Abstract
This study reports two synthetic approaches leading to 2-aminobenzoxazoles and their N-substituted analogues. Our first synthetic strategy involves a reaction between various o-aminophenols and N-cyano-N-phenyl-p-toluenesulfonamide as a nonhazardous electrophilic cyanating agent in the presence of Lewis acid. The second synthetic approach uses the Smiles rearrangement upon activation of benzoxazole-2-thiol with chloroacetyl chloride. Both developed synthetic protocols are widely applicable, afford the desired aminobenzoxazoles in good to excellent yields, and use nontoxic and inexpensive starting material.
Introduction
2-Aminobenzoxazoles and their N-substituted analogues play an important role in medicinal chemistry and chemical biology.2−8 They are described as potential therapeutic agents including various enzyme inhibitors (proteases, chymase, butyrylcholinesterase, topoisomerase II inhibitors, etc.).5,8,9 They also have applications in materials chemistry.10 In addition, aminobenzoxazoles serve as positron emission tomography probes.3 Therefore, the development of effective methods leading to 2-aminobenzoxazoles and their analogues has attracted great attention.
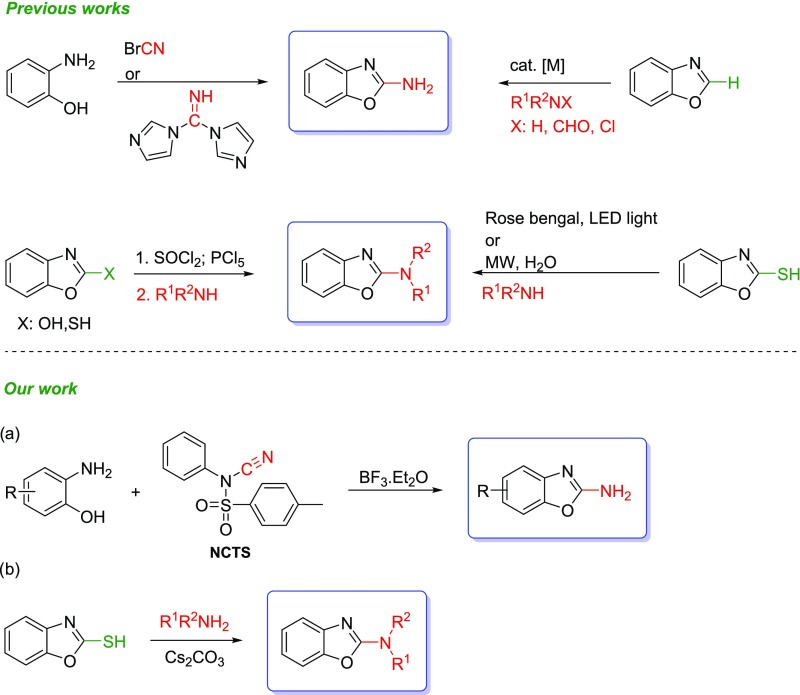
Several methods for synthesis of 2-aminobenzoxazoles have been reported (Scheme 1).9,11−21 However, most of these methods suffer from specific drawbacks. The most published protocol comprises the cyclization of 2-aminophenols using BrCN as a cyanating agent,9,15−19,22 but this BrCN reagent is highly toxic. The next reported approach is the direct 2-C amination of benzoxazoles,23−25 but its drawbacks include the use of transition metal catalysts, high temperatures, nitrogen atmosphere, or co-oxidants. In recent years, many efficient methods have been developed for the synthesis of N-substituted aminobenzoxazoles.26−33 Most of these methods suffer from low yields and use toxic precursors or expensive reagents. N-substituted aminobenzoxazoles can be prepared from corresponding hydroxyl/thiol scaffolds through the aryl halides (Scheme 1).31 Unfortunately, this method requires toxic and acidic halogenating agents. Amination using Rose Bengal as a photocatalyst has been recently reported.29 Despite being efficient and the low cost of Rose Bengal catalyst, this protocol needs a strong organic base (DBU) and has long reaction time that downgrade its advantages. Microwave-enhanced on-water amination of 2-mercaptobenzoxazoles has also been recently introduced.30 However, its drawbacks include an inert atmosphere or co-oxidants. In addition, transition metal catalysts used in this method may be expensive and higher temperatures are sometimes inevitable.
Scheme 1. Aminobenzoxazole Synthesis.
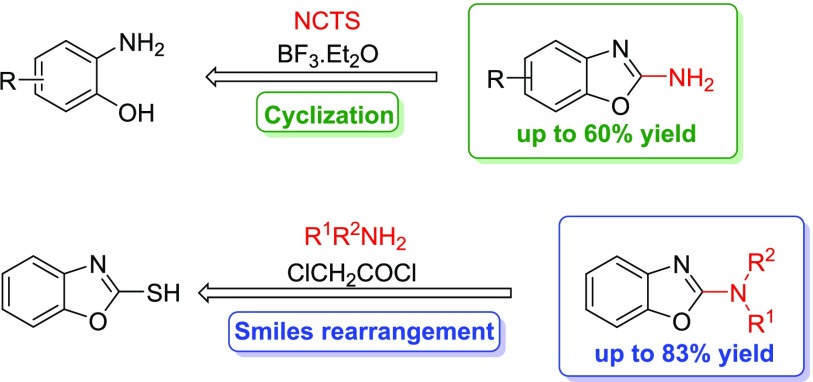
Given the shortcomings of the abovementioned methods, we attempted to develop a new, nontoxic, and efficient protocol relying on an inexpensive starting material. Thus, accordingly, we designed two independent protocols leading to 2-aminobenzoxazoles (Scheme 1, path a) and their N-mono or disubstituted analogues (Scheme 1, path b). The first protocol is based on the use of N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) as a nonhazardous electrophilic cyanating agent. It combines operational simplicity, a nontoxic reagent, and wide substrate scope. The second approach is based on the Smiles rearrangement that enables the functionalization of heteroaromatic rings under economic conditions. Both the developed protocols are widely applicable and afford the desired compounds in good yields, thus indicating their efficacy.
Results and Discussion
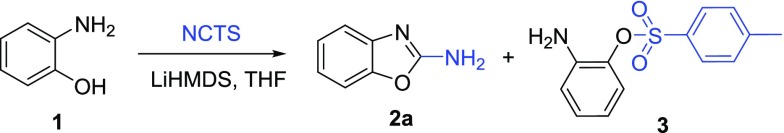
In 2015, Kasthuri et al. reported the synthesis of 2-aminobenzoxazoles using an efficient, air-stable, and nonhazardous electrophilic cyanating agent, i.e., NCTS.14 NCTS can be easily synthesized from inexpensive and commercially available phenyl urea by dehydrative tosylation in pyridine in high yields.34 The synthetic protocol described by Kasthuri et al.14 required 1 equiv of LiHMDS to accomplish the reaction. Unfortunately, despite our efforts to acquire additional optimization, we found the reaction to be irreproducible in our hands as it yielded only up to 11% of the desired product 2a (Table 1, entry 6).35 Compound 3 was always detected as the major product according to liquid chromatography–mass spectrometry (LC–MS) traces.
Table 1. Optimization of the Reaction Conditionsa.
| entry | NCTS (equiv) | LiHMDS (equiv) | T (°C) | resultsb (% of 2a) |
|---|---|---|---|---|
| 1 | 1 | 1 | 0 to rt | 9 |
| 2 | 1 | 3 | 0 to rt | 9 |
| 3 | 1.2 | 1 | 0 to rt | 6 |
| 4 | 1.5 | 1 | 0 to rt | 4 |
| 5 | 1.2 | 3 | 0 to rt | 9 |
| 6 | 1 | 3 | 0 to 60 | 11 |
Reaction conditions: o-aminophenol (0.18 mmol, 20 mg), NCTS, LiHMDS, tetrahydrofuran (THF; 1 mL), 2 h.
Conversion estimated from LC–MS traces at 210–500 nm.
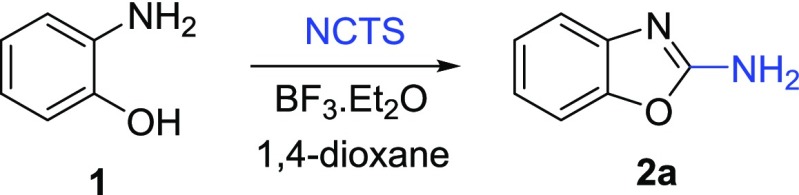
Following these results, we changed the reaction conditions keeping NCTS as a cyanating agent. Initial investigations were aimed at the direct use of NCTS without any activation. Unfortunately, NCTS was found to be ineffective in this case. Thus, we suggested that the use of a strong Lewis acid as BF3·Et2O in combination with NCTS could successfully activate the CN group toward a nucleophilic attack34 and afford the desired product 2a. Indeed, our expectations met with successful results, and after some optimization (Table 2), the desired product 2a was obtained in good yield. Using these optimal conditions, the reaction can proceed even at a 5 mmol scale. This result suggested that the process might be scaled up with only a slightly lower yield.
