Abstract
Background and Aims:
Haemodynamic changes during endotracheal intubation are major concerns in general anaesthesia This study compared the efficacy of intranasal and intravenous dexmedetomidine (DEX) to attenuate the stress response of laryngoscopy and endotracheal intubation.
Methods:
In this prospective, randomised, double-blinded study, 70 adults were divided into two groups [Group DIV(n=35) and Group DIN(n=35)]. DIV group received intravenous dexmedetomidine (DEX) infusion (0.5 μg/kg) over 40 min and DIN group received intranasal dexmedetomidine (1 μg/kg) 40 min before induction. The primary objective was the comparison the mean arterial pressure (MAP) between two groups from 40 min before induction at every 10 min intervals till induction of anaesthesia, at the time of intubation, thereafter every 1 min interval till 5 min, at 7 min and 10 min after intubation. The secondary outcomes were comparison of heart rate, systolic and diastolic blood pressure along with sedation and other adverse effects. Statistical analysis was with Statistica 6.0 and Graph Pad prism version 5.
Results:
In both the groups, all the haemodynamic parameters were maintained within (20% of baseline values) throughout the study period. There was no statistically significant difference in MAP between two groups (P>0.05). Preoperative sedation score was significantly higher in the DIV group than the DIN group (P = 0.014).
Conclusion:
Like IV DEX, intranasal DEX can also attenuate the haemodynamic stress responses of laryngoscopy and endotracheal intubation without significant differences in MAP between two groups.
Key words: Intranasal dexmedetomidine, intravenous dexmedetomidine, laryngoscopy and intubation
INTRODUCTION
Induction of general anaesthesia, laryngoscopy, tracheal intubation, and extubation are associated with various haemodynamic changes. Laryngoscopy and tracheal intubation may be associated with sympathetic stimulation and lead to tachycardia and hypertension. These haemodynamic changes may predispose to myocardial ischaemia.[1] Therefore, there is a need to blunt these noxious responses effectively. Various drug combinations have been used with variable success to attenuate the sympathetic responses during laryngoscopy and intubation (L-I).
Premedication is usually administered to reduce anxiety, easy parental separation, amnesia and to reduce anaesthetic requirements. An ideal premedication should have anxiolytic, sedative, analgesic and antisialagogue property. It preferably should be short acting, rapid onset, administered non-parenterally and devoid of any adverse haemodynamic or respiratory effect.[2]
Dexmedetomidine (DEX), a highly selective, short-acting, alpha2-adrenoreceptor agonist, has sedative, analgesic and anxiolytic property without any respiratory depressive action. It is an ideal agent for relieving anxiety or nervousness before anaesthesia. It is established that preoperative intravenous (IV) DEX can successfully attenuate the laryngoscopic stress response.[3] However, adverse haemodynamic complications like hypotension, bradycardia and even cardiac arrest might have hindered the widespread use of IV DEX.[4] Delayed recovery with IV DEX is also documented due to its sedative effect.[4] It has been suggested that alternative routes other than rapid intravenous delivery may help to minimise the adverse effects of DEX.
Beside IV route, DEX is also effective through intramuscular, oral and intranasal (IN) routes. The intranasal route is more convenient and effective than others.[5] Intranasal DEX has been shown to have a high rate of patient acceptance. Recently, several studies in paediatric age group have reported beneficial perioperative outcomes of intranasal DEX premedication as an alternative to traditional premedication.[5,6] Till now to the best of our knowledge, there is no reported study which has compared the efficacy of preoperative IV DEX with IN DEX for attenuation of haemodynamic responses during L-I.
The primary objective of the study was to compare the mean arterial pressure (MAP) between two groups in pre-induction and post-intubation period. The secondary outcomes were comparison of heart rate, systolic and diastolic blood pressure along with sedation scores and other adverse effects within the study period.
The present study hypothesised that like IV DEX, the preoperative IN Dexmedetomidine will also attenuate the haemodynamic responses to laryngoscopy and intubation.
METHODS
After Institutional Ethical Committee approval [obtained on 23/4 2018 –IPGMRER Research Oversight Committee (Memo No: IPGMER/IEC/2018/231)]and with appropriate informed consent from the participants this double blinded, randomised control study was conducted in a tertiary care teaching institute from April 2018 to September 2018. The study was registered in the clinical trial registry of India prospectively (CTRI/2018/03/012556).
The study was conducted according to the declaration of Helsinki principles.
Seventy adults ASA (American Society of Anaesthesiologists physical status) I and II, aged 18-60 years, undergoing elective lumbar spine surgery under general anaesthesia with endotracheal intubation, were included in this study.
Patients who refused to participate, had known allergy or hypersensitivity to dexmedetomidine, suffered from significant cardiac and respiratory disease and predicted difficult airway were excluded from this study. During the preoperative visit, all the patients were examined for any intranasal pathology. Any Patient with nasal ulcers, polyps, nasal septum deviation was excluded from the study.
