Abstract
Intraoperative hypotension (IOH) very commonly accompanies general anaesthesia in patients undergoing major surgical procedures. The development of IOH is unwanted, since it is associated with adverse outcomes such as acute kidney injury and myocardial injury, stroke and mortality. Although the definition of IOH is variable, harm starts to occur below a mean arterial pressure (MAP) threshold of 65 mmHg. The odds of adverse outcome increase for increasing duration and/or magnitude of IOH below this threshold, and even short periods of IOH seem to be associated with adverse outcomes. Therefore, reducing the hypotensive burden by predicting and preventing IOH through proactive appropriate treatment may potentially improve patient outcome. In this review article, we summarise the current state of the prediction of IOH by the use of so-called machine-learning algorithms. Machine-learning algorithms that use high-fidelity data from the arterial pressure waveform, may be used to reveal 'traits' that are unseen by the human eye and are associated with the later development of IOH. These algorithms can use large datasets for 'training', and can subsequently be used by clinicians for haemodynamic monitoring and guiding therapy. A first clinically available application, the hypotension prediction index (HPI), is aimed to predict an impending hypotensive event, and additionally, to guide appropriate treatment by calculated secondary variables to asses preload (dynamic preload variables), contractility (dP/dtmax), and afterload (dynamic arterial elastance, Eadyn). In this narrative review, we summarise the current state of the prediction of hypotension using such novel, automated algorithms and we will highlight HPI and the secondary variables provided to identify the probable origin of the (impending) hypotensive event.
Key words: Blood pressure, hemodynamic monitoring, hypotension prediction index, machine learning, predictive analysis
INTRODUCTION
'Prediction is very difficult, especially about the future' is a very true quote from the Danish physicist Niels Bohr (1885-1962)1. Although Bohr used this statement in the context of quantum physics, it is highly applicable in the complex world of perioperative medicine, where data from different monitoring modalities, besides clinical examination(s) and events, are to be integrated and interpreted by the attending anaesthetist for guiding perioperative therapy, particularly haemodynamic management, in an attempt to ultimately reduce the incidence of major (postoperative) morbidity and mortality.
Intra-operative hypotension (IOH) has been associated with adverse outcomes[1] in terms of kidney[2,3,4,5] and myocardial injury,[3,4,6,7] as well as mortality,[8,9] and probably also stroke.[10,11] Therefore, prevention of IOH may be a key in further improving patient outcome.
Machine-learning algorithms, introduced in recent years also in clinical medicine, allow the analysis of complex data streams in order to associate these data with outcome for its subsequent prediction. The use of such algorithms in perioperative medicine may make the future prediction of unwanted events, such as IOH, somewhat easier.
In this paper, we will discuss the relevance of predicting hypotension and how it may be predicted using machine-learning algorithms.
SEARCH METHODOLOGY
For performing this narrative review on intra-operative hypotension and its prediction, the electronic databases from PubMed and the Web of Science were used as source. Terms included in the search were: ('blood pressure' OR 'hypotension') AND ('prediction' OR 'machine-learning'). The search was performed in August 2019 and was restricted to studies published in English, Dutch and German language between January 2000 and August 2019. Importantly, since this review focuses solely on intra-operative hypotension, studies that did not include this particular instance were rendered non-eligible.
INTRA-OPERATIVE HYPOTENSION: INCIDENCE AND DEFINITION
The development of hypotension (or: intra-operative hypotension, IOH) at some point during or after surgery, is a commonly encountered problem[1] with an incidence estimated between 5-99%,[12,13] depending on the definition of IOH. The extremely varying incidence of IOH is secondary to substantial differences in the definition of IOH; some define IOH based on absolute threshold values of systolic blood pressure (SBP), while others use mean arterial pressure (MAP) instead. Likewise, the threshold value differs markedly between studies (e.g., MAP <65 mmHg versus SBP <100 mmHg). Others use relative decreases in blood pressure values compared to 'baseline' as a threshold for the definition of IOH, instead of using absolute values. Here, baseline is typically defined using either pre-induction blood pressure values or using (single) measurements at the pre-operative ward. Importantly though, these blood pressure values were only weakly correlated with mean daytime blood pressure measured using automated ambulatory monitoring pre-operatively[14] and may therefore not serve as an adequate reference value for defining IOH.
Despite the lack of consensus for the definition of IOH, a MAP <65 mmHg is, at least in terms of population harm, most frequently considered nowadays as appropriate threshold value for defining IOH.[15,16]
The incidence of IOH is probably even underestimated, since in the majority of cases blood pressure is only measured intermittently (every 3-5 min), so that short periods of IOH may go undetected. A recent study showed that the application of continuous non-invasive haemodynamic blood pressure monitoring could nearly halve the amount of intraoperative hypotension.[17]
Furthermore, the problem of hypotension extends to the postoperative period: a recent study in patients after major abdominal surgery, in which blood pressure was measured continuously and non-invasively in the initial 48 hours after surgery, revealed that 24% experienced an episode of MAP <70 mmHg for ≥30 min, 18% had an episode of MAP <65 mmHg for ≥15 min, and that about 50% of these events were undetected by routine vital-sign assessments.[18] Most of these events are preventable, e.g., by continuous ward monitoring, which has led to the concept of 'failure to rescue'.[19]
INTRA-OPERATIVE HYPOTENSION: WHY IS IT A PROBLEM?
