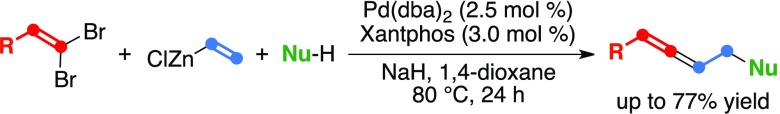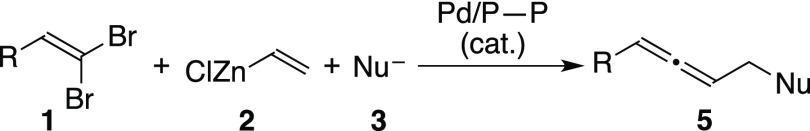Abstract
The three-component coupling reaction of 1,1-dibromoalkenes 1, vinylzinc chloride 2, and carbon soft nucleophiles 3 was realized in the presence of the catalytic palladium/Xantphos species, and 1,3-disubstituted allenic products 5 were obtained in yields of up to 77%. The successive two palladium-catalyzed processes, namely the cross-coupling reaction and the allylic substitution, assembled 5 from the easily accessible starting compounds.
Introduction
Allenes have emerged as unique building blocks in synthetic organic chemistry,1 and their convenient synthetic methods have been highly desirable. Construction of allenic C=C=C frameworks by multicomponent coupling reactions of easily accessible compounds is an attractive procedure for preparing allenic compounds.
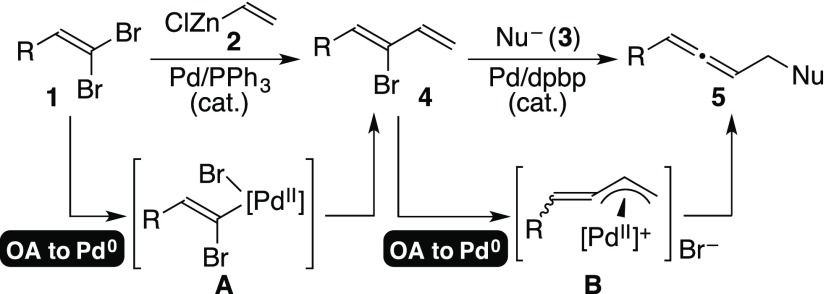
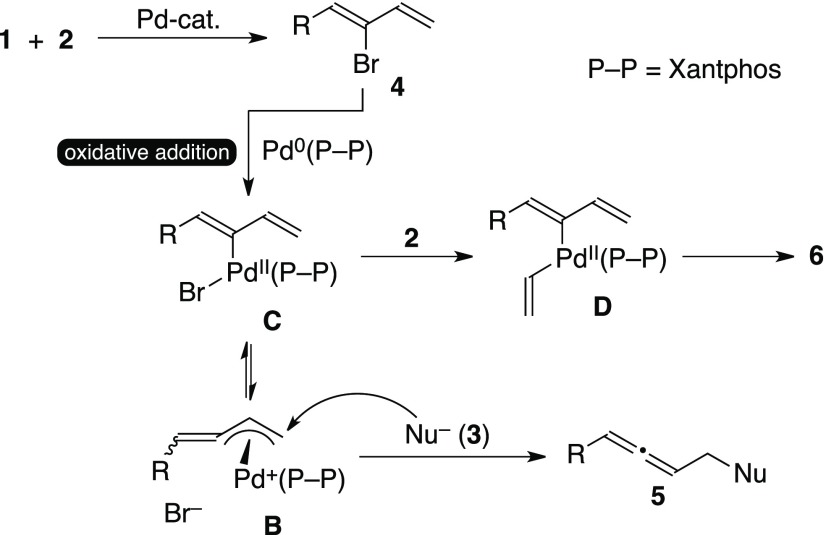
In 2000, we developed a palladium-catalyzed reaction for preparing various functionalized allenes 5 starting with 1-hydrocarbyl-2-bromo-1,3-dienes 4 and appropriate soft nucleophiles 3 (Scheme 1).2 By the use of a chiral palladium catalyst, the reaction could provide scalemic axially chiral allenes 5 in up to 94% ee.3 The reaction proceeds via (alkylidene-π-allyl)palladium intermediate B,4 that is somewhat similar to the widely accepted intermediates in the Tsuji–Trost reaction.5 An analogous (alkylidene-π-allyl)palladium species can be generated by the addition of an allenylmethyl ester to a zero-valent palladium species and its reaction with a soft nucleophile gives a comparable allenic product as well.6 Bromodiene substrates 4 were prepared by the regioselective cross-coupling reaction between 1,1-dibromoalkenes 1 and vinylzinc chloride 2 in the presence of catalytic Pd(PPh3)4.2a The two reaction steps shown in Scheme 1 are analogous to each other; both are palladium-catalyzed processes which involve the oxidative addition of an olefinic C–Br bond to a zero-valent palladium species, and generated palladium(II) intermediates A or B react with nucleophilic reagents subsequently (Scheme 1).
Scheme 1. Reported “Stepwise” Process of Preparing 1,3-Disubstituted Allenes Using Two Different Palladium Precatalysts.
Due to the close similarity of the two palladium-catalyzed reactions, we envisioned that if we could manage both reaction steps in “one-pot” in the presence of a single palladium species starting with a mixture of three reactants 1, 2, and 3, the whole reaction could be a unique three-component coupling reaction giving allenic compounds 5 by a single operation (Scheme 2). After the extensive optimization of the reaction conditions, indeed, the three-component coupling reactions have been realized. Here, we report details of our efforts toward this goal.
Scheme 2. Palladium-Catalyzed Three-Component Coupling of Preparing 1,3-Disubstituted Allenes.