Table 2. Optimization of the Reaction Conditions with Lewis Acidsa.
| entry | BF3·Et2O (equiv) | NCTS (equiv) | T (°C) | resultsc (% of 2a) |
|---|---|---|---|---|
| 1 | 3 | 3 | reflux | 86 |
| 2 | 3 | 3 | 100b | 39 |
| 3 | 3 | 2 | reflux | 75 |
| 4 | 3 | 1.5 | reflux | 87 |
| 5 | 3 | 1.2 | reflux | 54 |
| 6 | 2 | 1.5 | reflux | 90 |
| 7 | 1 | 1.5 | reflux | 71 |
Reaction conditions: o-aminophenol (0.18 mmol, 20 mg), NCTS, BF3·Et2O, 1,4-dioxane (1 mL), 30 h.
External temperature of the bath.
Conversion estimated from LC–MS traces at 210–500 nm.
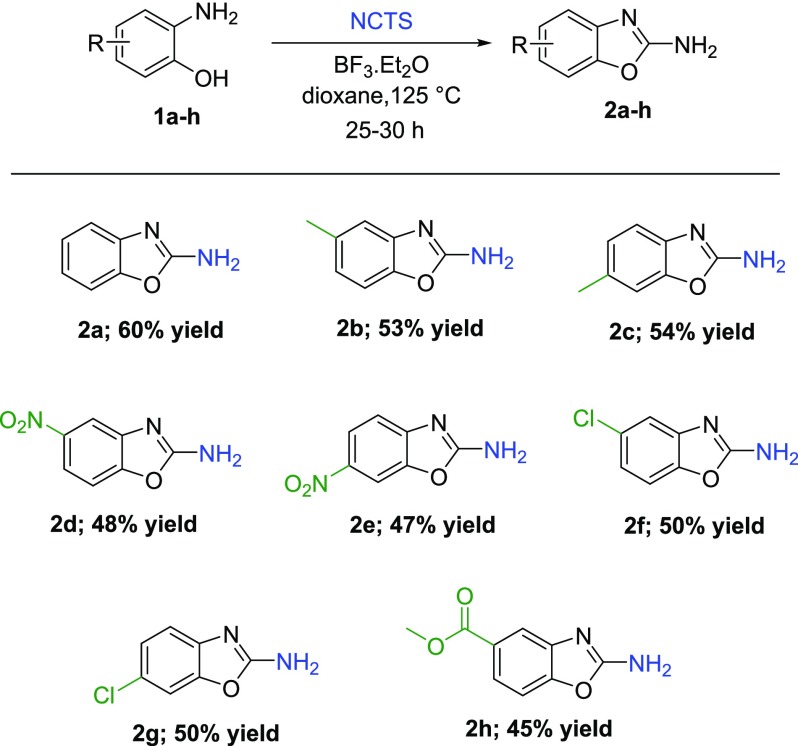
With optimum reaction conditions, the substrate scope was explored (Table 3). We found out that the substitution pattern does not affect the reaction yields. Both EWG and EDG substituents yielded the desired products in moderate to good yields (45–60%). Considering the satisfactory LC–MS conversions, we estimated that the multistep isolation and purification procedure resulted in lowered isolated yields. Hence, different solvents were tried to improve the workup and isolated yield (Supporting Information, Table S1). Unfortunately, none of the used solvents led to a higher yield.
Table 3. Substrate Scope of the Cyclization Reactiona.
Reaction conditions: o-aminophenol (0.9 mmol, 100 mg), NCTS (1.5 equiv), BF3·Et2O (2 equiv), 1,4-dioxane (5 mL), reflux, 25–30 h.
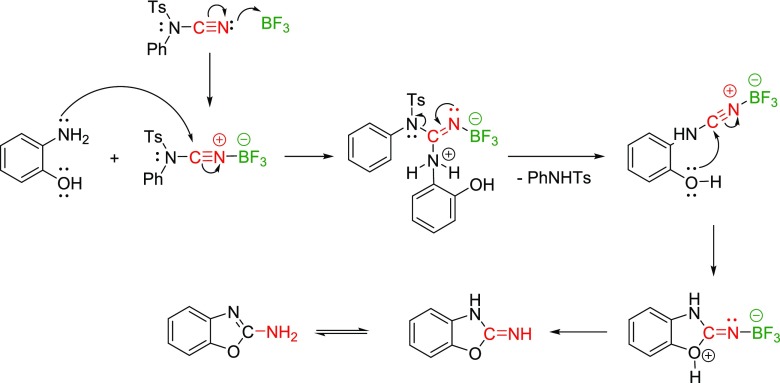
Based on the observations and prevalent literature,36 the reaction mechanism was proposed (Scheme 2). The reaction is initiated through the Lewis acidic activation of NCTS via the coordination of its cyano group to BF3·Et2O. The activation facilitates the subsequent nucleophilic attack of the amino group and the elimination of the sulfonamide residue. The hydroxy group then attacks the electron-deficient carbon. Finally, the desired product 2 is formed during the workup. We confirmed the eliminated sulfonamide by mass m/z = 246 [M – H], providing the next experimental support for our mechanistic proposal.
Scheme 2. Proposed Reaction Pathway.
We were also interested in the catalyst-free synthesis of N-substituted benzoxazole analogues. Recently, the Smiles rearrangement, an intramolecular SNAr reaction, has received renewed attention.37 It enables the functionalization of heteroaromatic rings via breaking a C–X single bond and forming a new C–X or C–C bond under economic conditions. In 2017, Wang et al. published an interesting synthesis of N-aryl-2-aminobenzoxazoles from substituted benzoxazole-2-thiol and 2-chloro-N-arylacetamides in a KOH–dimethylformamide (DMF) system.38 However, this method is described only for aromatic substrates. By taking inspiration from the capabilities of the Smiles rearrangement and commercially available heterocyclic thiols, we suggested a synthetic approach to N-substituted aminobenzoxazoles.
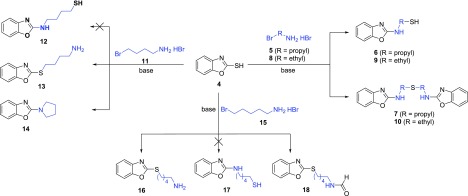
First, we focused on the reaction of benzoxazole-2-thiol with various aliphatic bromoamines and expected the Smiles rearrangement to result in the desired thiol (Scheme 3). Interestingly, some of the investigated substrates reacted giving the mixture of thiol and disulfide or other unexpected products.
Scheme 3. Reaction of Benzoxazole-2-thiol with Various Aliphatic Bromoamines.
The reaction of benzoxazole-2-thiol 4 with 3-bromopropylamine HBr 5 according to the conditions listed by Abdelazeem et al.4 gave a mixture of benzoxazole 6 and disulfide analogue 7 (Scheme 3). The reaction conditions were further optimized concerning the amine and base equivalents (Supporting Information, Table S2). If an excess of the base was used, disulfide was obtained as the main product of the reaction. However, 2 equiv of the amine together with 2 equiv of the base provided compound 6 selectively. Moreover, radical scavenger Et3N strongly suppressed disulfide 7 formation. Therefore, disulfide 7 might be probably formed by a radical mechanism. On the other hand, there was no effect of oxidation (Supporting Information, Table S2, entry 8).
Subsequently, we explored the reaction of benzoxazole-2-thiol with 2-bromoethylamine HBr (Scheme 3). Surprisingly, we observed a different reactivity compared to bromopropylamine (Supporting Information, Table S3). The reaction of benzoxazole-2-thiol started with 4 equiv of the amine and 3 equiv of K2CO3, giving a mixture of products 9 and 10. Disulfide 10 was formed selectively using just 2 equiv of the amine. The temperature was found to play a key role in the reaction. Heating at 70 °C resulted in a mixture of both compounds, and an increased temperature of 120 °C afforded disulfide 10 selectively. Changing the amount of K2CO3 did not provide the desired selectivity. Consequently, we tried Et3N instead and realized that 2 equiv of the amine and 1 equiv of Et3N are the most convenient conditions for the synthesis of compound 9.
We also studied the reactivity of benzoxazole-2-thiol with 4-bromobutylamine HBr 11 (Scheme 3, Supporting Information, Table S4). The bromoamine 11 was synthesized from commercially available aminoalcohol according to the previously described procedure followed by ionex workup.39 Surprisingly, the rapid 5-membered ring closure provided pyrrolidine 14 as the main product regardless of the reaction conditions. This ring-closing reaction is speeded up by negligible ring strain and insignificant entropy change. Lower temperature only slowed all of the conversion down. Finally, 2 equiv of the amine and 2 equiv of Et3N in refluxing toluene provided compound 14 within 4 h.
The reactivity of 5-bromopentylamine HBr 15 was also explored (Scheme 3, Supporting Information, Table S5). Bromoamine 15 was synthesized from 5-aminopentan-1-ol according to the previously described procedure followed by ionex workup.39 The substitution leading to product 16 proceeded smoothly after the careful optimizations using 2 equiv of Et3N in refluxing toluene. Harsher reaction conditions were tried to obtain the rearranged compound 17, but we observed the formylation in DMF instead. As a result, compound 18 was isolated after the microwave irradiation.