In recent years, evidence is growing that the development of IOH is associated with adverse postoperative outcomes in terms of both morbidity and mortality. In a large observational study in 104,401 adult patients undergoing non-cardiac surgery,[8] increased durations of IOH (defined by MAP thresholds between 50 and 80 mmHg) were strongly associated with 30-day mortality. In another large retrospective cohort study in 18,756 patients undergoing non-cardiac surgery,[9] these findings were confirmed, as an increase in 30-day mortality was found for patients with either SBP <70 mmHg, MAP <49 mmHg or DBP <30 mmHg for at least 5 minutes.
Multiple other studies have shown the association between IOH and postoperative morbidity in terms of myocardial[3,4,6,7] and acute kidney injury,[2,3,4,5] and possibly for the occurrence of ischaemic stroke[11] too. Importantly, these studies also demonstrated that even short instances of IOH (in some studies as short as one minute) were associated with adverse outcome. For example in one of these studies,[3] performed in 33,330 patients that underwent non-cardiac surgery, the odds ratio (OR) of postoperative myocardial injury and kidney injury when MAP was lower than 55 mmHg for only 1-5 minutes was still 1.30 (1.06-1.50, 95% confidence interval) and 1.18 (1.06-1.31), respectively. Moreover, even short periods of post-induction hypotension – before surgical incision – have been shown to be associated with postoperative acute kidney injury.[2]
It may be speculated that in patients with chronic hypertension, the attending anaesthesiologist should aim at higher intraoperative values of MAP to prevent the occurrence of adverse postoperative outcome. Unlike in critically ill patients requiring vasopressors during septic shock,[20] this assumption has not been well-funded in the literature concerning perioperative care in patients undergoing non-cardiac surgery and literature on beneficial effects on maintaining blood pressure above individualised is – at least until now – sparse.[21] Moreover, it is suggested that the association between IOH and adverse postoperative outcomes based on either absolute or relative values of MAP is comparable.[4]
In summary, it appears that not only the magnitude of hypotension but also its duration – the combination of which is frequently reflected by the time-weighted average – negatively affects postoperative outcome in perioperative patients. It may, therefore, sound highly reasonable to treat hypotension as early as possible, but it may even be better to prevent it from occurring.
INTRA-OPERATIVE HYPOTENSION: AETIOLOGY AND TREATMENT
Development of IOH may be seen as a symptom rather than a disease itself, and reflects an imbalance in cardiocirculatory regulation. Hence, it may be caused either by (a combination of) a reduction in either cardiac preload or afterload, or by an impairment in cardiac contractility. In Table 1, some typical examples for causes of IOH are given, many of which are associated with anaesthesia and are easily modifiable. Importantly, pressure does not equal flow, but the essence of maintaining an 'adequate' blood pressure is, that it determines the inflow perfusion pressure of most organs. Some of these organs (brain, heart, kidney) adjust vascular tone so that perfusion is maintained (autoregulation), but within certain limits, and in extremis of these limits, inflow pressure of these organs becomes linearly dependent on blood pressure and will thus be critically endangered in case of hypotension. Furthermore, the perfusion of other organs, e.g., those depending on the splanchnic circulation, may already be at risk of hypoperfusion when blood pressure is 'normal'.[22] The exact aetiology of IOH is multifactorial and is based on patient-specific factors and procedure-related features. In a recent study,[23] predictors were defined for the development of post-induction hypotension – i.e., immediately after induction of general anaesthesia – and early IOH within 30 minutes of surgery. A lower pre-induction SBP, older age and emergency surgery were independently associated with both post-induction hypotension and early IOH. In addition, supplementary neuraxial anaesthesia, male sex and ASA physical status IV were additionally associated with early IOH only. Obviously, other factors such as bleeding, (un)clamping of large arteries, may additionally lead to IOH in a later phase during surgery.
Table 1.
