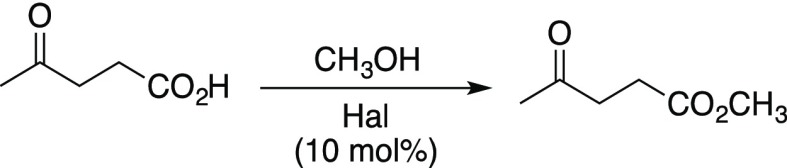
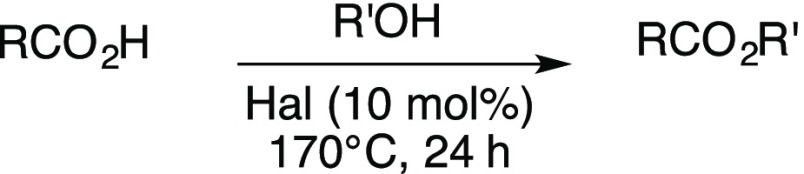
Abstract
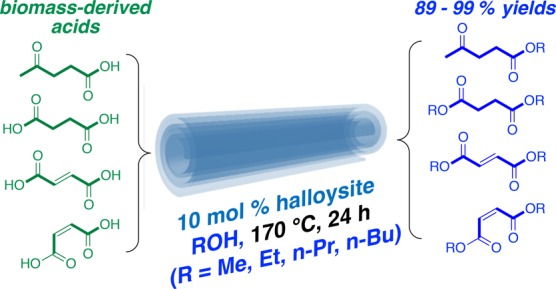
Halloysite, a natural clay with a hollow tubular structure, was studied as a catalyst for the esterification of biomass-derived carboxylic acids (levulinic acid, fumaric acid, maleic acid, and succinic acid) with four different alcohols (MeOH, EtOH, n-PrOH, and n-BuOH). Reaction conditions were optimized (10 mol % halloysite, 170 °C, 24 h) and gave high yields of the corresponding esters and diesters (>90%). The halloysite was easily recovered and recycled after washing and drying.
Introduction
Levulinic acid and succinic acid (including the unsaturated analogs fumaric acid and maleic acid) have been identified as among the top 10 value-added chemicals derived from biomass.1 Levulinic acid and succinic acid derivatives have broad application as chemical building blocks for fine chemicals.1,2 Biomass-derived succinates have been used as monomer precursors for polyether synthesis, while levulinate esters have been employed as octane boosters for gasoline and extenders for diesel fuel.2−8 Esters of levulinic and succinic acid can be readily prepared by a variety of esterification methods from simple alcohols catalyzed by both homogeneous and heterogeneous acid catalysts. Mineral acids are cost-effective homogeneous catalysts that produce alkyl levulinates and dialkyl succinates in high yields. However, heterogeneous acid catalysts offer the advantages of easy removal from the reaction media facilitating product purification and catalyst recyclability. Therefore, a variety of solid acid catalysts derived from a plethora of chemically modified solid supports have been investigated as catalysts for this conversion.9−17
Halloysite (Hal) is a natural 1:1 aluminosilicate clay which is commonly found in weathered rocks and soil. The clay is chemically similar to kaolinite; however, it is commonly found as a hollow tubular nanostructure.18,19 Because aluminosilicate chemistry is generally not toxic and is very durable, halloysite has many advantages in chemical processes.20,21 Studies have shown that halloysite has a high biocompatibility and a high thermal stability, making it much safer to work with than Brønsted or Lewis acids. Also, because of its abundance, halloysite is easily obtained, cheap, and even reusable.19 A recent study demonstrated the effectiveness of halloysite as a catalyst for the conversion of lauric acid to corresponding methyl laurate and ethyl laurate esters.22 With a conversion rate of over 90% for ester formation and an optimum catalyst loading of 12% (w/w), it was of interest to explore the scope and utility of this clay for the development of sustainable conversions of biomass-derived substrates into fine chemical building blocks and fuel additives. Therefore, as part of an ongoing study in our labs aimed at the development of halloysite-based materials, we sought to explore the use of raw halloysite as an esterification catalyst for the preparation of several important biomass-derived esters.
