Abstract
Background
Statin therapy reduces the risk of cardiovascular events across a broad spectrum of patients; however, it increases the risk of new-onset diabetes (NOD). Although the highest dose pitavastatin is considered to not be associated with NOD, there are limited data regarding the impact of long-term highest dose pitavastatin use on the development of NOD in patients at high risk of developing diabetes. Therefore, we prospectively compared the differences in the development of NOD between the lowest and the highest dose of pitavastatin in patients at high risk of developing diabetes during a 3-year follow-up.
Methods
This post hoc analysis of a prospective, single-blinded, randomized study compared the risk of NOD between the highest dose of pitavastatin (4 mg) and the lowest dose of pitavastatin (1 mg) over a 3-year follow-up in patients with acute coronary syndrome. Among 1044 patients of the original study, 667 patients at high risk of developing type 2 diabetes mellitus were in the subgroup analysis. The primary endpoint was a comparison of the differences in the cumulative incidence of NOD in the pitavastatin 1 mg and 4 mg groups during a 3-year follow-up.
Results
With propensity score matching, there were no significant differences in baseline demographic characteristics between the 2 groups. Incidence of NOD was similar between the pitavastatin 1 mg and 4 mg groups [12 of 289 patients (4.2%) and 8 of 289 patients (2.8%), respectively; p = 0.36]. In a prespecified analysis, there were no significant differences in NOD events according to sex, age, diagnosis, body mass index, glucose intolerance, or dyslipidemia.
Conclusions
Administration of highest-dose pitavastatin did not increase the risk of NOD in patients at high risk of developing diabetes during the 3-year follow-up. Moreover, various risk factors for NOD such as metabolic syndrome components, glucose intolerance, dyslipidemia, obesity, or hypertension did not affect the development of NOD during pitavastatin administration. Thus, the highest dose pitavastatin can be safely used in patients with metabolic syndrome who are at high risk of developing diabetes.
Trial registration Clinical Trial registration information. URL: https://clinicaltrials.gov/ct2/show/NCT02545231. Unique identifier: NCT02545231
Keywords: Acute coronary syndrome, New-onset diabetes, Metabolic syndrome, Pitavastatin
Background
Statins are well known to reduce the risk of cardiovascular events and improve clinical outcomes across a broad spectrum of patients [1, 2]. Moreover, higher dose statin therapy more significantly reduces cardiovascular events in high-risk patients [3]. Previous studies, however, have suggested a relationship between statin therapy and new-onset diabetes (NOD) [4–7]. A meta-analysis has shown that statin therapy increased the risk of NOD by 9% and that intensive therapy additionally increased the risk of NOD by 12% [6, 8].
Interestingly, the risk of NOD seems to vary according to the type and dose of statins. Previous studies reported that various types of statins, including atorvastatin, rosuvastatin, and simvastatin, increased the risk of NOD [6, 8, 9]. Higher doses of statin therapy further increased the hazard ratio of NOD [10]. However, high-dose pitavastatin is not considered to be associated with NOD [11]. Pitavastatin 2 mg showed a similar effectiveness in improving lipid profiles to that of atorvastatin 10 mg in Asian patients, with similar safety parameters [12]. When compared to other statins, the beneficial effects of pitavastatin on glucose metabolism or NOD may stem from the fact that it does not impair the differentiation and maturation of 3T3-L1 preadipocytes and does not suppress glucose transporter type 4 (GLUT4) expression [13].
Known risk factors for NOD during statin therapy are fasting blood glucose (FBG) ≥ 100 mg/dL, fasting triglycerides ≥ 150 mg/dL, body mass index (BMI) ≥ 30 kg/m2, and a history of hypertension (HTN) [14]. In the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, the risk factors for developing diabetes mellitus (DM) included at least 1 of the following: metabolic syndrome, impaired fasting glucose, BMI ≥ 30 kg/m2, or HbA1c > 6% [15]. Dyslipidemia can be controlled by the administration of relatively lower dose statins in Asian patients [16]. However, there are limited data regarding the impact of chronic pitavastatin use on the development of NOD in the Asian population [17, 18]. We prospectively compared the differences in the development of NOD between the lowest dose of pitavastatin and the highest dose of pitavastatin in patients at high risk of developing DM during a 3-year follow-up.
Methods
Study patients
Patients aged 30 to 79 years were eligible for the original trial of this study if they were (1) diagnosed with acute coronary syndrome (ACS) that was successfully treated with coronary stent implantation (TIMI flow grade 3 after the procedure). A total of 2463 consecutive patients were screened for inclusion at the Korea University Anam Hospital Cardiovascular Center between March 2013 and April 2015. Exclusion criteria were: (1) hypersensitivity to pitavastatin; (2) serum creatinine > 2.0 mg/dL; (3) hemoglobin A1c > 9%; (4) type 1 DM; (5) serum platelet level < 100,000/μL; (6) left main coronary artery lesion; (7) left ventricular ejection fraction < 40%; (8) hepatic dysfunction (aspartate aminotransferase or alanine aminotransferase > twice the upper limit); (9) gastrointestinal disorders, such as Crohn’s disease; (10) alcohol abuse; (11) steroid or hormone replacement therapy; (12) life expectancy less than 1 year; (13) those with a known pregnancy, breast feeding, or having an intention to become pregnant during the study period; (14) any condition that would make participation in this study unsafe or unsuitable in the opinion of the investigator or (15) a lack of follow-up data.