Results and Discussion
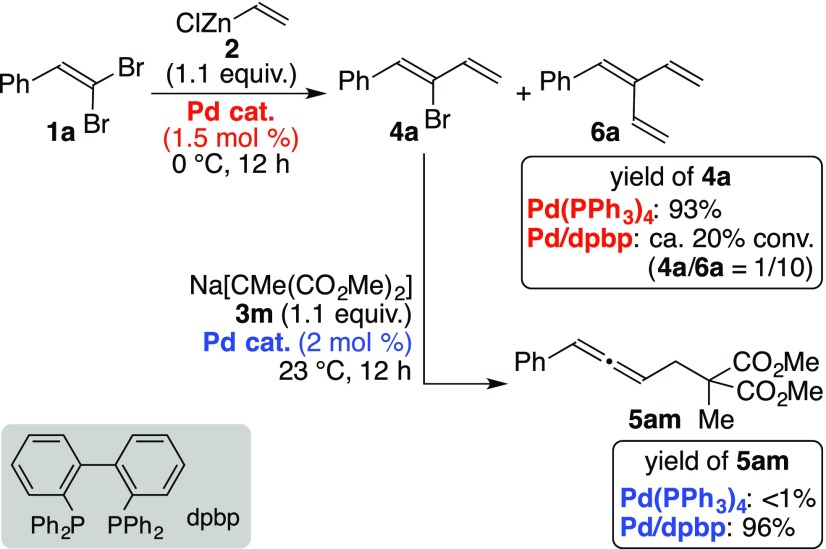
In the previous reports,2a the two different palladium precatalysts were employed in the respective reaction steps in Scheme 1. While Pd(PPh3)4 (1.5 mol %) was chosen as a suitable precatalyst in the regioselective cross-coupling reaction of 1 with 2, the reaction between 3 and 4 was catalyzed by a palladium species (2.0 mol %) generated in situ from [PdCl(π-allyl)]2 and 2,2′-bis(diphenylphosphino)-1,1′-biphenyl (dpbp).7 Under the reported conditions, the two palladium species were not interconvertible. That is, the cross-coupling reaction between 1a and 2 was much slower and much less regioselective in the presence of the Pd/dpbp species (1.5 mol %) instead of Pd(PPh3)4. The reaction showed ca. 20% conversion and the formation of triene 6a was dominant under the otherwise identical conditions. Alternatively, the use of Pd(PPh3)4 (2.0 mol %) in the reaction between 3m and 4a provided the desired allenic product 5am in less than a 1% yield (Scheme 3).
Scheme 3. Specificity of Palladium Precatalysts in Converting 1a to 5am via 4a.
At the outset, a possibility of the three-component coupling was investigated for the reaction between 1a, a vinyl nucleophile (2, 2′, or 2″), and 3m. The results of the screening are summarized in Table 1. A treatment of 1a with 2 (1.5 equiv) and 3m (1.5 equiv) in the presence of a palladium species generated from [Pd(π-allyl)Cl]2 and dpbp (2.5 mol %) at 60 °C in THF for 24 h gave a mixture of 1a, 4a, 6a, and 5am in 44:2:51:3 molar ratio (determined by 1H NMR; entry 1). The use of vinyltin reagent 2′ instead of 2 led to complete consumption of 1a, however, a considerable amount of uncharacterized species was formed together with 4a and 6a. Allene 5am was not detected in the reaction mixture (entry 2). The reaction with vinylmagnesium chloride 2″ was very messy and the formation of 5am was not observed (entry 3). From the results in entries 1–3, zinc reagent 2 was chosen as a vinyl nucleophile. Preformed Pd(PPh3)4 was slightly more effective than the Pd/dpbp species giving a mixture of 4a, 6a, and 5am in 28:60:12 molar ratio. Although 1a was completely consumed, the main product was triene 6a, and the formation of allene 5am was still minimal (entry 4). The Pd/dppf precatalyst8 gave 4a as a major product, with a small amount of 5am (entry 5). The yield of 5am was improved to 31% by the use of DPEphos9 as an ancillary ligand, and the formation of 6a was somewhat suppressed (entry 6). Reducing the amount of 2 (1.0 equiv) or lowering the reaction temperature (40 °C) did not improve the 6a/5m ratios, while the reactions were slower (entries 7 and 8). Running the reaction at 80 °C in 1,4-dioxane dramatically improved the yield of 5am to 62% (entry 9). The use of a palladium species generated from [Pd(π-allyl)Cl]2 and Xantphos9 further improved the selectivity of the reaction and 5am was isolated in a 77% yield (entry 10). The combination of Pd(dba)2 and Xantphos was equally effective for the reaction to give 5am in a 74% isolated yield (entry 11).
Table 1. Optimization of Reaction Conditions for Three-Component Coupling between 1a, 2, and 3ma.