Detailed pre-anaesthetic evaluation and necessary investigations were done for individual patients. Each patient of both groups fasted for 6 hours and received tablet ranitidine (150 mg) and alprazolam (0.5 mg) as premedication at night before surgery.
Randomisation was done on the basis of computer generated random number table. This was in the custody of senior anaesthesiologists who was not involved in day to day care and monitoring of study parameters. This randomisation schedule facilitated patient disposition into two equal groups (Group -DIV and Group DIN). The list was concealed in opaque sealed envelopes that was numbered and opened sequentially after obtaining the patient's consent.
On the day of operation, all the participants were shifted to preoperative area 2 h before the operation time. All standard monitors like pulse oximetry, non-invasive blood pressure (NIBP), electrocardiogram (ECG) were attached and baseline haemodynamic parameters were recorded in preoperative room. IV Ringer's lactate solution was administered as maintenance fluid (80ml/hr) through 18G peripheral venous canula.
Group DIV received IV DEX (0.50 μg/kg) [200 μg diluted in 50 ml syringe with normal saline (NS) =4 μg/ml] through an infusion pump over 40 min before induction. The equivalent volume of NS was administered intravenously to DIN group.
Group -DIN- patients received IN DEX (1 μg/kg) in undiluted form which was prepared from parenteral preparation (100 μg/ml). Intranasal drug was dripped into both nostrils in equal volume using a 1 ml syringe in supine head down position about 40 min before induction. The equivalent volume of NS was administered intranasally to DIV group. All the patients were instructed not to suck or sneeze after intranasal drug administration.
During the conduct of the study, a double blinding procedure was followed in which the person administering the drug and the patients both were unaware about group distribution.
In the preoperative room, haemodynamic parameters like heart rate (HR), mean arterial pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and SpO2 were noted at every 10 min intervals till induction of anaesthesia. In the operative room, haemodynamic parameters were noted at the time of intubation, thereafter every 1 min interval till 5 min, at 7 min and 10 min after intubation. Sedation status in both groups, were assessed by an observer using the Ramsay sedation scale (RSS) at baseline and then 40 min after study drug administration.
After shifting the patient in operative room, general anaesthesia techniques were standardised for both groups. Beside routine monitors, in operative room, additional monitors such as neuromuscular monitor, ETCO2, were attached and monitoring was continued till the end of operation. Haemodynamic monitoring was continued throughout the perioperative period. After preoxygenation with 100% oxygen for 3 min, all patients were induced with IV propofol (2 mg/kg) and fentanyl (1 μg/kg). IV rocuronium bromide (1 mg/kg) was administered to facilitate tracheal intubation. Laryngoscopy was performed with a Macintosh laryngoscope blade and endotracheal (ET) intubation was done with appropriate size cuffed- disposable armoured ET tube by an experienced anaesthesiologist when train of four (TOF) count was 0. L-I time was limited to15-20 seconds. If there was a failure to L and I within 15-20 seconds the data was excluded from study analysis. No surgical intervention was allowed till 10 min after intubation.
Anaesthesia was maintained with a low flow anaesthesia technique (50% O2—NO2 at 1 litre/min), propofol infusion (10-15 mg/kg/hr titrated to keep Bispectral Index between 40-60) and repeated intermittent bolus doses of rocuronium (0.1 mg/kg) as and when necessary. All patients were ventilated on volume controlled ventilation (Fabius plus–Dragger) using a closed circuit to maintain an EtCO2 level between 35-40 mm of Hg. Extubation timing was guided by neuromuscular monitoring (TOF watch).
The primary outcome of interest was a comparison of changes in mean arterial pressure (MAP), between two groups from pre induction period up to 40 min after study drug administration and in post intubation period up to 10 min after intubation at frequent intervals. The secondary outcomes were comparison of HR, SBP, DBP along withsedation score and other adverse effects within the study period.
Episodes of hypotension (MAP <20% of baseline), bradycardia (HR <50/min) and hypoxia (SPO2 <90%) within the study period were noted and treated accordingly.
After completion of surgery, neuromuscular block was reversed with appropriate dose of IV neostigmine (0.05 mg/kg) and glycopyrolate (0.01 mg/kg). After adequate recovery, the patient was shifted to the recovery room. When Alderate score >9, the patient was shifted to ward and his vitals were monitored there for 12 h.
A pilot study was conducted to calculate the sample size on the basis of difference in MAP between the two groups in post intubation period with standard deviation of 15 mmHg. It was calculated that 35 subjects were required per group in order to detect difference of 10 mmHg in MAP with 80% power and 5% type-1 error probability. Sample size calculation was done by nMaster 2.0 software. [Dept. of Biostatistics, CMC Vellore].