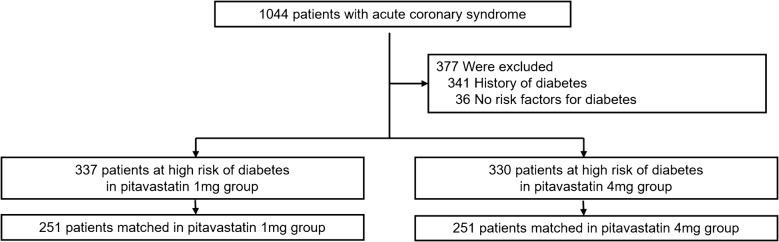
Among these patients, we enrolled the patients who fulfilled at least 1 of following criteria for a high risk of developing type 2 DM: FBG ≥ 100 mg/dL, fasting triglycerides ≥ 150 mg/dL, BMI ≥ 25 kg/m2 according to the Korean guidelines of obesity, or presence of HTN (Fig. 1) [19]. To investigate the impacts on NOD, we excluded the patients with an existing diagnosis of DM.
Fig. 1.
Study protocol. A total of 1044 patients underwent randomization in a 1:1 ratio to receive lowest dose (1 mg) or highest dose pitavastatin (4 mg) therapy for 3 years. After propensity score matching, 289 patients were enrolled in each group
Study design
This study was a post hoc analysis of the prospective, open-label, single-blinded, randomized trial. A total of 1044 patients received lowest-dose pitavastatin (1 mg) or highest-dose pitavastatin (4 mg) for 3 years in the original study. Among them, 667 patients were at high risk of developing type 2 DM in the subanalysis (Fig. 1). Patients received randomization numbers sequentially from a secret randomization list that was computer generated in blocks of 3 by individuals who had no contact with the persons who assigned the patients to study groups or performed any of the assessments. Participants were unaware of the randomization assignments until the final data were obtained. The study was approved by the Korea University Hospital Institutional Review Board, and written informed consent was obtained from all participants or their legal guardians before their inclusion in the study. All clinical investigations were conducted according to the principles of the Declaration of Helsinki.
Endpoints
The primary endpoint was a comparison of the differences in the cumulative incidence of NOD in the pitavastatin 1 mg and pitavastatin 4 mg groups during a 3-year follow-up. NOD was defined as ≥ HbA1c 6.5% or the current use of hypoglycemic agents according to the physician’s discretion. The secondary endpoints were the predictors of NOD and comparison of the changes in vascular function. Vascular function was evaluated by brachial-ankle pulse wave velocity (PWV), central blood pressure (BP), and augmentation index (AI). A patient was defined as an alcoholic when an average of ≥ 7 units of alcohol among men and ≥ 5 units among women were consumed for 2 days per week.
Pulse wave velocity
All patients were evaluated for PWV at baseline and at the 36-month follow-up. After 5 min of rest in the supine position, PWV was measured using a volume plethysmographic apparatus (BP-203 RPE II; Colin, Komaki, Japan), which simultaneously recorded the PWV and the brachial and ankle BP on the left and right sides.
Measurements of central BP and AI
Central BP recordings were obtained using the Omron HEM-9000AI cSBP device (Omron Healthcare, Kyoto, Japan) according to the manufacturer’s and user’s manuals. BP measurement was obtained via the digital oscillometric method using a BP cuff. The accompanying AI calculation was based on the patient’s pressure waveforms calibrated using the brachial systolic and diastolic BP. AI was determined by the change in pressure between the first and second peaks divided by the pulse pressure (AI = ΔP/PP). The first peak was obtained when the blood was ejected from the aorta. The second pressure peak occurred when the blood reflected at the aortic bifurcation. The pulse pressure was the overall peak pressure. All data were stored and analyzed off-line after the completion of testing.
Laboratory analysis
Venous blood samples were drawn from each patient after fasting for 8 h or overnight. Blood samples were centrifuged to obtain plasma that was stored at − 80 °C. Plasma glucose was measured using the glucose oxidase method, and serum insulin levels were measured using an immunoradiometric assay (Biosource, Nivelles, Belgium). Total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol levels were determined using enzymatic methods with standard biochemical procedures on a BM Hitachi automated clinical chemistry analyzer (Hitachi, Tokyo, Japan).
