Abstract
Background and Aims:
A previous meta-analysis reported that scalp block had limited benefits (low-quality evidence) compared to no-scalp block modalities for analgesia after craniotomy. However, it included studies using two different pain intensity measurement scales. Therefore, we performed another meta-analysis using a single scale.
Methods:
We conducted the search for all randomised controlled trials evaluating the effect of scalp block on postcraniotomy pain compared to no-scalp block in Cochrane Central Register of Controlled Trials and PubMed database. We assessed the quality of included studies employing GRADE approach. We performed random-effects inverse-variance weighted meta-analysis of outcomes including pain intensity assessed by a 0--10 visual analog scale and opioid consumption during the first 24 h postoperative period using RevMan 5.3.
Results:
A total of 10 studies (551 patients) were included. It revealed a statistically significant mean pain intensity reduction in scalp block group when compared to no-scalp block at very early and early 24 h period (seven trials, very low-quality evidence, mean difference (MD) = −1.37, 95% confidence interval (CI): −2.23 to -0.05, I2 = 70%; nine trials, very low-quality evidence, MD = −1.16, 95% CI: −2.09 to −0.24, I2 = 57%, respectively). There was also reduction in the opioid requirements over the first 24 h postoperatively.
Conclusion:
Scalp block might be useful at <6 h postcraniotomy with very-low quality evidence. Additionally, it had uncertain but moderate effect on reducing total 24 h opioid consumption. Therefore, more studies are needed to reach optimal information size.
Key words: Analgesia, craniotomy, pain, postsurgery, scalp block
INTRODUCTION
The best practice in pain control after craniotomy is debatable. Scalp block offers an advantage over systemic opioids because it does not obscure the assessment of neurological functions nor mask signs of neurological complications.[1] A previous meta-analysis published in 2013 reported limited benefits with low-quality evidence of scalp block compared to no-scalp block modalities for postcraniotomy analgesia.[2] However, they used mean differences for meta-analysis of studies using different pain intensity measurement scales: numeric rating scale (NRS) and visual analog scale (VAS). The outcomes measured using NRS were usually reported in median and interquartile range. Means and medians can be very different from each other if the data are skewed, and medians are often reported for skewed data. Thus, estimating means and standard deviations from reported medians and interquartile ranges could introduce bias to pooling. Therefore, we performed this meta-analysis of studies that used VAS for documenting pain intensity to evaluate effectiveness of scalp block for analgesia after craniotomy.
METHODS
Study selection
We reported this study in accordance with the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).[3] We selected randomised controlled trials (RCTs) that assessed either pain intensity with VAS or 24 h opioid consumption as primary or secondary outcomes. These studies compared pre- or postoperative scalp block to no-scalp block or placebo in adults undergoing craniotomy. No-scalp block included placebo or any analgesia modalities other than scalp block. We excluded studies that only examined the efficacy of local anaesthetic infiltration along a planned scalp incision (or postoperatively into the wound margin). Any study that had scalp block in all arms (i.e., studying adjuvant for scalp block regimen or comparing different doses or types of local anaesthetic) was also excluded.
We conducted the search using keywords of “scalp block” and “craniotomy” in the CENTRAL (Cochrane Central Register of Controlled Trials) database. The PubMed database was searched using a combination of medical subject headings (MeSH) terms (“craniotomy” or “neurosurgery”) and keyword (“scalp block”). We identified additional records through references of the included articles. We performed searching without language restriction on January 15, 2019. Two investigators (AW and SU) independently screened and assessed titles and abstracts before full-text retrieval. Then, the full papers that potentially met the inclusion/exclusion criteria were reviewed by two authors (AW and SU) for final inclusion.
Data extraction
Two investigators (AW and SU) extracted data, which included author, year of publication, number of patients, postcraniotomy pain treatment modality (timing and the dosage), pain intensity for all time points at which it was measured, total opioid consumption for 24 h, postoperative rescue analgesia. We recorded them using a dedicated data extraction form on an Excel spreadsheet.
