irregular hyperchromatic nuclei, few cells showing nucleoli [Figure 3].
Figure 1.
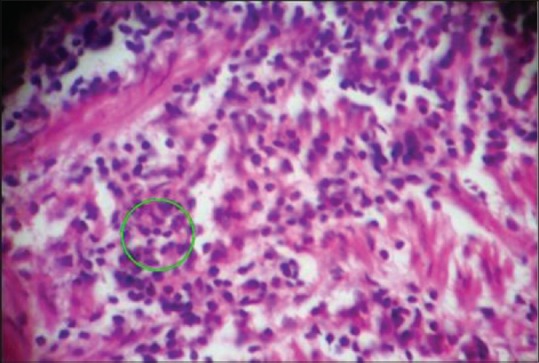
Histological section showing mild prostatitis with inflammatory cells (×100)
Figure 2.
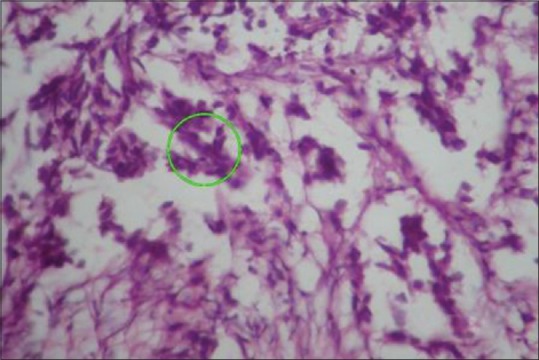
Histological section showing severe prostatitis with inflammatory cells, lymphocytes and plasma cells (×100)
Figure 3.
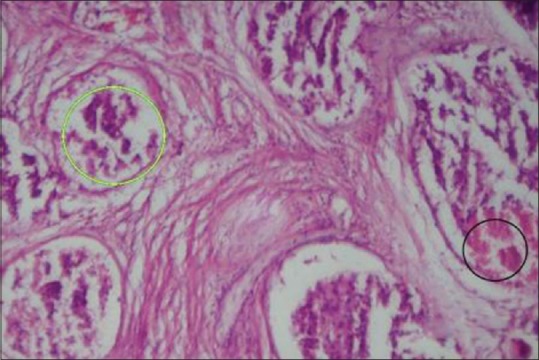
Histological section showing prostate carcinoma with hyperchromatic nuclei and necrosis (×100)
Discussion
The association between periodontitis and prostatitis is yet to be established. The role played by pro-inflammatory cytokines in the pathogenesis of periodontitis and prostatitis has already been recognized. There is substantial evidence to establish that periodontal disease leads to amplified levels of pro-inflammatory cytokines such as IL-1 β, IL-6, and TNF-α which were found to be prominent in the serum of men with prostatitis. Thus, a conclusion can be drawn that periodontitis indirectly contributes to prostatitis by increasing inflammatory response to prostatitis.[7,8] There is another possibility of inflammation of prostate gland resulting in increased PSA levels in the circulation. Periodontium, a distant nonprostatitis source of PSA may also be considered responsible for the increase in serum PSA levels.[6]
Park et al. carried out a study evaluating the optimal baseline PSA level at different ages to estimate the prostate cancer risk (CaP). It was inferred that optimal PSA value that distinguishes the threat of CaP was 2.0 ng/mL for 50- to 69-year-old. Patients having a baseline PSA level higher than the optimal value had a 27.78-fold increase of prostate cancer risk.[9] Retrospective studies by Lee et al. have evaluated the correlation between bone-metastasis serum PSA accumulation in diagnosed prostate cancer (Pca) through a bone scan and concluded that bone scans might be required in men with serum PSA between 10 and 20 ng/mL.[10]
Along with periodontal parameters like CAL, others like the GI and PI values have also been seriously taken into account and these values have also shown a positive correlation with PSA values. Similarly, when gingival, PI scores were compared between the groups, it was raised in 2B group (CAL ≥3 mm with moderate/severe prostatitis) in comparison to other groups, implying that PSA values go up with severe periodontitis and severe prostatitis. Joshi et al. studied the link between periodontal disease and PSA levels in chronic prostatitis patients and concluded that patients having comorbidity of CAL ≥2.7 mm and moderate/severe prostatitis had higher levels of PSA than those with one of these conditions.[6]
The present study was also in confirmatory with the findings of Kandirali et al., who showed, higher PSA levels in patients, suffering from moderate-to-severe prostatitis in comparison with those who suffered from no/mild condition of the same. They also proved the affirmative association between the serum PSA levels and the assertiveness of inflammation of prostate glands, through the histopathological findings from the biopsy.[11]