Some examples of contributing factors to the development of intra-operative hypotension
| Reduction in preload: Vasodilation/vasoplegia, e.g., induced by anaesthetic agents Hypovolaemia secondary to haemorrhage Venous pooling (e.g., patient positioning) Other causes, e.g., surgical great vein manipulation/compression |
| Reduction in cardiac contractility: Cardiac ischaemia Side-effect of anaesthetics Blunted response to sympathetic activation (e.g., due to bleeding) |
| Reduction in afterload: Anaesthetic agents (± ↓cardiac contractility and heart rate) Neuraxial anaesthesia Other, e.g., induced histamine-release (including anaphylaxis) |
| Other (mixed) causes: Pregnancy Positive pressure mechanical ventilation Pneumothorax Fat/CO2 embolism |
The treatment of IOH is usually reactive, i.e., it starts after hypotension has already ensued. A typical situation in which reactive treatment is initiated after hypotension has already ensued, is given in Figure 1. The treatment of hypotension is highly dependent on its cause. Simply stated, treatment of hypotension is a combination of administering either fluids (mainly optimising preload), vasopressors (mainly optimising afterload), or inotropes (optimising contractility and thus cardiac output), in combination with titrating the level of (general) anaesthesia, and compensating for surgery-related disturbances.
Figure 1.
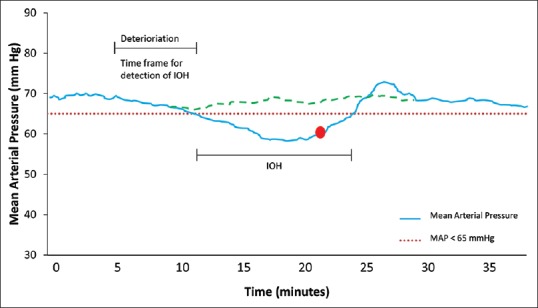
Graph showing changes in mean arterial pressure over time before, during and after the onset of intraoperative hypotension (IOH), when conventional haemodynamic monitoring is applied. Usually, reactive therapy is applied (red dot) after hypotension has occurred. Yet, if hypotension were predicted in the respective timeframe (e.g., by using the hypotension prediction index), it may have been prevented (green dotted line) by proactive treatment
An important consideration in the treatment of hypotension is the methods by which haemodynamics are actually monitored. Blood pressure itself can be monitored either non-invasively using automated cuff-oscillometry, or invasively using an indwelling arterial catheter, depending on the severity of patient co-morbidity and/or the type of surgical procedure performed. Monitoring other haemodynamic variables, e.g., heart rate, cardiac output and dynamic preload variables, may aid in choosing the right treatment modality for IOH.
While current monitoring modalities, whether basic or advanced, may provide in-depth knowledge on the current haemodynamic status of the patient – and will perfectly demonstrate the presence of hypotension – it will not provide a prediction whether or not hypotension is likely to happen while the patient is still haemodynamically stable. This issue was recently demonstrated in 255 patients undergoing major surgery, in which it was shown that conventional haemodynamic variables (e.g., pulse rate, MAP) and more advanced haemodynamic variables such as stroke volume and stroke volume variation, are not suitable to predict the occurrence of IOH.[24]
Given the substantial evidence that even short periods of IOH negatively affects outcome, its prediction and consequently prevention by proactive treatment [Figure 1] may prove beneficial for patients. Here, machine learning algorithms might come into play.
MACHINE LEARNING ALGORITHMS
Current monitoring modalities applied in patients under general anaesthesia undergoing (major) surgery, yield an enormous amount of data streams from different sources, of which continuous electrocardiography signals, invasive ABP monitoring-derived waveforms and (processed) EEG monitoring signals are examples. Many of these data are derived in a high frequency and may show 'subtle' changes, that are hardly visible by the human eye. These subtle signs may nevertheless have a physiologic meaning and relevance. Therefore, the use of 'artificial intelligence' by means of machine learning may be useful if such complex data were associated with clinically relevant outcome variables, both on the short- and long-term.
Machine-learning entails a very different methodology in structuring and programming artificially intelligent algorithms than traditional rule-based programming. In general, in machine learning, an algorithm is developed that uses multiple input variables (features) to associate it with output variable(s). So, the algorithm can learn from observations and improve the recognition of features – and their interrelationship – with the subsequent output variables. Different types of machine learning algorithms exist, of which supervised machine learning may be the most well-known. Here, after a task for the algorithm has been assigned including the definition of both input and output variables, data will be collected upon which the algorithm will be trained and tested, which will be done by splitting the data into a training and validation cohort. In the training cohort, the features, as well as their interaction, will be mathematically coupled to the outcome variable. The most important step in this process is the actual training itself: repetitive testing of the association of features and outcome variable(s) will be done by the algorithm, aimed to optimise the overall model prediction. Finally, the model that has been built will be tested in the validation cohort of the data. Recently, the theoretical concepts on machine learning in medicine have been excellently described elsewhere,[25] just as its application in anaesthetic and critical care practice has been described in detail too.[26,27,28]
PREDICTION OF INTRA-OPERATIVE HYPOTENSION BY MACHINE LEARNING ALGORITHMS
Currently, there are 2 machine learning algorithms developed that are aimed to predict either the occurrence of post-induction hypotension, or the occurrence of intraoperative hypotension.