The primary outcome was pain intensity assessed via VAS and total opioid consumption within 24 h postoperative period. We performed two comparisons including scalp block vs. no-scalp block and scalp block vs. placebo. We rescaled the interval of 0 to 100 pain intensity of VAS to a standard interval of 0 to 10. Pain intensity measurement was categorised into five periods (very early: ≥0.5 h but <2 h; early: ≥2 h but <6 h; intermediate: ≥6 h but 12 h; late: ≥12 h but <20 h; very late: ≥20 h but ≤24 h. When there were multiple intervention groups, each of all relevant experimental intervention groups of the study or each of all relevant control intervention groups were combined into a single group and the average of their mean and standard deviation were calculated using the formula in [Table 7].7.a of the Cochrane Handbook.[4] We combined the data in a similar manner when there was more than one measured pain intensity of each study in one defined period.
We contacted corresponding authors to obtain any incomplete data. If the results were presented only in graphical form and there was no further information from the corresponding authors, the relevant data were extracted using ImageJ (ImageJ v1.52k January 2019: http://wsr.imagej.net/distros/win/ij152-win-java8.zip). Two independent authors graded each included study for methodological quality by assessing risk of bias using the Cochrane Collaboration Risk of Bias Tool and employed the GRADE approach to assess the overall quality of the evidence.[5,6]
Data analysis
We conducted the meta-analysis using mean difference (MD) for pain intensity, whereas standard mean difference (SMD) was used for 24 h total opioid consumption. SMD was chosen because the specific opioid and route of administration varied among trials, and calculation of morphine equivalents may have introduced bias. We employed a random-effects and inverse-variance weighting using Review Manager (RevMan v5.3 2014). We conducted subgroup analysis based on timing of scalp block performed (pre- or postoperative). We evaluated between and within-study heterogeneity using the I2 statistic.
RESULTS
Search results and description of included trials
We identified a total of 48 studies from the CENTRAL database, 51 studies from PUBMED database, and 7 studies from other sources as shown in Figure 1. A total of 14 duplicated studies and 46 non-RCTs studies were excluded following screening of titles and abstracts. Thirty-three other studies were excluded because of no any pain intensity outcome (13 studies), no suitable nonscalp block in control group (9), no relevant intervention (6), using NRS for the pain intensity measurement (4), and not craniotomy procedure (1 study). A study reporting 24 h opioid consumption without the relevant pain intensity was also included.[7] Later, we excluded three studies because there was not sufficient information in the abstract and no email nor response from the author/contacted corresponding author. However, we did not exclude two other in-abstract studies because they reported sufficient data to allow assessment of outcome.[8,9] Totally, we included only 10 studies (551 patients) in the analysis.
Figure 1.
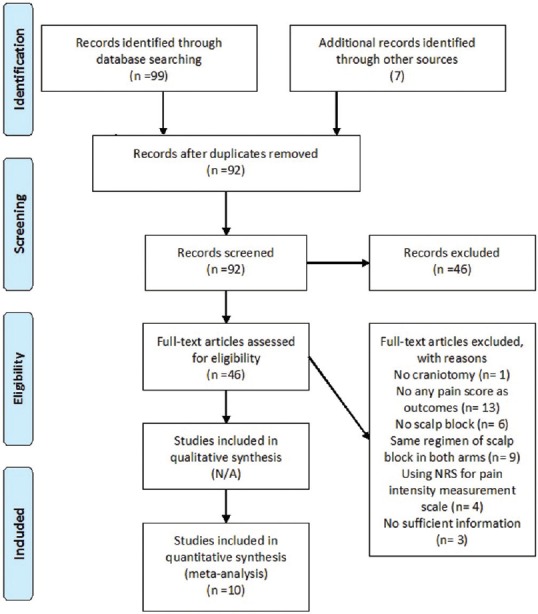
Study flow chart (as per PRISMA guideline)
Details of the included trials are displayed in Table 1. Two of their data[8,10] were extracted using ImageJ and two studies had missing data in some period of measurement.[9,11] Because our attempts to obtain further data from the corresponding authors failed, we performed sensitivity analysis based on removing those studies.
Table 1.