| entry | Pd precursor | ligand | vinyl-[M] | x | T (°C) | solvent | 1a/4a/6a/5amb | 5am (%)c |
|---|---|---|---|---|---|---|---|---|
| 1 | [Pd(π-allyl)Cl]2 | dpbp | 2 | 1.5 | 60 | THF | 44/2/51/3 | |
| 2 | [Pd(π-allyl)Cl]2 | dpbp | 2′ | 1.5 | 60 | THF | 0/48/52/0d | |
| 3 | [Pd(π-allyl)Cl]2 | dpbp | 2″ | 1.5 | 60 | THF | messy | |
| 4e | Pd(PPh3)4 | 2 | 1.5 | 60 | THF | 0/28/60/12 | ||
| 5 | [Pd(π-allyl)Cl]2 | dppf | 2 | 1.5 | 60 | THF | 1/60/31/8 | |
| 6 | [Pd(π-allyl)Cl]2 | DPEphos | 2 | 1.5 | 60 | THF | 0/51/16/33 | 31 |
| 7 | [Pd(π-allyl)Cl]2 | DPEphos | 2 | 1.0 | 60 | THF | 0/72/9/19 | |
| 8 | [Pd(π-allyl)Cl]2 | DPEphos | 2 | 1.5 | 40 | THF | 0/82/7/11 | |
| 9 | [Pd(π-allyl)Cl]2 | DPEphos | 2 | 1.5 | 80 | 1,4-dioxane | 0/0/34/66 | 62 |
| 10 | [Pd(π-allyl)Cl]2 | Xantphos | 2 | 1.5 | 80 | 1,4-dioxane | 0/0/10/90 | 77 |
| 11 | Pd(dba)2 | Xantphos | 2 | 1.5 | 80 | 1,4-dioxane | 0/0/12/88 | 74 |
The reaction was carried out with 1a (0.50 mmol), 2, and 3m (0.75 mmol) in the presence of a palladium catalyst (2.5 mol %) generated in situ from a palladium precursor and a bisphosphine ligand.
Determined by the 1H NMR measurement of the crude reaction mixture.
Isolated yield by silica gel chromatography.
Uncharacterized species were detected together with 4a and 6a.
Preformed tetrakis(triphenylphosphine)palladium(0) was used.
Due to air-stability and easier handling of Pd(dba)2, the conditions of entry 11 in Table 1 were chosen as the optimized conditions, and the reactions with various dibromoolefins 1 and nucleophiles 3 were conducted in the same way. The results of the three-component coupling are summarized in Table 2. The reaction works well with various 1-aryl-2,2-dibromoethylenes 1a–f (entries 1–6). A wide range of aryl substituents, such as electron-rich (1b, 1d, and 1f), electron-poor (1c), bulky (1d–f), and organometallic (1f) aryl groups, can be employed in 1, and their reactions with 2 and 3m afforded the corresponding allenic products in good yields ranging 51–74%. On the other hand, aliphatic dibromoolefins 1g–i showed different results in the reaction with 2 and 3m, depending on the alkyl substituents. While substrate 1g, which is with a bulky tert-butyl substituent, gave allene 5gm in a 62% yield (entry 7), 1h (with a sec-alkyl, cyclohexyl, group) provided allene 5hm in a 14% yield (entry 8) and the reaction of 1i (with a primary alkyl, n-octyl, group) did not give allenic products (entry 9). The major products in the reactions of 1h or 1i were the corresponding bromodienes 4 and trienes 6.
Table 2. Scope of Dibromoolefins 1 and Nucleophiles 3 in the Palladium-Catalyzed Three-Component Couplinga.
| entry | R in 1 | nucleophile 3 | 1/4/6/5b | 5 (%)c |
|---|---|---|---|---|
| 1 | Ph (1a) | Na[CMe(CO2Me)2] (3m) | 0/0/12/88 | 74 (5am) |
| 2 | 4-MeO-C6H4 (1b) | Na[CMe(CO2Me)2] (3m) | 0/0/22/78 | 64 (5bm) |
| 3 | 4-F3C-C6H4 (1c) | Na[CMe(CO2Me)2] (3m) | 0/0/11/89 | 71 (5cm) |
| 4 | 2,4,6-Me3-C6H2 (1d) | Na[CMe(CO2Me)2] (3m) | 0/0/36/64 | 51 (5dm) |
| 5 | 1-naphthyl (1e) | Na[CMe(CO2Me)2] (3m) | 0/0/32/68 | 63 (5em) |
| 6 | ferrocenyl (1f) | Na[CMe(CO2Me)2] (3m) | 0/8/26/66 | 53 (5fm) |
| 7 | tBu (1g) | Na[CMe(CO2Me)2] (3m) | trace/5/5/90 | 62 (5gm) |
| 8 | Cy (1h) | Na[CMe(CO2Me)2] (3m) | 6/38/38/18 | 14 (5hm) |
| 9 | nC8H17 (1i) | Na[CMe(CO2Me)2] (3m) | 3/37/60/0 | ND |
| 10 | Ph (1a) | Na[CEt(CO2Et)2] (3n) | 0/0/15/85 | 68 (5an) |
| 11 | Ph (1a) | Na[CPh(CO2Et)2] (3o) | 0/0/14/86 | 69 (5ao) |
| 12 | Ph (1a) | Na[CH(CO2Me)2] (3p) | 0/5/52/43 | 33 (5ap) |
| 13 | Ph (1a) | Na[CH(SO2Ph)2] (3q) | 0/0/32/68 | 49 (5aq) |
| 14 | Ph (1a) | Na[BDT] (3r) | 0/0/10/90 | 61 (5ar) |
| 15 | Ph (1a) | K[N(boc)2] (3s) | messy | ND |
The reaction was carried out with 1 (0.45–0.50 mmol), 2 (1.5 equiv to 1), 3 (1.5 equiv to 1), and a palladium catalyst (2.5 mol %) generated in situ from Pd(dba)2 and Xantphos.
Determined by the 1H NMR measurement of the crude reaction mixture.
Isolated yield by silica gel chromatography.