The first algorithm, published recently,[29] was constructed to predict the development of post-induction hypotension following institution of general anaesthesia. As input variables for the algorithm, a combination of variables, such as patient co-morbidity, pre-operative vital signs and medication(s). A training set of data from 13,323 patients from electronic health records was used, and outcome was defined as a MAP value <55 mmHg within 10 minutes after induction of general anaesthesia. The authors used different machine learning techniques, and the best model was 'tuned' and used for validation. Post-induction hypotension developed in 8.9% of patients. The authors observed substantial differences in performance of these different machine learning techniques and observed that 'gradient boosting' had the highest area under the operating receiver curve (0.76), with associated negative and positive predictive values of 19% and 96%, respectively.
Although this (experimental) algorithm is not publicly available and data was gathered from one institution only, it elegantly demonstrates the feasibility and potential of machine learning algorithms in the perioperative management of patients undergoing surgery. Of note, this study shows some similarity to a recent, non-machine learning based study in patients undergoing spinal anaesthesia for caesarean section, in whom subsequent IOH could be predicted by analysing heart rate variability using dedicated software from the ANIscope™ monitor.[30]
The second algorithm is another good example of the evolution of (supervised) machine learning algorithms in perioperative medicine. The commercially available Hypotension Prediction Index (HPI; Edwards Lifesciences, Irvine, USA) algorithm[31] is a variable that represents a unitless number, ranging from 0 to 100, and indicates the likelihood that hypotension will occur in the next 5–15 minutes while the patient is still haemodynamically stable. The HPI algorithm was based on a supervised machine learning algorithm with the arterial pressure waveform as input and occurrence of hypotension (MAP <65 mmHg) and non-hypotension (MAP >75 mmHg) for at least 1 minute, as output variables. The algorithm has been developed using data from a mixed OR/ICU population (n = 1334 patients),[31] partly from the Multiparameter Intelligent Monitoring in Intensive Care II database and contained a total of 25461 episodes of hypotension. Using this database, the algorithm was set up to detect waveform characteristics that were associated with the defined time phrases in which either hypotension or non-hypotension was present. The algorithm first uses the FloTrac algorithm for the detection of individual pulse waves and elimination of artefacts and splits incoming data in time frames of 20 seconds. Then, the algorithm divides the recognised individual beats in 5 distinct phases and detects multiple characteristics of the pulse wave that will be used as features later on, such as time(s) and amplitude(s) of the waveform morphology, but also other features like baroreflex and variability variables. This way, a total of 3022 individual characteristics of the waveform were identified, of which 51 were selected as 'base features' based on their ability to predict the outcome variables. Data from 5, 10 and 15 minutes before the occurrence of hypotension was used for developing the model. The sensitivity and specificity of predicting hypotension was 92% and 92% at 5 minutes, 89% and 90% at 10 minutes, and 88% and 87% at 15 minutes before the onset of hypotension. In the subsequent external validation cohort (n = 204 patients from the OR, with a total of 1923 episodes of hypotension), sensitivity and specificity were 87%/89%, 84%/84% and 84%/83%, respectively, at 5, 10 and 15 minutes before onset of hypotension.
Hence, it was shown that a (supervised) machine learning, based on a retrospective, offline analysis of complex arterial pressure waveform signals was able to predict occurrence of hypotension. Importantly, the algorithm obviously does not take factors into account such as the administration of relevant anaesthetic drugs and the sometimes (imminent) influence of surgical manipulations. Also, MAP values between 65 mmHg and 75 mmHg were treated as a 'grey zone' and therefore excluded from analysis in order to create a binary model with increased precision.
The clinical performance of the HPI algorithm has been further evaluated in two studies. In one of these studies,[24] the HPI algorithm analysed arterial pressure waveform data from 255 patients offline. This cohort of patients underwent major surgery, and the ability of HPI to predict hypotension (MAP <65 mmHg for >1 min) was compared with that of more conventional haemodynamic variables such as MAP, the change in MAP (ΔMAP) and stroke volume. Interestingly, HPI performed superior at 5, 10 and 15 minutes in comparison to all other variables; the associated values of sensitivity and specificity were 86%/86%, 82%/82% and 81%/81%, respectively. For example, ΔMAP only had sensitivity and specificity values between 55% and 60%.
In another recent study,[32] HPI performance was investigated in patients (n = 23) undergoing vascular or cardiac surgery. The authors determined an optimal threshold value of HPI for predicting hypotension 5–7 minutes prior to the event. It was found that a HPI value of 56 provided the highest cumulative sensitivity and specificity (79% and 63%, respectively). Interestingly, a HPI value of 85 – which is preset by the manufacturer to provide the clinician a warning on the monitoring screen – provided a sensitivity of 62% and specificity of 78%, with only an associated 13% positive predictive value. Hence, the authors suggested to determine HPI values <85 as 'safe', and further suggest that indeed, higher HPI values give a warning of potentially developing hypotension, but might not (yet) require further therapeutic actions.