Included trials
| Author | Year | Timing | Scalp block | Volume (ml) | n | Control | n | Pain intensity assessment | Scale | Rescue analgesia | Adverse outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gazoni et al.[13] | 2008 | Preoperative | 0.5% ropivacaine | 30 | 14 | Placebo | 16 | 1, 2, 4 h | VAS 1-10 | Morphine | No report |
| Choi et al.[16] | 2009 | Postoperative | 0.75% ropivacaine | 16 | Placebo | 16 | 0.5, 1, 2, 4, 6, 12, 24, and 48 h | VAS 1-100 | Tramadol | No report | |
| Jayaram et al.[14] | 2016 | Preoperative | 0.5% bupivacaine/2% lidocaine | 30 | 20 | Bilateral maxillary block bupivacaine 0.5%/lidocaine 2% | 20 | 1, 2, 4, 12 h | VAS 1-10 | Diclofenac | None |
| Nguyen et al.[15] | 2001 | Postoperative | 0.75% ropivacaine | 20 | 20 | Placebo | 20 | 4, 8, 12, 16, 20, 24, and 48 h | VAS 1-10 | Codeine | No report |
| Tuchinda et al.[12] | 2010 | Preoperative | 0.25% bupivacaine with 1:200.000 adrenaline | 20 | Placebo | 20 | 0.5, 1, 1.5, 2, 6, 12, and 24 | VAS 1-10 | Morphine PCA | None | |
| 0.5% bupivacaine with 1:200.000 adrenaline | 20 | ||||||||||
| Akcil et al.[10] | 2017 | Preoperative | 0.5% bupivacaine | 20 | 15 | Scalp infiltration 0.5% bupivacaine | 15 | 10 m, 1, 2, 6, 12, and 24 h | VAS 1-10 | Morphine PCA | No report |
| Placebo | 15 | ||||||||||
| Can and Bilgin[11] | 2017 | Preoperative | 0.5% bupivacaine | 20 | 30 | Placebo | 30 | 2, 4, 8, 16, and 24 h | VAS 1-10 | Meperidine | None |
| 0.5% levobupivacaine | 20 | 30 | |||||||||
| Rigamonti et al.[8] | 2013 | Postoperative | 0.5% bupivacaine with 1:200.000 adrenaline | 20 | 44 | Placebo | 45 | 1,2,4,8,12,18,24 | VAS 1-10 | Hydromorphone PCA | No report |
| Dudko et al.[9] | 2014 | Postoperative | 0.25% bupivacaine/1% lidocaine with 1:200.000 adrenaline | 20 | 25 | Systemic paracetamol 1 g/Ketoprofen 2 mg/kg | 25 | 1, 3, 6, and 24 h | VAS 1-100 | Ketorolac and Paracetamol | No report |
| Scalp infiltration 0.25% bupivacaine/1% lidocaine with 1:200.000 adrenaline | 25 | 1, 3, 6, and 24 h | |||||||||
| Ayoub et al.[7] | 2006 | Post-operative | 0.5% bupivacaine/2% lidocaine | 20 | 25 | Systemic morphine 0.1 mg/kg | 25 | 1,2,4,8,12,16, and 24 h | NRS 1-10 | Codeine | No report |
Nine studies[8,9,10,11,12,13,14,15,16] were analysed for pain intensity outcome and seven trials[7,8,10,12,13,15,16] for 24 h total opioid consumption outcome. The largest of the trials involved 90 patients.[11] Six studies included fewer than 50 patients. Four trials had three-arm design.[9,10,11,12] Data from systemic analgesia or scalp infiltration were combined with placebo into single no-scalp block group. However, they were excluded when comparing scalp block to placebo.[9] Data from scalp block using various agents were treated as single treatment group.[11,12] There were various opioids administered postoperatively including morphine, codeine, hydromorphone, and tramadol. Three trials used patient-controlled analgesia (PCA) device,[8,10,12] while the others administered rescue opioid as requested by patient or defined by high VAS.[7,13,15,16]
Methodological quality assessment
Two in-abstract studies were considered as high risk for all aspects of risk of bias assessment because risk of bias could not be ascertained. All trials did not report details of their randomisation strategy. One trial had different numbers of participants in each group suggesting a randomisation bias as shown in Figure 2.[13] Three trials had high risk of adhering-to-intervention bias because of no blinding to the anaesthesiologist who performed the intervention.[13,14,15] Measurement bias was suggested in five trials because there were no blinding to assessor.[11,12,13,15,16]
Figure 2.
