Abstract
Marcetia taxifolia is a neotropical plant present in South America and it has been evaluated in several biological models due to the presence of active metabolites. Nevertheless, there is a limited quantity of studies related to the antiviral activity of the compounds present in this genus. In our work, the antiviral effect of the compounds isolated from the aerial parts of Marcetia taxifolia was evaluated against Hepatitis B virus (HBV), Herpes Simplex Virus type 1 (HSV-1), and Poliovirus type 1 (PV-1). The cytopathic effect and viral quantification by qPCR were determined as indicative of antiviral activity. Our data show that myricetin rhamnoside (MyrG), myricetin-3-α-O-ramnosil (1→6)-α-galactoside (MyrGG), 5,3'-dihydroxy-3,6,7,8,4'-pentamethoxyflavone (PMF), 5-hydroxy-3,6,7,3',4'pentamethoxyflavone (PMF-OH) had antiviral activity without cytotoxic effects. The methoxyflavones PMF and PMF-OH were the most active compounds, showing an antiviral effect against all the evaluated viruses. Computational studies showed that these compounds could interact with the Reverse Transcriptase. Altogether, these results suggest that the flavonoids (related to myricetin and methoxyflavones) are the main antiviral compounds present in the aerial parts of Marcetia taxifolia. Furthermore, our results showed that the methoxyflavones have a broad antiviral activity, which represents an opportunity to evaluate these flavonoids as lead molecules to develop new antiviral compounds.
Keywords: antiviral, flavonoids, Marcetia taxifolia, HBV, HSV, polio
Introduction
Emergence, resistance and spreading of viral strains promote research for new antiviral drugs. In fact, several biological compounds are found in plants and some of these plant extracts have shown broad antiviral properties. These inhibitory effects could be related to blocking main viral enzymes directly or in some cases, to be modulating a cellular pathway (Arena et al., 2004[1]; Jassim and Naji, 2003[16]; Sharma et al., 2009[40]). These molecular mechanisms are similar to the drugs used in antiviral therapy: direct-acting antiviral and drugs that block a host cellular process avoiding viral replication (Ortega et al., 2013[32]).
Marcetia is a Neotropical genus distributed from Venezuela to Uruguay (Berry and Luckana, 2001[4]). Baptista et al. (2016[3]) described for the first time the compounds present in the aerial parts of Marcetia taxifolia. These compounds are: marcetol (1), 5-α-cholestan-3-β-ol (2), 5-α-cholestan-7-en3-β-ol (3), 24,25-dehydropollinastanol (4), lupa-12,20(29)-dien-3-ol (5), 5,3'- dihydroxy-3,6,7,8,4'-pentamethoxyflavone (6), 5-hydroxy-3,6,7,3',4'pentamethoxyflavone (7), myricetrine (8) and myricetin-3-α-O-ramnosil (1→6)-α-galactoside (9). Previous reports have shown that these methoxyflavones and myricetrine have antiviral activity against HIV-1 (Ortega et al., 2017[34][33]). Additionally, Leite et al. have reported the antibacterial and antifungal activities of Marcetia taxifolia extract. These activities are principally associated with polymethoxylated flavonoids (Leite et al., 2012[21]). However, the antiviral activity of these compounds has been scarcely evaluated. Thus, in this study the compounds obtained from the aerial parts of Marcetia taxifolia were evaluated against three different viral families of public health concern: Hepatitis B virus (HBV), Herpes simplex virus (HSV) and Poliovirus (PV). HBV affects around 240 million people worldwide and is related to hepatic cirrhosis and hepatocellular carcinoma (WHO, 2019[46]). HSV may produce oral or genital lesion depending on the virus type (HSV-1 and HSV-2 respectively) (Whitley and Baines, 2018[45]) and Poliovirus is associated with paralysis in unvaccinated children (Menant and Gandevia, 2018[26]). These viruses exhibit many differences in the replicative cycle. Therefore, the aim of this study was to evaluate the antiviral activity of compounds isolated from Marcetia taxifolia against these different viral families.
Material and Methods
Cell culture, viruses, and compounds
HepG2.2.15 cells were kindly donated by Dr. Isabelle Chemin (Team leader Hepatocarcinogenesis and viral infections) from INSERM, France. These cells were grown in RPMI medium supplemented with 10 % fetal bovine serum (Gibco), 100 IU/mL penicillin G, 100 µg/mL streptomycin, 0.25 µg/mL amphotericin B and 200 µg/mL G418 and Vero cells in Eagle medium supplemented with 10 % fetal bovine serum (Gibco), 100 IU/ml penicillin G, 100 µg/mL streptomycin at 37 °C under 5 % CO2 in a CO2 incubator. Herpes simplex type 1 F strain (HSV-1), poliovirus type 1 Sabin strain (PV-1) of viral repository stocks from “Cátedra Virología, Facultad de Farmacia y Bioquímica, UBA, Argentina”.