Nevertheless, these studies demonstrate that the analysis of high-fidelity data such as delivered by an arterial pressure waveform, can be associated with clinically relevant outcome variables (hypotension), and outperform both static (e.g., MAP) and dynamic (e.g., ΔMAP) haemodynamic variables that are usually to be interpreted by the attending clinician. In an ongoing, prospective randomised study,[33] the influence of using HPI in the context of intraoperative haemodynamic management will be assessed in 213 patients undergoing major non-cardiac surgery. This study may show whether HPI monitoring – and acting upon it – actually reduces the duration of IOH.
HPI AND THE CAUSE OF HYPOTENSION
The cause of a hypotensive event may have (one or several) different origins [Table 1], which must be identified in order to deliver the appropriate treatment. Along with the commercially available HPI comes a secondary screen that will help finding the most probable reason underlying the (upcoming) hypotensive event. As stated earlier in this review, the development of IOH should be seen as a symptom rather than as a disease, and requires optimising either preload, afterload or cardiac contractility. Many of these factors can be modified by the attending anaesthetist, yet in order to do this properly, this requires the monitoring of more haemodynamic variables than blood pressure alone. The EV1000 monitor helps in directing treatment by providing a decision tree in case HPI exceeds 85 [Figure 2] to determine (surrogates of) preload, afterload, and cardiac contractility. In case cardiac output (CO) is also low, CO can be optimised either by optimising preload or by increasing cardiac contractility. First, to determine preload, fluid responsiveness is assessed using stroke volume variation (SVV; lower left side of the decision tree, Figure 2), which is a well-established dynamic (preload) variable. In essence, SVV relies on the heart-lung interaction during mechanical ventilation and reflects the ventilation-induced changes in venous return (and thus, stroke volume) over time.[34] If the patient is preload-dependent (and SVV is high, i.e., >12%), the administration of fluids will likely increase CO. Consequently, circulating volume can be optimised, assuming all requirements are met for a valid interpretation of dynamic preload variables, such as sinus rhythm and the application of volume-controlled positive pressure mechanical ventilation with tidal volumes >7 ml kg-1.[35]
Figure 2.
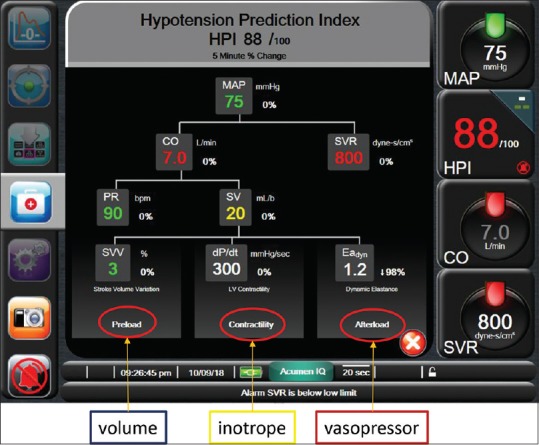
Screenshot of the “secondary screen” that is shown in case HPI exceeds 85. Here, a decision tree is provided in order to treat the underlying cause of (impending) hypotension, either by optimising preload (volume administration), by optimising cardiac contractility (inotropic support) or by optimising afterload (administration of vasopressors). These factors are reflected either by stroke volume variation (SVV), by dP/dtmax, or by dynamic arterial elastastance (Eadyn). Additionally, given is cardiac output (CO), systemic vascular resistance (SVR), pulse rate (PR) and stroke volume (SV)
Another variable that is proposed by the decision tree is dP/dtmax, a variable that is assumed to help in a further optimisation of CO by estimating cardiac contractility (middle lower arm of decision tree, Figure 2). In short, dP/dtmax represents the maximal change in pressure over time in the left ventricle and represents the state of inotropy of the left side of the heart. Unfortunately, measuring dP/dtmax requires left ventricular catheterisation, making it not feasible for daily clinical practice. The arterial pressure waveform, however, also permits measuring dP/dtmax in a minimally invasive fashion, as the 'steepness' of the upslope, systolic part of the pressure waveform was shown to correlate highly with invasive measurement of dP/dtmax, not only in animal studies[36,37] but also in anaesthetised patients undergoing coronary artery bypass surgery.[38] Hence, dP/dtmax may, at least theoretically, help the clinician in guiding the administration of inotropes for the correction of hypotension, mainly by assessing relative changes in dP/dtmax(from individual baseline) as there is no true absolute value that should be targeted. Importantly, further studies are warranted to demonstrate benefits on patient outcome, but simply because most of our anaesthetic drugs have negative inotropic effects, dP/dtmax guided care may prove highly beneficial in further optimising perioperative haemodynamic care.