The present protocol could be applicable to the other carbon soft nucleophiles as well. Sodium salts 3n and 3o, which are from ethylmalonate and phenylmalonate, reacted with 1a in the same way to give the allenic products in 68 and 69% yields, respectively (entries 10 and 11). The reaction of 3p, that is an anionic derivative from parent malonate, was sluggish and the yield of 5ap was 33% (entry 12). Bis(sulphonyl)methane-derived nucleophiles, 3q and 3r, could be used in the present protocol affording the allenic products in 49 and 61% yields, respectively (entries 13 and 14). Allenes 5ap, 5aq, and 5ar possess acidic hydrogen in them, and thus, they are capable of functioning as carbon soft nucleophiles, which may lead to the formation of bis-allenes 7 by the second three-component coupling with 1 and 2. However, bis-allenes 7 were not detected in the 1H NMR spectra nor isolated in all of the cases (entries 12–14). The reaction with nitrogen-nucleophile 3s was messy and the desired allenic product was not obtained, although 1a was completely consumed (entry 15). It should be mentioned that the reaction between preformed 4a and 3s catalyzed by Pd/dpbp gave the allenic product in an 89% yield.2a
Plausible reaction pathways in the present three-component coupling reaction are outlined in Scheme 4. Whereas 1a did not react with 3m in the absence of 2 under the optimized conditions, the initial formation of bromodiene 4 from 1 and 2 is almost certain. Indeed, a reaction of 1a with 2 (1.0 equiv to 1a) without 3m under the same conditions afforded 4a, together with unreacted 1a and 6a (1a/4a/6a = 14:77:9 molar ratio determined by the 1H NMR measurement). The oxidative addition of 4 to the Pd(0) species forms C, which is in equilibrium with B with the dissociation of the bromide ligand from the palladium center.4a Intermediate C reacts with 2 to give triene 6, the major side-product of the present protocol, via D by the standard cross-coupling process. On the other hand, (alkylidene-π-allyl)palladium intermediate B reacts with 3, just like the Tsuji–Trost reaction, to give allene 5. Due to the wide bite-angle of Xantphos,9 the palladium center in C is rather congested. Whereas the alkylidene-π-allyl ligand in B is much more compact4a than the combination of the σ-butadienyl/bromo ligands in C, the congestion at the palladium atom is somewhat diminished in B. As a consequence, Xantphos in B/C might drive the equilibrium toward B, leading to a higher yield of allene 5.
Scheme 4. Plausible Reaction Pathways of Three-Component Coupling Reaction.
Conclusions
We have established the three-component coupling reaction of 1,1-dibromoalkenes 1, vinylzinc chloride 2, and carbon soft nucleophiles 3 leading to various 1,3-disubstituted allenes 5 in yields of up to 77%. The reaction is effectively catalyzed by the palladium/Xantphos species, and the successive two palladium-catalyzed processes, namely the cross-coupling reaction and the allylic substitution like the Tsuji–Trost reaction, assembled the allenic products in good yields from the easily accessible starting compounds.
Experimental Section
General Information
All anaerobic and/or moisture-sensitive manipulations were carried out with standard Schlenk techniques under predried nitrogen or with glove box techniques under prepurified argon. 1H NMR (at 400 MHz) and 13C NMR (at 101 MHz) chemical shifts are reported in ppm downfield of internal tetramethylsilane. Tetrahydrofuran and 1,4-dioxane were distilled from benzophenone-ketyl under nitrogen prior to use. Pd(PPh3)4,10 [Pd(π-allyl)Cl]2,11 Pd(dba)2,12 dpbp,7 DPEphos,9 and 1,3-benzodithiole-1,1,3,3-tetraoxide (BDT)13 were prepared as reported. All other chemicals were obtained from commercial sources and used without additional purification.
1,1-Dibromo-1-alkenes (1a–i)
These compounds were prepared from the commercially available aldehydes by the reported methods14 and were characterized by comparison of their NMR spectra with those reported previously (1a,151b,15,161c,15,161d,16,171e,16,181f,181g,191h,20 and 1i(3b)).
Palladium-Catalyzed Three-Component Coupling Reaction of 1, 2, and 3
The reaction conditions and the results are summarized in Table 2. A general procedure is given below. To a mixture of 1 (0.45–0.50 mmol), 3-H (1.5 equiv to 1), NaH (1.6 equiv to 1), Pd(dba)2 (2.5 mol %), and Xantphos (3.0 mol %) in 1,4-dioxane (5 mL) was added a suspension of vinylzinc chloride, which was prepared from vinylmagnesium chloride (1.4 mol/L solution in THF; 1.5 equiv to 1) and dry ZnCl2 (1.6 equiv to 1) in 1,4-dioxane (2 mL), and the mixture was stirred for 24 h at 80 °C. The reaction mixture was filtrated through a short pad of silica gel, and the silica gel pad was washed with a small amount of Et2O three times. The combined organic solution was evaporated to dryness under reduced pressure. The residue was chromatographed on silica gel to give allene 5 in the pure form. The characterization data of the allenic products are listed below.
Dimethyl 2-Methyl-2-(4-phenylbuta-2,3-dienyl)propane-1,3-dioate (5am)2a
Pale-yellow oil. Yield: 101 mg (0.37 mmol; 74%) starting with 1a (131 mg; 0.50 mmol). This is a known compound and was characterized by a comparison of its NMR spectra with those reported previously.
Dimethyl 2-[4-(4-Methoxyphenyl)buta-2,3-dienyl]-2-methylpropane-1,3-dioate (5bm)
Pale-yellow oil. Yield: 87 mg (0.29 mmol; 64%) starting with 1b (131 mg; 0.45 mmol). 1H NMR (CDCl3): δ 1.51 (s, 3H), 2.68–2.71 (m, 2H), 3.70 (s, 3H), 3.72 (s, 3H), 3.80 (s, 3H), 5.42 (td, J = 7.2 and 6.4 Hz, 1H), 6.10 (dt, J = 6.4 and 2.4 Hz, 1H), 6.84 (d, J = 8.8 Hz, 2H), 7.18 (d, J = 8.8 Hz, 2H). 13C{1H} NMR (CDCl3): δ 19.9, 35.7, 52.58, 52.61, 53.8, 55.3, 89.2, 94.0, 114.1, 126.3, 127.9, 158.7, 172.1, 172.2, 206.3. ESI-HRMS calculated for C17H20O5Na (M + Na): 328.1242; found: 328.1251.