Statistical analysis was done by using Statistica version 6 [Tulsa, Oklahoma: StatSoft Inc., 2001) and GraphPad Prism version 5 [San Diego, California: GraphPad Software Inc., 2007). All the numerical variables in the descriptive statistics were normally distributed (by Kolmogorov-Smirnov goodness-of-fit test), except RSS and SpO2 values. For statistical analysis, RSS score 2 (awake, oriented and cooperative) was considered as satisfactory.
Comparisons of numerical variables between two groups were analyzed by Student's unpaired t test for normally distributed data, and by Mann-Whitney U test for skewed data. Intergroup comparison was done by repeated measures analysis of variance (ANOVA) followed by Tukey's test as a post hoc test, if normally distributed, and for skewed data by Friedman's analysis of variance (ANOVA) followed by Dunn's test as a post hoc test. A P value <0.05 was considered as significant.
RESULTS
Out of initial 80 adult patients, 10 patients were excluded as they did not meet the inclusion criteria or declined to participate in the study. Finally 70 patients were randomised for assessment and none of these patients was lost during the follow up [Figure 1].
Figure 1.
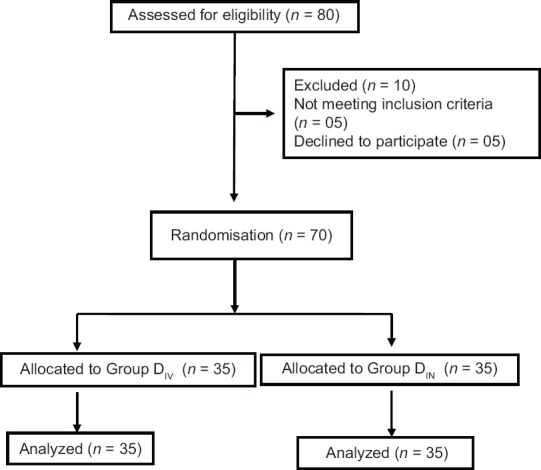
Consort transparent reporting of trial -- Flow of patients in the trial. (DIV= Intravenous dexmedetomidine DIN= Intranasal dexmedetomidine,)
The groups were well matched for their demographic data. The baseline haemodynamic parameters were similar in both the groups [Table 1]. All the patients of both groups were alert and awake at the beginning of the study (RSS2).
Table 1.
Demographic characters and basal haemodynamics of the patients in two groups
| Demography and other parameters of patients | Group DIV (n=35) Mean±SD | Group DIN (n=35) Mean±SD | P |
|---|---|---|---|
| Age in year | 42.03±12.50 | 40.71±10.91 | 0.64 |
| Sex (M/F) | 19/16 | 22/13 | 0.64 |
| Weight in kg | 61.03±7.35 | 60±6.89 | 0.54 |
| Height in meter | 1.62±0.06 | 1.62±0.07 | 0.89 |
| BMI in kg/m2 | 23.10±2.38 | 22.75±1.70 | 0.98 |
| SBP in mm of Hg | 126.94±10.51 | 128.20±11.74 | 0.63 |
| DBP in mm of Hg | 78.74±8.96 | 79.74±7.59 | 0.61 |
| MAP in mm of Hg | 93.06±9.36 | 94.63±8.12 | 0.45 |
| Heart Rate in beats/min | 82.43±8.46 | 87.06±11.33 | 0.05 |
P<0.05 - significant, BMI – Body mass index, SBP – Systolic blood pressure, DBP – Diastolic blood pressure, MAP – Mean arterial pressure, DIV – Intravenous dexmedetomidine, DIN – Intranasal dexmedetomidine
During pre-induction period, after the administration of the study drug, it was observed that SBP, DBP and MAP gradually decreased from baseline values in both DIV and DIN groups. In both groups, maximum reduction of BP was noted at 40 min after study drug administration. It was seen that in the DIV group BP was slightly lower than the DIN group at all time intervals. However, during intergroup comparisons, differences in SBP, DBP and MAP were statistically insignificant at all time intervals (P>0.05). None of the patient in both groups has clinically significant hypotension [Table 2].
Table 2.
