Abstract
Background and Aims:
The patients undergoing total knee and hip replacement surgeries are mostly obese, more than 50 years of age with osteophytic spine and spine deformities making the blind conventional technique of regional anaesthesia more difficult. The aim of the study was to compare the role of preprocedural ultrasound scan to conventional blind technique in obese patients with osteophytic spines undergoing total knee or hip replacement surgeries in terms of technical difficulty, clinical efficacy, safety and patient comfort.
Methods:
A prospective, randomised controlled trial was conducted in which 210 consenting American Society of Anesthesiologists (ASA) grade III patients, age >50 years, Body Mass Index (BMI) ≥30 kg/m2 with osteophytic spines including abnormalities undergoing joint replacement surgeries were randomised in two groups. Ultrasound group (“B”) received Combined Spinal Epidural Anaesthesia (CSEA) after preprocedural lumbar ultrasound scan. In control group (“A”), CSEA was given by blind conventional technique. The primary objective was to compare the rate of successful epidural block on 1st needle insertion attempts in both the groups. The secondary objectives were to compare both groups in terms of ease, success, comfort and safety of epidural block.
Results:
Ultrasound improved success of CSEA at 1st attempt from 74.3% in control group (“A”) to 85.7% in Ultrasound group (“B”) (P = 0.038). Fewer needle insertion attempts, passes and anaesthesiologist were required in ultrasound group. Pearson correlation coefficient was 0.976 using both views.
Conclusion:
Preprocedural ultrasound scan is a useful adjunct to lumbar epidural blocks in obese patients with osteophytic abnormal spines.
Key words: Obese, preprocedural ultrasound scan, useful adjunct
INTRODUCTION
Joint replacement surgeries of hip and knee joint are one of the common orthopaedic surgeries nowadays.[1] Combined Spinal Epidural Anaesthesia (CSEA) using blind conventional single segment needle through needle technique is the most widely accepted regional anaesthetic technique.[2]
Patients undergoing total hip and knee replacement surgeries are mostly obese, more than fifty years of age, having arthritic joints with osteophytic spine, narrowed intervertebral spaces, indistinct anatomical landmarks and spine deformities. Variable epidural space and suboptimal position adds to the difficult access to epidural space leading to multiple attempts. Failure to identify the epidural space can be hazardous for the patient leading to inadvertent dural puncture and other complications.[3,4] Methods should thus be adopted to reduce the technical difficulty in these patients so as to prevent procedural complications and patient discomfort. Nasser et al. demonstrated the use of preprocedural ultrasound for epidural catheterisation in obstetric population.[5] To the best of our knowledge, utility of preprocedural ultrasound use for epidural catheterisation is not yet demonstrated in patients with osteophytic spines posted for joint replacement surgeries.
The aim of the study was to compare the utility of the preprocedural use of an ultrasound with the conventional blind technique for the epidural neuraxial block in obese patients with osteophytic spines undergoing orthopaedic joint replacement surgeries in terms of technical difficulty, clinical efficacy, safety and patient comfort.
METHODS
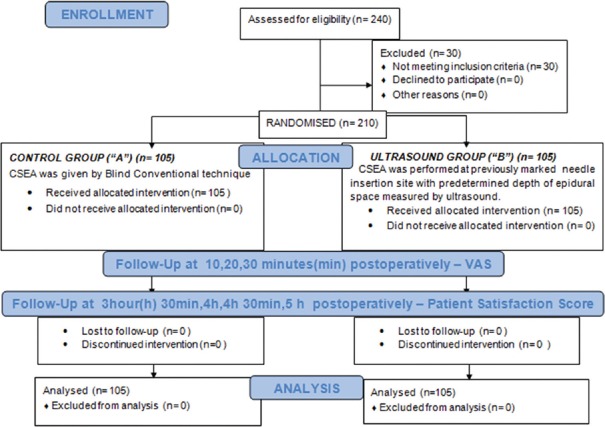
This prospective, randomised controlled study was conducted from August 2016 to March 2017 in tertiary research institute after approval by the Ethical Committee (TS/MSSH/BMDRC/ANESTH/IEC16-02) (14 june 2016) [CTRI/2017/07/009137]. The study was performed according to the principles of the Declaration of Helsinki. Participants were eligible for inclusion if they were more than 50 years of age with Body Mass Index (BMI) ≥30 kg/m2 and American Society of Anesthesiologists (ASA) physical grade I-III undergoing joint replacement surgeries. We prospectively recruited 210 consenting patients as per inclusion criteria after 40 pilot cases. Patients with known hypersensitivity to local anaesthetics or contraindication of neuraxial blockade were excluded from the study. After screening patients for eligibility, they were given an information sheet detailing the purpose, benefits and risks of the study [Figure 1].