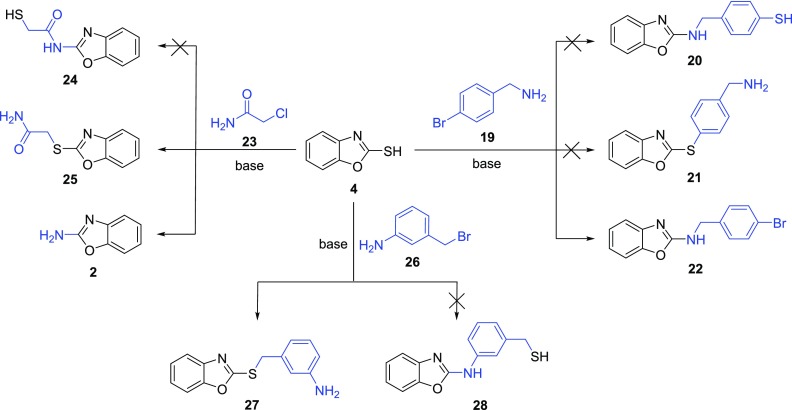
We next investigated the behavior of chloroacetamide as well as aromatic substrates in the Smiles reaction (Scheme 4). For this, we studied the reactivity of aromatic 4-bromobenzylamine 19 (Scheme 4, Supporting Information, Table S6). Lower susceptibility to nucleophilic attack usually makes aromatic halides rather unreactive. However, the unexpected reaction occurred on using 2 equiv of Et3N in refluxing toluene. Under these conditions, the amino group was found to be sufficiently nucleophilic to substitute the thiol, providing benzoxazolamine 22.
Scheme 4. Reaction of Benzoxazole-2-thiol with 4-Bromobenzylamine/2-Chloroacetamide/3-(Bromomethyl)aniline.
The investigation of more challenging substrates such as amides and benzylic bromides gave only the expected substitution products. Their rearrangement was probably prevented by sterically and energetically demanding transition states. First, we examined the reactivity of 2-chloroacetamide 23 (Scheme 4, Supporting Information, Table S7). All of the optimizations gave mostly compound 25 probably due to lower acidic nitrogen nucleophilicity as well as an unfavorable transition state. Moreover, microwave irradiation afforded a mixture of 25 and 2-aminobenzoxazole 2. We also explored the reactivity of 3-(bromomethyl)aniline (Scheme 4, Supporting Information, Table S8). There was no rearrangement regardless of the reaction conditions. We always obtained only the substitution product 27. Finally, reactions with various aromatic bromoanilines were attempted, but no reaction occurred as expected.
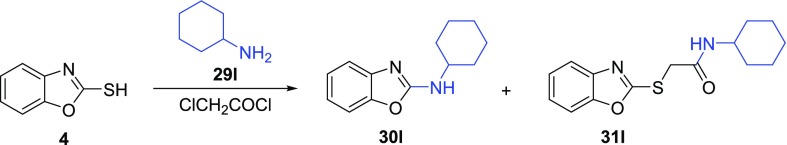
Since we aimed at a wide substrate scope, a universal method was needed. Thus, a more challenging intramolecular variant of the Smiles rearrangement using chloroacetyl chloride was explored (Table 4). First, reaction conditions described by Tian et al.40 applied on cyclohexylamine as a model substrate were attempted (Table 4, entry 1). This one-pot reaction was performed under microwave irradiation as well as under conventional heating in an oil bath at 160 °C. The second reaction provided a slightly better result. Excess chloride or Cs2CO3, as well as changing the solvent, prolonged the reaction time, or the higher temperature gave worse conversion. On the other hand, no rearrangement occurred using different bases. Since bromoacetyl bromide showed better activating properties, milder reaction conditions were considered for aniline (Supporting Information, Table S9). The reaction proceeded even at 85 °C without compromising the isolated yield. Different bases provided only the substitution product 31c. Unfortunately, these milder conditions failed with other amines giving the substitution product 31 only.
Table 4. Optimization of Reaction Conditions with Cyclohexylaminea.
| entry | chloride (equiv) | base (equiv) | T (°C) | time (h) | solvent | ratio of 30l:31l (%)b |
|---|---|---|---|---|---|---|
| 1 | 1.2 | Cs2CO3 (3.2) | 160 (MW) | 1/2 | DMF | 57:0 |
| 2 | 1.2 | Cs2CO3 (3.2) | 160 | 8 | DMF | 58:0 |
| 3 | 1.7 | Cs2CO3 (3.2) | 160 (MW) | 1/2 | DMF | 31:0 |
| 4 | 1.2 | Cs2CO3 (3.7) | 160 (MW) | 1/2 | DMF | 48:0 |
| 5 | 1.2 | Cs2CO3 (3.2) | 160 (MW) | 1/2 | N,N-DMA | 25:0 |
| 6 | 1.2 | Cs2CO3 (3.2) | 160 (MW) | 1/2 | N,N-DMA | 47:0 |
| 7 | 1.2 | DBU (3.2) | reflux | 2 | MeCN | 0:95 |
| 8 | 1.2 | NaH (3.2) | 150 | 2 | N,N-DMA | 0:42 |
Reaction conditions: Benzoxazole-2-thiol (0.16 mmol, 25 mg), cyclohexylamine (0.16 mmol), base, ClCH2COCl, solvent (1 mL).
Conversion estimated from LC–MS traces at 210–500 nm.
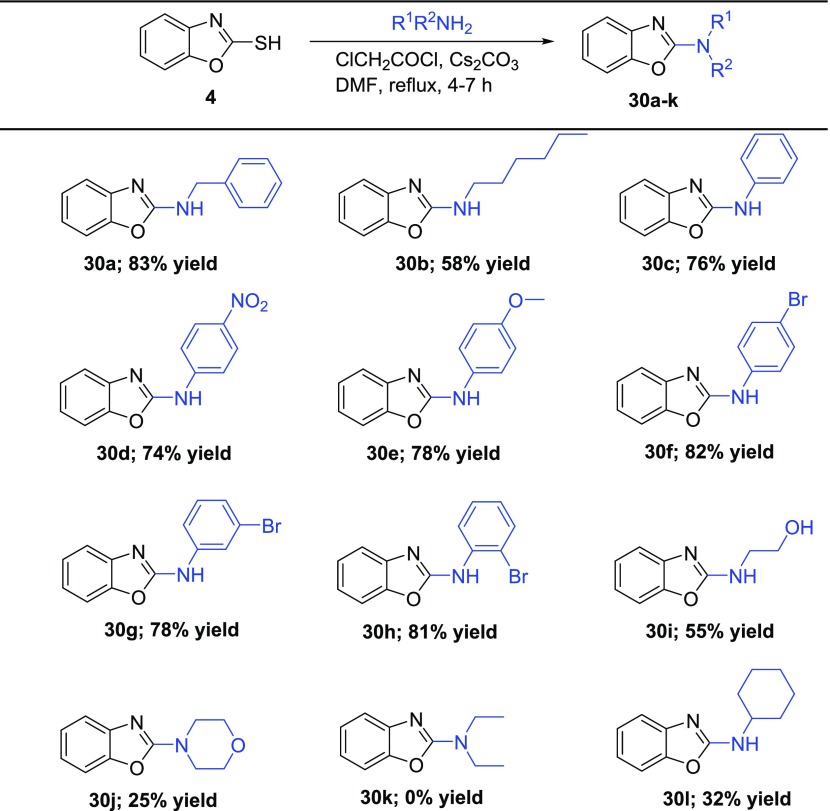
With the optimized reaction conditions, we studied the scope of the rearrangement (Table 5). Various types of amines, including aromatic, aliphatic, alicyclic (both primary and secondary), and bisnucleophilic ethanolamine, were tested. Regardless of the electron effects and substitution patterns, the aromatic and primary amines reacted smoothly, giving the desired products 30a–h in good yields (58–83%). On the other hand, a steric hindrance on the amine lowered the yield significantly. The reaction resulted in a moderate isolated yield of benzoxazole 30l (32%), while the reaction with morpholine gave low yield of 30j (25%). The rearrangement of diethylamine completely failed. Finally, we upscaled the reaction of benzoxazole-2-thiol 4 with aniline to 3.5 mmol of 4, which required a slightly prolonged reaction time (8 h), giving 30c in a 71% isolated yield. This result suggested that the process also can be upscaled with only a slightly lower yield.
Table 5. Substrate Scope of the Rearrangement.
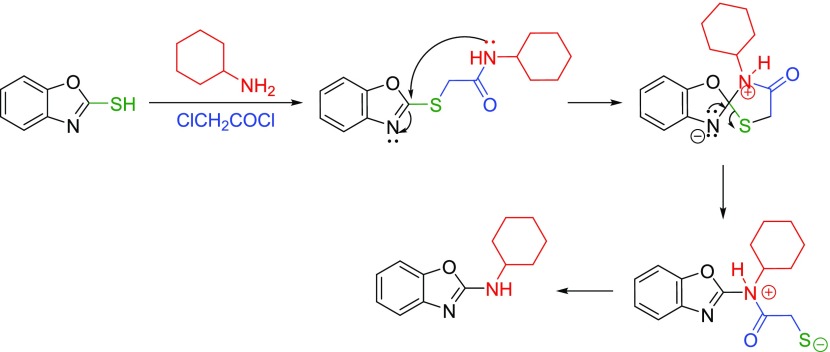
Based on the above observations and the existing literature, the reaction mechanism set out in Scheme 5 was suggested. First, S-alkylated thiol undergoes the Smiles rearrangement by the nucleophilic attack of the nitrogen at the benzoxazole ring carbon, forming a new C–N bond to give the spiro intermediate. Second, rearomatization and alkaline hydrolysis in the presence of Cs2CO3 afford the N-substituted benzoxazole 30.
Scheme 5. Proposed Reaction Pathway.