The rationale for the enhanced volume of serum PSA was related to breach in the epithelial integrity of the cells of prostate glands, rather than increased production of the same as proven by Hasui et al.[12] Wayne Kuzner accomplished that amplified PSA levels in prostatitis patients were due to response to the inflammatory reaction which disrupts glandular epithelium and causes leakage of PSA into the blood stream.[13]
Furthermore, studies by Morote et al. showed that serum PSA levels were directly related to the prostate volume and not the extent or type of inflammatory infiltrate.[14] This study was in unison with Nadler et al. who confirmed that prostate volume and inflammation are the most important factors contributing to serum PSA elevation in men devoid of any clinically diagnosed prostate cancer.[15] Because of this disparity in the increased levels of PSA levels, it was assumed that there could be a distant possible nonprostatitis basis of PSA such as periodontium which is responsible for the increasing serum PSA levels.[14] Diamandis conducted immunological and molecular techniques to demonstrate the presence of PSA protein in various nonprostatic tissues, also its proportionality with steroid hormones and its receptors, and showed that PSA regulation is under the control of steroid hormones and their receptors, thus throwing light on the realization that PSA could also be formed by normal, hyperplastic, and even malignant cells.[16]
The limitations of the study are portrayed by the lack of supportive evidence which fails to provide a causal affiliation to periodontitis and high serum PSA levels, due to the size of the sample and manner of the cross-sectional study, in spite of deducing a favorable association between periodontitis and prostatitis. The prostate needle biopsy sample being non-representative of the pathology of the whole gland was not dependable for a confirmatory diagnosis. Furthermore, an estimation of prostate volume by transrectal ultrasonography was not done in this study. Hence, longitudinal studies with larger sample size are required to establish association between periodontitis and prostatitis. Substantial efforts are underway to improve the reliability of the study by aiming principally at minimizing the false-positive test results, thereby improving the specificity of the test. Alwithanani et al. have conducted a study to assess the changes in serum PSA and inflammatory cytokine levels such as CRP and IL-1 β after nonsurgical periodontal treatment in men with chronic periodontitis, and concluded that the periodontal treatment improved prostate symptom score and lowered PSA value in men afflicted with chronic periodontitis.[17] In the 12 years longitudinal study conducted by Lee et al. in South Korea to assess the relationship between periodontal disease and prostate cancer, using data in the National Health Insurance Service–Health Examinee Cohort, have inferred that periodontal disease is significantly and positively associated with prostate cancer.[18]
Conclusion
Patients with moderate/severe prostatitis and the same grades of the severity of periodontitis were found to have higher PSA levels. The clinical parameters of periodontitis were also found to be significantly increased in patients with moderate-to-severe prostatitis, revealing a probable pathological link between the two.