Figure 1.
Consort flowchart
After obtaining informed consent, demographic variables including age, gender, weight, BMI of patients were noted. Preoperatively, the ease of palpation of the spine surface landmarks on 4 grade scale (grade 1- easy, grade 2 – moderate, grade 3- difficult and grade 4 – impossible) and lumbar spine X-ray findings (osteophytes, reduced intervertebral spaces and ligament calcification) were also noted for all the patients.
After confirming nil per oral status, patients were taken to operation threatre. In the operation theatre, after securing intravenous line and applying standard ASA monitors, patients were positioned in the sitting arched back position. Then, they were randomised by computer generated random sequence programme at random.org. and allocated by sealed envelopes to either receive preprocedural ultrasound scan (Ultrasound group) (“B”) or not to receive ultrasound scan (Control group) (“A”).
In both the groups (“A” and “B”), under all aseptic precautions, CSEA was given by different anaesthesiologist with experience ranging from 2-30 years using midline approach and needle through needle technique (27/18 G CSEA set).
In Control group (“A”), the epidural component of CSEA was given by conventional blind technique i.e., anatomical surface landmark guided technique. The level of intervertebral spaces was based on the Tuffier's line and confirmation of epidural space was done by loss of resistance (LOR) to air.
In ultrasound group (“B”), detailed preprocedural lumbar ultrasound scan was done using a 2-5 MHz curvilinear ultrasound (FUJIFILM SonoSite, USA) by the principal investigator. The level of intervertebral space and needle insertion site was marked. The depth of epidural space was measured.
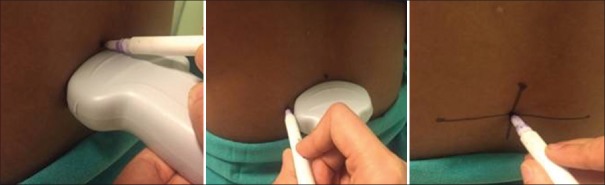
The following are the steps of lumbar ultrasound scan: The intervertebral spaces L4-L5/L3-L4/L2-L3 were marked by “counting up method” along with measurement of depth of epidural space in median longitudinal view. Then, the transducer was moved 90° to obtain the transverse position. The probe was inclined slightly in a cephalad or a caudal direction to obtain a better image of the epidural space. The best intervertebral space was identified based on visualisation of anterior and posterior complexes and marked with skin marker. Then, after freezing the image, the depth of the epidural space was measured with the help of an electronic caliper of ultrasound machine from the skin point to the midpoint surface of the posterior complex seen. With the transducer on the same position, the midpoint of horizontal i.e upper and lower surfaces and lateral i.e. left and right surfaces of the probe were marked by a skin marker. Two lines were drawn joining the respective marks. The needle insertion site was determined by the intersection of both the lines [Figure 2].
Figure 2.
Marking of skin to determine the needle insertion site with help of preprocedural lumbar ultrasound scan
In Ultrasound group (“B”), the epidural component of CSEA was performed at previously marked needle insertion site with a predetermined depth of epidural space measured by ultrasound. To confirm the epidural space, LOR to air was started at 0.5 cm less than the predetermined epidural depth till the epidural space was encountered. The needle was marked close to the skin with the marker to measure the depth of epidural space.
In both the groups (“A” and “B”), the spinal anaesthesia was given through Tuohy's needle using the spinal needle of the CSEA 27/18 G set and 2.5 ml (12.5 mg) of 0.5% Bupivacaine (heavy) was administered. The epidural catheter was inserted in the epidural space and aspirated for blood or cerebrospinal fluid. In case of negative aspiration, the epidural catheter was flushed with normal saline and fixed at a depth equal to the depth of epidural space plus 5 cm. In case of positive aspiration, the catheter was repositioned or the procedure was performed again.