Results and Discussion
Initially, esterification of levulinic acid with methanol to form methyl levulinate was explored with commercially available raw halloysite. This system was used to identify optimized molar ratios for the reactants/catalyst as well as identify optimized reaction conditions (°C, h) because of the ease with which the reaction could be monitored by simple thin-layer chromatography and the product could be isolated and characterized by NMR. The molar amount of the halloysite used in the reaction was based upon the unit cell of Al2Si2O5(OH)4·2H2O (MW 294.19). In a typical reaction, the acid, alcohol, and halloysite were combined in a sealed stainless steel reaction vessel and heated to an elevated temperature in a silicon oil bath. The reaction vessel was then allowed to cool, and the product ester was isolated and characterized. An optimum molar ratio of acid/alcohol/halloysite of 1:24:0.1 was found to be ideal for the easy handling of the reaction slurry. Although the esterification could be successfully executed with lower alcohol (1:12:0.1) concentrations, less alcohol gave poor dispersion of the halloysite and made isolation of the product and the recovery of the halloysite more difficult. Alternatively, increasing the amount of halloysite did not significantly improve yields of the ester. As summarized in Table 1, temperature and time seemed to have the most significant effect on the yield of the ester. Consistent with the earlier report, high temperature was required to drive the halloysite-catalyzed esterification to completion. In our system, we found it necessary to heat the reaction vessel in an oil bath set to a temperature of 170 °C. A reaction time of 24 h gave the maximum acid conversion and product yield (99%), while shorter reaction times led to incomplete conversion of the acid.
Table 1. Optimization of Esterification Conditions for Levulinic Acid in Methanol.
| entry | T (°C) | t (h) | yield (%)a |
|---|---|---|---|
| 1 | 150 | 24 | 30 |
| 2 | 150 | 48 | 48 |
| 3 | 150 | 72 | 46 |
| 4 | 160 | 48 | 68 |
| 5 | 160 | 72 | 66 |
| 6 | 170 | 12 | 72 |
| 7 | 170 | 24 | 99 |
Isolated yields.
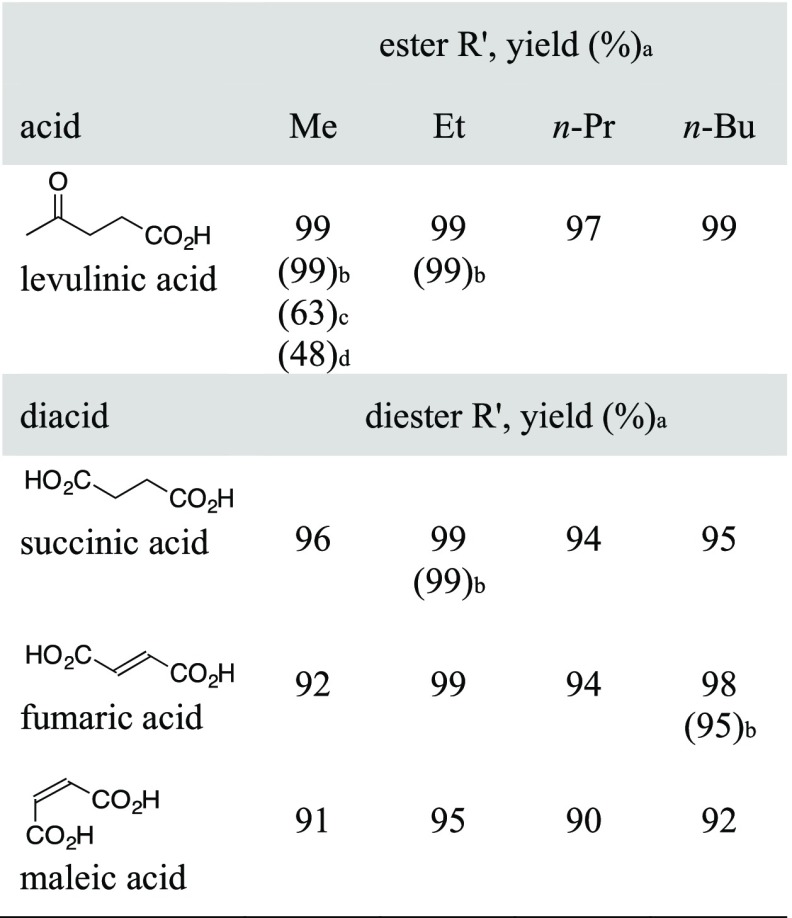
With optimized conditions established for levulinic acid in methanol, the esterification of levulinic acid with other short chain alcohols (EtOH, n-PrOH, and n-BuOH) was investigated (Table 2). Using the optimized conditions established for the methyl ester, the yields of the corresponding ethyl, n-propyl, and n-butyl levulinates were nearly quantitative. Moreover, the esters exhibited a high degree of purity with little more than filtration required for work-up. The success of this