The Marcetia taxifolia (A.St.-Hil.) DC., plant collection, verification, and compound isolation were previously described (Baptista et al., 2016[3]). The Marcetia taxifolia (A.St.-Hil.) DC. (Melastomataceae) was collected in the Amazonanian state in Venezuela. The chemical constituents were separated through organic extraction, further isolation was developed by chromatographic techniques and the compounds were characterized using spectroscopic and spectrometric methods.
Cytotoxicity assay
The cytotoxicity assays were determined by the MTT method (Mosmann, 1983[28]). Also, the cells were seeded in 96-wells plate at a density of 1.5 x 104 cells/well. The next day, different concentrations of the flavonoid compounds were added to the cells and 24 h later, the cell viability was evaluated using the MTT (ThermoFisher, USA) cell proliferation assay as previously described (Ortega et al., 2017[34]).
HBV antiviral assay
HepG2.2.15 constitutively produces HBV particles and is broadly used to evaluate the antiviral activity (Sells et al., 1988[39]). The HBV production was quantified through a real-time PCR methodology, using as target the X gene. The sequence of specific primers was forwarded-1608-1627: ATG GAG ACC ACC GTG AAC GC and reversed-1887-1868 AGG CAC AGC TTG GTG GCT TG. The amplification product was of approximately 260 bp. Briefly, 24 h prior to the antiviral assay, the cells were seeded at a density of 1.2 x 104 cells / well for each condition. The cells were incubated with “Infection medium” (RPMI with 2 % FBS and 1 % DMSO) (Quintero et al., 2011[38]) with and without the compounds at the described concentrations. After 72 h, the medium and the compounds were refreshed by replacing them and incubated by 72 h. The supernatant was collected and viral DNA extracted using High Pure Viral Nucleic Acid Kit (Roche Life Science, Germany). The development of qPCR was done on Applied Biosystems 7300 by using a Sybr green commercial kit (Luna® Universal qPCR, Kit NEB Co. USA). The amplification conditions were the following: 3 min at 95 °C, 40 cycles of 15 sec. at 95 °C, 30 sec. at 61 °C and 30 sec. at 72 °C. The viral inhibition relative to control (%) was calculated. Statistical analyses of at least three independent experiments were performed.
Herpes virus and polio antiviral assay
To determine the antiviral activity against Herpes and Polio, a plaque reduction assay, on 24 well culture plates was performed. The Vero cells were infected with 100 plaque forming units (PFU) of HSV-1 or PV-1. After 60 min adsorption at 37 °C and 5 % CO2 the virus was removed. Then, the monolayers were washed twice with PBS and overlaid with plaque medium (0.5 % Methylcellulose). Next, the compounds were added at different concentrations in each well and incubated by 24 or 48 h for PV-1 and HSV-1 respectively. Finally, cells were fixed with 10 % formalin and stained with 0.4 % crystal violet. Viral infection, cellular morphology, and cytotoxic controls in each assay were all performed.
3D model building and refinement
Homology modeling
A homology model of the Hepatitis B virus Reverse Transcriptase (HBV-RT) was generated by using the crystallographic structure of HIV-1 Reverse Transcriptase (HIV-1-RT) (PDB 3V81) as the template (Daga et al., 2010[8]; Das et al., 2012[9]). The initial model was obtained with the SWISS-MODEL modeling server (Arnold et al., 2006[2]) and the tools of DeepView/Swiss-PdbViewer 4.01 software (Guex and Peitsch, 1997[13]). In this model, hydrogen atoms were added, and the partial charges were assigned for energy refinement. After that, the protein model was embedded in a 100 Å water box. Then, energy minimization preserving the enzyme folding and optimizing the relative position between the water molecules and the fixed backbone atoms was performed. The obtained system underwent a one nanosecond (ns) molecular dynamic (MD) simulation with the following characteristics: (a) Periodic Boundary Conditions (PBC) were introduced to stabilize the simulation space; (b) the long-range electrostatic potential was treated by the Particle Mesh Ewald summation method (PME) (Toukmaji et al., 2000[42]); (c) Newton's equation using the r-RESPA method (Masella, 2006[25]) (every 4 femtoseconds (fs) for long-range electrostatic forces, 2 fs for short-range non-bonded forces, and 1 fs for bonded forces) was integrated; (d) the temperature was maintained 300 ± 5 K by means of Langevin's algorithm (Izaguirre et al., 2012[15]); (e) Lennard-Jones (L-J) interactions were calculated with a cut-off of 14 Å, the switching distance was 10 Å, and the non-bonded pair list distance was 14 Å; (f) a frame every 5 pico seconds (ps) was stored, yielding 2000 frames; (g) constraints with a force constant of 1000kcal/mol/Å2 were applied to the backbone atoms (N, Ca, C, O) and gradually decreased during the simulation. The simulations were carried out in three phases: an initial period of heating from zero to 300 K over nine ps; an equilibration MD simulation of 0.1 ns, and monitored MD phase of simulation of one ns. The lowest energy frame of the MD simulation was further minimized and represented in the final structure. MD simulations described in the study were performed with NAMD 2.8 (Phillips et al., 2005[37])/Vega ZZ 3.1.0.21, using the CHARMM force field (Vanommeslaeghe et al., 2010[44]) and the Gasteiger charges. All reported minimizations were performed using the conjugate gradient algorithm until a root-median square (rms) gradient was smaller than 0.01 kcal/mol/Å. The final model validation was carried out with ProSA (Wiederstein and Sippl, 2007[47]) and PROCHECK programs (Laskowski et al., 1993[20]).