The operator was given the discretion to use a 16 gauge Tuohy's needle for the initial attempt, to make subsequent changes in the needle, length of needle or to attempt a different lumbar intervertebral space, if deemed necessary. If epidural anaesthesia was unsuccessful after two or more needle insertion attempts, the operator was allowed to use other means to locate a lumbar interlaminar space, including a para median needle approach or a second anaesthesiologist was called in.
In both the groups (“A” and “B”), intraoperatively, after negative test dose, infusion Bupivacaine 0.5% via the epidural catheter at the rate of 5 ml/hour-8 ml/hour was initiated after 90 minutes of initiation of spinal anaesthesia and was stopped with the completion of the surgery. During the postoperative period, when the patient moved his/her toes, epidural analgesia was started with infusion Bupivacaine 0.125% with 2 mcg/ml of Fentanyl at the rate of 5 ml/hour-8 ml/hour.
Technical difficulty was measured by the number of puncture attempts required for successful catheterisation as compared to the time taken for the procedure. Multiple attempts is an independent predictor of procedural complications compared to time taken for the procedure which is heterogenous.[6] In both the groups (“A” and “B”), number of puncture attempts, passes and number of anaesthesiologist required for successful catheterisation were noted. The number of puncture attempts was defined as needle insertion preceded by complete withdrawal of the Tuohy's needle from the patient's skin and puncture passes was defined either as needle insertion or redirection attempt. A needle redirection attempt was defined as any change in needle insertion trajectory that did not involve complete withdrawal of the needle from the patient's skin. In Ultrasound group (“B”), epidural depth measured by ultrasound and LOR technique were also noted.
The pain was assessed by the anaesthesiologist blinded to group allocation using 11 point Visual Analogue Scale (VAS) after 10, 20 and 30 minutes of initiation of epidural catheter infusion postoperatively. Patient satisfaction score was noticed after 3 h 30 min, 4 h, 4 h 30 min and 5 h from insertion of the epidural catheterisation on a 5-point scale (5- excellent, 4 – very good, 3- good, 2- fair, 1- poor) so that till then spinal anaesthesia effect weans off.[7] To maintain uniformity, efficacy of the epidural component of CSEA was determined by pain relief after 30 minutes of initiation of epidural infusion postoperatively and patient satisfaction after 5 hours of epidural catheterisation.
Complications like inadvertent dural puncture by Tuohy's needle, multiple needle attempts and failed block were recorded in both the groups. A failed epidural block was defined as a block providing inadequate analgesia (VAS ≥4) despite two boluses of 10 ml of 0.5% Bupivacaine at 15 minutes interval and one bolus of 5 ml of 2% lignocaine. Multiple needle attempts and passes were defined as needle insertion attempts and passes exceeding two and four respectively for the successful catheterisation.
In both the groups, the primary outcome of interest was rate of successful epidural block on the 1st needle insertion attempt. The secondary outcomes included number of needle insertion attempts, the needle passes required for successful epidural block, correlation of the depth of the epidural space measured with an Ultrasound and LOR technique, efficacy of epidural component of CSEA measured by VAS, patient satisfaction score and safety in terms of number of complications like inadvertent dural puncture by Tuohy's needle and failed block.
Taking number of insertion attempts in the ultrasound group and control group as primary outcome from the study by MC Vallejo et al. and power of 80% at 5% significance level with mean difference of 0.5, the sample size came to 98 in each group.[8] It was therefore proposed to cover at least 100 cases in each group during the study period so that this power was achieved.
Statistical testing was conducted with the Statistical Package for the Social Science (SPSS software Version 20.0). Quantitative and qualitative variables were presented as mean ± SD, frequencies and percentages respectively. Statistical testing for qualitative and quantitative data was done by chi square test and student t test, respectively. In data where the cell frequency was extremely small (less than 5) like in a failed epidural block, Fisher exact test was used. Bland–Altman method analysis was done to find the agreement between the depth of epidural space measured by ultrasound and LOR technique. In addition, we estimated the 95% limit of agreement for the differences, which represent differences likely to arise between the two measures with a 95% probability. The Pearson's correlation coefficient was also used to determine the degree of agreement between epidural depth measured by Ultrasound and LOR technique. For all the statistical tests, the 2 tailed P value was calculated and P value less than 0.05 was considered as significant difference.