Pre induction SBP, DBP and MAP variation in mm of Hg (mean±SD) from time Of intranasal and intravenous drug administration till 40 min
| Group | Basal | 10 min | 20 min | 30 min | 40 min |
|---|---|---|---|---|---|
| SBP | |||||
| DIV | 126.94±10.51 | 123.71±9.05 | 118.89±10.41 | 116.23±10.67 | 111.80±9.71 |
| DIN | 128.20±11.74 | 125.03±11.44 | 120.20±11.48 | 117.26±12.79 | 113.54±12.18 |
| P | 0.63 | 0.59 | 0.61 | 0.71 | 0.51 |
| DBP | |||||
| DIV | 78.74±8.96 | 75.97±9.59 | 72.91±9.44 | 70.60±8.24 | 66.77±7.62 |
| DIN | 79.74±7.59 | 75.29±7.45 | 71.89±7.94 | 68.91±8.42 | 66.00±6.96 |
| P | 0.61 | 0.74 | 0.62 | 0.40 | 0.66 |
| MAP | |||||
| DIV | 93.06±9.36 | 90.37±9.34 | 86.94±9.17 | 84.57±7.86 | 80.74±7.91 |
| DIN | 94.63±8.12 | 90.83±8.62 | 87.00±8.88 | 84.20±9.33 | 81.06±7.67 |
| P | 0.45 | 0.83 | 0.97 | 0.85 | 0.86 |
P<0.05- significant, SBP – Systolic blood pressure, DBP – Diastolic blood pressure, MAP – Means arterial pressure, DIV – Intravenous dexmedetomidine, DIN – Intranasal dexmedetomidine
In the pre-induction period in both groups, it was observed that HR decreased from the baseline value. However, in DIV group HR was lower than DIN group. During intergroup comparison, statistically significant differences in HR between two groups were shown at 30 and 40 min after study drug administration (P < 0.05). None of the patient in both groups had clinically significant bradycardia [Table 3].
Table 3.
Comparison of pre and postinduction heart rate (HR) between two groups
| Time in min | Group DIV HR (Mean±SD) | Group DIN HR (Mean±SD) | P |
|---|---|---|---|
| Basal | 82.43±8.46 | 87.06±11.33 | 0.05 |
| 10 min | 78.80±8.21 | 82.31±10.71 | 0.12 |
| 20 min | 73.09±7.35 | 76.80±10.23 | 0.08 |
| 30 min | 69.86±6.72 | 73.86±9.37 | 0.04 |
| 40 min | 66.60±5.55 | 71.23±9.48 | 0.01 |
| Induction | 65.40±5.77 | 68.23±9.16 | 0.12 |
| L-I | 79.03±5.87 | 84.40±9.43 | 0.00 |
| 1 min | 80.54±6.48 | 82.06±8.73 | 0.41 |
| 2 min | 77.94±6.06 | 79.74±9.08 | 0.33 |
| 3 min | 76.29±5.45 | 78.54±9.93 | 0.24 |
| 4 min | 75.60±5.32 | 77.94±10.83 | 0.25 |
| 5 min | 74.63±5.32 | 76.46±9.66 | 0.33 |
| 7 min | 75.09±5.88 | 76.09±9.63 | 0.60 |
| 10 min | 76.09±5.40 | 76.71±9.16 | 0.72 |
P<0.05 - significant. Bold values Statistically significant at P value. DIV – Intravenous dexmedetomidine, DIN – Intranasal dexmedetomidine
It was found that in both the groups, the maximal increase in HR and BP were noted during L-I and then it gradually decreased to basal preoperative value within 10 min after intubation.
In post-intubation period, HR was slightly higher in the DIN group than DIV group. During intergroup comparison, statistically significant difference in HR between two groups was found only during L-I (P < 0.05). None of the patient in both groups had clinically significant tachycardia during the study period [Table 3].
Similarly, after L-I in DIN group SBP, DBP and MAP all were slightly higher than DIV group, but there was no statistical significant differences in BP in post-intubation period during intergroup comparison (P>0.05). None of the patient in both groups had clinically significant hypertension during the study period [Table 4].
Table 4.