Molecular docking with HBV-RT
The 3D structure of each inhibitor was obtained from the PubChem database (Kim et al., 2016[18]) or generated by parent structure modification with VegaZZ 3.1.0.21 (Pedretti et al., 2004[36]). The molecular docking was performed with AutoDock 4/VegaZZ 3.1.0.21 and 30 runs conducted for each compound. Sampling in a cube of 10 Å sides around the ligand was carried out. The results were prioritized according to the predicted free energy of binding. Then the final complexes, keeping the backbone atoms of the protein fixed and improving the mutual adaptability between ligand and enzyme were minimized.
Results
The antiviral activity of the compounds isolated from the aerial parts of M. taxifolia in in vitro models of HBV, HSV-1, and PV-1 was assayed. Table 1(Tab. 1) summarizes the results for cytotoxicity and viral inhibition for the evaluated compounds. As indicated in this table, only: myricetin rhamnoside (MyrG), myricetine-3α-O-rhamnosyl (1→6)-α-galactoside (MyrGG), 5,3'-dihydroxy-3,6,7,8,4'-pentamethoxyflavone (PMF) and 5-hydroxy-3,6,7,3',4'-pentamethoxyflavone (PMF-OH), were active against at least one of the evaluated viruses. Additionally, these compounds showed a scarce or non-cytotoxic effect.
Table 1. Cytotoxic concentration and Inhibitory concentration of the tested compounds.
Antiviral activity on HSV-1 and PV-1 models
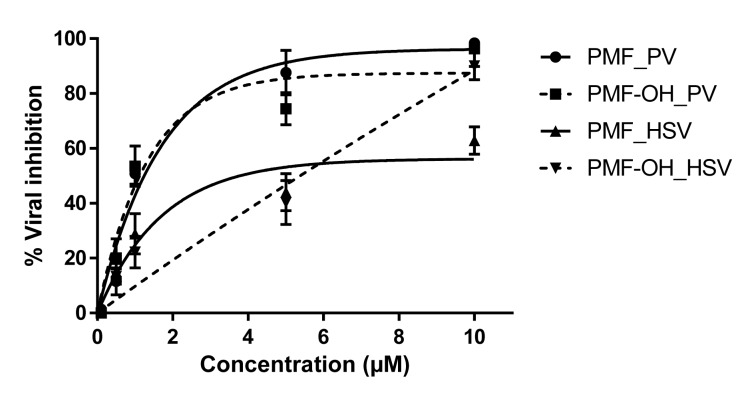
The antiviral activity of each compound against Polio-1 and HSV-1 was assayed. The myricetin related compounds did not show antiviral activity against these viruses. However, methoxyflavones exhibited activity with an EC50 near to 1 µM against PV-1 (Figure 1(Fig. 1)). An inhibitory effect of 90 % (PMF) and 60 % (PMF-OH) against HSV-1 was observed at a concentration of 10 μM (Figure 1(Fig. 1)).
Figure 1. Antiviral activity of methoxyflavones against PV-1 and HSV-1.
Antiviral activity on the HBV model
The selectivity index (SI) of the methoxyflavones (PMF and PMF-OH) against HBV was 10,000 and 102 respectively, with EC50 values of 0.001 µM for PMF and 0.098 µM for PMF-OH. These compounds showed the highest antiviral activity against HBV (Figure 2A(Fig. 2)). Additionally, MyrG presents an SI of 6667 and an EC50= 0.015 µM while MyrGG showed an SI of 8.3 and EC50= 12 µM (Figure 2B(Fig. 2)).
Figure 2. Antiviral activity of Marcetia taxifolia flavonoids against HBV. (A) methoxyflavones: PMF and PMF-OH, (B) Myricetin glycosides: MyrG and MyrGG.
Previous reports show that glycosylated flavonoids related to myricetin, as well as methoxyflavones, can inhibit the HIV-1-RT (Ortega et al., 2017[33]). Thus, the interaction of these compounds with the HBV reverse transcriptase (HBV-RT) as a potential target was evaluated in silico. To date, there is no experimental crystal structure of HBV-RT available. For this reason, a homology model of the HBV-RT was constructed by using the crystal structure of HIV-1-RT as a template.