Dimethyl 2-Methyl-2-[4-(4-trifluoromethylphenyl)buta-2,3-dienyl]propane-1,3-dioate (5cm)
Pale-yellow oil. Yield: 109 mg (0.32 mmol; 71%) starting with 1c (149 mg; 0.45 mmol). 1H NMR (CDCl3): δ 1.51 (s, 3H), 2.73 (dd, J = 7.8 and 2.5 Hz, 2H), 3.71 (s, 3H), 3.73 (s, 3H), 5.54 (td, J = 7.8 and 6.4 Hz, 1H), 6.17 (dt, J = 6.4 and 2.5 Hz, 1H), 7.35 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 8.2 Hz, 2H). 13C{1H} NMR (CDCl3): δ 20.1, 35.5, 52.81, 52.84, 53.8, 90.2, 93.9, 123.0, 125.6 (q, JCF = 3.8 Hz), 127.0, 129.0 (q, JCF = 32.5 Hz), 138.3 (q, JCF = 1.5 Hz), 172.13, 172.14, 207.7. 19F NMR (CDCl3): δ −62.3. ESI-HRMS calculated for C17H17F3O4Na (M + Na): 365.0977; found: 365.0970.
Dimethyl 2-Methyl-2-[4-(2,4,6-trimethylphenyl)buta-2,3-dienyl]propane-1,3-dioate (5dm)
Pale-yellow oil. Yield: 73 mg (0.23 mmol; 51%) starting with 1d (137 mg; 0.45 mmol). 1H NMR (CDCl3): δ 1.46 (s, 3H), 2.25 (s, 3H), 2.30 (s, 6H), 2.66 (dt, J = 7.6 and 2.4 Hz, 2H), 3.66 (s, 3H), 3.70 (s, 3H), 5.18 (td, J = 7.6 and 6.8 Hz, 1H), 6.20 (dt, J = 6.8 and 2.4 Hz, 1H), 6.84 (s, 2H). 13C{1H} NMR (CDCl3): δ 19.9, 20.9, 21.1, 35.6, 52.478, 52.483, 53.9, 85.9, 89.8, 128.4, 128.9, 136.19, 136.24, 172.1, 172.2, 207.7. ESI-HRMS calculated for C19H24O4Na (M + Na): 339.1572; found: 339.1565.
Dimethyl 2-Methyl-2-[4-(1-naphthyl)buta-2,3-dienyl]propane-1,3-dioate (5em)
Pale-yellow oil. Yield: 103 mg (0.32 mmol; 63%) starting with 1e (156 mg; 0.50 mmol). 1H NMR (CDCl3): δ 1.53 (s, 3H), 2.76–2.78 (m, 2H), 3.67 (s, 3H), 3.71 (s, 3H), 5.51 (td, J = 7.3 and 6.4 Hz, 1H), 6.84 (dt, J = 6.4 and 2.4 Hz, 1H), 7.42–7.54 (m, 4H), 7.73 (d, J = 8.0 Hz, 1H), 7.84 (d, J = 8.2 Hz, 1H), 8.17 (d, J = 8.0 Hz, 1H). 13C{1H} NMR (CDCl3): δ 20.0, 35.6, 52.57, 52.59, 53.8, 88.3, 91.0, 123.4, 125.4, 125.6, 125.7, 126.0, 127.5, 128.6, 130.3, 130.7, 133.8, 172.10, 172.12, 208.2. ESI-HRMS calculated for C20H20O4Na (M + Na): 347.1259; found: 347.1259.
Dimethyl 2-(4-Ferrocenylbuta-2,3-dienyl)-2-methylpropane-1,3-dioate (5fm)
Red-orange oil. Yield: 101 mg (0.26 mmol; 53%) starting with 1f (185 mg; 0.50 mmol). 1H NMR (CDCl3): δ 1.51 (s, 3H), 2.58–2.70 (m, 2H), 3.72 (s, 3H), 3.74 (s, 3H), 4.11 (s, 5H), 4.15–4.17 (m, 2H), 4.20–4.24 (m, 2H), 5.15 (td, J = 7.3 and 6.4 Hz, 1H), 5.84 (dt, J = 6.4 and 2.4 Hz, 1H). 13C{1H} NMR (CDCl3): δ 19.9, 35.8, 52.57, 52.62, 53.8, 67.0, 67.1, 68.35, 68.36, 69.2, 80.3, 87.8, 90.8, 172.1, 172.2, 205.8. ESI-HRMS calculated for C20H22FeO4Na (M + Na): 405.0765; found: 407.0765.
Dimethyl 2-(5,5-Dimethyl-2,3-hexadienyl)-2-methylpropane-1,3-dioate (5gm)3c
Colorless oil. Yield: 79 mg (0.31 mmol; 62%) starting with 1g (121 mg; 0.50 mmol). This is a known compound and was characterized by a comparison of its NMR spectra with those reported previously.
Dimethyl 2-(4-Cyclohexyl-2,3-butadienyl)-2-methylpropan-1,3-dioate (5hm)2b
Colorless oil. Yield: 20 mg (0.071 mmol; 14%) starting with 1h (134 mg; 0.50 mmol). This is a known compound and was characterized by a comparison of its NMR spectra with those reported previously.