RESULTS
210 patients were randomised in two groups. Both the groups were comparable in physical characteristics [Table 1]. They were compared in terms of technical difficulty, efficacy, patient comfort and safety [Table 2 and Figure 3]. Preprocedural use of ultrasound increased the success rate of CSEA at first attempt from 74.28% in group “A” to 85.71% in group “B”. (P value - 0.034) Fewer needle attempts (P value - 0.013) and passes (0.022) were required in group “B” as compared to group “A” for placement of epidural catheter. In ultrasound group, successful needle insertions at predetermined puncture site identified using ultrasound scan was in 97.14% patients. Ultrasound precisely determined the depth of epidural space using two views (P-0.66). Bland altman analysis revealed mean difference of 0.007 cm [-0.044,0.030]. Using both views, Pearson coefficient increased from 88.9% in median longitudinal view or 94.9% in transverse view to 97.6% in both views [Figure 4].
Table 1.
Physical characterstics of patients in both groups
| Characteristics | Group A (n - 105) | Group B (n - 105) | P |
|---|---|---|---|
| Age (years) | 66.3 (±7.8) | 64.7 (±7.5) | 0.147 |
| Height (cm) | 154.7 (±8.0) | 154.4 (±7.6) | 0.771 |
| Weight (kg) | 77.9 (±8.4) | 78.4 (±9.7) | 0.698 |
| BMI (kg/m2) | 32.5 (±2.4) | 32.9 (±2.3) | 0.246 |
| Spine deformities | 20 (19.1%) | 21 (20%) | |
| Scoliosis | 17 (16.1%) | 17 (16.1%) | 0.627 |
| Kyphosis | 1 (1%) | 1 (1%) | |
| Spine surgery | 1 (1%) | 0 (0%) | |
| Lordosis | 1 (1%) | 3 (2.9%) | |
| Palpation of anatomical landmarks | |||
| Easy | 10 (9.5%) | 4 (3.8%) | 0.100 |
| Moderate | 68 (64.8%) | 62 (59.1%) | |
| Difficult | 27 (25.7%) | 39 (37.1%) | |
| X Ray findings | |||
| All 3 | 30 (28.6%) | 32 (30.5%) | 0.786 |
| 2 | 61 (58.1%) | 60 (57.1%) | |
| 1 | 14 (13.3%) | 13 (12.4%) |
Table 2.
Comparison of both groups in terms of technical difficulty, efficacy, safety and comfort
| Outcome | Group A | Group B | P | |
|---|---|---|---|---|
| Attempts | Mean | 1.5±0.9 | 1.2±0.6 | 0.013 |
| 1st attempt | 78 (74.3%) | 90 (85.7%) | 0.038 | |
| More than one attempts | 2 attempts | 10 (9.5%) | 08 (7.6%) | 0.013 |
| 3 attempts | 11 (10.5%) | 06 (5.7%) | 0.030 | |
| More than 3 attempts | 06 (5.7%) | 1 (1%) | ||
| Passes | Mean | 2.2±1.7 | 1.8±1.2 | 0.022 |
| 1st pass | 52 (49.52%) | 64 (60.9%) | 0.095 | |
| More than one pass | 2ND pass | 22 (20.9%) | 19 (18.1%) | 0.022 |
| 3RD pass | 10 (9.5%) | 12 (11.4%) | ||
| 4TH pass | 7 (6.7%) | 5 (4.8%) | ||
| >4TH pass | 14 (13.3%) | 5 (4.8%) | 0.030 | |
| Experience of anaesthesiologist | Mean | 13.7±6.1 | 13.2±7.2 | 0.660 |
| 2-5 years | 15 (14.3%) | 26 (24.8%) | ||
| 6-10 years | 4 (3.8%) | 8 (7.6%) | ||
| 11-15 years | 52 (49.5%) | 34 (32.4%) | ||
| 16-20 years | 21 (20%) | 21 (20%) | ||
| 21-30 years | 13 (12.4%) | 16 (15.2%) | ||
| No of anaesthesiologist | Mean | 1.1±0.4 | 1.0±0.2 | 0.044 |
| One | 96 (91.4%) | 102 (97.1%) | ||
| More than 1 | 9 (8.6%) | 3 (2.9%) | ||
| Pain scores | Patient satisfaction score | 4.1±0.8 | 4.4±0.6 | 0.019 |
| Vas score | 1.0±0.1 | 1.0±0.1 | 0.157 | |
| Complications | Total | 8 (7.6%) | 0 | 0.003 |
| Failed block | 2 (1.9%) | 0 | 0.498 | |
| Accidental dural puncture | 6 (5.7%) | 0 | 0.013 |
Group A - Control group , Group B – Ultrasound group
Figure 3.