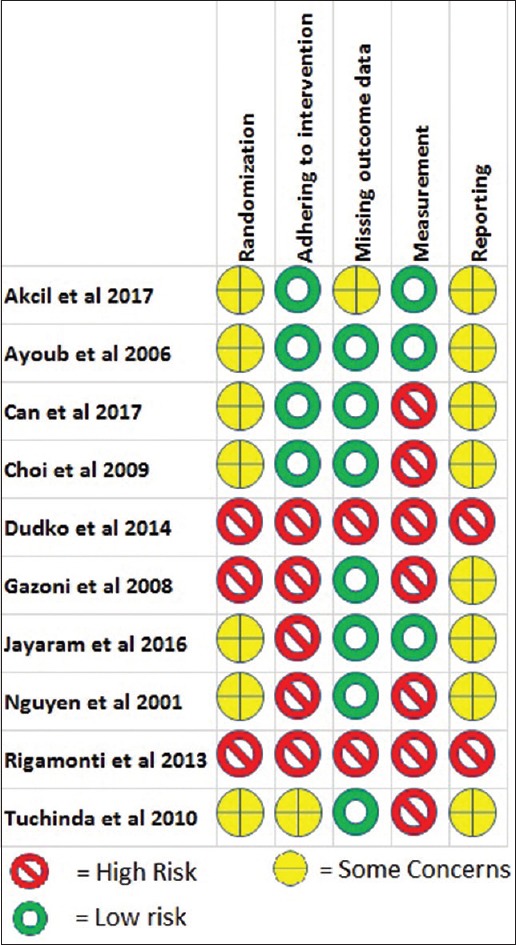
Risk of bias assessment summary
We downgraded a starting rating of 'high-quality' evidence by one level for serious concerns about risk of bias, inconsistency, indirectness, imprecision, or publication bias. We considered there was serious risk of bias in the included trials for every outcome. Outcomes with low heterogeneity (I2< 40%) suggested no inconsistency issue, while outcomes with substantial heterogeneity (I2 >60%) had severe inconsistency issue. We considered that the imprecision was present if the outcomes had less than 400 participants in our meta-analysis.[6] We considered there was a publication bias because our results came from small study populations. Funnel plots also showed an asymmetrical appearance indicating publication bias [Figure 3]. We did not conduct test for funnel plot asymmetry because there were less than ten included studies.[4] We understand that there was indication of publication bias. However, there was no diversity in the comparisons being made by the primary studies. Therefore, we considered that we can derive meaningful conclusions from our meta-analysis. To know the quality of evidence, we performed GRADE evaluation. Based on GRADE evaluation in Table 2, we determined that the evidences for each outcome were at most low quality.
Figure 3.
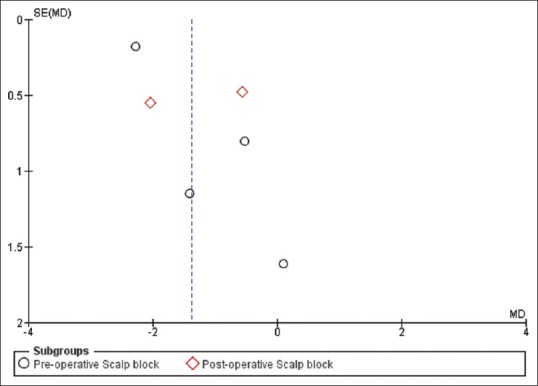
Funnel plot of comparison Scalp block vs. no-scalp block, outcome at very early 24 h period postoperatively
Table 2.