Homology modeling
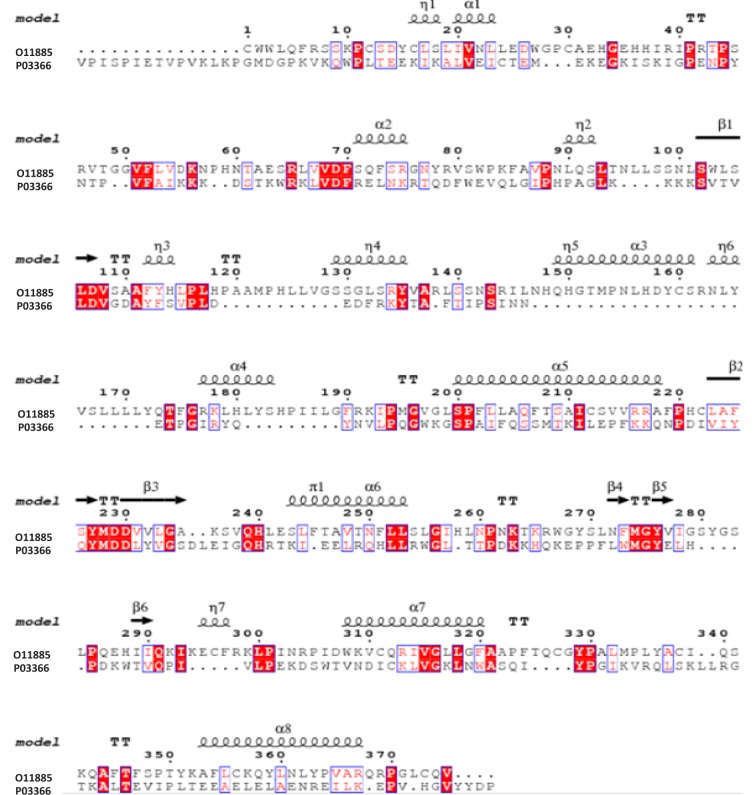
The alignment between HBV-RT and HIV-1-RT (UniProtKB O11885 and P03366) was performed using the structural alignment tool of Swiss-PdbViewer/DeepView (Figure 3(Fig. 3)). The HIV-1-RT and HBV-RT share many of the residues involved in key protein-ligand interactions (Daga et al., 2010[8]). These include the YMDD motif and the catalytic triad of aspartic acid residues, Asp107, Asp229, Asp230. Some differences in the putative hydrophobic NNRTI-binding pocket were found. However, the insertion of 4 amino acids in the HBV enzyme was the major difference observed. Altogether, data suggest that HIV-1-RT and HBV-RT share enough structural and functional similarity to serve as a putative predictor for the interaction with RT inhibitors.
Figure 3. Sequence alignment of HBV-RT and HIV-1-RT polymerases. Secondary structure prediction of HBV-RT. Predicted amino acid sequences of HBV-RT (O11885) and HIV-1-RT PDB code 3v81 (P03366) were accordingly to Daga et al. (2010) conserved residues, which are highlighted with a red background.
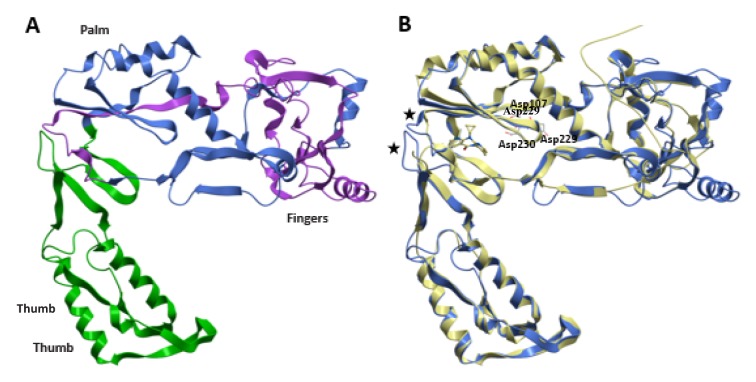
A structural model of the HBV-RT using the SWISS-MODEL package was generated (Figure 4(Fig. 4)). Three main subdomains in the HBV-RT structure were identified: the finger, residues 1-49 and 90-172, the palm, residues 50-89 and 173-267 and the thumb, residues 268-351. Nevertheless, differences between the models were found (Figure 4(Fig. 4)). Homology comparison between HBV-RT model and HIV-1-RT template suggested a similar structural conformation with a root mean square deviation (rmsd) of 2.1 Å for the Cα atoms (Table 2(Tab. 2)).
Figure 4. Comparison between the homology model of HBV-RT and the crystal structure of HIV-1-RT. (A) Ribbon diagram of HBV-RT. Three subdomains are described: Fingers (residues 1-49 and 90-172, purple), palm (residues 50-89 and 173-267, blue), and thumb (residues 268-351, green). (B) HBV-RT (blue) and HIV-1-RT (yellow) are superimposed; regions showing significant differences are marked (*). Amino acids of the catalytic triad and Nevirapine are displayed in sticks.
Table 2. Structural comparison between the HBV-RT model and HIV-1-RT.