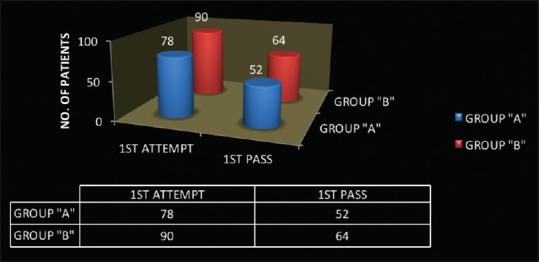
Success of Combined Spinal Epidural Anaesthesia at 1st attempt and pass in both the groups
Figure 4.
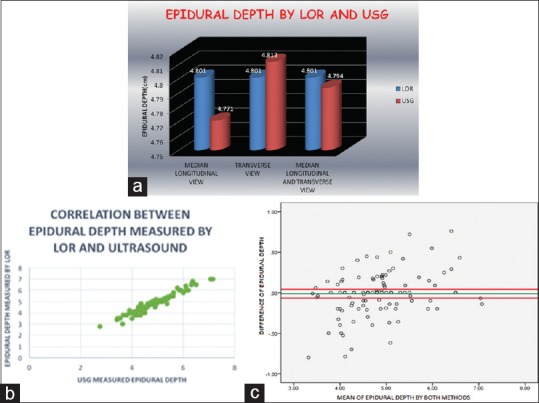
(a) Comparison of epidural depth measured by ultrasound and LOR technique using different views of ultrasound. (b). Correlation of epidural depth measured by LOR and ultrasound. (c). Bland Altman Analysis Epidural depth by both methods had mean difference of -0.007 cm ranging from -0.044 cm and 0.030 cm within 95% confidence limits i.e the differences can lie between the two measurement with 95% probability
In group “A”, 8 patients (7.61%) of 105 patients had procedural complications as compared to zero patients in group “B”. (P value – 0.003) In group “A”, 6 patients (5.71%) (P value – 0.013) had accidental dural puncture and 2 patients (1.9%) (P value – 0.498) had failed epidural block as compared to group “B”.
Patient satisfaction score after 5 hours of epidural catheter insertion was significantly higher in group “B”. (P value – 0.019) Although there was no significant difference in VAS score measured after 30 minutes of initiation of epidural infusion postoperatively in both the groups (P value – 0.157) except two patients (1.90%) had VAS >5 in group “A”.