GRADE assessment results
| Comparison | Outcomes | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication of bias | GRADE |
|---|---|---|---|---|---|---|---|
| Vs. no-scalp block | Very Early | Serious | Severe | No at all | No | Presence | Very low |
| Early | Serious | Some | No at all | Some | Presence | Very Low | |
| Intermediate | Serious | Some | No at all | No | Presence | Low | |
| Late | Serious | No | No at all | No | Presence | Low | |
| Very Late | Serious | Some | No at all | No | Presence | Very Low | |
| Vs. placebo | Very Early | Serious | Severe | No at all | No | Presence | Very low |
| Early | Serious | No | No at all | No | Presence | Low | |
| Intermediate | Serious | No | No at all | Some | Presence | Low | |
| Late | Serious | No | No at all | Some | Presence | Low | |
| Very Late | Serious | Some | No at all | Some | Presence | Very low | |
| Vs. no-scalp block | Opioid consumption | Serious | Some | No at all | Some | Presence | Very low |
| Vs. placebo | Opioid consumption | Serious | Severe | No at all | Some | Presence | Very low |
Pain intensity at the very early 24 h period
Random-effects meta-analysis revealed a mean pain intensity reduction in patients receiving scalp block when compared to no-scalp block (seven trials) and placebo (five trials) at the very early 24 h period as shown in Figure 4 (mean difference (MD) = −1.37, 95% confidence interval (CI): −2.23 to −0.05, I2 = 70%, and MD = −1.39, 95% CI: −2.88 to 0.11, I2 = 87%, respectively) as shown in Table 3. We detected substantial statistical heterogeneity. However, when including only the trials with placebo as control, the heterogeneity increased. Sensitivity analysis in group comparison to no-scalp block showed a mean difference of −0.61 with 95% CI −1.34 to 0.13 and I2 = 0%.
Figure 4.
Forest plot of pain intensity outcome
Table 3.
Summary of meta-analysis result
| Vs. no-scalp block | All studies |
I2 | Preoperative |
I2 | Postoperative |
I2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Subgroup | n | Effect size (95% CI) | n | Effect size (95% CI) | n | Effect size (95% CI) | |||
| Pain intensity after surgery | |||||||||
| Very early period | 7 (371) | −1.37 [−2.23, −0.50] | 70% | 4 (175) | −1.40 [−2.59, −0.21] | 57% | 3 (196) | −1.27 [−2.72, 0.17] | 76% |
| Early period | 9 (501) | −1.16 [−2.09, −0.24] | 57% | 5 (265) | −1.11 [−2.36, 0.15] | 63% | 4 (236) | −1.15 [−2.97, 0.66] | 56% |
| Intermediate | 7 (431) | −0.44 [−1.03, 0.15] | 43% | 3 (195) | −0.12 [−0.62, 0.37] | 17% | 4 (236) | −1.03 [−1.77, −0.29] | 0% |
| Late period | 7 (396) | −0.50 [−1.06, 007] | 25% | 4 (235) | −0.43 [−0.71, −0.15] | 0% | 3 (161) | −1.24 [−2.91, 0.43] | 57% |
| Very late period | 7 (431) | −0.54 [−1.37, 0.29] | 77% | 3 (195) | −0.50 [−1.79, 0.78] | 78% | 4 (236) | −0.62 [−1.94, 0.70] | 77% |
| 24 h total opioid consumption | 7 (346) | −0.36 [−0.76, 0.04] | 68% | 3 (135) | −0.61 [−1.24, 0.01] | 65% | 4 (211) | −0.18 [−0.67, 0.31] | 66% |
| Vs. placebo | All studies |
I2 | Preoperative |
I2 | Postoperative |
I2 | |||
| Subgroup | n | Effect size (95% CI) | n | Effect size (95% CI) | n | Effect size (95% CI) | |||
| Pain intensity after surgery | |||||||||
| Very early period | 5 (241) | −1.39 [−2.88, 0.11] | 87% | 3 (120) | −1.73 [−3.37, −0.09] | 76% | 2 (121) | −0.56 [−1.50, 0.38] | NA |