Statistical analysis
Data were expressed as mean ± standard deviation for the continuous variables, and as number and percentage of patients for the categorical variables. Fisher’s exact test or Chi-square test was used for categorical variables. The change from baseline was calculated as the value obtained at the end of treatment subtracted from the pre-treatment value. The results of the 2 groups were compared using the unpaired Student’s t test, and the comparisons of the results obtained before and after the treatment were analyzed using the paired t test. To balance the distribution of baseline characteristics, we used propensity score matching. We estimated a propensity score for each study participant using the multivariable logistic regression model. In the model, potential confounders and variables, such as age, sex, alcohol intake and smoking status, BMI, HTN, DM, and medication history were included. We then created an exchangeable comparison group of patients receiving pitavastatin 1 mg by matching each with a patient in the pitavastatin 4 mg group. The model was fit to the data during all steps of the regression analyses (Hosmer and Lemeshow goodness-of-fit test χ2 = 6.30, p = 0.85, and relative multivariate imbalance L1 after matching = 0.96). Using the propensity scores, we matched 251 of those patients receiving pitavastatin 1 mg to another 251 patients receiving pitavastatin 4 mg who had a similar propensity score. Our assessment of the covariate balance after matching focused on these standardized differences. p value < 0.05 was considered statistically significant. SAS software (version 9.3; SAS Institute, Cary, NC, USA) was used for the statistical analyses.
Results
Patient characteristics
Baseline patient characteristics between the pitavastatin 1 mg (n = 337) and pitavastatin 4 mg (n = 330) groups were significantly different. Mean age, body mass index, medications on admission, and lipid profiles showed statistically significant differences between the 2 groups (Table 1). After propensity score matching to balance the distribution of the baseline characteristics, there were no significant differences in baseline demographic characteristics between the 2 groups (Table 2). Follow-up was done 929.4 ± 312.5 and 940.2 ± 293.0 days after the index procedure in the pitavastatin 1 mg and pitavastatin 4 mg groups, respectively (p = 0.70).
Table 1.
Baseline demographic and angiographic characteristics
| Variable | Pitavastatin 1 mg (n = 337) | Pitavastatin 4 mg (n = 330) | p-value |
|---|---|---|---|
| Age (years) | 64.1 ± 11.9 | 62.2 ± 11.8 | 0.04 |
| Male sex | 230 (68.2) | 253 (76.7) | 0.02 |
| Body mass index (kg/m2) | 24.3 ± 3.0 | 24.8 ± 3.1 | 0.03 |
| Diagnosis | 0.99 | ||
| Unstable angina | 210 (72.7) | 209 (72.3) | |
| Non-ST elevation MI | 45 (15.6) | 45 (15.6) | |
| ST elevation MI | 34 (11.8) | 35 (12.1) | |
| Risk factors | |||
| Hypertension | 194 (57.6%) | 183 (55.5%) | 0.59 |
| Smoking status | 0.01 | ||
| Current smoker | 66 (19.6%) | 98 (29.7%) | |
| Ex-smoker | 64 (19.0%) | 61 (18.5%) | |
| Never smoker | 207 (61.4%) | 171 (51.8%) | |
| Alcoholics | 54 (16.0%) | 63 (19.1%) | 0.31 |
| Prior MI | 19 (5.4%) | 13 (3.8%) | 0.29 |
| Medication on admission | |||
| Aspirin | 335 (99.4%) | 330 (100%) | 0.50 |
| Clopidogrel | 295 (87.5%) | 263 (79.7%) | 0.01 |
| Ticagrelor | 49 (14.5%) | 63 (19.1%) | 0.12 |
| Prasugrel | 10 (3.0%) | 29 (8.8%) | 0.001 |
| ACE inhibitor | 69 (20.5%) | 60 (18.2%) | 0.49 |
| ARB | 114 (33.8%) | 94 (28.5%) | 0.16 |
| β-blocker | 142 (42.1%) | 158 (47.9%) | 0.14 |
| CCB | 110 (32.6%) | 116 (35.2%) | 0.51 |
| Diuretics | 61 (18.1%) | 54 (16.4%) | 0.61 |
| Laboratory findings | |||
| Total cholesterol (mg/dL) | 174.2 ± 41.7 | 185.2 ± 49.7 | < 0.01 |
| Triglyceride (mg/dL) | 144.0 ± 105.9 | 164.5 ± 175.0 | 0.08 |
| HDL cholesterol (mg/dL) | 43.8 ± 11.2 | 42.9 ± 9.9 | 0.28 |
| LDL cholesterol (mg/dL) | 119.6 ± 34.5 | 127.5 ± 37.1 | 0.01 |
| Creatinine (mg/dL) | 1.1 ± 1.5 | 0.9 ± 0.2 | 0.06 |
| Uric acid (mg/dL) | 5.5 ± 1.5 | 5.8 ± 1.6 | 0.09 |
| hsCRP (mg/L) | 5.9 ± 12.6 | 7.4 ± 17.5 | 0.26 |
| Fasting glucose (mmol/L) | 113.6 ± 27.8 | 116.4 ± 28.4 | 0.28 |
| HbA1c (%) | 5.8 ± 0.4 | 5.8 ± 0.3 | 0.89 |
Values are presented as mean ± standard deviation or n (%)
ACE angiotensin converting enzyme, ARB angiotensin receptor blocker, CCB calcium channel blocker, HbA1c hemoglobin A1c, HDL high-density lipoprotein, hsCRP high-sensitivity C-reactive protein, LDL low-density lipoprotein, MI myocardial infarction
Table 2.