DISCUSSION
For decades, anaesthesiologists have striven to perfect identification and cannulation of the epidural space using skills learned, i.e. knowledge of the relevant anatomy and detection of tactile clues during training and early clinical practice. This randomised controlled trial was done to compare CSEA after preprocedural ultrasound scan or by blind conventional technique. It showed statistically significant improvement in the first attempt success rate of epidural catheterisation in ultrasound group (“B”) to 85.7% as compared to 74.3% in control group (“A”) (P – 0.038) and other studies.[5,9,10,11,12]
Similar to Nasser et al., Vallejo et al., Grau et al. and others, preprocedural use of ultrasound scan decreased the technical difficulty and increased the ease of performance in patients with difficult and osteophytic spine as compared to blind conventional technique.[5,8,9,10,11,12,13,14,15,16,17]
Similar to Grau et al., preprocedural use of ultrasound, also significantly reduced the total number of passes (P-0.022) and attempts (P-0.013) in the ultrasound group (“B”) [Table 2].[13,14] The discrepancy in result of success rate at 1st pass (P- 0.095) as compared to others could be due to the fact that the insertion angle of the needle was not measured in our study.[9,10,15]
In our study, the successful insertion rate at a predetermined puncture site by ultrasound scan was in 97.1% cases whereas in 3 cases (2.9%), a second anaesthesiologist with more experience had to intervene. This is in contrast to Wang Q et al. and Pablo et al. with 100% successful insertion rate at a predetermined puncture site.[12,16] This can occur because of inaccuracy due to change of position at the time of skin markings by ultrasound and needle placement. Other causes include patient profile, misidentification of midline, movement of the skin and subcutaneous tissue during probe placement due to loose and elastic skin of the patients. Therefore, meticulous care during skin markings with the help of an ultrasound is important as it can otherwise, lead to multiple attempts and an unsuccessful block.
Our study also demonstrated that the preprocedural use of ultrasound significantly decreases the multiple attempts (P – 0.030) and number of anaesthesiologist (P – 0.044) required in obese patients with osteophytic spine. Hence, preprocedural ultrasound use can decrease the learning curve of residents and improves patient safety as demonstrated by Vallejo and Grau et al.[8,18]
Use of preprocedural lumbar ultrasound scan improved the efficacy, patient comfort and safety of CSEA significantly by higher patient satisfaction score (P–0.019) and decreased procedural complications (P- 0.003) like inadvertent dural puncture (0.013) and failed epidural block (P-0.498) respectively.
Previous evidence has suggested that ultrasound can accurately measure the depth of the epidural space prior to the procedure.[8,10,15,19,20] This is of valuable importance as this helps in identification of epidural space, selection of needle of appropriate length and may prevent inadvertent dural puncture [Figure 5].
Figure 5.
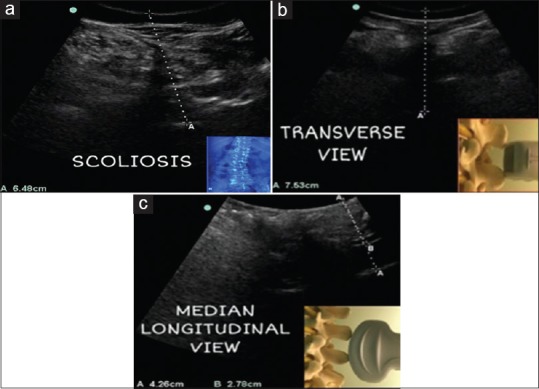
Case 1 (a): Scoliosis, the posterior complex is deviated from midline depicting rotated vertebrae in ultrasound transverse view Case 2,3 (b,c): Variability in epidural depth irrespective of obesity. Use of ultrasound in measuring epidural depth in both transverse and median longitudinal view and preventing accidental dural puncture in case 3 (c) as epidural depth was only 2.7 cm
In our study, the epidural depth was measured from skin to the midpoint of the posterior complex in both median longitudinal and transverse view. This is in contrast to others who either measured the distance from skin to ventral or dorsal surface of ligamentum – dura complex in transverse view or lamina in sagittal view or both.[8,10,15,19,20] Ultrasound precisely determined the depth of the epidural space with mean difference of 0.007 cm (Bland altman analysis) using both views (P – 0.66) [Figure 4].
Slight difference in epidural depth by both methods could be due to different trajectories of the ultrasound beam while marking the needle insertion and Tuohy's needle while its insertion or tissue compression either by probe compression during ultrasound scanning leading to 5 mm change in depth or by Tuohy's needle during its insertion.