Finally, afterload should be optimised in case of hypotension, and this is also reflected in the HPI decision tree (right lower corner, Figure 2) using another novel variable, dynamic arterial elastance (Eadyn). Eadyn is constituted from two different dynamic preload variables, i.e., pulse pressure variation (PPV) and SVV, and is calculated by the quotient of the two (i.e., PPV/SVV). While for the prediction of fluid responsiveness these two variables can be used interchangeably,[39] their origin is different. SVV is mainly a flow-based variable, originating from changes in stroke volume, and may thus be considered a 'true' measure of fluid responsiveness. PPV instead, is a pressure-based variable, and originates from changes in pulse pressure, i.e., the differences between systolic and diastolic pressure. The distinction between SVV and PPV is mainly from different effects of aortic compliance on stroke volume and pulse pressure.[40] Therefore, changes in aortic elastance result in differential changes in the ratio between PPV and SVV. Importantly, it is a functional variable, representing the actual position on the pressure-volume curve, not a direct measure of afterload or SVR. In preload-dependent patients,[41] it may predict whether blood pressure will increase along with cardiac output following fluid administration, or it may predict whether vasopressors may be reduced whilst maintaining blood pressure.[39,42] As such, it may guide in functionally assessing afterload and may discriminate between hypotension secondary to either hypovolaemia (Eadyn high, fluids probably beneficial), or hypovolaemia secondary to vasoplegia (Eadyn low), requiring vasopressor(s). Figuratively, we might consider Eadyn the SVV of the pressure world. For this, an 'optimal' cut-off value is around 1.0 with a 'gray zone' of possible inconclusive values between 0.8 and 1.2, for which a decision on whether or not fluid(s) or vasopressor(s) should be given is at the discretion of the attending anaesthetist.[39,43,44,45,46,47]
As with dP/dtmax, the value of Eadyn in optimising patient outcome is to be established and requires further prospective studies in various patient population(s). Nevertheless, the “secondary” screen of HPI, allows the clinician to treat the symptom of (impending) hypotension according to its cause by identifying the probable causes as a prerequisite for optimising the three cardinal features of cardiocirculatory physiology effectively.
Together with advances in other parts of anaesthesia, e.g., the digital data analysis on the recognition of a difficult airway[48] and the prediction of bispectral index during target-controlled propofol-remifentanil anaesthesia,[49] these studies on the prediction of hypotension – and treating it according to its cause – will only the first from many to follow that use novel machine learning approaches to associate clinically relevant outcome with a multitude of complex variables and characteristics of the many data we are faced with in our daily clinical life.
SUMMARY
Intraoperative hypotension is common, and of relevance to the attending anaesthetist given its association with adverse outcome and the possibility to prevent or treat it appropriately. Hypotension is usually a late sign and is preceded by alterations in cardiocirculatory state, that may be used as input for machine-learning algorithms in order to predict the development of hypotension. The hypotension prediction index, which reliably predicts hypotension up to 15 minutes before its actual occurrence, has the potential to change our practice from reactive to proactive blood pressure management [Figure 1]. The secondary screen variables offered with this index may help identifying the probable cause underlying the hypotensive event and finding the appropriate treatment. The value of such novel algorithms in optimising postoperative patient outcome remains to be established.
Financial support and sponsorship
Nil.
Conflicts of interest
TWLS received research grants and honoraria from Edwards Lifesciences (Irvine, CA, USA) and Masimo Inc. (Irvine, CA, USA) for consulting and lecturing and from Pulsion Medical Systems SE (Feldkirchen, Germany) for lecturing.
Footnotes
Of note: although this citation is generally attributed to Niels Bohr, it is also attributed to fellow Danish country-men.
REFERENCES
- 1.Wesselink EM, Kappen TH, Torn HM, Slooter AJ, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br J Anaesth. 2018;121:706–21. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 2.Maheshwari K, Turan A, Mao G, Yang D, Niazi AK, Agarwal D, et al. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: A retrospective cohort analysis. Anaesthesia. 2018;73:1223–8. doi: 10.1111/anae.14416. [DOI] [PubMed] [Google Scholar]
- 3.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 4.Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis. Anesthesiology. 2017;126:47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 5.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–23. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 6.Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44:811–22. doi: 10.1007/s00134-018-5224-7. [DOI] [PubMed] [Google Scholar]
- 7.Hallqvist L, Martensson J, Granath F, Sahlen A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: An observational study. Eur J Anaesthesiol. 2016;33:450–6. doi: 10.1097/EJA.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 8.Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91. doi: 10.1097/ALN.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 9.Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping ST, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–19. doi: 10.1097/ALN.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 10.Nagre AS. Perioperative stroke-prediction, prevention, and protection. Indian J Anaesth. 2018;62:738–42. doi: 10.4103/ija.IJA_292_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bijker JB, Persoon S, Peelen LM, Moons KG, Kalkman CJ, Kappelle LJ, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology. 2012;116:658–64. doi: 10.1097/ALN.0b013e3182472320. [DOI] [PubMed] [Google Scholar]
- 12.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107:213–20. doi: 10.1097/01.anes.0000270724.40897.8e. [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Physicians and the Association of Anaes-thetists of Great Britain and Ireland. National Hip Fracture Database. Anaesthesia Sprint Audit of Practice. [Last accessed on 2019 Jan 06; Updated 2014]. Available from: https://www.nhfd.co.uk/20/hipfractureR.nsf/4e9601565a8ebbaa802579ea0035b25d/f085c664881d370c80257cac00266845/$FILE/onlineASAP.pdf .