procedure prompted further investigation to confirm halloysite-catalyzed esterification as a viable method for the conversion of other biomass-derived acids into esters. As summarized in Table 2, methyl esters of the C6-carbohydrate-derived succinic acid and fumaric acid could be prepared using the optimized conditions in high yields. In a similar fashion to levulinic acid, the dimethyl esters of succinic acid and fumaric acid were obtained in 96 and 92% yield, respectively. For the diacids, the alcohol concentration was maintained and the same molar ratio of diacid/alcohol/halloysite (1:24:0.1) was used. As with levulinic acid, the diacids were readily converted into the corresponding dimethyl, diethyl, di-n-propyl, and di-n-butyl succinates and fumarates in excellent yield and purity. These conditions were also suitable for conversion of the cis-isomer, maleic acid, into dialkyl maleates in 90–95% yield.23 It was notable that despite the elevated temperature, isomerization of the carbon double bond to give the trans fumarate esters was not observed.
Table 2. Biomass-Derived Esters and Diesters.
Isolated yield.
Isolated yield of 100 mmol scale reaction.
Isolated yield; reaction with kaolinite.
Isolated yield; reaction with montorillonite.
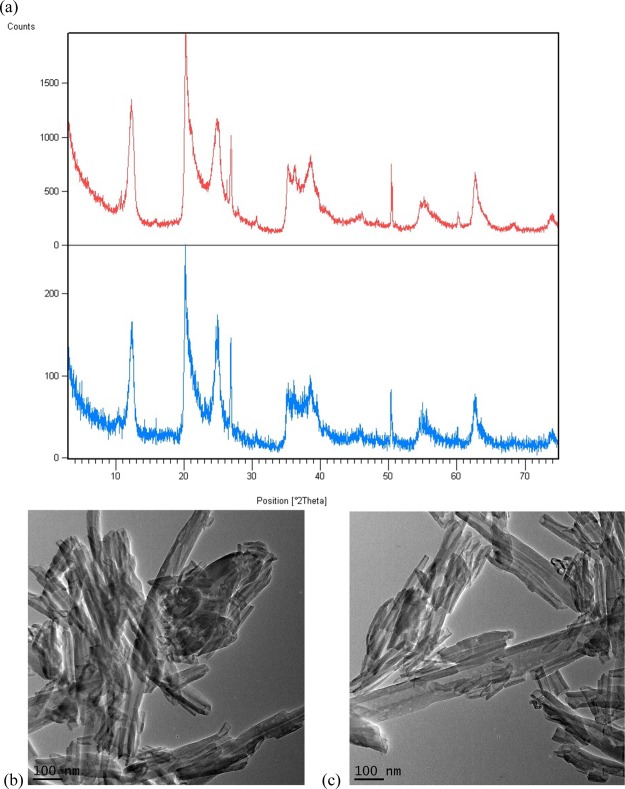
The halloysite used in this study has origins from geological deposits at Dragon Mine, Utah (purchased from Sigma-Aldrich). The morphology of this polydispersed nanoclay has been well characterized and reported to be 90% halloysite with the remaining 10% of the material consisting of kaolinite, quartz and gibbsite.24 As a catalyst, this halloysite was found to be durable and chemically robust. It was superior to other clay catalysts like kaolite25 and montorillonite26 which under the same conditions gave inferior yields of methyl levulinate (<65%, Table 2). The halloysite could be recovered from the reaction mixture by simple vacuum filtration and reused without loss of activity. There was no apparent structural change to the halloysite after the esterification reaction. Based upon X-ray diffraction (XRD) profiles (Figure 1a), the positions of the indexed basal reflections (001, 002, and 003) of the raw halloysite (7.23, 3.59 and 2.33 Å) remained unchanged in the recycled halloysite (7.18, 3.57, and 2.34 Å). These results were consistent with the diffraction pattern of the dehydrated form of halloysite (7 Å form) as opposed to the hydrated form of the clay (10 Å form).27 Further visual inspection of the raw and recycled materials by transmission electron microscopy (TEM) (Figure 1b) revealed that there was no apparent change to the tubular structure of the catalyst.