The estimation of epidural depth measured by preprocedural ultrasound scan, also increases safety outcomes by decreasing procedural complication like accidental dural puncture (P-0.013). Our results are comparable with previous evidence presented on safety outcomes of ultrasound in Nassar et al. and NICE UK guidelines.[5,21] In our study, the incidence of accidental dural puncture (5.7%) in these patients with blind conventional technique was also comparable with the incidence reported in obstetric population.[3]
Ultrasound is of great help in neuraxial procedures, especially in patients with challenging anatomy [Figure 5]. In scoliosis, it helps to determine the most neutral space and its orientation. It also helps to determine the midline by identifying spinous processes in median longitudinal view and needle insertion site. Ultrasound has been shown to be more accurate in the identification of intervertebral level than clinical assessment.[22,23,24] In our study, we identified the intervertebral level by counting the spinous processes upward from sacrum in the median longitudinal view in ultrasound group. This is in contrast to counting up method of lamina in Para Saggital Oblique view as described by Ki chin et al. and Srinivasan et al.[19,25] To reduce the errors due to misidentification of lumbosacral junction, “counting down method” was also used.[19]
To the best of our knowledge, this is the first randomised controlled study to compare preprocedural ultrasound scan to blind conventional technique for epidural catheterisation in the patients with osteophytic spine undergoing joint replacement surgeries.
Ultrasound is a noninvasive, safe, easily accessible and portable machine. It can unblind the spinal structures and give crucial information on the structure of the spine in different planes. Although, detailed training, efficiency and technical difficulty limit its use for neuraxial blockade.[26] Therefore, educational and learning strategies should be followed to attain this useful skill. In our study, before enrolling the subjects, 40 pilot cases were done after reviewing reference articles and hands on practice on human volunteers as suggested by Halpern et al. and Margarido et al.[27,28] Ghosh et al. has recommended learning strategies for neuraxial ultrasound scan.[29]
Limitation of the study includes non-blinding of the subjects and observer as it was infeasible. The power of the study and the magnitude of observed difference will eliminate the risk of therapeutic personality bias or expectation bias. Other parameters like trajectory of the probe used for guiding trajectory of the needle while insertion, time taken for the duration of the procedure and efficacy of different USG view and CSEA approaches were also not studied.
CONCLUSION
Use of preprocedural ultrasound scan significantly increases the success rate of epidural cathterisation at first attempt in patients with difficult spine.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I deeply appreciate the help and cooperation of O.T staff and technicians including Mr. G.G. Sharma, Mr. Ratan Bisht, Mr. Naresh Kumar, Mr Vikas Deepak, Mr Vikas, Mr Bheem, Mr Himanshu, Mr Vikas Arora, Mr Manoj and Mr Shafiq.
Presentation: The preliminary data for this study was presented as a poster presentation at the Euroanaesthesia meeting, 1-4 June 2018, Copenhagen.
REFERENCES
- 1.Frost & Sullivan Research Service. Overview of Orthopedic Joint Replacement Market in India [Internet] 2011. [Last accessed on 2018 may 10]. Available from: https://www.frost.com/prod/servlet/report-brochure.pag?id=P54D-01-00-00-00 .
- 2.Kar-binh Ong BA, Sashidharan R. Combined spinal-epidural techniques. Contin Educ Anaesth Crit Care Pain. 2007;7:38–41. [Google Scholar]
- 3.Wenk M, Gurlit S, Pöpping DM, Möllmann M. Teaching epidural insertion: A modified approach to combined spinal–epidural anaesthesia. Br J Anaesth. 2011;106:420–1. doi: 10.1093/bja/aer021. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Kishore K. Complications and controversies of regional anaesthesia: A Review. Indian J Anaesth. 2009;53:543–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Nassar M, Abdelazim IA. Pre- puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29:573–7. doi: 10.1007/s10877-014-9634-y. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh F, Brzezinski J, Alexander S, Arzola C, Carvalho JC, Jose CA, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: Systemic review and meta analysis. BMJ. 2013;346:172. doi: 10.1136/bmj.f1720. [DOI] [PubMed] [Google Scholar]