Diethyl 2-Ethyl-2-(4-phenylbuta-2,3-dienyl)propane-1,3-dioate (5an)
Pale-yellow oil. Yield: 107 mg (0.34 mmol; 68%) starting with 1a (131 mg; 0.50 mmol). 1H NMR (CDCl3): δ 0.85 (t, J = 8.0 Hz, 3H), 1.22 (t, J = 7.2 Hz, 3H), 1.25 (t, J = 7.2 Hz, 3H), 2.05 (q, J = 8.0 Hz, 2H), 2.74 (dd, J = 8.0 and 2.4 Hz, 2H), 4.13–4.23 (m, 4H), 5.38 (td, J = 8.0 and 6.4 Hz, 1H), 6.12 (dt, J = 6.4 and 2.4 Hz, 1H), 7.17–7.20, (m, 1H), 7.25–7.31 (m, 4H). 13C{1H} NMR (CDCl3): δ 8.5, 14.1, 14.2, 25.3, 31.8, 58.0, 61.30, 61.32, 89.3, 94.6, 126.9, 127.0, 128.6, 134.3, 171.175, 171.179, 206.8. ESI-HRMS calculated for C19H24O4: 317.1753; found: 317.1758.
Diethyl 2-Phenyl-2-(4-phenylbuta-2,3-dienyl)propane-1,3-dioate (5ao)
Pale-yellow oil. Yield: 125 mg (0.34 mmol; 69%) starting with 1a (131 mg; 0.50 mmol). 1H NMR (CDCl3): δ 1.21 (t, J = 7.2 Hz, 3H), 1.23 (t, J = 7.2 Hz, 3H), 3.15–3.18 (m, 2H), 4.17–4.30 (m, 4H), 5.50 (q, J = 7.2 Hz, 1H), 6.04 (dt, J = 6.8 and 2.4 Hz, 1H), 7.10–7.17 (m, 3H), 7.22–7.26 (m, 2H), 7.30–7.37 (m, 3H), 7.44–7.47 (m, 2H). 13C{1H} NMR (CDCl3): δ 14.06, 14.09, 36.1, 61.85, 61.87, 62.8, 90.0, 94.6, 126.9 (2C), 127.7, 128.3 (2C), 128.6, 134.2, 136.5, 170.22, 170.24, 207.0. ESI-HRMS calculated for C23H25O4Na (M + H): 365.1753; found: 365.1759.
Dimethyl 2-(4-Phenylbuta-2,3-dienyl)propane-1,3-dioate (5ap)2a
Colorless oil. Yield: 43 mg (0.165 mmol; 33%) starting with 1a (131 mg; 0.50 mmol). This is a known compound and was characterized by comparison of its NMR spectra with those reported previously.
1-Phenyl-5,5-bis(phenylsulfonyl)penta-1,2-diene (5aq)
Pale-yellow solid. Yield: 104 mg (0.245 mmol; 49%) starting with 1a (131 mg; 0.50 mmol). 1H NMR (CDCl3): δ 2.93–3.08 (m, 2H), 4.65 (t, J = 5.4 Hz, 1H), 5.69 (q, J = 6.4 Hz, 1H), 6.20 (dt, J = 6.4 and 3.2 Hz, 1H), 7.22–7.26 (m, 3H), 7.30–7.34 (m, 2H), 7.46–7.50 (m, 2H), 7.53–7.57 (m, 2H), 7.63–7.70 (m, 2H), 7.85–7.89 (m, 2H), 7.94–7.97 (m, 2H). 13C{1H} NMR (CDCl3): δ 24.7, 82.2, 91.3, 98.0, 127.0, 127.5, 128.6, 129.03, 129.05, 129.4, 129.5, 133.1, 134.5, 134.6, 137.6, 137.8, 204.8. ESI-HRMS calculated for C23H20O4S2Na (M + Na): 447.0701; found: 447.0695.
2-(4-Phenylbuta-2,3-dienyl)benzodithiole 1,1,3,3-Tetraoxide (5ar)
Pale-yellow solid. Yield: 106 mg (0.306 mmol; 61%) starting with 1a (131 mg; 0.50 mmol). 1H NMR (CDCl3): δ 3.06–3.21 (m, 2H), 4.57 (t, J = 7.2 Hz, 1H), 5.82 (dt, J = 6.8 and 6.4 Hz, 1H), 6.41 (dt, J = 6.8 and 2.8 Hz, 1H), 7.21–7.26 (m, 1H), 7.30–7.35 (m, 4H), 7.89–7.94 (m, 2H), 7.99–8.05 (m, 2H). 13C{1H} NMR (CDCl3): δ 22.6, 73.0, 88.0, 98.1, 122.59, 122.61, 127.2, 127.6, 128.7, 133.0, 135.19, 135.20, 137.5, 137.6, 206.2. ESI-HRMS calculated for C17H14O4S2Na (M + Na): 369.0231; found: 369.0239.
Acknowledgments
This work was supported by a Grant-in-Aid for Challenging Exploratory Research (16K13989) to M.O. from JSPS, Japan. We are grateful to Dr. Yen-Chou Chen for his assistance in data collection.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b03444.