Postinduction SBP, DBP and MAP (mm of Hg) from time of induction to 10 min after Intubation
| Group | induction | L-I | 1 | 2 | 3 | 4 | 5 | 7 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| SBP | |||||||||
| DIV | 108.63±8.95 | 121.83±9.20 | 122.43±8.99 | 119.66±9.75 | 117.46±9.40 | 116.40±9.21 | 114.51±8.80 | 114.60±8.80 | 115.83±8.82 |
| DIN | 108.46±9.20 | 125.83±8.74 | 124.97±1.33 | 121.03±11.15 | 118.51±11.04 | 116.63±10.94 | 116.09±10.28 | 115.66±9.81 | 115.29±9.10 |
| P | 0.93 | 0.06 | 0.30 | 0.58 | 0.66 | 0.92 | 0.49 | 0.63 | 0.80 |
| DBP | |||||||||
| DIV | 66.03±7.99 | 75.03±8.23 | 74.74±7.75 | 72.00±7.45 | 69.54±7.47 | 68.71±7.16 | 68.20±6.20 | 67.71±5.84 | 68.14±7.10 |
| DIN | 63.49±6.42 | 77.09±7.48 | 75.43±6.81 | 72.29±6.13 | 70.60±6.68 | 70.34±6.68 | 69.66±6.20 | 69.26±6.56 | 67.54±5.70 |
| P | 0.14 | 0.27 | 0.69 | 0.86 | 0.53 | 0.32 | 0.32 | 0.30 | 0.69 |
| MAP | |||||||||
| DIV | 80.80±8.14 | 90.91±8.33 | 91.26±6.85 | 88.17±7.81 | 86.40±7.53 | 85.20±7.30 | 83.97±6.64 | 83.71±6.09 | 84.66±7.55 |
| DIN | 78.74±6.41 | 93.63±7.08 | 91.97±7.83 | 88.83±6.85 | 86.89±7.69 | 86.23±6.78 | 85.49±7.08 | 85.54±6.63 | 83.26±5.61 |
| P | 0.24 | 0.14 | 0.68 | 0.70 | 0.79 | 0.54 | 0.36 | 0.23 | 0.38 |
P<0.05- significant, SBP – Systolic blood pressure, DBP – Diastolic blood pressure, MAP – Mean arterial pressure, DIV – Intravenous dexmedetomidine, DIN – Intranasal dexmedetomidine
Considering the sedation status, most of the patients (57.1%) in DIN group remained in RSS stage II [(oriented, cooperative, tranquil),], whereas 77.14%, patients of DIV group remained in RSS stage III [(response to command only)] at 40 min intervals of study drug administration. During intergroup comparison sedation score was significantly higher in the DIV group than the DIN group at 40 min interval (P 0.014).
There was no incidence of significant bradycardia, tachycardia, hyper or hypotension in either group. None of the patients experienced nausea, vomiting or respiratory depression in the study period.
DISCUSSION
Attenuation of laryngoscopic stress response is a major challenge for anaesthesiologist. The satisfactory role of preoperative DEX for attenuation of laryngoscopic stress responses is well established. Nowadays besides IV route, use of IN DEX as premedication is becoming popular, specially in paediatric population. In the present study, we compared the efficacy of IV and IN dexmedetomidine on the stress responses of L-I.
From this study it was found that if intranasal 1 μg/kg DEX was administered as premedication 40 min before induction, the effect was comparable with preoperative IV DEX infusion (0.5 μg/kg) for the prevention of stress responses of L-I. Both intranasal and intravenous DEX attenuated successfully the laryngoscopic stress responses without significant hypertension and tachycardia. In both the groups, all the haemodynamic parameters (HR, SBP, DBP, MAP) were maintained in normal limit (±20% of basal values) before and during L-I. However, in DIV group, preoperative sedation score was significantly higher than the DIN group.
During general anaesthesia, L-I cause noxious stimulation that leads to significant increase in HR and MAP. This is caused by sympathetic stimulation with an increase in the circulating catecholamines levels.[7] The response is initiated within 5 s of laryngoscopy, peaks in 1–2 min and returns to normal levels by 5-10 min. Raid and Brace first described the haemodynamic changes of L-I.[8] To prevent this sympathetic stimulation a proper sympatholytic agent is necessary. Various pharmacological agents like opioids (fentanyl), adrenergic blocking agents (esmolol), vasodilating agents (sodium nitropruside) or local anaesthetics drug (IV lidocaine) have been tried to attenuate these haemodynamic effects, but none of them can completely attenuate these responses.[9,10]
DEX, a centrally acting α2agonists, is widely used in the intensive care unit for its unique sedative, hypnotic, anxiolytic, sympatholytic, antisecretory and analgesic properties. It has unique pharmacological property of conscious sedation and is devoid of any respiratory depression. It is responsible for producing dose dependant co-operative sedation that allows early interaction and early postoperative neurological assessment.[11] Dex also has a reversal drug for its sedative effect called as atipamizole, which acts by increasing the central turnover of noradrenaline.[12] Due to all of these specific characteristics, nowadays DEX become popular as an ideal premedication agent.[13,14]
DEX inhibits noradrenaline release and causes sedation and hypnosis through presynaptic central α2 receptor in the locus ceruleus. The sympatholytic activity of DEX is mediated through postsynaptic α2 receptor which prevents tachycardia and hypertension.[15] Due to this sympatholytic property, both IV and IN DEX in our study can successfully attenuate the laryngoscopic stress responses.