Figure 1.
(a) XRD of raw halloysite (red) and recycled halloysite (blue). (b) TEM image of raw halloysite. (c) TEM image of recycled halloysite.
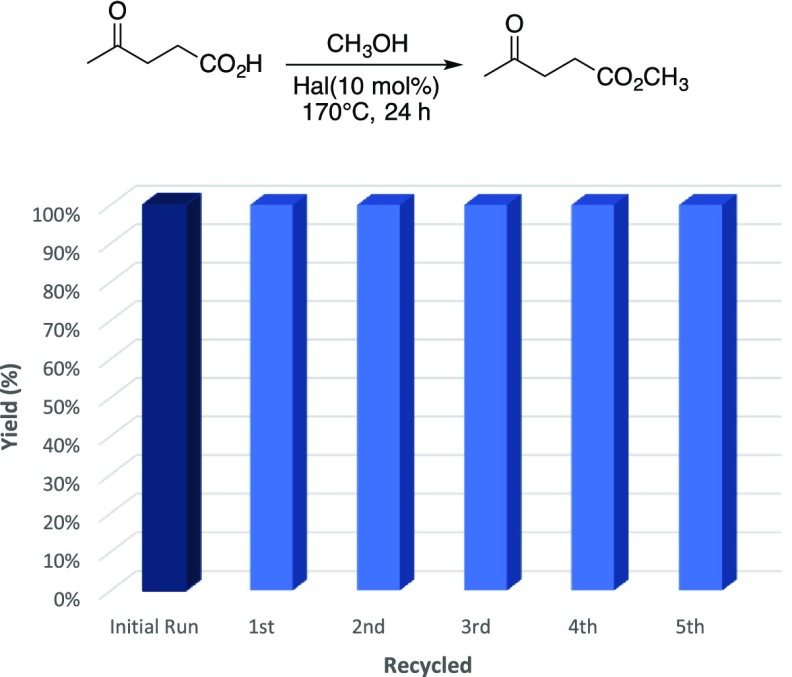
As illustrated in Figure 2, recycling studies with halloysite showed that the catalyst could be cycled through at least six consecutive esterification reactions with no loss of activity and excellent isolated yields of the methyl levulinate in each trial. In these studies, the halloysite was collected by vacuum filtration and washed with the corresponding alcohol used in the esterification. The recovered clay was then further rinsed with deionized water and dried at 120 °C overnight. This afforded the recovered halloysite as a free-flowing powder that would be used in subsequent reactions. Using this method of recovery, the halloysite could be used in different reaction systems with no observed cross-contamination of esters.
Figure 2.
Halloysite recycling studies.