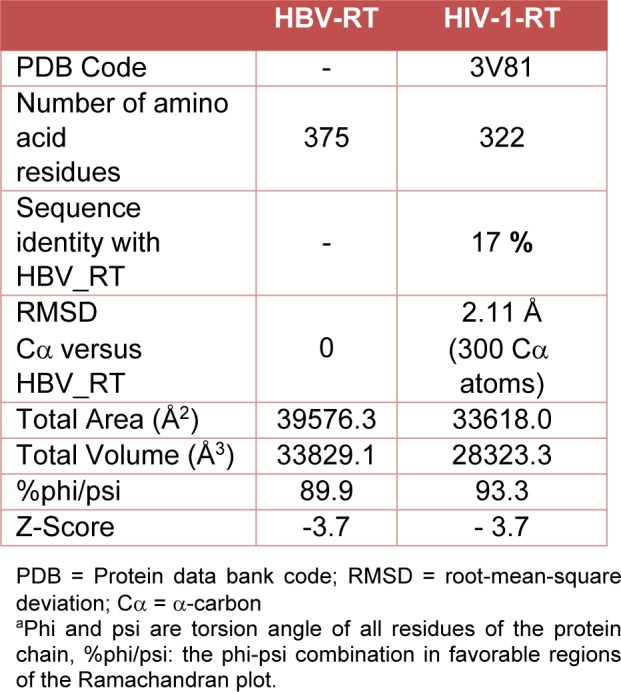
The conformational differences related to the secondary structure between HIV-RT and HBV-RT were determined. The main HBV features were the elongations in 4 and 5 residues occurring in the protein loops (Figure 4 B(Fig. 4)). The palm contains the polymerase active site and the non-nucleoside pocket located ~10 Å apart. No differences were observed around the three aspartate residues of the catalytic triad: Asp107, Asp229, and Asp230, in HBV and corresponding in HIV-1 RT to Asp110, Asp185, and Asp186.
Docking at the NNRTI binding pocket
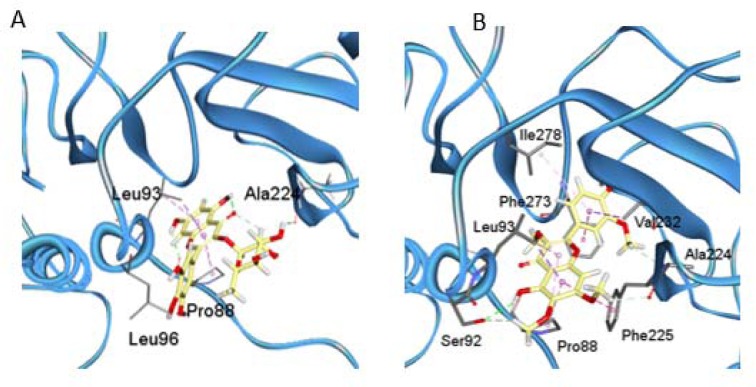
Some flavonoids have been characterized as HIV-1-RT inhibitors. Furthermore, previous reports showed that these compounds, particularly myricetin and myricetin 3-rhamnoside, could occupy the NNRTI pocket (Li et al., 2011[22]; Ortega et al., 2017[34]). Thus, molecular docking study of glycosylated flavonoids (MyrG and MyrGG) and methoxyflavones (PMF and PMF-OH) into the NNRTI pocket on the HBV-RT model were carried out. Figure 5(Fig. 5) shows the docking representation of the MyrG (A) and PMF (B) on the HBV-RT model, and Table 3(Tab. 3) summarizes the docking scores for the ligands evaluated in this study. The compounds that best interact with the NNRTI pocket were the methoxyflavones. However, the best-docked compound was PMF-OH, with a binding energy of -5.59 kcal/mol. The compound PMF shows binding energy of -5.33 kcal/mol. However, the glycosylated flavonoids showed lower binding energy than methoxyflavones. The best ranked binding energy was for MyrG with a binding energy of -4.61 Kcal/mol. All these results are summarized in Table 3(Tab. 3).
Figure 5. Docking results of MyrG (A) and PMF (B) over the HBV-RT model. 3D representations of the complex interactions categorized by hydrogen bonds (green dashed lines), π-stacks (pink-dashed lines).
Table 3. Docking results of the evaluated compounds using the HBV-RT model.
For more results see the Supplementary material.
Discussion
Plant-derived compounds represent a broad variety of chemical families. However, flavonoids have been the most widely studied as possible antivirals. Indeed, some of these compounds have been active against viruses such as HBV, Polio, and Herpes virus (Zhang et al., 2014[49]; Eggers, 1985[11]; Parvez et al., 2016[35]; Huang et al., 2017[14]). Marcetia genus plants are a source of flavonoids and have been barely studied. Thus, to determine the antiviral activity of the nine compounds isolated from the aerial parts of M. taxifolia, we selected three different in vitro models: HBV, HSV-1, and PV-1.