| Early period | 7 (371) | −1.33 [−2.53, −0.13] | 74% | 4 (210) | −1.38 [−3.12, 0.35] | 80% | 3 (161) | −1.15 [−2.97, 0.66] | 56% |
| Intermediate | 6 (341) | −0.09 [−0.61, 0.44] | 14% | 3 (180) | 0.06 [−0.39, 0.51] | 0% | 3 (161) | −0.77 [−1.86, 0.33] | 1% |
| Late period | 6 (341) | −0.53 [−1.23, 0.17] | 39% | 3 (180) | −0.33 [−0.78, 0.12] | 0% | 3 (161) | −1.24 [−2.91, 0.43] | 57% |
| Very late period | 6 (341) | −0.22 [−1.01, 0.56] | 67% | 3 (180) | −0.37 [−1.35, 0.60] | 60% | 3 (161) | −0.19 [−1.47, 1.10] | 67% |
| 24 h total opioid consumption | 6 (281) | −0.53 [−1.07, 0.02] | 78% | 3 (120) | −0.80 [−1.74, 0.14] | 81% | 3 (161) | −0.28 [−0.98, 0.42] | 77% |
Pain intensity at the early 24 h period
At early 24 h period postcraniotomy, scalp block was more effective than no-scalp block (MD of pain intensity score −1.16, 95% CI −2.09 to −0.24, I2 = 57%, nine trials) and placebo (MD of pain intensity score −1.33, 95% CI −2.53 to −0.13, I2 = 74%, nine trials) as shown in Figure 4. The heterogeneity increased even though we included only trials with placebo as control. Sensitivity analysis in group comparison to no-scalp block showed a mean difference of −0.76 with 95% CI −1.64 to 0.13 and I2 = 10%.
Pain intensity at the intermediate 24 h period
Random-effects meta-analysis showed that scalp block reduced mean pain intensity at intermediate 24 h period when compared to no-scalp block (seven trials) and placebo (six trials) as shown in Figure 4 (MD = −0.44, 95% CI: −1.03 to −0.15, I2 = 43%, and MD = −0.09, 95% CI: −0.61 to 0.44, I2 = 14%, respectively). Sensitivity analysis in group comparison to no-scalp block showed a mean difference of −0.27 with 95% CI −1.35 to 0.81 and I2 = 38%.
Pain intensity at the late 24 h period
Patients in scalp block group at late 24 h period reported a pooled lower mean difference as shown in Figure 4 (MD = −0.50, 95% CI: −1.06 to 0.07, I2 = 25%, and MD = −0.54, 95% CI: −1.37 to 0.29, I2 = 77%) in pain intensity than in the no-scalp block group and placebo group, respectively. There was substantial statistical heterogeneity in comparison between scalp block and placebo. Sensitivity analysis in group comparison to no-scalp block showed a mean difference of −0.56 with 95% CI −1.68 to 0.56 and I2 = 44%.
Pain intensity at the very late 24 h period
Scalp block has some effect (MD = −0.54) on reducing pain intensity with 95% confidence of −1.37 to 0.29 compared to no-scalp block and effect of MD −0.22 with 95% confidence of −1.01 to 0.56 compared to placebo at very late 24 h period as shown in Figure 4. There was substantial statistical heterogeneity. Sensitivity analysis in group comparison to no-scalp block showed a mean difference of −0.37 with 95% CI −1.22 to 0.49 and I2 = 12%.
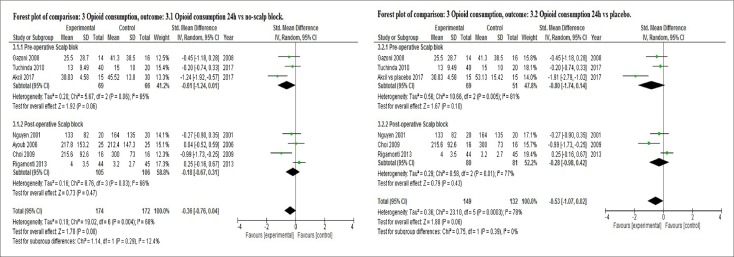
Opioid consumption within 24 h period
Administration of scalp block reduced total opioid consumption within 24 h when compared to no-scalp block and placebo in a random-effects meta-analysis as shown in Figure 5 (SMD = −0.36 with 95% CI −0.76 to 0.04, I2 = 68% and SMD = −0.53 with 95% CI −1.07 to 0.02, I2 = 78%, respectively). However, there were no statistically significant differences. There was evidence of substantial heterogeneity in both comparisons.