1H NMR spectra, 13C NMR spectra, and 19F NMR spectrum of allenic products 5 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Krause N., Ed. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations; Georg Thieme Verlag: Stuttgart, 2008; Vol. 44 (Cumulenes and Allenes). [Google Scholar]; b Brummond K. M.; DeForrest J. E. Synthesizing Allenes Today (1982-2006). Synthesis 2007, 2007, 795–818. 10.1055/s-2007-965963. [DOI] [Google Scholar]; c Ogasawara M. Catalytic Enantioselective Synthesis of Axially Chiral Allenes. Tetrahedron: Asymmetry 2009, 20, 259–271. 10.1016/j.tetasy.2008.11.039. [DOI] [Google Scholar]; d Yu S.; Ma S. How Easy Are the Syntheses of Allenes?. Chem. Commun. 2011, 47, 5384–5418. 10.1039/c0cc05640e. [DOI] [PubMed] [Google Scholar]; e Yu S.; Ma S. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]; f Ye J.; Ma S. Conquering Three-Carbon Axial Chirality of Allenes. Org. Chem. Front. 2014, 1, 1210–1224. 10.1039/C4QO00208C. [DOI] [Google Scholar]
- a Ogasawara M.; Ikeda H.; Hayashi T. π-Allylpalladium-Mediated Catalytic Synthesis of Functionalized Allenes. Angew. Chem., Int. Ed. 2000, 39, 1042–1044. . [DOI] [PubMed] [Google Scholar]; b Ogasawara M.; Ge Y.; Uetake K.; Fan L.; Takahashi T. Preparation of Multisubstituted Allenes from Allylsilanes. J. Org. Chem. 2005, 70, 3871–3876. 10.1021/jo050142h. [DOI] [PubMed] [Google Scholar]; c Ogasawara M.; Suzuki M.; Takahashi T. Preparation of C2-Symmetric Allenes by Palladium-Catalyzed Double-Nucleophilic Substitution on 3-Bromopenta-2,4-dienyl Acetate. J. Org. Chem. 2012, 77, 5406–5410. 10.1021/jo300511e. [DOI] [PubMed] [Google Scholar]
- a Ogasawara M.; Ikeda H.; Nagano T.; Hayashi T. Palladium-Catalyzed Asymmetric Synthesis of Axially Chiral Allenes: A Synergistic Effect of Dibenzalacetone on High Enantioselectivity. J. Am. Chem. Soc. 2001, 123, 2089–2090. 10.1021/ja005921o. [DOI] [PubMed] [Google Scholar]; b Ogasawara M.; Nagano T.; Hayashi T. A New Route to Methyl (R,E)-(−)-Tetradeca-2,4,5-trienoate (Pheromone of Acanthoscelides obtectus) Utilizing a Palladium-Catalyzed Asymmetric Allene Formation Reaction. J. Org. Chem. 2005, 70, 5764–5767. 10.1021/jo050684z. [DOI] [PubMed] [Google Scholar]; c Ogasawara M.; Ngo H. L.; Sakamoto T.; Takahashi T.; Lin W. Applications of 4,4’-(Me3Si)2-BINAP in Transition-Metal-Catalyzed Asymmetric Carbon-Carbon Bond-Forming Reactions. Org. Lett. 2005, 7, 2881–2884. 10.1021/ol050834s. [DOI] [PubMed] [Google Scholar]; d Ogasawara M.; Okada A.; Subbarayan V.; Sörgel S.; Takahashi T. Palladium-Catalyzed Asymmetric Synthesis of Axially Chiral Allenylsilanes and Their Application to SE2’ Chirality Transfer Reactions. Org. Lett. 2010, 12, 5736–5739. 10.1021/ol102554a. [DOI] [PubMed] [Google Scholar]
- a Ogasawara M.; Okada A.; Watanabe S.; Fan L.; Uetake K.; Nakajima K.; Takahashi T. Synthesis, Structure, and Reactivity of (1,2,3-η3-Butadien-3-yl)palladium Complexes. Organometallics 2007, 26, 5025–5029. 10.1021/om700538p. [DOI] [Google Scholar]; b Zeng X.; Hu Q.; Qian M.; Negishi E. Clean Inversion of Configuration in the Pd-Catalyzed Cross-Coupling of 2-Bromo-1,3-dienes. J. Am. Chem. Soc. 2003, 125, 13636–13637. 10.1021/ja0304392. [DOI] [PubMed] [Google Scholar]
- a Tsuji J. Carbon-Carbon Bond Formation via Palladium Complexes. Acc. Chem. Res. 1969, 2, 144–152. 10.1021/ar50017a003. [DOI] [Google Scholar]; b Trost B. M.; Van Vranken D. L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; c Trost B. M.; Chulbom L.. Asymmetric Allylic Alkylation Reaction. In Catalytic Asymmetric Synthesis, 2nd ed.; Ojima I., Ed.; VCH: New York, 2000; pp 593–649. [Google Scholar]; d Consiglio G.; Waymouth M. Enantioselective Homogeneous Catalysis Involving Transition-Metal-Allyl Intermediates. Chem. Rev. 1989, 89, 257–276. 10.1021/cr00091a007. [DOI] [Google Scholar]