Conclusions
In conclusion, synthetic strategies affording various 2-aminobenzoxazoles and their N-substituted analogues from readily available starting materials have been developed. Our first approach to substituted 2-aminobenzoxazoles is based on the nonhazardous cyanating agent NCTS, activated with BF3·Et2O. This newly developed synthetic protocol combines operational simplicity, a nontoxic and readily available reagent, and wide substrate scope. Moreover, an efficient one-pot amination of benzoxazole-2-thiol by various amines mediated with chloroacetyl chloride via the intramolecular Smiles rearrangement was developed. Our methodology stands out because of the wide amine scope, short reaction time, and a metal-free approach. The obtained results also indicated that the reactions can be scaled up, providing an alternative process for the chemical industry. Finally, we believe that our methodologies represent a straightforward way to various aminobenzoxazoles and their analogues as important building blocks in organic and medicinal chemistry.
Experimental Section
General Information
Solvents and chemicals were purchased from Sigma-Aldrich (www.sigmaaldrich.com) and Fluorochem (www.fluorochem.co.uk). All reactions were carried out at ambient temperature (21 °C) unless stated otherwise. Analytical thin-layer chromatography (TLC) was performed using aluminum plates precoated with silica gel (silica gel 60 F254).
The LC–MS analyses were carried out on an UHPLC–MS system consisting of UHPLC chromatograph Accela with a photodiode array detector and triple quadrupole mass spectrometer TSQ Quantum Access (Thermo Scientific, CA), using a Nucleodur Gravity C18 column (dimensions 1.8 μm, 2.1 × 50 mm2 at 30 °C and a flow rate of 800 μL/min (Macherey-Nagel, Germany)). The mobile phase was (A) 0.1% ammonium acetate in water and (B) 0.1% ammonium acetate in acetonitrile, linearly programmed from 10 to 80% B over 2.5 min, kept for 1.5 min. The column was re-equilibrated with 10% of solution B for 1 min. The APCI source operated at a discharge current of 5 μA, vaporizer temperature of 400 °C, and a capillary temperature of 200 °C.
NMR 1H/13C spectra were recorded on a JEOL ECX-500SS (500 MHz) or JEOL ECA400II (400 MHz) spectrometer at magnetic field strengths of 11.75 T (with operating frequencies 500.16 MHz for 1H and 125.77 MHz for 13C) and 9.39 T (with operating frequencies 399.78 MHz for 1H and 100.53 MHz for 13C) at ambient temperature (∼21 °C). Chemical shifts (δ) are reported in parts per million (ppm), and coupling constants (J) are reported in hertz (Hz). NMR spectra were recorded at ambient temperature (21 °C) in DMSO-d6 and referenced to the resonance signal of the solvent.
HRMS analysis was performed with LC–MS and an Orbitrap high-resolution mass spectrometer (Dionex, Ultimate 3000, Thermo Exactive plus, MA) operating in positive full scan mode in the range of 80–1200 m/z. The settings for electrospray ionization were as follows: 150 °C oven temperature and 3.6 kV source voltage. The acquired data were internally calibrated with phthalate as a contaminant in methanol (m/z 297.15909). Samples were diluted to a final concentration of 0.1 mg/mL in a solution of water and acetonitrile (50:50, v/v). The samples were injected into the mass spectrometer following HPLC separation on a Kinetex C18 column (2.6 μm, 100 A, 50 × 3.0 mm2) using an isocratic mobile phase of 0.01 M MeCN/ammonium acetate (80/20) at a flow rate of 0.3 mL/min.
All reactions carried out under microwave irradiation were performed with the CEM Discover SP microwave synthesizer, using the dynamic mode in the following settings: maximum amount of microwave power (150 W), premixing time (1 min), and stirring speed (high). A simultaneous cooling of the reaction vessel provided by compressed air (24 psi) was applied during the entire experiment (PowerMax option “ON”). All 0.5 mmol scale reactions were performed in a 10 mL borosilicate glass reaction vessel closed with a disposable silicon cap and equipped with a Teflon-coated egg-shaped magnetic stir bar. The temperature was monitored by an external infrared sensor.
General Procedures and Characterization of Individual Compounds
Cyclization of Aminophenols with NCTS
O-aminophenol 1 (0.9 mmol; 1 equiv) and NCTS (1.35 mmol; 1.5 equiv) were dissolved in 1,4-dioxane (4 mL). BF3·Et2O (1.8 mmol; 2 equiv) was added dropwise. The mixture was refluxed overnight (monitored by TLC, 24–30 h). After that, the cooled (rt) mixture was quenched with sat. NaHCO3 (pH ∼ 7), diluted with H2O (30 mL), and extracted with EtOAc (3 × 30 mL). Combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
Benzo[d]oxazol-2-amine (2a)
Brown solid. Yield: 60% (72 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.35 (s, 2H), 7.30 (d, J = 7.8 Hz, 1H), 7.19 (d, J = 7.3 Hz, 1H), 7.08 (t, J = 7.2 Hz, 1H), 6.95 (t, J = 7.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 162.70, 147.92, 143.61, 123.45, 119.92, 115.25, 108.38. HRMS: m/z: calcd for C7H6N2O: 135.0553 [M + H]+; found: 135.0554.
5-Methylbenzo[d]oxazol-2-amine (2b)
Brown solid. Yield: 53% (71 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.29 (s, 2H), 7.16 (d, J = 8.0 Hz, 1H), 7.00 (s, 1H), 6.75 (d, J = 7.9 Hz, 1H), 2.30 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 162.89, 146.09, 143.78, 132.47, 120.46, 115.69, 107.80, 21.10. HRMS: m/z: calcd for C8H8N2O: 149.0709 [M + H]+; found: 149.0709.
6-Methylbenzo[d]oxazol-2-amine (2c)
Brown solid. Yield: 54% (72 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.29 (s, 2H), 7.14 (s, 1H), 7.06 (d, J = 7.9 Hz, 1H), 6.90 (d, J = 7.6 Hz, 1H), 2.32 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 162.30, 148.06, 140.89, 129.38, 124.09, 114.69, 108.9, 20.97. HRMS: m/z: calcd for C8H8N2O: 149.0709 [M + H]+; found: 149.0710.
5-Nitrobenzo[d]oxazol-2-amine (2d)
Yellow solid. Yield: 48% (77 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.98–7.96 (m, 1H), 7.95–7.90 (m, 3H), 7.55 (d, J = 8.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 164.80, 152.31, 144.65, 144.36, 116.65, 109.98, 108.64. HRMS: m/z: calcd for C7H5N3O3: 178.0258 [M – H]+; found: 178.0242.
6-Nitrobenzo[d]oxazol-2-amine (2e)
Yellow solid. Yield: 47% (75 mg). 1H NMR (400 MHz, DMSO-d6): δ 8.29–8.17 (m, 3H), 8.10 (dd, J = 8.7, 2.3 Hz, 1H), 7.32 (d, J = 8.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 166.35, 150.99, 147.19, 140.29, 121.09, 114.13, 104.65. HRMS: m/z: calcd for C7H5N3O3: 178.0258 [M – H]+; found: 178.0241.
5-Chlorobenzo[d]oxazol-2-amine (2f)
Brown solid. Yield: 50% (76 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.58 (s, 2H), 7.32 (d, J = 8.4 Hz, 1H), 7.22 (d, J = 2.1 Hz, 1H), 6.97 (dd, J = 8.4, 2.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 163.86, 146.73, 145.30, 127.65, 119.43, 114.88, 109.39. HRMS: m/z: calcd for C7H5ClN2O: 169.0163 [M + H]+; found: 169.0165.
6-Chlorobenzo[d]oxazol-2-amine (2g)
Red solid. Yield: 50% (75 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.52 (s, 2H), 7.48 (d, J = 1.8 Hz, 1H), 7.17 (d, J = 8.3 Hz, 1H), 7.12 (dd, J = 8.3, 2.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 163.31, 148.27, 142.80, 123.69, 123.54, 115.85, 109.13. HRMS: m/z: calcd for C7H5ClN2O: 169.0163 [M + H]+; found: 169.0164.
Methyl 2-Aminobenzo[d]oxazol-5-carboxylate (2h)
Yellow solid. Yield: 45% (49 mg). 1H NMR (500 MHz, DMSO-d6): δ 7.72 (d, J = 1.7 Hz, 1H), 7.65 (dd, J = 8.3, 1.7 Hz, 1H), 7.63 (s, 2H), 7.43 (d, J = 8.3 Hz, 1H), 3.84 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 166.36, 163.71, 151.30, 144.02, 125.32, 122.17, 115.78, 108.47, 52.05. HRMS: m/z: calcd for C9H8N2O3: 193.0608 [M + H]+; found: 193.0607.
Cyclization of Aminophenols with LiHMDS
O-Aminophenol 1 (1.61 mmol; 1 equiv) and NCTS were dissolved in THF (dry, 2.5 mL). The mixture was cooled down to 5 °C, and 1 M LiHMDS in hexane (1.61 mmol; 1 equiv) was added dropwise. It was stirred at 5 °C to rt for 1 h. Then, the reaction mixture was poured into ice water (50 mL) and stirred for 15 min. This was followed by an extraction with EtOAc (3 × 50 mL), and the organic layers were washed with brine (1×), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
2-Aminophenyl-4-methylbenzenesulfonate (3)
Brown solid. Yield: 35% (148 mg). 1H NMR (500 MHz, DMSO-d6): δ 9.28 (s, 2H), 7.63 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.2 Hz, 2H), 7.13 (dd, J = 8.0, 1.6 Hz, 1H), 6.91 (td, J = 7.9, 1.6 Hz, 1H), 6.72 (dd, J = 8.0, 1.1 Hz, 1H), 6.68 (td, J = 7.9, 1.4 Hz, 1H), 2.33 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 150.19, 142.89, 137.82, 129.42, 126.86, 126.15, 124.40, 119.04, 115.59, 21.05. HRMS: m/z: calcd for C13H13NO3S: 264.0689 [M + H]+; found: 264.0689.