Dex can be administered through various routes like intravenous, intramuscular, intranasal or intraoral.[16,17] The intranasal route is more convenient as it is painless, odourless and tasteless without need of any intravenous infusion. Intranasal drug can penetrate the blood–brain barrier and reach the central nervous system directly.[18] Due to the higher vascularity of the nasal mucosa, DEX may access the systemic circulation rapidly, bypassing the first-pass metabolism of liver.[19]
In various clinical studies, it has been proved that preoperative IV Dex can successfully attenuate the L-I stress responses.[3,20,21,22] In a study, Bon Sebastian et al. have compared between IV dexmedetomidine and normal saline for attenuation of the haemodynamic stress response to laryngoscopy and endotracheal intubation. The intergroup comparison reveal a statistically significant reduction in HR and MAP by dexmedetomidine than normal saline.[3] In another study by Keniya VM et al., it has been proved that perioperative infusion of dexmedetomidine is effective in attenuating sympathoadrenal response to tracheal intubation. After tracheal intubation, maximal average increase are 8% in systolic and 11% in diastolic blood pressure in dexmedetomidine group, as compared to 40% and 25%, respectively, in the control group. Similarly, average increase in heart rate are 7% and 21% in the dexmedetomidine and control groups, respectively.[20]
In another study where role of dexmedetomidine as an anaesthetic adjuvant in intracranial tumour surgery is evaluated-- it has been proved that IV DEX can blunt the hypertensive and tachycardic responses to intubation and extubation (P < 0.01).[22] These findings are closely correlate with findings in our study.
The main disadvantages of IV Dex, is that sedative action is more pronounced than analgesic effect with profound bradycardia and hypotension.[21] Moreover, rapid IV Dex infusion may cause biphasic alteration of MAP which is undesirable in anaesthesia.[22] To minimise these adverse effects, alternative routes of DEX are under trial.
All the adverse effects of IV DEX are mainly dose dependent and higher IV (>0.5 μg/kg) dose is associated with marked sedation and haemodynamic instability.[21,22,23] To avoid any adverse haemodynamic effect and excessive sedation, we administered the effective lower dose of IV DEX (0.5 μg/kg). In a similar study, 60 adult patients scheduled for elective off-pump coronary artery bypass surgery have been randomly allocated to receive dexmedetomidine (0.5 mcg/kg) or normal saline 15 min before intubation. Patients have been compared for haemodynamic changes (heart rate, arterial blood pressure and pulmonary artery pressure) at baseline, 5 min after drug infusion, before intubation and 1, 3 and 5 min after intubation. The dexmedetomidine group has a better control of haemodynamics during laryngoscopy and endotracheal intubation.[24]
Nowadays, in some paediatric clinical trials, intranasal DEX has been successfully used as a premedication.[6,25] It was proved that. intranasal administration of dexmedetomidine is more effective at inducing sleep and a useful alternative premedication in children.[6] Intranasal dexmedetomidine also provide a reliable and effective method of providing sedation during CT scan.[25]
In adult patients, the efficacy of IN DEX has also been proved during both local and general anaesthesia.[26,27] In a comparative study, Jayaraman L et al. evaluated the effect of intranasal dexmedetomidine versus oral alprazolam as a premedication agent in morbidly obese patients undergoing bariatric surgery. It is documented that intranasal dexmedetomidine can obtund haemodynamic response to laryngoscopy and tracheal intubation in adult obese patients.[27]
In a recent study by HrishiPA et al., it has been well proved that IN DEX (1 μg/kg) provides good surgical field condition along with the added advantages of lesser haemodynamic fluctuation during transnasal transphenoidal skull base surgery.[28] There were no statistically significant variations in heart rate and blood pressure with reduced anesthetic requirement in IN DEX group. IN DEX also provides considerable role to attenuate the increase in MAP during intubation response.
In another study by Wang SS et al. it is concluded that IN DEX (1 μg/kg) provides considerable effect to attenuate the increase in MAP caused by intubation response. Changes in HR and BIS also demonstrate that this kind of premedication provides effective attenuation of intubation responses.[29]
Yuen, et al. proved that with IN DEX the time for onset of sedation is 25 (25-30) minutes, which is last for 85 (35-100) minutes. Based on these features, it can be speculated that intranasal dexmedetomidine 25 to 40 minutes before surgery can provide desirable effect.[30] Yuen et al. have found that when the preoperative administration has been extended to 40-45 minutes, 91% of the children have achieved satisfactory result. Correlate with that in our study, the time of preoperative administration was 40 minutes before induction.[30]
In a prospective randomised controlled trial by ChengxiangLu et al. on 81 adult patients scheduled for elective direct laryngoscopy receive intranasal dexmedetomidine (1 μg/kg) or placebo 40-45 min before anaesthetic induction. It has been document that episodes of tachycardia and hypertension after tracheal intubation and extubation are less in dexmedetomidine group.[31] This findings are closely correlated with our study.
In our study, when we compared the haemodynamic effects of intranasal and intravenous dexmedetomidine during laryngoscopy and intubation, it was documented that dexmedetomine, through both IV and IN route, can attenuate the stress responses and we did not find any significant difference in haemodynamics between two groups. In both groups all the haemodynamic parameters remained within 20% of basal values without significant changes in MAP and HR.