To demonstrate further the utility of halloysite as a catalyst for the esterification reaction, 100 mmol–scale reactions were performed with levulinic acid, succinic acid, and fumaric acid with selected alcohols. The scaled optimized conditions afforded the monoesters methyl and ethyl levulinate in 99% yield. In addition, the diesters, diethyl succinate (99% yield), and dibutyl fumarate (95% yield) were obtained in nearly quantitative yields.
In summary, we have shown that the natural clay, halloysite, is a cost-effective, highly efficient, and easily recyclable catalyst for the esterification of bio-mass-derived carboxylic acids (levulinic acid, fumaric acid, maleic acid, and succinic acid). The optimized reaction conditions (10 mol % halloysite, 170 °C, 24 h) were scalable and represent a green process for the preparation of levulinate and succinate esters. Halloysite, with its unique tubular nanostructure, exhibited remarkable chemical reactivity superior to other clays. Further development of halloysite-based reactions and halloysite-based nanomaterials is under investigation and will be reported in due course.
Experimental Section
Materials and Methods
All reactions were carried out in a stainless-steel reaction vessel suspended in a stirred silicon oil bath. Halloysite clay was purchased from Sigma-Aldrich (source: Applied Minerals, Inc. Dragon Mine, Utah USA) and was used without modification. All other chemicals were purchased from Alfa Aesar, Sigma-Aldrich and VWR. All chemicals were used as received without further purification or modification. Reaction mixtures were filtered prior to work-up using Fisherbrand filter paper, P2-grade [porosity: fine (particle retention: 1–5 μm)]. 1H NMR spectra were recorded at r.t. in DMSO-d6 or CDCl3 on a Bruker 400 MHz instrument operating at a frequency of 300 MHz for 1H NMR. 1H chemical shifts were referenced to the DMSO solvent signal (2.50 ppm) or the CHCl3 solvent signal (7.26 ppm). Halloysite XRD measurements were performed on a Philips X’Pert diffractometer utilizing Cu Kα radiation (λ = 1.5418 Å) and a curved graphite monochromator at a voltage of 45 kV and a current of 40 mA. Halloysite TEM images were obtained on a JEOL 2010 equipped with an EDAX genesis energy-dispersive spectroscopy system, operated at an accelerating voltage of 200 kV and an emission current of 109 μA.
General Procedure
To a clean dry stainless-steel reaction pressure vessel was added the carboxylic acid (10.0 mmol), alcohol (240 mmol), halloysite (0.249 g, 1.00 mmol), and a stir bar. The reaction vessel was sealed and placed in an oil bath at 170 °C and allowed to stir for 24 h. The reaction mixture was then allowed to cool to room temperature for 30 min. The halloysite was filtered using vacuum filtration. The halloysite filter cake was washed with alcohol (10 mL) and set aside for later use. The alcohol from the combined filtrates was removed under reduced pressure. Saturated sodium bicarbonate solution (10 mL) was added to the resulting oil residue, and the mixture was extracted with EtOAc (3 × 20 mL). The combined organic portions were washed with brine, dried over Na2SO4, and filtered, and the solvent was removed under vacuum to afford the ester (or diester) in pure form as determined by 1H NMR.
General Procedure: Large Scale
To a clean dry stainless reaction pressure vessel was added the carboxylic acid (100.0 mmol), alcohol (2400 mmol), halloysite (2.50 g, 10.00 mmol), and a stir bar. The reaction vessel was sealed and placed in an oil bath at 170 °C and allowed to stir for 24 h. The reaction mixture was then allowed to cool to room temperature for 30 min. The halloysite was filtered using vacuum filtration. The halloysite filter cake was washed with alcohol (30 mL) and set aside for later use. The excess alcohol from the combined filtrates was removed and recovered on a rotoevaporator under reduced pressure. Saturated sodium bicarbonate solution (100 mL) was added to the resulting oil residue, and the mixture was extracted with EtOAc (3 × 100 mL). The combined organic portions were washed with brine, dried over Na2SO4, and filtered, and the solvent was removed under vacuum to afford the ester (or diester) in pure form as determined by 1H NMR.