The results showed that only the glycosylated flavonoids related to myricetin and the methoxyflavones exhibited antiviral activity. Myricetin related compounds and methoxyflavones were active against HBV (Figure 2(Fig. 2)). However, the most potent compounds were the methoxyflavones (Figure 2A(Fig. 2)). In our best knowledge, this is the first report that indicates the antiviral activity of these compounds on HBV. Interestingly, other flavonoids related to myricetin such as quercetin have exhibited an antiviral effect against HBV (Cheng et al., 2015[7]). The methoxyflavones have been barely studied and there are just a few reports related to their antiviral activity. (Ortega et al., 2017[34]; Nagai et al., 1995[29]; Eggers, 1985[11]; De Meyer et al., 1991[10]).
Flavonoids and related compounds can interact with polymerases, especially with the reverse transcriptase (Kuete et al., 2010[19]; Ono et al., 1990[31]). Ortega et al. (2017[34][33]) described that myricetin and methoxyflavones may act as non-nucleoside Reverse Transcriptase inhibitors (NNRTI). Indeed, a Reverse Transcriptase is involved in the HBV replication cycle and this enzyme has a high similarity with the HIV-1-RT. The binding of MyrG and MyrGG on the reported NNRTI binding pocket was shown by molecular docking. Moreover, the binding energy was inversely proportional to glycosylation grade (Figure 5(Fig. 5) and Table 3(Tab. 3)). The methoxyflavones showed lower binding energies compared to the glycosylated compounds. Additionally, these compounds produce more hydrogen bonds, which suggest a more stable interaction with the HBV-RT. These docking results are in concordance with the antiviral activities obtained in the in vitro model of HBV. Residues that consistently within 5 Å from docked ligands were Val87, Pro88, Asn89, Leu90, Ser92, Leu 93, Trp 103, Leu223, Ala224, Phe225, Val232, and Phe273. As shown, these results suggest the hydrophobic NNRTI pocket of HBV-RT as a potential target for new inhibitors.
To determine the antiviral spectrum of these flavonoids, two additionally viral species were selected as models. PV-1 (a single strain RNA virus) and HSV-1 (a DNA virus), which diverged from HBV in the replication mechanisms. Our data show that the glycosylated flavonoids did not have an inhibitory effect against Polio nor Herpes. These observations are in concordance with previous reports for these myricetin related compounds (Lin et al., 2000[23]; Castrillo et al., 1986[6]). The methoxyflavones displayed a potent effect against Polio and these results are in agreement with previous reports of similar compounds versus this virus (Van Hoof et al., 1984[43]; De Meyer et al., 1991[10]). However, the effect of the methoxyflavones against HSV-1 (Figure 1(Fig. 1)) was modest compared to those observed against HBV (Figure 2a(Fig. 2)). Altogether, these results showed that the methoxyflavones are active against Polio, Herpes, and Hepatitis B. Additionally, as previously mentioned the methoxyflavones can inhibit influenza virus and HIV-1. These viruses do not share any replicative step thus; the broad range of antiviral activity of methoxyflavones may suggest two possible inhibitory mechanisms: a) inhibiting viral polymerases by acting as non-selective inhibitors, or b) modulating a cellular process related to the viral replication cycle. Indeed, methoxyflavones have been described as inhibitors of several cellular processes, such as the cell cycle, apoptosis, kinases pathway, NFKb/AKT pathway and others (Wudtiwai et al., 2011[48]; Bhardwaj et al., 2016[5]; Faqueti et al., 2016[12]; Kim et al., 2014[17]; Ma et al., 2015[24]; Merlo et al., 2015[27]; Shi et al., 2013[41]; Nakao et al., 2011[30]).
In conclusion, the methoxyflavones showed antiviral activity against all the evaluated viruses, while the glycosylated flavonoids related to myricetin were active only on HBV. In the HBV model, the possible antiviral mechanism could be related to HBV-RT inhibition. On the other hand, the results obtained for PMF against HSV-1 and PV-1 suggest that these compounds could have another mechanism related to a cellular process modulation. However, additional pharmacological studies to establish the target of the methoxyflavones and further evaluations of glycosylated flavonoids as RT inhibitors are needed. Altogether, these results show the potential of flavonoids as antiviral lead compounds.
Notes
Héctor Rodolfo Campos and Héctor Rafael Rangel (Laboratorio de Virología Molecular, Centro de Microbiología y Biología Celular, Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela; Tel: 58 212 5041874, E-mail: hrangel@ivic.gob.ve, hrangel2006@gmail.com) contributed equally as corresponding authors.
Acknowledgements
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; PICT2014-1672) to RC at Cátedra de Virología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, IVIC project number 495. To HR at Laboratorio de Virología Molecular, Caracas, Venezuela, and UNU Biotechnology Program for Latin America and the Caribbean UNU-BIOLAC to OJT, FP and RC.