In the study about pharmacokinetic and pharmacodynamic of intranasal DEX by Li et al., it has been documented that intranasal dexmedetomidine is associated with a slower and more gradual onset than IV administration.[32] Rapid IV administration results in much higher peak plasma concentrations and earlier onset than IN route. A more gradual onset may actually be desirable in avoiding the alpha 1 agonist effects seen with rapid IV administration (hypertension and bradycardia). Similar haemodynamic effects were documented in our study with both slow IV DEX infusion and intranasal DEX.
Intravenous dexmedetomidine is thought to provide significant sedation without any respiratory adverse effect.[23] On the other hand, another study with intranasal dexmedetomidine as a sedative premedication induced a favourable perioperative anxiolysis without prolongation in anesthesia recovery.[31] Intranasal dexmedetomidine is also a safe and effective agent for procedural sedation in paediatric dental patients with good patient compliance and early recovery. There were no documented episodes of oxygen desaturation or apnoea.[6,26] Similar effect was noted in our study, at 40 min intervals of study drug administration, the sedation score was significantly higher in DIV group than DIN group. Most of the patients in the DIN group remained in RSS stage II and in the DIV group remained in RSS stage III.
From the present study, it is proved that both intranasal and intravenous dexmedetomidine can be used as a premedication for attenuation of haemodynamic surges during L-I with more or less same efficacy. This finding can be attributed to the fact that both IV and IN DEX prevent the escalation of central catecholamine level.
The limitations in the study included failure to correlate the effects of IV and IN dexmedetomidine premedication on the analgesic and anaesthetic requirements during intra and postoperative period. As in both groups, dexmedetomidine was administered 40 min before induction – a longer premedication time was required. In future studies, the recovery characteristic of both IV and IN DEX in postoperative period has to be evaluated.
CONCLUSION
From this study, it is concluded that both intravenous infusion of DEX (0.5 μg/kg in 40 min) and intranasal dexmedetomidine (1μg/kg) when administered 40 min before induction, are equally effective for attenuation of haemodynamic surges during laryngoscopy and intubation. Therefore like IV DEX, intranasal DEX can be used as a safer alternative premedication for adequate control of haemodynamic responses [mean arterial pressure (MAP)]during laryngoscopy and endotracheal intubation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Abhijit Hazra, Professor, Department of Pharmacology, IPGMER, Kolkata.
REFERENCES
- 1.Mahajon L, Kaur M, Gupta R, Aujila KS, Singh A, Kaur A. Attenuation of the pressor responses to laryngoscopy and endotracheal intubation with intravenous dexmedetomidine versus magnesium sulphate under bispectral index-controlled anaesthesia: A placebo-controlled prospective randomised trial. Indian J Anaesth. 2018;62:337–43. doi: 10.4103/ija.IJA_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maldhe AD. Dexmedetomidine as premedication in children: Status at the beginning of 2017. Indian J Anaesth. 2017;61:101–2. doi: 10.4103/ija.IJA_61_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastian B, Talikoti AT, Krishnamurthy D. Attenuation of haemodynamic responses to laryngoscopy andendotracheal intubation with intravenous dexmedetomidine: A comparison between two doses. Indian J Anaesth. 2017;61:48–54. doi: 10.4103/0019-5049.198404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharati S, Pal A, Biswas C, Biswas R. Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: A case series and review of published case reports. Acta Anaesthesiol. 2011;49:165–7. doi: 10.1016/j.aat.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LH. A double-blind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg. 2007;105:374–80. doi: 10.1213/01.ane.0000269488.06546.7c. [DOI] [PubMed] [Google Scholar]
- 6.Talon MD, Woodson LC, Sherwood ER, Aarsland A, McRae L, Benham T. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res. 2009;30:599–605. doi: 10.1097/BCR.0b013e3181abff90. [DOI] [PubMed] [Google Scholar]
- 7.Joffe AM, Deem SA. Physiologic and pathophysiologic responses to intubation. In: Benumof J, Hagberg CA, editors. Benumof and Hagberg's Airway Management. 3rd ed. Philadelphia: Elsevier Saunders; 2012. pp. 184–95. [Google Scholar]
- 8.Reid LC, Brace DE. Irritation of respiratory tract and its reflex effect upon heart. Surg Gynae and Obst. 1940;70:157–62. [Google Scholar]