Acknowledgments
Support from the Office of Research and Sponsored Programs and the Department of Chemistry at the University of New Orleans are gratefully acknowledged. The authors wish to thank Alexis Blanco of the UNO Advanced Material Research Institute for providing the TEM images of halloysite.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02870.
1H NMR spectra of esters and diesters (PDF)
Author Present Address
† Department of Chemistry Texas A&M University, Chemistry Building, 3255 TAMU, 580 Ross St, College Station, TX, 77843.
The authors declare no competing financial interest.
Supplementary Material
References
- Bozell J. J.; Petersen G. R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. 10.1039/b922014c. [DOI] [Google Scholar]
- Balat M.; Balat H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. 10.1016/j.apenergy.2010.01.012. [DOI] [Google Scholar]
- Davis R.; Tao L.; Biddy M. J.; Beckham G. T.; Scarlata C.; Jacobson J.; Cafferty K.; Ross J.; Lukas J.; Knorr D.; Schoen P.. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons. NREL Technol. Rep., 2013; pp 88–101.
- Wu L.; Moteki T.; Gokhale A. A.; Flaherty D. W.; Toste F. D. Production of fuels and chemicals from biomass: Condensation reactions and beyond. Chem 2016, 1, 32–58. 10.1016/j.chempr.2016.05.002. [DOI] [Google Scholar]
- Delhomme C.; Weuster-Botz D.; Kühn F. E. Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media. Green Chem. 2009, 11, 13–26. 10.1039/b810684c. [DOI] [Google Scholar]
- Haas T.; Jaeger B.; Weber R.; Mitchell S. F.; King C. F. New diol processes: 1,3-propanediol and 1,4-butanediol. Appl. Catal., A 2005, 280, 83–88. 10.1016/j.apcata.2004.08.027. [DOI] [Google Scholar]
- Démolis A.; Essayem N.; Rataboul F. Synthesis and applications of alkyl levulinates. ACS Sustainable Chem. Eng. 2014, 2, 1338–1352. 10.1021/sc500082n. [DOI] [Google Scholar]
- Ahmad E.; Alam M. I.; Pant K. K.; Haider M. A. Catalytic and mechanistic insights into the production of ethyl levulinate from bio-renewable feedstocks. Green Chem. 2016, 18, 4804–4823. 10.1039/c6gc01523a. [DOI] [Google Scholar]
- Fernandes D. R.; Rocha A. S.; Mai E. F.; Mota C. J. A.; Teixeira da Silva V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal., A 2012, 425-426, 199–204. 10.1016/j.apcata.2012.03.020. [DOI] [Google Scholar]
- Valle-Vigón P.; Sevilla M.; Fuertes A. B. Sulfonated mesoporous silica–carbon composites and their use as solid acid catalysts. Appl. Surf. Sci. 2012, 261, 574–583. 10.1016/j.apsusc.2012.08.059. [DOI] [Google Scholar]
- Melero J. A.; Morales G.; Iglesias J.; Paniagua M.; Hernández B.; Penedo S. Efficient conversion of levulinic acid into alkyl levulinates catalyzed by sulfonic mesostructured silicas. Appl. Catal., A 2013, 466, 116–122. 10.1016/j.apcata.2013.06.035. [DOI] [Google Scholar]
- Pileidis F. D.; Tabassum M.; Coutts S.; Titirici M.-M. Esterification of levulinic acid into ethyl levulinate catalysed by sulfonated hydrothermal carbons. Chin. J. Catal. 2014, 35, 929–936. 10.1016/s1872-2067(14)60125-x. [DOI] [Google Scholar]
- An S.; Song D.; Lu B.; Yang X.; Guo Y.-H. Morphology tailoring of sulfonic acid functionalized organosilica nanohybrids for the synthesis of biomass-derived alkyl levulinates. Chem. —Eur. J. 2015, 21, 10786–10798. 10.1002/chem.201501219. [DOI] [PubMed] [Google Scholar]