Figure 5.
Forest plot of opioid consumption outcome
DISCUSSION
Meta-analysis was performed separately for pain intensity assessed at very early, early, intermediate, late, and very late 24 h postoperative periods. Our analysis favored the scalp block at all periods compared to either no-scalp block or placebo, although we were certain the results only at up to the 6 h postoperative period. However, the results were graded as very low-quality evidence based on GRADE approach. In subgroup analysis, meta-analysis favored the preoperative scalp block group more than the no-scalp block group at the very early and late periods. Postoperative scalp block group had certain pain intensity reduction only at the intermediate period.
There was significant heterogeneity in the treatment effect at all periods except the intermediate and late period assessment. The sensitivity analysis showed improvement in heterogeneity. The sensitivity analysis demonstrated no significant difference in both comparisons at all periods. This suggested that the statistically significant pain intensity reductions of scalp block at up to the 6 h postoperative period could be affected by the decision to include trials which had incomplete data.
A systematic review by Tsaousi et al.[17] put scalp infiltration and scalp block into one category and the conclusion of beneficial scalp infiltration/block in the early postoperative pain management came from only one scalp infiltration trial,[18] not from the two included scalp block trials.[14,19] Another systematic review by Akhigbe and Zolnourian[20] involving five trials[7,12,15,19,21] concluded that scalp block provided analgesia after craniotomy. However, the authors mentioned that the evidence was limited because most data were confounded by weaknesses in methodology and most of the studies have small sample sizes.
Previous meta-analysis combining trials using NRS and VAS reported significant reduction in pain intensity up to 6--8 h postoperatively, however, with high heterogeneity.[2] Our study included only studies measuring pain score in VAS, but not NRS. We excluded trials using NRS because SMD may be used in meta-analysis for combining continuous data only, not ordinal one.[22]
Sise of the effect in pain intensity was around only 1-point. It probably indicates little to no implication in practice. However, scalp block had moderate effect in reducing 24 h total opioid consumption which reflects more a quantitative, not self-reporting outcome.
Included studies are insufficient to address the objective because their quality at most is graded as low. Wide confidence interval in our meta-analysis was due to increased heterogeneity because our analysis used random-effects models. Variation in local anaesthetic or postoperatively opioid management and timing may contribute to the wide CI.
There were some limitations in this meta-analysis. First, we searched studies only in two databases. Second, we included four trials whose data were incomplete. Removal of those studies from further meta-analysis made differences in estimates of effect for pain intensity reduction. This finding may generate for further research.
CONCLUSION
We conclude that the scalp block might be useful at the earliest period (<6 hour postcraniotomy) with very low-quality evidence and substantial statistical heterogeneity from nine trials. In addition, scalp block had uncertain but moderate effect on reducing total 24 h opioid consumption compared to no-scalp block. Therefore, more studies are needed with more participants to reach optimal information size for benefit assessment of scalp block.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Quiney N, Cooper R, Stoneham M. Pain after craniotomy? A time for reappraisal. Br J Neurosurg. 1996;10:295–9. doi: 10.1080/02688699650040179. [DOI] [PubMed] [Google Scholar]
- 2.Guilfoyle MR, Helmy A, Duane D, Hutchinson PJA. Regional scalp block for postcraniotomy analgesia: A systematic review and meta-analysis. Anesth Analg. 2013;116:1093–102. doi: 10.1213/ANE.0b013e3182863c22. [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med. 2009;3:123–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. [cited 2019 Mar 10]. Available from: https://handbook-5-1.cochrane.org/
- 5.Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Methods. Cochrane Database of Systematic Reviews. 2016. p. 10. [Google Scholar]
- 6.Ryan R, Hill S. How to GRADE the quality of the evidence Version 3.0. Cochrane Consumers and Communication Group. 2016. Dec, [[cited 2019 Mar 13]: [about 25 p.]]. Available from: https://cc.cochrane.org/sites/cc.cochrane.org/files/public/uploads/how_to_grade.pdf .