- 9.Khan FA, Ullah H. Pharmacological agents for preventing morbidity associated with the haemodynamic response to tracheal intubation. Cochrane Database Syst Rev. 2013;7:CD004087. doi: 10.1002/14651858.CD004087.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Figueredo E, Garcia-Fuentes EM. Assessment of the efficacy of esmolol on the haemodynamic changes induced by laryngoscopy and tracheal intubation: A meta-analysis. Acta Anaesthesiol Scand. 2001;11:1011–22. doi: 10.1034/j.1399-6576.2001.450815.x. [DOI] [PubMed] [Google Scholar]
- 11.Yazbek-Karam VG, Aouad MM. Perioperative uses of dexmedetomidine. Middle East J Anaesthesiol. 2006;18:1043–58. [PubMed] [Google Scholar]
- 12.Karhuvaara S, Kallio A, Salonen M, Tuominen J, Scheinin M. Rapid reversal of alpha 2-adrenoceptor agonist affects by atipamizole in human volunteers. Br J Clin Pharmacol. 1991;31:160–5. doi: 10.1111/j.1365-2125.1991.tb05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maksimow A, Snaoir A, Sarkela M, Kentala E, Koskenvuo J, Posti J, et al. Assessing the depth of dexmedtomidine-induced sedation with electroencephalogram (EEG)-based spectral entropy. Acta Anaesthesiol Scand. 2007;51:222–30. doi: 10.1111/j.1399-6576.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Liu C, Zhang Y, Luo B, She S, Xu L, et al. Low-dose intramuscular dexmedetomidine as premedication: A randomized controlled trial. Med Sci Monit. 2014;20:2714–9. doi: 10.12659/MSM.891051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavya Prabhu M, Mehandale SG. Comparison of oral dexmedetomidine versus oral midazolam as premedication to prevent emergence agitation after sevoflurane anaesthesia in paediatric patients. Indian J Anaesth. 2017;61:131–6. doi: 10.4103/0019-5049.199852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talgaonkar S, Mishra PR. Intranasal delivery: An approach to bypass the blood brain barrier. India J Pharmacol. 2004;36:140–7. [Google Scholar]
- 19.Kumar L, Kumar A, Panikkaveetil R, Vasu KB, Rajan S, Nair GS. Efficacy of intranasal dexmedetomidine versus oral midazolam for paediatric premedication. Indian J Anaesth. 2017;61:125–30. doi: 10.4103/0019-5049.199850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 22.Mustafa A, Isik B, Ozsoylar O, Akcabay M. The effects of alfa2 adrenargic agonist dexmedetomidine on haemodybamic response to direct laryngoscopy. Open Otorhinolaryngol J. 2007;107:157. [Google Scholar]
- 23.Saǧıroǧlu AE, Celik M, Orhon Z, Yüzer S, Sen B. Dıfferent doses of dexmedetomidine on controlling haemodynamic responses to tracheal intubation. Internet J Anesthesiol. 2010;27:2. [Google Scholar]
- 24.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of Dexmedetomidine on attenuation os stress response to Patients undergoing elective off pump CABG. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 25.Mason KP, Zgleszewski SE, Dearden JL, Dumont RS, Pirich MA, Stark CD, et al. Dexmedetomedine for pediatric sedation for computed tomography imaging studies. Anesth Analg. 2006;103:57–62. doi: 10.1213/01.ane.0000216293.16613.15. [DOI] [PubMed] [Google Scholar]
- 26.Nooh N, Sheta SA, Abdullah WA, Abdelhalim AA. Intranasal atomized dexmedetomidine for sedation during third molar extraction. Int J Oral Maxillofac Surg. 2013;42:857–62. doi: 10.1016/j.ijom.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman L, Sinha A, Punhani D. A comparative study to evaluate the effect of intranasal dexmedetomidine versus oral alprazolam as a premedication agent in morbidly obese patients undergoing bariatric surgery. J Anaesth Clin Pharmacol. 2013;29:179–82. doi: 10.4103/0970-9185.111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hrishi PA, Ruby K, Nair P. A novel use of a novel drug: Preoperative nasalpreparation with dexmedetomidine fortransnasal transsphenoidal neurosurgeryapproach in skull base neurosurgery. Indian J Neurosurg. 2017;6:170–5. [Google Scholar]
- 29.Wang SS, Zhang MZ, Sun Y, Wu C, Xu WY, Bai J, et al. The sedative effects and the attenuation of cardiovascular and arousal responses during anesthesia induction and intubation in pediatric patients: A randomized comparison between two different doses of preoperative intranasal dexmedetomidine. Paediatr Anesth. 2014;24:275–81. doi: 10.1111/pan.12284. [DOI] [PubMed] [Google Scholar]
- 30.Yuen VM, Hui TW, Irwin MG, Yao TJ, Wong GL, Yuen MK. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia. 2010;65:922–9. doi: 10.1111/j.1365-2044.2010.06453.x. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Zhang LM, Zhang Y, Ying Y, Li L, Xu L, et al. Intranasal dexmedetomidine as a sedative premedication for patients undergoing suspension laryngoscopy: A randomized double-blind study. PLoS One. 11:e0154192. doi: 10.1371/journal.pone.0154192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A, Yuen VM, Goulay-Dufaÿ S, Sheng Y, Standing JF. Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth. 2018;12:960–8. doi: 10.1016/j.bja.2017.11.100. [DOI] [PubMed] [Google Scholar]