- 14.Saugel B, Reese PC, Sessler DI, Burfeindt C, Nicklas JY, Pinnschmidt HO, et al. Automated ambulatory blood pressure measurements and intraoperative hypotension in patients having noncardiac surgery with general anesthesia: A prospective observational study. Anesthesiology. 2019;131:74–83. doi: 10.1097/ALN.0000000000002703. [DOI] [PubMed] [Google Scholar]
- 15.Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44:811–22. doi: 10.1007/s00134-018-5224-7. [DOI] [PubMed] [Google Scholar]
- 16.Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–74. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Maheshwari K, Khanna S, Bajracharya GR, Makarova N, Riter Q, Raza S, et al. A Randomized trial of continuous noninvasive blood pressure monitoring during noncardiac surgery. Anesth Analg. 2018;127:424–31. doi: 10.1213/ANE.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turan A, Chang C, Cohen B, Saasouh W, Essber H, Yang D, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: A prospective blinded observational study. Anesthesiology. 2019;130:550–9. doi: 10.1097/ALN.0000000000002626. [DOI] [PubMed] [Google Scholar]
- 19.Sessler DI, Saugel B. Beyond 'failure to rescue': The time has come for continuous ward monitoring. Br J Anaesth. 2019;122:304–6. doi: 10.1016/j.bja.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–93. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 21.Futier E, Lefrant JY, Guinot PG, Godet T, Lorne E, Cuvillon P, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial. JAMA. 2017;318:1346–57. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boston US, Slater JM, Orszulak TA, Cook DJ. Hierarchy of regional oxygen delivery during cardiopulmonary bypass. Ann Thorac Surg. 2001;71:260–4. doi: 10.1016/s0003-4975(00)01883-x. [DOI] [PubMed] [Google Scholar]
- 23.Sudfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57–64. doi: 10.1093/bja/aex127. [DOI] [PubMed] [Google Scholar]
- 24.Davies SJ, Vistisen ST, Jian Z, Hatib F, Scheeren TW. Ability of an arterial waveform analysis-derived hypotension prediction index to predict future hypotensive events in surgical patients. Anesth Analg. 2019 doi: 10.1213/ANE.0000000000004121. Epub ahead of print. doi: 10.1213/ANE.0000000000004121. [DOI] [PubMed] [Google Scholar]
- 25.Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347–58. doi: 10.1056/NEJMra1814259. [DOI] [PubMed] [Google Scholar]
- 26.Connor CW. Artificial intelligence and machine learning in anesthesiology. Anesthesiology. 2019 doi: 10.1097/ALN.0000000000002694. Epub ahead of print. doi:10.1097/ALN.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vistisen ST, Johnson AE, Scheeren TW. Predicting vital sign deterioration with artificial intelligence or machine learning. J Clin Monit Comput. 2019 doi: 10.1007/s10877-019-00343-7. Epub ahead of print. doi: 10.1007/s10877-019-00343-7. [DOI] [PubMed] [Google Scholar]
- 28.Saugel B, Kouz K, Hoppe P, Maheshwari K, Scheeren TW. Predicting hypotension in perioperative and intensive care medicine. Best Pract Res Clin Anaesth. 2019;33:189–97. doi: 10.1016/j.bpa.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Kendale S, Kulkarni P, Rosenberg AD, Wang J. Supervised machine-learning predictive analytics for prediction of postinduction hypotension. Anesthesiology. 2018;129:675–88. doi: 10.1097/ALN.0000000000002374. [DOI] [PubMed] [Google Scholar]
- 30.Prashanth A, Chakravarthy M, George A, Mayur R, Hosur R, Pargaonkar S. Sympatho-vagal balance, as quantified by ANSindex, predicts post spinal hypotension and vasopressor requirement in parturients undergoing lower segmental cesarean section: A single blinded prospective observational study. J Clin Monit Comput. 2017;31:805–11. doi: 10.1007/s10877-016-9906-9. [DOI] [PubMed] [Google Scholar]
- 31.Hatib F, Jian Z, Buddi S, Lee C, Settels J, Sibert K, et al. Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology. 2018;129:663–74. doi: 10.1097/ALN.0000000000002300. [DOI] [PubMed] [Google Scholar]
- 32.Ranucci M, Barile L, Ambrogi F, Pistuddi V. Surgical and Clinical Outcome Research (SCORE) Group. Discrimination and calibration properties of the hypotension probability indicator during cardiac and vascular surgery. Minerva Anestesiol. 2019;85:724–30. doi: 10.23736/S0375-9393.18.12620-4. [DOI] [PubMed] [Google Scholar]