Baseline demographic characteristics after propensity score matching
| Variable | Pitavastatin 1 mg (n = 251) | Pitavastatin 4 mg (n = 251) | p-value |
|---|---|---|---|
| Age (years) | 63.3 ± 12.2 | 62.6 ± 11.9 | 0.54 |
| Male sex | 177 (70.5%) | 185 (73.7%) | 0.49 |
| Body mass index (kg/m2) | 24.4 ± 3.0 | 24.6 ± 3.0 | 0.66 |
| Diagnosis | 0.88 | ||
| Unstable angina | 184 (73.3%) | 181 (72.1%) | |
| Non-ST elevation MI | 37 (14.7%) | 41 (16.3%) | |
| ST elevation MI | 30 (12.0%) | 29 (11.6%) | |
| Risk factors | |||
| Hypertension | 142 (56.6%) | 144 (57.4%) | 0.93 |
| Smoking status | 0.94 | ||
| Current smoker | 64 (25.5%) | 65 (25.9%) | |
| Ex-smoker | 46 (18.3%) | 43 (17.1%) | |
| Never smoker | 141 (56.2%) | 143 (57.0%) | |
| Alcoholics | 46 (18.3%) | 44 (17.5%) | 0.91 |
| Prior stroke | 0 (0.0%) | 0 (0.0%) | – |
| Medication on admission | |||
| Aspirin | 251 (100.0%) | 251 (100.0%) | – |
| Clopidogrel | 215 (85.7%) | 204 (81.3%) | 0.23 |
| Ticagrelor | 40 (15.9%) | 48 (19.1%) | 0.41 |
| Prasugrel | 10 (4.0%) | 20 (8.0%) | 0.09 |
| ACE inhibitor | 41 (16.3%) | 49 (19.5%) | 0.42 |
| ARB | 82 (32.7%) | 78 (31.1%) | 0.77 |
| β-blocker | 117 (46.6%) | 118 (47.0%) | 0.99 |
| CCB | 86 (34.3%) | 84 (33.5%) | 0.93 |
| Diuretics | 49 (19.5%) | 44 (17.5%) | 0.65 |
| Laboratory findings | |||
| Total cholesterol (mg/dL) | 177.9 ± 41.4 | 180.8 ± 46.6 | 0.46 |
| Triglyceride (mg/dL) | 145.7 ± 109.7 | 162.6 ± 182.6 | 0.21 |
| HDL cholesterol (mg/dL) | 43.4 ± 10.6 | 42.9 ± 9.9 | 0.60 |
| LDL cholesterol (mg/dL) | 122.9 ± 34.2 | 123.4 ± 34.2 | 0.88 |
| Creatinine (mg/dL) | 1.1 ± 1.5 | 0.9 ± 0.2 | 0.08 |
| Uric acid (mg/dL) | 5.6 ± 1.5 | 5.8 ± 1.6 | 0.23 |
| hsCRP (mg/L) | 5.6 ± 11.8 | 6.7 ± 15.9 | 0.43 |
| Fasting glucose (mmol/L) | 115.4 ± 29.3 | 114.9 ± 26.3 | 0.85 |
| HbA1c (%) | 5.8 ± 0.4 | 5.8 ± 0.3 | 0.36 |
Values are presented as mean ± standard deviation or n (%)
ACE angiotensin converting enzyme, ARB angiotensin receptor blocker, CCB calcium channel blocker, HbA1c hemoglobin A1c, HDL high-density lipoprotein, hsCRP high sensitivity C-reactive protein, LDL low-density lipoprotein, MI myocardial infarction
NOD and clinical outcomes during the 3-year follow-up
The incidence of NOD was similar between the pitavastatin 1 mg and the pitavastatin 4 mg groups during the 3-year follow-up [14 of 251 patients (5.6%) and 9 of 251 patients (3.6%), respectively; p = 0.39] (Table 3 and Fig. 2). In a prespecified analysis, there were no significant differences in NOD events that occurred at less than 1 year or more than 1 year after baseline randomization between the pitavastatin 1 mg and the pitavastatin 4 mg groups [1 (0.4%) and 0 (0.0%), p = 0.99 during the first year of follow-up and 13 (5.2%) and 9 (3.6%), p = 0.39 more than 1 year after baseline randomization, respectively]. In the analyses of the separate clinical events, the incidences of each event were similar between the 2 groups.