- 7.Ayoub C, Girard F, Boudreault D, Chouinard P, Ruel M, Moumdjian R. A comparison between scalp nerve block and morphine for transitional analgesia after remifentanil-based anesthesia in neurosurgery. Anesth Analg. 2006;103:1237–40. doi: 10.1213/01.ane.0000244319.51957.9f. [DOI] [PubMed] [Google Scholar]
- 8.Rigamonti A, Garavaglia M, Hanlon J, Cusimano MD, Macdonald RL, Hare GM, et al. Scalp nerve blocks for post-operative supra-tentorial craniotomy analgesia. J Neurosurg Anesthesiol. 2013;25:469. [Google Scholar]
- 9.Dudko J, Juske M, Banevicius G. Postoperative pain management after craniotomy: 14AP9-11. Eur J Anaesthesiol. 2014;31:241. [Google Scholar]
- 10.Akcil EF, Dilmen OK, Vehid H, Ibısoglu LS, Tunali Y. Which one is more effective for analgesia in infratentorial craniotomy? The scalp block or local anesthetic infiltration. Clin Neurol Neurosurg. 2017;154:98–103. doi: 10.1016/j.clineuro.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Can BO, Bilgin H. Effects of scalp block with bupivacaine versus levobupivacaine on haemodynamic response to head pinning and comparative efficacies in postoperative analgesia: A randomized controlled trial. J Int Med Res. 2017;45:439–50. doi: 10.1177/0300060516665752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuchinda L, Somboonviboon W, Supbornsug K, Worathongchai S, Limutaitip S. Bupivacaine scalp nerve block: Hemodynamic response during craniotomy, intraoperative and post-operative analgesia. Asian Biomed. 2010;4:243–51. [Google Scholar]
- 13.Gazoni FM, Pouratian N, Nemergut EC. Effect of ropivacaine skull block on perioperative outcomes in patients with supratentorial brain tumors and comparison with remifentanil: A pilot study. J Neurosurg. 2008;109:44–9. doi: 10.3171/JNS/2008/109/7/0044. [DOI] [PubMed] [Google Scholar]
- 14.Jayaram K, Srilata M, Dilipkumar K, Ramachandran G. Regional anesthesia to the scalp for craniotomy: Innovation with innervation. J Neurosurg Anesthesiol. 2017;29:72–3. doi: 10.1097/ANA.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen A, Girard F, Boudreault D, Fugère F, Ruel M, Moumdjian R, et al. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001;93:1272–6. doi: 10.1097/00000539-200111000-00048. [DOI] [PubMed] [Google Scholar]
- 16.Choi EM, Choi SH, Lee NH, Min KT. Effect of scalp nerve blocks on post-craniotomy pain in the patients undergoing craniotomy. Anesth Pain Med. 2009;4:142–5. [Google Scholar]
- 17.Tsaousi GG, Logan SW, Bilotta F. Postoperative pain control following craniotomy: A systematic review of recent clinical literature. Pain Pract. 2017;17:968–81. doi: 10.1111/papr.12548. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Li L, Yu P, Gao T, Liu K. Preemptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine reduces postoperative pain after craniotomy. Acta Neurochir. 2015;157:993–8. doi: 10.1007/s00701-015-2394-8. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JY, Bang JS, Oh CW, Joo JD, Park SJ, Do S, et al. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: A randomized, double-blind, and controlled study. World Neurosurg. 2015;83:108–13. doi: 10.1016/j.wneu.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Akhigbe T, Zolnourian A. Use of regional scalp block for pain management after craniotomy: Review of literature and critical appraisal of evidence. J Clin Neurosci. 2017;45:44–7. doi: 10.1016/j.jocn.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Bala I, Gupta B, Bhardwaj N, Ghai B, Khosla VK. Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesth Intensive Care. 2006;34:224–7. doi: 10.1177/0310057X0603400203. [DOI] [PubMed] [Google Scholar]
- 22.Cummings P. Meta-analysis based on standardized effects is unreliable. Arch Pediatr Adolesc Med. 2004;158:595–7. doi: 10.1001/archpedi.158.6.595. [DOI] [PubMed] [Google Scholar]