- 33.Maheshwari K, Shimada T, Fang J, Ince I, Mascha EJ, Turan A, et al. Hypotension prediction index software for management of hypotension during moderate- to high-risk noncardiac surgery: Protocol for a randomized trial. Trials. 2019;20:255. doi: 10.1186/s13063-019-3329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinsky MR. Heart lung interactions during mechanical ventilation. Curr Opin Crit Care. 2012;18:256–60. doi: 10.1097/MCC.0b013e3283532b73. [DOI] [PubMed] [Google Scholar]
- 35.Sondergaard S. Pavane for a pulse pressure variation defunct. Crit Care. 2013;17:327. doi: 10.1186/cc13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morimont P, Lambermont B, Desaive T, Janssen N, Chase G, D'Orio V. Arterial dP/dtmax accurately reflects left ventricular contractility during shock when adequate vascular filling is achieved. BMC Cardiovasc Disord. 2012;12:13. doi: 10.1186/1471-2261-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monge Garcia MI, Jian Z, Settels JJ, Hunley C, Cecconi M, Hatib F, et al. Performance comparison of ventricular and arterial dP/dtmax for assessing left ventricular systolic function during different experimental loading and contractile conditions. Crit Care. 2018;22:325. doi: 10.1186/s13054-018-2260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Hert SG, Robert D, Cromheecke S, Michard F, Nijs J, Rodrigus IE. Evaluation of left ventricular function in anesthetized patients using femoral artery dP/dt(max) J Cardiothorac Vasc Anesth. 2006;20:325–30. doi: 10.1053/j.jvca.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Vos JJ, Kalmar AF, Struys MM, Wietasch JK, Hendriks HG, Scheeren TW. Comparison of arterial pressure and plethysmographic waveform based dynamic preload variables in assessing fluid responsiveness and dynamic arterial tone in patients undergoing major hepatic resection. Br J Anaesth. 2013;110:940–6. doi: 10.1093/bja/aes508. [DOI] [PubMed] [Google Scholar]
- 40.Pinsky MR. Functional haemodynamic monitoring. Curr Opin Crit Care. 2014;20:288–93. doi: 10.1097/MCC.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monge Garcia MI, Saludes Orduna P, Cecconi M. Understanding arterial load. Intensive Care Med. 2016;42:1625–7. doi: 10.1007/s00134-016-4212-z. [DOI] [PubMed] [Google Scholar]
- 42.Bar S, Leviel F, Abou Arab O, Badoux L, Mahjoub Y, Dupont H, et al. Dynamic arterial elastance measured by uncalibrated pulse contour analysis predicts arterial-pressure response to a decrease in norepinephrine. Br J Anaesth. 2018;121:534–40. doi: 10.1016/j.bja.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Cecconi M, Monge Garcia MI, Gracia Romero M, Mellinghoff J, Caliandro F, Grounds RM, et al. The use of pulse pressure variation and stroke volume variation in spontaneously breathing patients to assess dynamic arterial elastance and to predict arterial pressure response to fluid administration. Anesth Analg. 2015;120:76–84. doi: 10.1213/ANE.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 44.Monge Garcia MI, Gil Cano A, Gracia Romero M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care. 2011;15:R15. doi: 10.1186/cc9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia MI, Romero MG, Cano AG, Aya HD, Rhodes A, Grounds RM, et al. Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: A validation study. Crit Care. 2014;18:626. doi: 10.1186/s13054-014-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo H, Kong YG, Jin SJ, Chin JH, Kim HY, Lee YK, et al. Dynamic arterial elastance in predicting arterial pressure increase after fluid challenge during robot-assisted laparoscopic prostatectomy: A prospective observational study. Medicine (Baltimore) 2015;94:e1794. doi: 10.1097/MD.0000000000001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guinot PG, Bernard E, Levrard M, Dupont H, Lorne E. Dynamic arterial elastance predicts mean arterial pressure decrease associated with decreasing norepinephrine dosage in septic shock. Crit Care. 2015;19:14. doi: 10.1186/s13054-014-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connor CW, Segal S. Accurate classification of difficult intubation by computerized facial analysis. Anesth Analg. 2011;112:84–93. doi: 10.1213/ANE.0b013e31820098d6. [DOI] [PubMed] [Google Scholar]
- 49.Lee HC, Ryu HG, Chung EJ, Jung CW. Prediction of bispectral index during target-controlled infusion of propofol and remifentanil: A deep learning approach. Anesthesiology. 2018;128:492–501. doi: 10.1097/ALN.0000000000001892. [DOI] [PubMed] [Google Scholar]