Table 3.
Incidence of new-onset diabetes and clinical events during the 3-year follow-up
| Variable | Pitavastatin 1 mg (n = 251) | Pitavastatin 4 mg (n = 251) | p-value |
|---|---|---|---|
| New-onset diabetes | 14 (5.6%) | 9 (3.6%) | 0.39 |
| Baseline ~ 1 year | 1 (0.4%) | 0 (0.0%) | 0.99 |
| > 1 year | 13 (5.2%) | 9 (3.6%) | 0.39 |
| All cause death | 9 (3.6%) | 8 (3.2%) | 0.99 |
| Cardiac death | 3 (1.2%) | 1 (0.4%) | 0.62 |
| Non-fatal myocardial infarction | 14 (5.6%) | 8 (3.2%) | 0.28 |
| Ischemic stroke | 5 (2.0%) | 2 (0.8%) | 0.45 |
| Target lesion revascularization | 12 (4.8%) | 9 (3.6%) | 0.66 |
| Target vessel revascularization | 18 (7.2%) | 13 (5.2%) | 0.46 |
| Non-target lesion/vessel revascularization | 7 (2.8%) | 15 (6.0%) | 0.13 |
Values are presented as n (%)
Fig. 2.
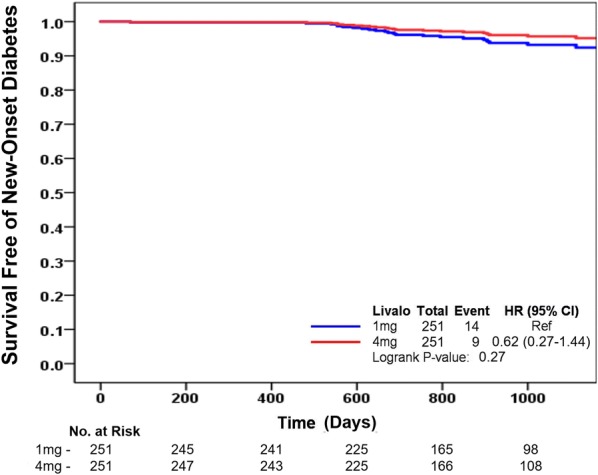
Survival without new-onset diabetes in the pitavastatin 1 mg and pitavastatin 4 mg groups during the 3-year follow-up
Predictors of NOD
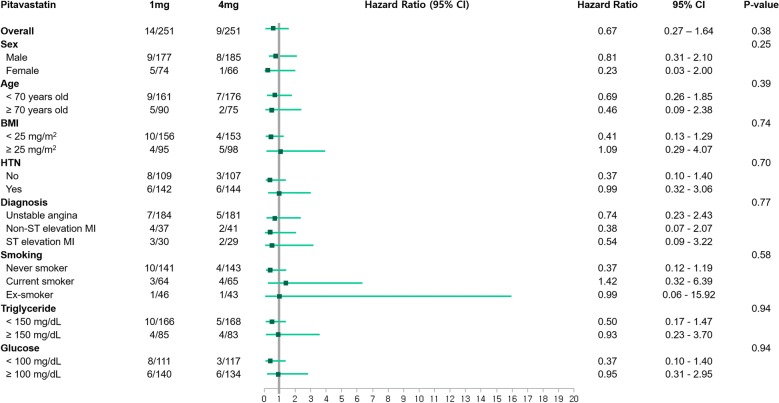
The number of metabolic syndrome components did not affect the occurrence of NOD (Table 4). Total occurrences of NOD did not increase along with the increased number of metabolic syndrome components. Moreover, administration of pitavastatin 4 mg did not increase the risk of NOD compared to pitavastatin 1 mg. Incidences of NOD according to sex, age, BMI, level of TG and glucose, HTN, initial diagnosis at randomization, and smoking status were comparable between the 2 groups (Fig. 3). In the univariate analysis with the propensity score-matched population, pitavastatin 4 mg did not significantly increase the risk of NOD (Table 5). The number of metabolic syndrome components and other factors did not show significant differences in terms of the development of NOD.
Table 4.
Incidence of new-onset diabetes according to the number of metabolic syndrome components during the 3-year follow-up
| Number of metabolic syndrome components | Incidence of new-onset diabetes | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Pitavastatin 1 mg | Pitavastatin 4 mg | |||
| 1 | 4/50 | 1/51 | 0.23 (0.03, 2.13) | 0.20 |
| 2 | 5/96 | 4/100 | 0.75 (0.20, 2.91) | 0.69 |
| 3 | 4/73 | 2/74 | 0.48 (0.09, 2.70) | 0.40 |
| 4 | 1/32 | 2/26 | 2.58 (0.22, 30.20) | 0.45 |
| Overall | 14/251 | 9/251 | 0.67 (0.27, 1.64) | 0.38 |
Fig. 3.