Smiles Rearrangement with 3-Bromopropylamine HBr
Benzoxazole-2-thiol 4 (1.61 mmol, 1 equiv), K2CO3 (3.2 mmol; 2 equiv/4.8 mmol; 3 equiv), and 3-bromopropylamine HBr 5 (3.2 mmol; 2 equiv/1.61 mmol; 1 equiv) were suspended in DMF (10 mL). The reaction mixture was stirred at 70 °C for 2 h. It was followed by dilution with H2O (50 mL) and extraction with EtOAc (3 × 50 mL). Organic layers were washed with brine (1×), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
3-(Benzo[d]oxazol-2-ylamino)propane-1-thiol (6)
White solid. Yield: 67% (222 mg). 1H NMR (500 MHz, DMSO-d6): δ 7.90 (t, J = 5.6 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.23 (d, J = 7.1 Hz, 1H), 7.10 (td, J = 7.6, 1.0 Hz, 1H), 6.96 (td, J = 7.7, 1.2 Hz, 1H), 3.39 (dd, J = 12.6, 6.7 Hz, 2H), 2.56 (dd, J = 13.8, 6.8 Hz, 2H), 2.40 (t, J = 7.3 Hz, 1H), 1.86 (p, J = 6.9 Hz, 2H). 13C NMR (126 MHz, DMSO-d6): δ 162.31, 147.98, 143.24, 123.51, 120.02, 115.36, 108.42, 40.74, 32.96, 21.11. HRMS: m/z: calcd for C10H12N2OS: 209.0743 [M + H]+; found: 209.0743.
N,N′-(Disulfanediylbis(propane-3,1-diyl)bis(benzo[d]-oxazol-2-amine)) (7)
Yellow solid. Yield: 66% (219 mg). 1H NMR (500 MHz, DMSO-d6): δ 7.94 (t, J = 5.6 Hz, 2H), 7.31 (d, J = 7.8 Hz, 2H), 7.23 (d, J = 7.4 Hz, 2H), 7.09 (td, J = 7.7, 0.9 Hz, 2H), 6.96 (td, J = 7.8, 1.1 Hz, 2H), 3.39 (dd, J = 12.7, 6.6 Hz, 4H), 2.81 (t, J = 7.1 Hz, 4H), 2.03–1.90 (m, 4H). 13C NMR (126 MHz, DMSO-d6): δ 162.25, 147.99, 143.23, 123.51, 120.04, 115.39, 108.43, 40.89, 35.02, 28.38. HRMS: m/z: calcd for C20H22N4O2S2: 415.1257 [M + H]+; found: 415.1258
Smiles Rearrangement with 2-Bromoethylamine HBr
Benzoxazole-2-thiol 4 (1.61 mmol, 1 equiv), K2CO3 (4.8 mmol; 3 equiv), and 2-bromoethylamine HBr 8 (3.2 mmol; 2 equiv) were suspended in DMF (10 mL). The reaction mixture was stirred at 120 °C for 2 h. This was followed by dilution with H2O (50 mL) and extraction with EtOAc (3 × 50 mL). Organic layers were washed with brine (1×), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
N,N′-(Disulfanediylbis(ethane-2,1-diyl)bis(benzo[d]-oxazol-2-amine)) (10)
White solid. Yield: 71% (220 mg). 1H NMR (400 MHz, DMSO-d6): δ 8.14 (t, J = 5.7 Hz, 2H), 7.33 (d, J = 7.8 Hz, 2H), 7.25 (d, J = 7.6 Hz, 2H), 7.10 (td, J = 7.5, 0.9 Hz, 2H), 6.97 (td, J = 7.7, 1.1 Hz, 2H), 3.61 (dd, J = 12.9, 6.4 Hz, 4H), 3.01 (t, J = 6.7 Hz, 4H). 13C NMR (101 MHz, DMSO-d6): δ 162.03, 148.02, 143.08, 123.62 120.24, 115.56, 108.58, 41.45, 36.86. HRMS: m/z: calcd for C18H18N4O2S2: 387.0944 [M + H]+; found: 387.0944.
Smiles Rearrangement with 4-Bromobutylamine HBr
Benzoxazole-2-thiol 4 (0.6 mmol, 1 equiv) and Et3N (1.2 mmol, 2 equiv) were dissolved in toluene (5 mL) and premixed at rt for 10 min. Subsequently, 4-bromobutylamine HBr 11 (1.2 mmol, 2 equiv) was added. The reaction mixture was refluxed for 4 h. It was cooled to room temperature, diluted with H2O (20 mL), and extracted with EtOAc (3 × 20 mL). Organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
2-(Pyrrolidin-1-yl)benzo[d]oxazole (14)
White solid. Yield: 54% (60 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.37 (dd, J = 7.9, 0.5 Hz, 1H), 7.25 (dd, J = 7.8, 0.8 Hz, 1H), 7.12 (td, J = 7.7, 1.1 Hz, 1H), 6.97 (td, J = 7.7, 1.3 Hz, 1H), 3.54 (ddd, J = 6.7, 4.3, 2.6 Hz, 4H), 2.00–1.93 (m, 4H). 13C NMR (101 MHz, DMSO-d6): δ 160.51, 148.54, 143.59, 123.72, 119.77, 115.36, 108.63, 47.16, 25.03. HRMS: m/z: calcd for C11H12N2O: 189.1022 [M + H]+; found: 189.1022.
Smiles Rearrangement with 5-Bromopentylamine HBr
Benzoxazole-2-thiol 4 (0.6 mmol, 1 equiv) and Et3N (1.2 mmol, 2 equiv) were dissolved in toluene (5 mL) and premixed at rt for 10 min. Subsequently, 5-bromopentylamine HBr 15 (1.2 mmol, 2 equiv) was added. The reaction mixture was refluxed for 4 h. It was cooled to room temperature, diluted with H2O (20 mL), and extracted with EtOAc (3 × 20 mL). Organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo.
5-(Benzo[d]oxazol-2-ylthio)pentan-1-amine HBr (16)
White solid. Yield: 95% (134 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.68–7.59 (m, 2H), 7.37–7.28 (m, 2H), 3.54 (br s, 3H), 3.37–3.28 (m, 2H), 2.60 (t, J = 6.5 Hz, 2H), 1.85–1.71 (m, 2H), 1.53–1.35 (m, 4H). 13C NMR (101 MHz, DMSO-d6): δ 164.47, 151.18, 141.31, 124.52, 124.12, 118.15, 110.09, 41.04, 31.77, 31.68, 28.75, 25.30. HRMS: m/z: calcd for C12H16N2OS: 237.1056 [M + H]+; found: 237.1056.
Smiles Rearrangement with 16
Compound 16 (0.42 mmol, 1 equiv) and Et3N (0.84 mmol, 2 equiv) were dissolved in DMF (4 mL). The reaction mixture was stirred under microwave irradiation at 120 °C for 2 h. After that, it was cooled to room temperature, diluted with H2O (20 mL), and extracted with EtOAc (3 × 20 mL). Organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
N-(5-(Benzo[d]oxazol-2-ylthio)pentyl)formamide (18)
Brown oil. Yield: 58% (64 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.99 (s, 1H), 7.97 (br s, 1H), 7.68–7.59 (m, 2H), 7.36–7.28 (m, 3H), 3.35–3.28 (m, 2H, overlapped with H2O), 3.09 (q, J = 6.3 Hz, 2H), 1.84–1.73 (m, 2H), 1.50–1.38 (m, 4H). 13C NMR (101 MHz, DMSO-d6): δ 164.43, 160.87, 151.18, 141.31, 124.52, 124.12, 118.16, 110.10, 36.83, 31.59, 28.51, 28.39, 25.30. HRMS: m/z: calcd for C13H16N2O2S: 265.1005 [M + H]+; found: 265.1006.
Smiles Rearrangement with 4-Bromobenzylamine
Benzoxazole-2-thiol 4 (0.6 mmol, 1 equiv) and Et3N (1.2 mmol, 2 equiv) were dissolved in toluene (5 mL) and premixed at rt for 10 min. Subsequently, 4-bromobenzylamine 19 (1.2 mmol, 2 equiv) was added. The reaction mixture was refluxed overnight (monitored by TLC, 18 h). It was cooled to room temperature, diluted with H2O (20 mL), and extracted with EtOAc (3 × 20 mL). Organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
N-(4-Bromobenzyl)benzo[d]oxazol-2-amine (22)
Brown solid. Yield: 69% (125 mg). 1H NMR (400 MHz, DMSO-d6): δ 8.48 (t, J = 6.1 Hz, 1H), 7.56–7.49 (m, 2H), 7.34 (d, J = 8.6 Hz, 3H), 7.24 (dd, J = 7.8, 0.6 Hz, 1H), 7.10 (td, J = 7.6, 1.0 Hz, 1H), 6.98 (td, J = 7.7, 1.2 Hz, 1H), 4.50 (d, J = 6.0 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 162.29, 148.11, 143.01, 138.54, 131.21, 129.39, 123.61, 120.28, 120.02, 115.58, 108.58, 45.02. HRMS: m/z: calcd for C14H11BrN2O: 303.0128 [M + H]+; found: 303.0126.