Supplementary Material
References
- 1.Arena A, Bisignano G, Pavone B, Tomaino A, Bonina FP, Saija A, et al. Antiviral and immunomodulatory effect of a lyophilized extract of Capparisspinosa L. buds. Phytother Res. 2008;22:313–317. doi: 10.1002/ptr.2313. [DOI] [PubMed] [Google Scholar]
- 2.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 3.Baptista J, Chavez K, Torrico F, Trejo E, Garca C, Urbina J, et al. Constituyentes qumicos y actividad antiinflamatoria de Marcetia taxifolia. Ciencia. 2016;24:8294. [Google Scholar]
- 4.Berry PE, Luckana N. Melastomataceae. In: Berry PE, Yatskievych K, Holst BK, editors. Flora of the Venezuelan Guayana. Liliaceae-Myrsinaceae. 6th ed. St. Louis, MI: Missouri Botanical Garden; 2001. pp. 263–383. [Google Scholar]
- 5.Bhardwaj M, Kim NH, Paul S, Jakhar R, Han J, Kang SC. 5-Hydroxy-7-methoxyflavone triggers mitochondrial-associated cell death via reactive oxygen species signaling in human colon carcinoma cells. PLoS One. 2016;11(4):e0154525. doi: 10.1371/journal.pone.0154525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castrillo JL, VandenBerghe D, Carrasco L. 3-Methylquercetin is a potent and selective inhibitor of poliovirus RNA synthesis. Virology. 1986;152:219–227. doi: 10.1016/0042-6822(86)90386-7. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Sun G, Guo W, Huang Y, Sun W, Zhao F, et al. Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol Sin. 2015;30:261–268. doi: 10.1007/s12250-015-3584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daga PR, Duan J, Doerksen RJ. Computational model of hepatitis B virus DNA polymerase: Molecular dynamics and docking to understand resistant mutations. Protein Sci. 2010;19:796–807. doi: 10.1002/pro.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das K, Martinez SE, Bauman JD, Arnold E. HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism. Nat Struct Mol Biol. 2012;19:2539. doi: 10.1038/nsmb.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Meyer N, Haemers A, Mishra L, Pandey HK, Pieters LA, VandenBerghe DA, et al. 4'-Hydroxy-3-methoxyflavones with potentantipicornavirus activity. J Med Chem. 1991;34:736–746. doi: 10.1021/jm00106a039. [DOI] [PubMed] [Google Scholar]
- 11.Eggers HJ. Antiviral agents against picornaviruses. Antiviral Res. 1985;(Suppl 1):57–65. doi: 10.1016/s0166-3542(85)80009-7. [DOI] [PubMed] [Google Scholar]
- 12.Faqueti LG, Brieudes V, Halabalaki M, Skaltsounis AL, Nascimento LF, Barros WM, et al. Antinociceptive and anti-inflammatory activities of standardized extract of polymethoxyflavones from Ageratum conyzoides. J Ethnopharmacol. 2016;194:369–377. doi: 10.1016/j.jep.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Zhou W, Zhu H, Zhou P, Shi X. Baicalin benefits the anti-HBV therapy via inhibiting HBV viral RNAs. Toxicol Appl Pharmacol. 2017;15;323:36–43. doi: 10.1016/j.taap.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Izaguirre JA, Catarello DP, Wozniak JM, Skeel RD. Langevin stabilization of molecular dynamics. J Chem Phys. 2012;114:2090. [Google Scholar]
- 16.Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim HR, Park CG, Jung JY. Acacetin (5,7-dihydroxy-4'-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-?B/Akt signaling in prostate cancer cells. Int J Mol Med. 2014;33:317–324. doi: 10.3892/ijmm.2013.1571. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44:1202–1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuete V, Ngameni B, Mbaveng AT, Ngadjui B, Meyer JJ, Lall N. Evaluation of flavonoids from Dorstenia barteri for their antimycobacterial, antigonorrheal and anti-reverse transcriptase activities. Acta Trop. 2010;116:100–104. doi: 10.1016/j.actatropica.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK - a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:28391. [Google Scholar]
- 21.Leite TC, de Sena AR, Dos Santos Silva TR, Dos Santos AK, Uetanabaro AP, Branco A. Antimicrobial activity of Marcetia DC species (Melastomataceae) and analysis of its flavonoids by reverse phase-high performance liquid chromatography coupled-diode array detector. Pharmacogn Mag. 2012;8(31):209–214. doi: 10.4103/0973-1296.99286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Hattori T, Kodama EN. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antivir Chem Chemother. 2011;21:239–243. doi: 10.3851/IMP1774. [DOI] [PubMed] [Google Scholar]
- 23.Lin LC, Kuo YC, Chou CJ. Anti-herpes simplex virus type-1 flavonoids and a new flavanone from the root of Limonium sinense. Planta Med. 2000;66:333–336. doi: 10.1055/s-2000-8540. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Feng S, Yao X, Yuan Z, Liu L, Xie Y. Nobiletin enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cancer cells. Sci Rep. 2015;5:18789. doi: 10.1038/srep18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masella M. The multiple time step r-RESPA procedure and polarizable potentials based on induced dipole moments. Mol Phys. 2006;104:41528. [Google Scholar]