Incidences of new-onset diabetes in selected, prespecified subgroups
Table 5.
Predictors for new-onset diabetes
| Risk factor | Univariate analysis | |||
|---|---|---|---|---|
| HR | 95% CI | p-value | ||
| Lower | Upper | |||
| Pitavastatin 4 mg | 0.63 | 0.27 | 1.48 | 0.29 |
| Number of metabolic syndrome components | ||||
| 1 | – | |||
| 2 | 0.92 | 0.30 | 2.83 | 0.89 |
| 3 | 0.82 | 0.24 | 2.75 | 0.74 |
| 4 | 1.05 | 0.24 | 4.55 | 0.95 |
| Age | 0.99 | 0.65 | 1.03 | 0.63 |
| Female | 0.91 | 0.35 | 2.35 | 0.84 |
| Body mass index | 1.01 | 0.88 | 1.16 | 0.91 |
| Hypertension | 0.82 | 0.35 | 1.89 | 0.64 |
| Total cholesterol | 1.01 | 0.99 | 1.01 | 0.86 |
| Triglyceride | 1.00 | 0.99 | 1.01 | 0.82 |
| HDL-cholesterol | 0.96 | 0.92 | 1.01 | 0.11 |
| LDL-cholesterol | 1.00 | 0.99 | 1.02 | 0.58 |
| hsCRP | 1.01 | 0.99 | 1.04 | 0.23 |
| Fasting glucose | 1.01 | 0.99 | 1.02 | 0.15 |
| HbA1c | 6.61 | 0.69 | 63.18 | 0.10 |
HbA1c hemoglobin A1c, HDL high-density lipoprotein, hsCRP high sensitivity C-reactive protein, LDL low-density lipoprotein
Changes in inflammatory markers, lipid profiles, and vascular function during the 3-year follow-up
Decreases in LDL cholesterol levels from baseline were significantly greater in the pitavastatin 4 mg group than in the pitavastatin 1 mg group during the 3-year follow-up (− 37.5 ± 37.6 mg/dL and − 15.2 ± 39.3 mg/dL, respectively; p < 0.05) (Additional file 1: Table S1). The percent reduction of LDL cholesterol from baseline was 12.0% in the pitavastatin 1 mg group and 31.0% in the pitavastatin 4 mg group. No significant differences in the PWV, ankle-brachial index, central BP, or AI were detected between the groups (Additional file 1: Table S2).
Discussion
This post hoc analysis of a prospective, single-blinded, randomized study compared the risk of NOD between the highest dose of pitavastatin (4 mg) and the lowest dose of pitavastatin (1 mg) over a 3-year follow-up in patients with ACS. Administration of pitavastatin, including the highest-dose pitavastatin (4 mg), did not increase the risk of NOD during the 3-year follow-up; moreover, this study demonstrated that the patients with metabolic syndrome who were at high risk of NOD could safely receive the highest-dose pitavastatin without further increasing the risk of NOD.
Statin therapy and NOD risk
The JUPITER trial revealed that the use of rosuvastatin significantly increased the risk of NOD [20]. In a meta-analysis of randomized controlled trials, the administration of statins increased the risk of NOD by 10–13% [6]. More importantly, higher doses of statins considerably amplified the NOD risk [8]. Despite this less good glycemic control and NOD risk, various guidelines have recommended the use of statin therapy to reduce future major adverse cardiovascular events (MACEs), because the beneficial effects of statin were significantly greater than the known side effects of statins, especially in ACS patients [21]. However, individual assessment to minimize the risk of NOD when administering statins is needed. Although epicardial adipose tissue thickness at systole can be a useful marker for predicting NOD during high‑intensity statin therapy in a previous study, there are inconsistent findings with the pitvastatin [22].
Mechanisms of pitavastatin
Previous studies have suggested several mechanisms of the occurrence of NOD after statin therapy [23]. Although the strong LDL cholesterol lowering effects of statins are driven by the inhibition of HMG-CoA reductase, the isoprenoid levels are down-regulated during this process. Consequently, glucose uptake through GLUT4 in adipocytes may be decreased. In addition, statins directly block glucose-induced calcium channels in pancreatic β-cells and reduce insulin signal transduction. Unlike other statins, pitavastatin has shown at least neutral effects on the development of NOD. It was reported that pitavastatin significantly increased adiponectin levels, which may contribute to its anti-inflammatory and anti-diabetic properties [24]. A pharmacokinetic study of pitavastatin revealed higher systemic bioavailability, leading to increased extrahepatic effects on adipose tissue and circulating adiponectin levels [25]. In previous in vivo studies, adiponectin-deficient mice showed insulin-resistant and glucose-intolerant characteristics and supplementation of adiponectin improved glucose metabolism [26]. It has been well known that the expression of adiponectin receptors is decreased in obese patients, which is associated with the development of DM and atherosclerosis [27]. In addition, significant increases in HDL cholesterol level by pitavastatin could beneficially influence glucose metabolism by facilitating glucose uptake and enhanced insulin sensitivity [28]. Pitavastatin may also preserve adipocyte maturation and glucose transporter GLUT4 expression, which could be decreased with the use of other statins [29].