Smiles Rearrangement with 2-Chloroacetamide
Benzoxazole-2-thiol 4 (1.61 mmol, 1 equiv), K2CO3 (4.8 mmol; 3 equiv), and 2-chloroacetamide 23 (3.2 mmol; 2 equiv) were suspended in DMF (10 mL). The reaction mixture was stirred at 70 °C for 4 h. It was cooled to room temperature, diluted with H2O (50 mL), and extracted with EtOAc (3 × 50 mL). Organic layers were washed with brine (1×), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
2-(Benzo[d]oxazol-2-ylthio)acetamide (25)
Yellow solid. Yield: 46% (152 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.75 (s, 1H), 7.63 (ddd, J = 7.0, 3.1, 1.2 Hz, 2H), 7.39–7.27 (m, 3H), 4.12 (s, 2H). 13C NMR (101 MHz, DMSO-d6): δ 168.00, 164.10, 151.28, 141.27, 124.63, 124.25, 118.19, 110.19, 35.82. HRMS: m/z: calcd for C9H8N2O2S: 209.0379 [M + H]+; found: 209.0378.
Smiles Rearrangement with 3-(Bromomethyl)aniline
Benzoxazole-2-thiol 4 (0.6 mmol, 1 equiv), 3-(bromomethyl)aniline 26 (1.2 mmol, 2 equiv), and K2CO3 (1.2 mmol, 2 equiv) were suspended in DMF (5 mL). The reaction mixture was stirred at 70 °C for 2 h. It was cooled to room temperature, diluted with H2O (20 mL), and extracted with EtOAc (3 × 20 mL). Organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
3-((Benzo[d]oxazol-2-ylthio)methyl)aniline (27)
White solid. Yield: 80% (123 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.65 (m, 2H), 7.37–7.29 (m, 2H), 6.97 (t, J = 7.7 Hz, 1H), 6.66 (t, J = 1.9 Hz, 1H), 6.59 (d, J = 7.5 Hz, 1H), 6.47 (m, 1H), 5.13 (br s, 2H), 4.47 (s, 2H). 13C NMR (101 MHz, DMSO-d6): δ 164.04, 151.22, 148.92, 141.28, 136.52, 129.10, 124.60, 124.27, 118.27, 116.19, 114.01, 113.35, 110.18, 36.00. HRMS: m/z: calcd for C14H12N2OS: 257.0743 [M + H]+; found: 257.0742.
Smiles Rearrangement with Amines
To a stirred solution of amine 29 (0.48 mmol, 1 equiv) and Cs2CO3 (1.54 mmol, 3.2 equiv) in DMF (3 mL) cooled to −5 °C were added chloroacetyl chloride (0.58 mmol, 1.2 equiv) and benzoxazole-2-thiol 4 (0.48 mmol, 1 equiv). The reaction mixture was refluxed (monitored by TLC, 4–7 h). After that, the cooled (rt) mixture was diluted by H2O (20 mL), extracted with CH2Cl2 (3 × 30 mL), and washed with brine (50 mL). Combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc and Tol/MeCN.
N-Benzylbenzo[d]oxazol-2-amine (30a)
Yellow solid. Yield: 83% (89 mg). 1H NMR (500 MHz, DMSO-d6): δ 8.45 (t, J = 6.1 Hz, 1H), 7.41–7.30 (m, 5H), 7.25 (dd, J = 15.1, 7.8 Hz, 2H), 7.10 (td, J = 7.7, 1.0 Hz, 1H), 6.98 (td, J = 7.8, 1.2 Hz, 1H), 4.52 (d, J = 6.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 162.46, 148.13, 143.15, 139.06, 128.39, 127.19, 127.06, 123.64, 120.24, 115.54, 108.60, 45.69. HRMS: m/z: calcd for C9H12N2O4S: 225.1022 [M + H]+; found: 225.1023.
N-Hexylbenzo[d]oxazol-2-amine (30b)
Orange solid. Yield: 58% (60 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.89 (t, J = 5.6 Hz, 1H), 7.31 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 7.6 Hz, 1H), 7.09 (t, J = 7.6 Hz, 1H), 6.95 (td, J = 7.8, 0.9 Hz, 1H), 3.28 (dd, J = 13.0, 6.8 Hz, 2H), 1.62–1.51 (m, 2H), 1.35–1.25 (m, 6H), 0.86 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, DMSO-d6): δ 162.40, 147.99, 143.40, 123.52, 119.94, 115.32, 108.42, 42.29, 30.98, 28.88, 25.96, 22.10, 13.93. HRMS: m/z: calcd for C13H18N2O: 219.1492 [M + H]+; found: 219.1491
N-Phenylbenzo[d]oxazol-2-amine (30c)
Yellow solid. Yield: 76% (76 mg). 1H NMR (400 MHz, DMSO-d6): δ 10.61 (s, 1H), 7.76 (d, J = 7.8 Hz, 2H), 7.47 (dd, J = 13.3, 7.7 Hz, 2H), 7.37 (t, J = 7.9 Hz, 2H), 7.22 (td, J = 7.7, 0.9 Hz, 1H), 7.13 (td, J = 7.8, 1.2 Hz, 1H), 7.03 (t, J = 7.4 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 157.97, 146.99, 142.43, 138.73, 129.00, 124.01, 122.12, 121.68, 117.55, 116.62, 108.97. HRMS: m/z: calcd for C13H10N2O: 211.0866 [M + H]+; found: 211.0864
N-(4-Nitrophenyl)benzo[d]oxazol-2-amine (30d)
Yellow solid. Yield: 74% (90 mg). 1H NMR (400 MHz, DMSO-d6): δ 11.45 (s, 1H), 8.30 (d, J = 9.1 Hz, 2H), 7.98 (d, J = 8.8 Hz, 2H), 7.60–7.54 (m, 2H), 7.29 (t, J = 7.6 Hz, 1H), 7.22 (t, J = 7.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 156.87, 147.00, 144.93, 141.67, 141.27, 125.37, 124.38, 122.68, 117.30, 117.15, 109.41. HRMS: m/z: calcd for C13H9N3O3: 256.0717 [M + H]+; found: 256.0719.
N-(4-Methoxyphenyl)benzo[d]oxazol-2-amine (30e)
Orange solid. Yield: 78% (90 mg). 1H NMR (500 MHz, DMSO-d6): δ 10.36 (s, 1H), 7.65 (d, J = 9.1 Hz, 2H), 7.45 (d, J = 7.8 Hz, 1H), 7.41 (d, J = 7.1 Hz, 1H), 7.19 (td, J = 7.6, 1.1 Hz, 1H), 7.09 (td, J = 7.7, 1.2 Hz, 1H), 6.96 (d, J = 9.1 Hz, 2H), 3.74 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 158.36, 154.65, 147.08, 142.64, 131.92, 123.91, 121.34, 119.15, 116.34, 114.23, 108.82, 55.25. HRMS: m/z: calcd for C14H12N2O2: 241.0972 [M + H]+; found: 241.0970.
N-(4-Bromophenyl)benzo[d]oxazol-2-amine (30f)
Yellow solid. Yield: 82% (113 mg). 1H NMR (500 MHz, DMSO-d6): δ 10.77 (s, 1H), 7.74 (d, J = 8.9 Hz, 2H), 7.55 (d, J = 8.9 Hz, 2H), 7.48 (dd, J = 14.3, 7.7 Hz, 2H), 7.23 (td, J = 7.7, 1.0 Hz, 1H), 7.15 (td, J = 7.8, 1.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 157.63, 146.97, 142.19, 138.14, 131.77, 124.11, 121.94, 119.46, 116.77, 113.58, 109.09. HRMS: m/z: calcd for C13H9BrN2O: 288.9971 [M + H]+; found: 288.9969.
N-(3-Bromophenyl)benzo[d]oxazol-2-amine (30g)
Yellow solid. Yield: 78% (108 mg). 1H NMR (400 MHz, DMSO-d6) δ 10.82 (s, 1H), 8.11 (t, J = 1.9 Hz, 1H), 7.67 (dd, J = 8.2, 1.2 Hz, 1H), 7.51 (d, J = 8.9 Hz, 2H), 7.33 (t, J = 8.1 Hz, 1H), 7.23 (m, 2H), 7.16 (td, J = 7.6, 1.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 157.44, 146.94, 142.07, 140.34, 130.97, 124.66, 124.16, 122.08, 121.93, 119.62, 116.94, 116.46, 109.14. HRMS: m/z: calcd for C13H9BrN2O: 288.9971 [M + H]+; found: 288.9971.
N-(2-Bromophenyl)benzo[d]oxazol-2-amine (30h)
White solid. Yield: 81% (112 mg). 1H NMR (400 MHz, DMSO-d6): δ 9.87 (s, 1H), 7.93 (s, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.45 (t, J = 7.2 Hz, 2H), 7.37 (d, J = 7.6 Hz, 1H), 7.25–7.08 (m, 3H). 13C NMR (101 MHz, DMSO-d6): δ 133.00, 128.48, 126.53, 125.46, 124.07, 121.55, 117.20, 109.10. HRMS: m/z: calcd for C13H9BrN2O: 288.9971 [M + H]+; found: 288.9969.