Financial support and sponsorship
This was a self funded study.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Armitage GC. Research, Science and Therapy Committee of the American Academy of Periodontology. Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–47. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 2.Günhan O, Günhan M, Berker E, Gürgan CA, Yildirim H. Destructive membranous periodontal disease (Ligneous periodontitis) J Periodontol. 1999;70:919–25. doi: 10.1902/jop.1999.70.8.919. [DOI] [PubMed] [Google Scholar]
- 3.Thin RN. The diagnosis of prostatitis: A review. Genitourin Med. 1991;67:279–83. doi: 10.1136/sti.67.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos CG, Carvahal GF, Mager DE, Haberer B, Catalona WJ. The effect of high grade prostatic intraepithelial neoplasia on serum total and percentage of free prostate specific antigen levels. J Urol. 1999;162:1587–90. [PubMed] [Google Scholar]
- 5.Amling CL. Prostate-specific antigen and detection of prostate cancer: What have we learned and what should we recommend for screening? Curr Treat Options Oncol. 2006;7:337–45. doi: 10.1007/s11864-006-0001-1. [DOI] [PubMed] [Google Scholar]
- 6.Joshi N, Bissada NF, Bodner D, Maclennan GT, Narendran S, Jurevic R, et al. Association between periodontal disease and prostate-specific antigen levels in chronic prostatitis patients. J Periodontol. 2010;81:864–9. doi: 10.1902/jop.2010.090646. [DOI] [PubMed] [Google Scholar]
- 7.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E, et al. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–7. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 8.Jang TL, Schaeffer AJ. The role of cytokines in prostatitis. World J Urol. 2003;21:95–9. doi: 10.1007/s00345-003-0335-2. [DOI] [PubMed] [Google Scholar]
- 9.Park KK, Lee SH, Choi YD, Chung BH. Optimal baseline prostate-specific antigen level to distinguish risk of prostate cancer in healthy men between 40 and 69 years of age. J Korean Med Sci. 2012;27:40–5. doi: 10.3346/jkms.2012.27.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SH, Chung MS, Park KK, Yom CD, Lee DH, Chung BH, et al. Is it suitable to eliminate bone scan for prostate cancer patients with PSA≤20 ng/mL? World J Urol. 2012;30:265–9. doi: 10.1007/s00345-011-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandirali E, Boran C, Serin E, Semercioz A, Metin A. Association of extent and aggressiveness of inflammation with serum PSA levels and PSA density in asymptomatic patients. Urology. 2007;70:743–7. doi: 10.1016/j.urology.2007.06.1102. [DOI] [PubMed] [Google Scholar]
- 12.Hasui Y, Marutsuka K, Asada Y, Ide H, Nishi S, Osada Y, et al. Relationship between serum prostate specific antigen and histological prostatitis in patients with benign prostatic hyperplasia. Prostate. 1994;25:91–6. doi: 10.1002/pros.2990250206. [DOI] [PubMed] [Google Scholar]
- 13.Kuznar W. Leak phenomenon explains PSA rise in prostatitis. Urology Times. 1995;23:11. [Google Scholar]
- 14.Morote J, Lopez M, Encabo G, de Torres IM. Effect of inflammation and benign prostatic enlargement on total and percent free serum prostatic specific antigen. Eur Urol. 2000;37:537–40. doi: 10.1159/000020190. [DOI] [PubMed] [Google Scholar]
- 15.Nadler RB, Humphrey PA, Smith DS, Catalona WJ, Ratliff TL. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995;154:407–13. doi: 10.1097/00005392-199508000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Diamandis EP. Prostate specific antigen – New applications in breast and other cancers. Anticancer Res. 1996;16:3983–4. [PubMed] [Google Scholar]
- 17.Alwithanani N, Bissada NF, Joshi N, Bodner D, Demko D, MacLennan GT, et al. Periodontal treatment improves prostate symptoms and lowers serum PSA in men with high PSA and chronic periodontitis. Dentistry. 2015;5:2–4. [Google Scholar]
- 18.Lee JH, Kweon HH, Choi JK, Kim YT, Choi SH. Association between periodontal disease and prostate cancer: Results of a 12-year longitudinal cohort study in South Korea. J Cancer. 2017;8:2959–65. doi: 10.7150/jca.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]