Pitavastatin and NOD risk
Recently, a study reported that the administration of higher dose pitavastatin significantly decreased the incidence of future MACEs without increasing the risk of NOD compared to a lower dose of pitavastatin [30]. In that study, the overall incidence rate of NOD with pitavastatin was 4.3%. However, the risk of NOD may escalate according to patients’ baseline levels of FBG and triglycerides or BMI. In our study, we enrolled patients who were at high risk of developing NOD and specifically compared the development of NOD according to the number of risk factors for DM. We found that a higher dose of pitavastatin did not increase the risk of NOD. In addition, the number of metabolic syndrome components did not affect the risk of NOD with pitavastatin administration. Although previous studies have reported that the NOD risk was significantly elevated in older patients and women, there were no significant differences in the NOD incidences among those groups in our study [6, 31]. Moreover, any spike in NOD incidence within 1 year after the administration of statins should be carefully evaluated to assess whether it can be causally linked to statin exposure [10]. In our study, comparable incidences of NOD before and after 1 year were observed.
In a previous study, while the administration of statins definitely reduced the incidences of cardiovascular events, pitavastatin was found to increase the NOD risk or worsen the hyperglycemia [32, 33]. However, the baseline characteristics of the retrospectively enrolled patients were heterogeneous and the number of cases was limited to accurately assess the effects on NOD. According to other Asian data, higher dose of pitavastatin improved the FBG level in contrast to other statins [34]. There were no ethnic differences between the efficacy or safety of pitavastatin in Asian and European [35]. Moreover, a meta-analysis demonstrated that pitavastatin did not elevate the FBG and HbA1c levels or the incidence of DM compared to placebo or to other statins [36]. Another study reported that early pitavastatin therapy in patients with metabolic syndrome could be safely administered without any deterioration in glucose intolerance [37]. These results were meaningful in that highest dose pitavastatin could be safely administered in patients at high risk of developing DM, such as those with metabolic syndrome.
The present study has a few limitations. This study was originally designed to investigate the differences in the occurrence of cardiovascular events between pitavastatin 1 mg and 4 mg groups. Moreover, since this study was confined to patients at high risk of developing type 2 DM, our findings should not be extrapolated to a broad spectrum of patients.
Conclusions
In conclusion, when compared to the lowest dose of pitavastatin (1 mg), administration of the highest dose of pitavastatin (4 mg) did not increase the risk of NOD during the 3-year follow-up. Various risk factors for NOD, such as the number of metabolic syndrome components, glucose intolerance, dyslipidemia, obesity, and HTN, did not affect the development of NOD during pitavastatin therapy. Thus, patients with metabolic syndrome who are at high risk of NOD can safely receive the highest dose pitavastatin without increasing their risk of NOD.
Supplementary information
Additional file 1: Table S1. Comparison of the changes in laboratory findings during the 3-year follow-up between the pitavastatin 1 mg and 4 mg groups. Table S2. Comparison of the changes in vascular function during the 3-year follow-up between the pitavastatin 1 mg and 4 mg groups.
Acknowledgements
None.
Abbreviations
- ACS
acute coronary syndrome
- AI
augmentation index
- BMI
body mass index
- BP
blood pressure
- DM
diabetes mellitus
- FBG
fasting blood glucose
- HTN
hypertension
- JUPITER
Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin trial
- NOD
new-onset diabetes
- PWV
pulse wave velocity
Authors’ contributions
HSJ wrote the draft of the manuscript and performed the analyses. SJH designed the study and enrolled the patients. SS and HA performed the analyses. KK, HJJ, JHP, CWY, and D-SL enrolled the patients. All authors read and approved the final manuscript.
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2018M3A9A8017949). The authors are solely responsible for the study design, all analyses, and the drafting of the manuscript.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All patients provided written informed consent. The study was approved by the Korea University Hospital Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-019-0969-z.