- 26.Menant JC, Gandevia SC. Poliomyelitis. Handb Clin Neurol. 2018;159:337–344. doi: 10.1016/B978-0-444-63916-5.00021-5. [DOI] [PubMed] [Google Scholar]
- 27.Merlo S, Basile L, Giuffrida ML, Sortino MA, Guccione S, Copani A. Identification of 5-methoxyflavone as a novel DNA polymerase-beta inhibitor and neuroprotective agent against beta-amyloid toxicity. J Nat Prod. 2015;78:2704–2711. doi: 10.1021/acs.jnatprod.5b00621. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann T. MTT rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Nagai T, Suzuki Y, Tomimori T, Yamada H. Antiviral activity of plant flavonoid, 5,7,4'-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis against influenza A (H3N2) and B viruses. Biol Pharm Bull. 1995;18:295–299. doi: 10.1248/bpb.18.295. [DOI] [PubMed] [Google Scholar]
- 30.Nakao K, Murata K, Deguchi T, Itoh K, Fujita T, Higashino M, et al. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol Pharm Bull. 2011;34:1143–1146. doi: 10.1248/bpb.34.1143. [DOI] [PubMed] [Google Scholar]
- 31.Ono K, Nakane H, Fukushima M, Chermann JC, Barr-Sinoussi F. Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. Eur J Biochem. 1990;190:469–476. doi: 10.1111/j.1432-1033.1990.tb15597.x. [DOI] [PubMed] [Google Scholar]
- 32.Ortega JT, Rangel HR, Pujol FH. Cellular targets as an alternative mechanism in antiviral therapy. Interciencia. 2013;38:836–842. [Google Scholar]
- 33.Ortega JT, Serrano ML, Surez AI, Baptista J, Pujol FH, Rangel HR. Methoxyflavones from Marcetia taxifolia as HIV-1 reverse transcriptase inhibitors. Natural Product Commun. 2017;12:1677–1680. [Google Scholar]
- 34.Ortega JT, Surez AI, Serrano ML, Baptista J, Pujol FH, Rangel HR. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res Ther. 2017;14(1):57. doi: 10.1186/s12981-017-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parvez MK, Arbab AH, Al-Dosari MS, Al-Rehaily AJ. Antiviral natural products against chronic hepatitis B: Recent developments. Curr Pharm Des. 2016;22:286–293. doi: 10.2174/1381612822666151112152733. [DOI] [PubMed] [Google Scholar]
- 36.Pedretti A, Villa L, Vistoli G. VEGA - An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J Comput Aided Mol Des. 2004;18:16773. doi: 10.1023/b:jcam.0000035186.90683.f2. [DOI] [PubMed] [Google Scholar]
- 37.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintero A, Fabbro R, Maillo M, Barrios M, Milano MB, Fernndez A, et al. Inhibition of hepatitis B virus and human immunodeficiency virus (HIV-1) replication by Warscewiczia coccinea (Vahl) Kl. (Rubiaceae) ethanol extract. Nat Prod Res. 2011;25:1565–1569. doi: 10.1080/14786419.2010.535164. [DOI] [PubMed] [Google Scholar]
- 39.Sells MA, Zelent AZ, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988;62:2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma M, Anderson SA, Schoop R, Hudson JB. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antiviral Res. 2009;83:165–170. doi: 10.1016/j.antiviral.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Shi MD, Liao YC, Shih YW, Tsai LY. Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways in HGF-treated liver cancer HepG2 cells. Phytomedicine. 2013;20:743–752. doi: 10.1016/j.phymed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Toukmaji A, Sagui C, Board J, Darden T. Efficient particle-mesh Ewald based approach to fixed and induced dipolar interactions. J Chem Phys. 2000;113:1091327. [Google Scholar]
- 43.Van Hoof L, Berghe DA, Hatfield GM, Vlietinck AJ. 3-Methoxyflavones as potent inhibitors of viral-induced block of cell synthesis. Planta Med. 1984;50:513–517. doi: 10.1055/s-2007-969786. [DOI] [PubMed] [Google Scholar]
- 44.Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem. 2010;31:67190. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitley R, Baines J. Clinical management of herpes simplex virus infections: past, present, and future. F1000Res. 2018;7:F1000 Faculty Rev–F1000 Faculty1726. doi: 10.12688/f1000research.16157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. Geneva: WHO; 2019. Hepatitis B. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Google Scholar]
- 47.Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W40710. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wudtiwai B, Sripanidkulchai B, Kongtawelert P, Banjerdpongchai R. Methoxyflavone derivatives modulate the effect of TRAIL-induced apoptosis in human leukemic cell lines. J Hematol Oncol. 2011;4:52. doi: 10.1186/1756-8722-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang QG, Wei F, Liu Q, Chen LJ, Liu YY, Luo F, et al. The flavonoid from Polygonum perfoliatum L. inhibits herpes simplex virus 1 infection. ActaVirol. 2014;58:368–373. doi: 10.4149/av_2014_04_368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.