- 7.Otani K, Waterman B, Faulkner KM, Boslaugh S, Burroughs TE, Dunagan WC. Patient satisfaction: Focusing on “Excellent”. J Healthc Manag. 2009;54:93–102. [PubMed] [Google Scholar]
- 8.Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19:373–8. doi: 10.1016/j.ijoa.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anest Pain Med. 2001;26:64–7. doi: 10.1053/rapm.2001.19633. [DOI] [PubMed] [Google Scholar]
- 10.Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21:25–31. doi: 10.1017/s026502150400105x. [DOI] [PubMed] [Google Scholar]
- 11.Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: The correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108:1876–81. doi: 10.1213/ane.0b013e3181a323f6. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal – epidural puncture in obese parturients. Chin Med J (Engl) 2012;125:3840–3. [PubMed] [Google Scholar]
- 13.Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45:766–71. doi: 10.1034/j.1399-6576.2001.045006766.x. [DOI] [PubMed] [Google Scholar]
- 14.Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14:169–75. doi: 10.1016/s0952-8180(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 15.Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104:1188–92. doi: 10.1213/01.ane.0000250912.66057.41. [DOI] [PubMed] [Google Scholar]
- 16.Helavel PE, Conceiçao DB, Swarovsky C, Meurer G, de Oliveira Filho GM. Evaluating the depth of the epidural space with the use of ultrasound. Rev Bras Anestesiol. 2010;60:376–82. doi: 10.1016/S0034-7094(10)70046-5. [DOI] [PubMed] [Google Scholar]
- 17.Jain K, Jaiswal V, Puri A. Lumbar ultrasound scan : A guide to the epidural space. J Anaesthesiol Clin Pharmacol. 2019;35 doi: 10.4103/joacp.JOACP_293_18. Doi: 10.4103/joacp.JOACP_293_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grau T, Bartusseck E, Conradi R, Martin E, Motsch J. Ultrasound imaging improves learning curves in obstetric epidural anesthesia: A preliminary study. Can J Anaesth. 2003;50:1047–50. doi: 10.1007/BF03018371. [DOI] [PubMed] [Google Scholar]
- 19.Chin KJ, Karmakar MK, Peng P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology. 2011;114:1459–85. doi: 10.1097/ALN.0b013e318210f9f8. [DOI] [PubMed] [Google Scholar]
- 20.Sahota J, Carvalho J, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient. Anesth Analg. 2013;116:829–35. doi: 10.1213/ANE.0b013e31827f55f0. [DOI] [PubMed] [Google Scholar]
- 21.Ultrasound-guided Catheterisation of the Epidural Space. London: 2008. [Last accessed on 2018 Jun 01]. National Institute for Health and Care Excellence; pp. 1–4. Available from: http://www.nice.org.uk/nicemedia/pdf/ipg249guidance.pdf. ISBN1-84629-583-1. [Google Scholar]
- 22.Furness G, Reilly MP, Kuchi S. An evaluation of ultrasound imaging for identification of lumbar intervertebral level. Anaesth. 2002;57:277–80. doi: 10.1046/j.1365-2044.2002.2403_4.x. [DOI] [PubMed] [Google Scholar]
- 23.Watson MJ, Evans S, Thorp JM. Could ultrasonography be used by an anaesthetist to identify a specified lumbar interspace before spinal anaesthesia? Br J Anaesth. 2003;90:509–11. doi: 10.1093/bja/aeg096. [DOI] [PubMed] [Google Scholar]
- 24.Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: A systemic review and meta-analysis. Reg Anesth Pain Med. 2016;41:251–60. doi: 10.1097/AAP.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan KK, Lee PJ, Lohom G. Ultrasound for neuraxial blockade. Med Ultrason. 2014;16:356–63. [PubMed] [Google Scholar]
- 26.Sahin T, Belaban O. Lumbar ultrasonography for Obstetric Neuraxial blocks: Sonoanatomy and literature review. Turk J Anaesthesiol Reanim. 2018;46:257–67. doi: 10.5152/TJAR.2018.90277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harpern SH, Banerjee A, Stocche R, Glanc P. The use of ultrasound for lumbar spinous process identification: A pilot study. Can J Anaesth. 2010;57:817–22. doi: 10.1007/s12630-010-9337-x. [DOI] [PubMed] [Google Scholar]
- 28.Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists' learning curve for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57:120–6. doi: 10.1007/s12630-009-9219-2. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, Madjdpour C, Chin K. Ultrasound-guided lumbar central neuraxial block. BJA Educ. 2016;16:213–20. [Google Scholar]