- a Djahanbini D.; Cazes B.; Goré J. Reactive D’esters α-Alleniques. Synthese Regiospecifique de Diesters γ-Alleniques et de Dienes–1,3. Tetrahedron Lett. 1984, 25, 203–206. 10.1016/S0040-4039(00)99840-X. [DOI] [Google Scholar]; b Trost B. M.; Tour J. M. Synthesis of 4-(Dimethylphenylsilyl)buta-2,3-dien-1-ol and Its Use in Alkylation. J. Org. Chem. 1989, 54, 484–486. 10.1021/jo00263a042. [DOI] [Google Scholar]; c Imada Y.; Ueno K.; Kutsuwa K.; Murahashi S. Palladium-Catalyzed Asymmetric Alkylation of 2,3-Alkadienyl Phosphates. Synthesis of Optically Active 2-(2,3-Alkadienyl)malonates. Chem. Lett. 2002, 31, 140–141. 10.1246/cl.2002.140. [DOI] [Google Scholar]; d Trost B. M.; Fandrick D. R.; Dinh D. C. Dynamic Kinetic Asymmetric Allylic Alkylations of Allenes. J. Am. Chem. Soc. 2005, 127, 14186–14187. 10.1021/ja0543705. [DOI] [PubMed] [Google Scholar]; e Wan B.; Ma S. Enantioselective Decarboxylative Amination: Synthesis of Axially Chiral Allenyl Amines. Angew. Chem., Int. Ed. 2013, 52, 441–445. 10.1002/anie.201204796. [DOI] [PubMed] [Google Scholar]
- Ogasawara M.; Yoshida K.; Hayashi T. 2,2’-Bis(diphenylphosphino)-1,1’-biphenyl: New Entry of Bidentate Triarylphosphine Ligand to Transition Metal Catalysts. Organometallics 2000, 19, 1567–1571. 10.1021/om9909587. [DOI] [Google Scholar]
- Hayashi T.; Konishi M.; Kobori Y.; Kumada M.; Higuchi T.; Hirotsu K. Dichloro[1,1’-bis(diphenylphosphino)ferrocene]palladium(II): an Effective Catalyst for Cross-Coupling of Secondary and Primary Alkyl Grignard and Alkylzinc Reagents with Organic Halides. J. Am. Chem. Soc. 1984, 106, 158–163. 10.1021/ja00313a032. [DOI] [Google Scholar]
- Kranenburg M.; van der Burgt Y. E. M.; Kamer P. C. J.; van Leeuwen P. W. N. M.; Goubitz K.; Fraanje J. New Diphosphine Ligands Based on Heterocyclic Aromatics Inducing Very High Regioselectivity in Rhodium-Catalyzed Hydroformylation: Effect of the Bite Angle.. Organometallics 1995, 14, 3081–3089. 10.1021/om00006a057. [DOI] [Google Scholar]
- Coulson D. R. Tetrakis(triphenylphosphine)palladium(0). Inorg. Synth. 1972, 13, 121–124. 10.1002/9780470132449.ch23. [DOI] [Google Scholar]
- Tatsuno Y.; Yoshida T.; Otsuka S. (η3-Allyl)Palladium(II) Complexes. Inorg. Synth. 1979, 19, 220–223. 10.1002/9780470132500.ch51. [DOI] [Google Scholar]
- Ukai T.; Kawazura H.; Ishii Y.; Bonnet J. J.; Ibers J. A. Chemistry of Dibenzylideneacetone-Palladium(0) complexes: I. Novel Tris(dibenzylideneacetone)dipalladium(solvent) Complexes and Their Reactions with Quinones. J. Organomet. Chem. 1974, 65, 253–266. 10.1016/S0022-328X(00)91277-4. [DOI] [Google Scholar]
- Kündig E. P.; Cunningham A. F. Jr. 1,3-Benzodithiole Tetraoxide as a CH22– Synthon. Tetrahedron 1988, 44, 6855–6860. 10.1016/S0040-4020(01)86213-X. [DOI] [Google Scholar]
- a Ramirez F.; Desai N. B.; McKelvie N. A New Synthesis of 1,1-Dibromoölefins via Phosphine-Dibromomethylenes. The Reaction of Triphenylphosphine with Carbon Tetrabromide. J. Am. Chem. Soc. 1962, 84, 1745–1747. 10.1021/ja00868a057. [DOI] [Google Scholar]; b Corey E. J.; Fuchs P. L. A Synthetic Method for Formyl → Ethynyl Conversion (RCHO → RC≡CH or RC≡CR’). Tetrahedron Lett. 1972, 13, 3769–3772. 10.1016/S0040-4039(01)94157-7. [DOI] [Google Scholar]
- Huh D. H.; Jeong J. S.; Lee H. B.; Ryu H.; Kim Y. G. An Efficient Method for One-Carbon Elongation of Aryl Aldehydes via Their Dibromoalkene Derivatives. Tetrahedron 2002, 58, 9925–9932. 10.1016/S0040-4020(02)01324-8. [DOI] [Google Scholar]
- Ma X.; Herzon S. B. Synthesis of Ketones and Esters from Heteroatom-Functionalized Alkenes by Cobalt-Mediated Hydrogen Atom Transfer. J. Org. Chem. 2016, 81, 8673–8695. 10.1021/acs.joc.6b01709. [DOI] [PubMed] [Google Scholar]
- Theunissen C.; Métayer B.; Henry N.; Compain G.; Marrot J.; Martin-Mingot A.; Thibaudeau S.; Evano G. Keteniminium Ion-Initiated Cascade Cationic Polycyclization. J. Am. Chem. Soc. 2014, 136, 12528–12531. 10.1021/ja504818p. [DOI] [PubMed] [Google Scholar]
- Morri A. K.; Thummala Y.; Doddi V. R. The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. Org. Lett. 2015, 17, 4640–4643. 10.1021/acs.orglett.5b02398. [DOI] [PubMed] [Google Scholar]
- Fukudome Y.; Naito H.; Hata T.; Urabe H. Copper-Catalyzed 1,2-Double Amination of 1-Halo-1-alkynes. Concise Synthesis of Protected Tetrahydropyrazines and Related Heterocyclic Compounds. J. Am. Chem. Soc. 2008, 130, 1820–1821. 10.1021/ja078163b. [DOI] [PubMed] [Google Scholar]
- Rao M. L. N.; Jadhav D. N.; Dasgupta P. Pd-Catalyzed Domino Synthesis of Internal Alkynes Using Triarylbismuths as Multicoupling Organometallic Nucleophiles. Org. Lett. 2010, 12, 2048–2051. 10.1021/ol1004164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.