- Song D.; An S.; Sun Y.; Guo Y. Efficient conversion of levulinic acid or furfuryl alcohol into alkyl levulinates catalyzed by heteropoly acid and ZrO2 bifunctionalized organosilica nanotubes. J. Catal. 2016, 333, 184–199. 10.1016/j.jcat.2015.10.018. [DOI] [Google Scholar]
- Tejero M. A.; Ramírez E.; Fité C.; Tejero J.; Cunill F. Esterification of levulinic acid with butanol over ion exchange resins. Appl. Catal., A 2016, 517, 56–66. 10.1016/j.apcata.2016.02.032. [DOI] [Google Scholar]
- Ogino I.; Suzuki Y.; Mukai S. R. Esterification of levulinic acid with ethanol catalyzed by sulfonated carbon catalysts: Promotional effects of additional functional groups. Catal. Today 2018, 314, 62–69. 10.1016/j.cattod.2017.10.001. [DOI] [Google Scholar]
- Luan Q.-j.; Liu L.-j.; Gong S.-w.; Lu J.; Wang X.; Lv D.-m. Clean and efficient conversion of renewable levulinic acid to levulinate esters catalyzed by an organic-salt of H4SiW12O40. Process Saf. Environ. Prot. 2018, 117, 341–349. 10.1016/j.psep.2018.05.015. [DOI] [Google Scholar]
- Abdullayev E.; Lvov Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–2903. 10.1039/c3tb20059k. [DOI] [PubMed] [Google Scholar]
- Hillier S.; Brydson R.; Delbos E.; Fraser T.; Gray N.; Pendlowski H.; Phillips I.; Robertson J.; Wilson I. Correlations among the mineralogical and physical properties of halloysite nanotubes (HNTs). Clay Miner. 2016, 51, 325–350. 10.1180/claymin.2016.051.3.11. [DOI] [Google Scholar]
- Kirumakki S. R.; Nagaraju N.; Chary K. V. R. Esterification of alcohols with acetic acid over zeolites Hβ, HY and HZSM5. Appl. Catal., A 2006, 299, 185–192. 10.1016/j.apcata.2005.10.033. [DOI] [Google Scholar]
- Zhou C.-H. Emerging trends and challenges in synthetic clay-based materials and layered double hydroxides. Appl. Clay Sci. 2010, 48, 1–4. 10.1016/j.clay.2009.12.018. [DOI] [Google Scholar]
- Zatta L.; Gardolinski J. E. F. d. C.; Wypych F. Raw halloysite as reusable heterogeneous catalyst for esterification of lauric acid. Appl. Clay Sci. 2011, 51, 165–169. 10.1016/j.clay.2010.10.020. [DOI] [Google Scholar]
- Rodenas Y.; Mariscal R.; Fierro J. L. G.; Martín Alonso D.; Dumesic J. A.; López Granados M. Improving the production of maleic acid from biomass: TS-1 catalysed aqueous phase oxidation of furfural in the presence of γ-valerolactone. Green Chem. 2018, 20, 2845–2856. 10.1039/c8gc00857d. [DOI] [Google Scholar]
- Cavallaro G.; Chiappisi L.; Pasbakhsh P.; Gradzielski M.; Lazzara G. A structural comparison of halloysite nanotubes of different origin by Small-Angle Neutron Scattering (SANS) and electric birefringence. Appl. Clay Sci. 2018, 160, 71–80. 10.1016/j.clay.2017.12.044. [DOI] [Google Scholar]
- Konwar D.; Gogoi P. K.; Gogoi P.; Borah G.; Baruah R.; Hazarika N.; Borgohain R. Esterification of carboxylic acids by acid activated Kaolinite clay. Indian J. Chem. Technol. 2008, 15, 75–78. [Google Scholar]
- Kuma B. S.; Dhakshinamoorthy A.; Pitchumani K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. 10.1039/C4CY00112E. [DOI] [Google Scholar]
- Brindley G. W.; Brown G.. Crystal Structures of Clay Minerals and their X-ray Identification, 1st ed.; Mineralogical Society: London, 1980; pp 146–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.