2-(Benzo[d]oxazol-2-ylamino)ethan-1-ol (30i)
White solid. Yield: 55% (47 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.92 (t, J = 5.7 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 7.4 Hz, 1H), 7.09 (td, J = 7.7, 0.8 Hz, 1H), 6.96 (td, J = 7.7, 1.1 Hz, 1H), 4.80 (t, J = 5.5 Hz, 1H), 3.56 (q, J = 5.9 Hz, 2H), 3.35 (q, J = 5.8 Hz, 2H overlapped with H2O). 13C NMR (101 MHz, DMSO-d6): δ 162.49, 148.03, 143.31, 123.56, 120.01, 115.34, 108.47, 59.44, 44.99. HRMS: m/z: calcd for C9H10N2O2: 179.0815 [M + H]+; found: 179.0814.
2-Morpholinobenzo[d]oxazole (30j)
White solid. Yield: 31% (30 mg). 1H NMR (400 MHz, DMSO-d6) δ 7.42–7.40 (m, 1H), 7.33–7.28 (m, 1H), 7.16 (td, J = 7.6, 1.1 Hz, 1H), 7.03 (td, J = 7.7, 1.3 Hz, 1H), 3.76–3.69 (m, 4H), 3.62–3.55 (m, 4H).13C NMR (101 MHz, DMSO-d6): δ 161.85, 148.30, 142.79, 124.02, 120.69, 115.98, 108.98, 65.41, 45.37. HRMS: m/z: calcd for C11H12N2O2: 205.0972 [M + H]+; found: 205.0972.
N-Cyclohexylbenzo[d]oxazol-2-amine (30l)
Orange solid. Yield: 33% (34 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.84 (d, J = 7.7 Hz, 1H), 7.31 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 7.7 Hz, 1H), 7.08 (t, J = 7.6 Hz, 1H), 6.95 (t, J = 7.7 Hz, 1H), 3.54 (brs, 1H), 1.96 (m, 2H), 1.73 (m, 2H), 1.59 (m, 1H), 1.36–1.23 (m, 4H), 1.21–1.10 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 161.65, 147.85, 143.41, 123.50, 119.90, 115.27, 108.37, 51.56, 32.42, 25.20, 24.55. HRMS: m/z: calcd for C13H16N2O: 217.1335 [M + H]+; found: 217.1337.
Substitution with Cyclohexylamine
To a stirred solution of amine 29 (0.48 mmol, 1 equiv) and DBU (1.54 mmol, 3.2 equiv) in MeCN (3 mL) cooled to −5 °C were added chloroacetyl chloride (0.58 mmol, 1.2 equiv) and benzoxazole-2-thiol 4 (0.48 mmol, 1 equiv). The reaction mixture was refluxed (monitored by TLC, 3 h). After that, the cooled (rt) mixture was diluted by H2O (20 mL), extracted with CH2Cl2 (3 × 30 mL), and washed with brine (50 mL). Combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography using Hex/EtOAc.
2-(Benzo[d]oxazol-2-ylthio)-N-cyclohexylacetamide (31l)
White solid. Yield: 62% (86 mg). 1H NMR (400 MHz, DMSO-d6): δ 8.19 (d, J = 7.7 Hz, 1H), 7.66–7.58 (m, 2H), 7.38–7.29 (m, 2H), 4.11 (s, 2H), 3.62–3.48 (m, 1H), 1.79–1.63 (m, 4H), 1.53 (m, 1H), 1.32–1.11 (m, 5H). 13C NMR (101 MHz, DMSO-d6): δ 164.96, 163.94, 151.22, 141.24, 124.61, 124.24, 118.14, 110.12, 48.02, 35.83, 32.14, 25.11, 24.31. HRMS: m/z: calcd for C15H18N2O2S: 291.1162 [M + H]+; found: 291.1161.
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sport of the Czech Republic (project IGA_PrF_2019_027) and by grant no. JG_2019_002 from Palacký University in Olomouc.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02702.
Optimization of the reaction conditions with Lewis acids, 3-bromopropylamine HBr, 2-bromoethylamine HBr, 4-bromobutylamine HBr, 5-bromopentylamine HBr, 4-bromobenzylamine, 2-chloroacetamide, 3-(bromomethyl)aniline, and aniline; copies of 1H and 13C NMR spectra (Tables S1–S9) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Šlachtová V.; Brulikova L. Benzoxazole Derivatives as Promising Antitubercular Agents. ChemistrySelect 2018, 3, 4653–4662. 10.1002/slct.201800631. [DOI] [Google Scholar]
- Cui M.; Ono M.; Kimura H.; Ueda M.; Nakamoto Y.; Togashi K.; Okamoto Y.; Ihara M.; Takahashi R.; Liu B.; Saji H. Novel 18F-Labeled Benzoxazole Derivatives as Potential Positron Emission Tomography Probes for Imaging of Cerebral +--Amyloid Plaques in Alzheimer’s Disease. J. Med. Chem. 2012, 55, 9136–9145. 10.1021/jm300251n. [DOI] [PubMed] [Google Scholar]
- Abdelazeem A. H.; Khan S. I.; White S. W.; Sufka K. J.; McCurdy C. R. Design, synthesis and biological evaluation of bivalent benzoxazolone and benzothiazolone ligands as potential anti-inflammatory/analgesic agents. Bioorg. Med. Chem. 2015, 23, 3248–3259. 10.1016/j.bmc.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmange J. C.; Weiss M. M.; Germain J.; Polverino A. J.; Borg G.; Bready J.; Chen D.; Choquette D.; Coxon A.; DeMelfi T.; DiPietro L.; Doerr N.; Estrada J.; Flynn J.; Graceffa R. F.; Harriman S. P.; Kaufman S.; La D. S.; Long A.; Martin M. W.; Neervannan S.; Patel V. F.; Potashman M.; Regal K.; Roveto P. M.; Schrag M. L.; Starnes C.; Tasker A.; Teffera Y.; Wang L.; White R. D.; Whittington D. A.; Zanon R. Naphthamides as Novel and Potent Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors: Design, Synthesis, and Evaluation. J. Med. Chem. 2008, 51, 1649–1667. 10.1021/jm701097z. [DOI] [PubMed] [Google Scholar]
- Khajondetchairit P.; Phuangsawai O.; Suphakun P.; Rattanabunyong S.; Choowongkomon K.; Gleeson M. P. Design, synthesis, and evaluation of the anticancer activity of 2-amino-aryl-7-aryl-benzoxazole compounds. Chem. Biol. Drug Des. 2017, 90, 987–994. 10.1111/cbdd.13025. [DOI] [PubMed] [Google Scholar]
- Sweis R. F.; Hunt J. A.; Sinclair P. J.; Chen Y.; Eveland S. S.; Guo Q.; Hyland S. A.; Milot D. P.; Cumiskey A. M.; Latham M.; Rosa R.; Peterson L.; Sparrow C. P.; Anderson M. S. 2-(4-Carbonylphenyl)benzoxazole inhibitors of CETP: Attenuation of hERG binding and improved HDLc-raising efficacy. Bioorg. Med. Chem. Lett. 2011, 21, 2597–2600. 10.1016/j.bmcl.2011.02.049. [DOI] [PubMed] [Google Scholar]
- Potashman M. H.; Bready J.; Coxon A.; DeMelfi T. M. Jr; DiPietro L.; Doerr N.; Elbaum D.; Estrada J.; Gallant P.; Germain J.; Gu Y.; Harmange J. C.; Kaufman S. A.; Kendall R.; Kim J. L.; Kumar G. N.; Long A. M.; Neervannan S.; Patel V. F.; Polverino A.; Rose P.; Van der Plas S.; Whittington D.; Zanon R.; Zhao H. Design, Synthesis, and Evaluation of Orally Active Benzimidazoles and Benzoxazoles as Vascular Endothelial Growth Factor-2 Receptor Tyrosine Kinase Inhibitors. J. Med. Chem. 2007, 50, 4351–4373. 10.1021/jm070034i. [DOI] [PubMed] [Google Scholar]
- Chikhale R.; Thorat S.; Choudhary R. K.; Gadewal N.; Khedekar P. Design, synthesis and anticancer studies of novel aminobenzazolyl pyrimidines as tyrosine kinase inhibitors. Bioorg. Chem. 2018, 77, 84–100. 10.1016/j.bioorg.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Ko C. W.; Tao Y. T.; Danel A.; Krzeminska L.; Tomasik P. Organic Light-Emitting Diodes Based on 2-(Stilbene-4-yl)benzoxazole Derivatives: An Implication on the Emission Mechanism. Chem. Mater. 2001, 13, 2441–2446. 10.1021/cm010199u. [DOI] [Google Scholar]
- Grytsai O.; Druzhenko T.; Demange L.; Ronco C.; Benhida R. Cyanoguanidine as a versatile, eco-friendly and inexpensive reagent for the synthesis of 2-aminobenzoxazoles and 2-guanidinobenzoxazoles. Tetrahedron Lett. 2018, 59, 1642–1645. 10.1016/j.tetlet.2018.03.036. [DOI] [Google Scholar]
- Nagano T.; Itoh M.; Matsumura K. Preparation of certain derivatives of benzoxazole. J. Am. Chem. Soc. 1953, 75, 2770–2771. 10.1021/ja01107a511. [DOI] [Google Scholar]