References
- 1.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(1–160):3–4. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dormuth CR, Filion KB, Paterson JM, et al. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. doi: 10.1136/bmj.g3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 7.Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–124. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 8.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 9.Koh KK. Letter by Koh regarding article, “Statins and risk of new-onset diabetes mellitus”. Circulation. 2013;127:e837. doi: 10.1161/CIRCULATIONAHA.112.000524. [DOI] [PubMed] [Google Scholar]
- 10.Jones M, Tett S, Peeters GM, Mishra GD, Dobson A. New-onset diabetes after statin exposure in elderly women: the australian longitudinal study on Women’s health. Drugs Aging. 2017;34:203–209. doi: 10.1007/s40266-017-0435-0. [DOI] [PubMed] [Google Scholar]
- 11.Miyauchi K, Kimura T, Shimokawa H, et al. Rationale and design of randomized evaluation of aggressive or moderate lipid lowering therapy with pitavastatin in coronary artery disease (REAL-CAD) trial. Int Heart J. 2018;59:315–320. doi: 10.1536/ihj.17-557. [DOI] [PubMed] [Google Scholar]
- 12.Yokote K, Bujo H, Hanaoka H, et al. Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients: collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study) Atherosclerosis. 2008;201:345–352. doi: 10.1016/j.atherosclerosis.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Nakata M, Nagasaka S, Kusaka I, et al. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49:1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 14.Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57:1535–1545. doi: 10.1016/j.jacc.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee for the Korean Guidelines for the Management of D. 2015 Korean Guidelines for the Management of Dyslipidemia: executive Summary (English Translation) Korean Circ J. 2016;46:275–306. doi: 10.4070/kcj.2016.46.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang KL, Liu CJ, Chao TF, et al. Risk of new-onset diabetes mellitus versus reduction in cardiovascular events with statin therapy. Am J Cardiol. 2014;113:631–636. doi: 10.1016/j.amjcard.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Chen CW, Chen TC, Huang KY, et al. Differential impact of statin on new-onset diabetes in different age groups: a population-based case-control study in women from an asian country. PLoS ONE. 2013;8:e71817. doi: 10.1371/journal.pone.0071817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Anyanwagu U, Mamza J, Donnelly R, Idris I. Effects of background statin therapy on glycemic response and cardiovascular events following initiation of insulin therapy in type 2 diabetes: a large UK cohort study. Cardiovasc Diabetol. 2017;16:107. doi: 10.1186/s12933-017-0587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J, Kim YC, Park JJ, et al. Increased epicardial adipose tissue thickness is a predictor of new-onset diabetes mellitus in patients with coronary artery disease treated with high-intensity statins. Cardiovasc Diabetol. 2018;17:10. doi: 10.1186/s12933-017-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah RV, Goldfine AB. Statins and risk of new-onset diabetes mellitus. Circulation. 2012;126:e282–e284. doi: 10.1161/CIRCULATIONAHA.112.122135. [DOI] [PubMed] [Google Scholar]
- 24.Arnaboldi L, Corsini A. Could changes in adiponectin drive the effect of statins on the risk of new-onset diabetes? The case of pitavastatin. Atheroscler Suppl. 2015;16:1–27. doi: 10.1016/S1567-5688(14)70002-9. [DOI] [PubMed] [Google Scholar]
- 25.Corsini A, Ceska R. Drug-drug interactions with statins: will pitavastatin overcome the statins’ Achilles’ heel? Curr Med Res Opin. 2011;27:1551–1562. doi: 10.1185/03007995.2011.589433. [DOI] [PubMed] [Google Scholar]
- 26.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 28.Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8:237–245. doi: 10.1038/nrendo.2011.235. [DOI] [PubMed] [Google Scholar]
- 29.Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63:735–745. doi: 10.1016/j.metabol.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi I, Iimuro S, Iwata H, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation. 2018;137:1997–2009. doi: 10.1161/CIRCULATIONAHA.117.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172:144–152. doi: 10.1001/archinternmed.2011.625. [DOI] [PubMed] [Google Scholar]
- 32.Rha SW, Choi BG, Seo HS, et al. Impact of statin use on development of new-onset diabetes mellitus in asian population. Am J Cardiol. 2016;117:382–387. doi: 10.1016/j.amjcard.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Lee HS, Lee KY. Effect of statins on fasting glucose in non-diabetic individuals: nationwide population-based health examination in Korea. Cardiovasc Diabetol. 2018;17:155. doi: 10.1186/s12933-018-0799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong YJ, Jeong MH, Bae JH, et al. Efficacy and safety of pitavastatins in patients with acute myocardial infarction: livalo in Acute Myocardial Infarction Study (LAMIS) II. Korean J Intern Med. 2017;32:656–667. doi: 10.3904/kjim.2016.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada-Shiba M, Kastelein JJP, Hovingh GK, et al. Efficacy and safety of pitavastatin in children and adolescents with familial hypercholesterolemia in Japan and Europe. J Atheroscler Thromb. 2018;25:422–429. doi: 10.5551/jat.42242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallejo-Vaz AJ, Kondapally Seshasai SR, Kurogi K, et al. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015;241:409–418. doi: 10.1016/j.atherosclerosis.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Choi SH, Lim S, Hong ES, et al. PROPIT: a PROspective comparative clinical study evaluating the efficacy and safety of PITavastatin in patients with metabolic syndrome. Clin Endocrinol (Oxf) 2015;82:670–677. doi: 10.1111/cen.12580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparison of the changes in laboratory findings during the 3-year follow-up between the pitavastatin 1 mg and 4 mg groups. Table S2. Comparison of the changes in vascular function during the 3-year follow-up between the pitavastatin 1 mg and 4 mg groups.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.