- Wu Y. Q.; Limburg D. C.; Wilkinson D. E.; Hamilton G. S. Formation of nitrogen-containing heterocycles using di(imidazole-1-yl)methanimine. J. Heterocycl. Chem. 2003, 40, 191–193. 10.1002/jhet.5570400129. [DOI] [Google Scholar]
- Kasthuri M.; Babu H. S.; Kumar K. S.; Sudhakar C.; Kumar P. V. N. A Facile Synthesis of 2-Aminobenzoxazoles and 2-Aminobenzimidazoles Using N-Cyano-N-phenyl-p-toluenesulfonamide (NCTS) as an Efficient Electrophilic Cyanating Agent. Synlett 2015, 26, 897–900. 10.1055/s-0034-1380166. [DOI] [Google Scholar]
- Glotz G.; Lebl R.; Dallinger D.; Kappe C. O. Integration of Bromine and Cyanogen Bromide Generators for the Continuous-Flow Synthesis of Cyclic Guanidines. Angew. Chem., Int. Ed. 2017, 56, 13786–13789. 10.1002/anie.201708533. [DOI] [PubMed] [Google Scholar]
- Reddy L. M.; Prakash T. B.; Padmaja A.; Padmavathi V. Synthesis and antimicrobial activity of pyrazolyl benzoxazoles, benzothiazoles and benzimidazoles. Med. Chem. Res. 2015, 24, 970–979. 10.1007/s00044-014-1180-0. [DOI] [Google Scholar]
- Gudipati R.; Anreddy R. N. R.; Manda S. Synthesis, anticancer and antioxidant activities of some novel N-(benzo[d]oxazol-2-yl)-2-(7- or 5-substituted-2-oxoindolin-3-ylidene) hydrazinecarboxamide derivatives. J. Enzyme Inhib. Med. Chem. 2011, 26, 813–818. 10.3109/14756366.2011.556630. [DOI] [PubMed] [Google Scholar]
- Kaupp G.; Schmeyers J.; Boy J. Quantitative gas-solid reactions with ClCN and BrCN: synthesis of cyanamides, cyanates, thiocyanates, and their derivatives. Chem. - Eur. J. 1998, 4, 2467–2474. . [DOI] [Google Scholar]
- Schuart J.; Mueller H. K. Reactions with 2-aminobenzoxazoles. 8. 2-Aminooxazole and 2-iminooxazoline. Pharmazie 1975, 30, 155–157. [PubMed] [Google Scholar]
- Liu J.; Hoover J. M. Cobalt-Catalyzed Aerobic Oxidative Cyclization of 2-Aminophenols with Isonitriles: 2-Aminophenol Enabled O2 Activation by Cobalt(II). Org. Lett. 2019, 21, 4510–4514. 10.1021/acs.orglett.9b01384. [DOI] [PubMed] [Google Scholar]
- Lee S.; Lee Y. Copper-Catalyzed Electrophilic Amination of Benzoxazoles via Magnesation. Eur. J. Org. Chem. 2019, 2019, 3045–3050. 10.1002/ejoc.201900335. [DOI] [Google Scholar]
- Wu Y. Q.; Limburg D. C.; Wilkinson D. E.; Hamilton G. S. Formation of nitrogen-containing heterocycles using di(imidazole-1-yl)methanimine. J. Heterocycl. Chem. 2003, 40, 191–193. 10.1002/jhet.5570400129. [DOI] [Google Scholar]
- Kim J. Y.; Cho S. H.; Joseph J.; Chang S. Cobalt- and Manganese-Catalyzed Direct Amination of Azoles under Mild Reaction Conditions and the Mechanistic Details. Angew. Chem., Int. Ed. 2010, 49, 9899–9903. 10.1002/anie.201005922. [DOI] [PubMed] [Google Scholar]
- Cho S. H.; Kim J. Y.; Lee S. Y.; Chang S. Silver-mediated direct amination of benzoxazoles: tuning the amino group source from formamides to parent amines. Angew. Chem., Int. Ed. 2009, 48, 9127–9130. 10.1002/anie.200903957. [DOI] [PubMed] [Google Scholar]
- Wang J.; Hou J. T.; Wen J.; Zhang J.; Yu X. Q. Iron-catalyzed direct amination of azoles using formamides or amines as nitrogen sources in air. Chem. Commun. 2011, 47, 3652–3654. 10.1039/c0cc05811d. [DOI] [PubMed] [Google Scholar]
- Cioffi C. L.; Lansing J. J.; Yuksel H. Synthesis of 2-Aminobenzoxazoles Using Tetramethyl Orthocarbonate or 1,1-Dichlorodiphenoxymethane. J. Org. Chem. 2010, 75, 7942–7945. 10.1021/jo1017052. [DOI] [PubMed] [Google Scholar]
- Liu B.; Yin M.; Gao H.; Wu W.; Jiang H. Synthesis of 2-Aminobenzoxazoles and 3-Aminobenzoxazines via Palladium-Catalyzed Aerobic Oxidation of o-Aminophenols with Isocyanides. J. Org. Chem. 2013, 78, 3009–3020. 10.1021/jo400002f. [DOI] [PubMed] [Google Scholar]
- Liu M.; Zeng M. T.; Xu W.; Wu L.; Dong Z. B. Selective synthesis of 2-aminobenzoxazoles and 2-mercaptobenzoxazoles by using o-aminophenols as starting material. Tetrahedron Lett. 2017, 58, 4352–4356. 10.1016/j.tetlet.2017.09.092. [DOI] [Google Scholar]
- Rattanangkool E.; Sukwattanasinitt M.; Wacharasindhu S. Organocatalytic Visible Light Enabled SNAr of Heterocyclic Thiols: A Metal-Free Approach to 2-Aminobenzoxazoles and 4-Aminoquinazolines. J. Org. Chem. 2017, 82, 13256–13262. 10.1021/acs.joc.7b02357. [DOI] [PubMed] [Google Scholar]
- Tankam T.; Srisa J.; Sukwattanasinitt M.; Wacharasindhu S. Microwave-Enhanced On-Water Amination of 2-Mercaptobenzoxazoles To Prepare 2-Aminobenzoxazoles. J. Org. Chem. 2018, 83, 11936–11943. 10.1021/acs.joc.8b01824. [DOI] [PubMed] [Google Scholar]
- Wang G.; Peng Z.; Wang J.; Li J.; Li X. Synthesis, biological evaluation and molecular docking study of N-arylbenzo[d]oxazol-2-amines as potential +--glucosidase inhibitors. Bioorg. Med. Chem. 2016, 24, 5374–5379. 10.1016/j.bmc.2016.08.061. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Jia X.; Wang J.; Fan X. An economically and environmentally sustainable synthesis of 2-aminobenzothiazoles and 2-aminobenzoxazoles promoted by water. Green Chem. 2011, 13, 413–418. 10.1039/C0GC00418A. [DOI] [Google Scholar]
- Zhou Y.; Liu Z.; Yuan T.; Huang J.; Liu C. The synthesis of 2-aminobenzoxazoles using reusable ionic liquid as a green catalyst under mild conditions. Molecules 2017, 22, 576/1–576/9. 10.3390/molecules22040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo S. N-Cyano-N-phenyl-p-toluenesulfonamide. Synlett 2014, 25, 1337–1338. 10.1055/s-0033-1341246. [DOI] [Google Scholar]
- Yang Y.; Zhang Y.; Wang J. B. Lewis Acid Catalyzed Direct Cyanation of Indoles and Pyrroles with N-Cyano-N-phenyl-p-toluenesulfonamide (NCTS). Org. Lett. 2011, 13, 5608–5611. 10.1021/ol202335p. [DOI] [PubMed] [Google Scholar]
- Nagata T.; Matsubara H.; Kiyokawa K.; Minakata S. Catalytic Activation of 1-Cyano-3,3-dimethyl-3-(1H)-1,2-benziodoxole with B(C6F5)3 Enabling the Electrophilic Cyanation of Silyl Enol Ethers. Org. Lett. 2017, 19, 4672–4675. 10.1021/acs.orglett.7b02313. [DOI] [PubMed] [Google Scholar]
- Holden C. M.; Greaney M. F. Modern Aspects of the Smiles Rearrangement. Chem. - Eur. J. 2017, 23, 8992–9008. 10.1002/chem.201700353. [DOI] [PubMed] [Google Scholar]
- Wang G.; Wang J.; Li L.; Chen S.; Peng Y.; Xie Z.; Chen M.; Deng B.; Li W. Synthesis of N-aryl-2-aminobenzoxazoles from substituted benzoxazole-2-thiol and 2-chloro-N-arylacetamides in KOH-DMF system. Heterocycles 2017, 94, 1257–1268. 10.3987/COM-17-13712. [DOI] [Google Scholar]
- Minin P. L.; Walton J. C. Radical Ring Closures of 4-Isocyanato Carbon-Centered Radicals. J. Org. Chem. 2003, 68, 2960–2963. 10.1021/jo034002o. [DOI] [PubMed] [Google Scholar]
- Tian X.; Wu R. M.; Liu G.; Li Z. B.; Wei H. L.; Yang H.; Shin D. S.; Wang L. Y.; Zuo H. Smiles rearrangement for the synthesis of diarylamines. ARKIVOC 2011, 118–126. 10.3998/ark.5550190.0012.a10. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.