Abstract
Neurofibromatosis type 1 (NF1) is a common genetic condition associated with cognitive and social dysfunction as well as abnormal brain structure. The pathophysiology underlying social dysfunction in NF1 is poorly understood. Here, we investigate for the first time whether there is a broad deficit of social cognition in NF1 and explore the neural correlates for these deficits. Twenty‐nine adults with NF1 and 30 controls were administered an ecologically based test of social cognition, The Awareness of Social Inference Test (TASIT), to identify deficits in emotion recognition and sarcasm detection. We employed voxel‐based morphometry in a subset of NF1 patients (n = 16) and 16 additional controls to examine the neural correlates of these deficits. Results indicated that adults with NF1 were impaired in their ability to understand paradoxical sarcasm and their capacity to recognize emotion, particularly anger. TASIT performance was not associated with measures of attention, visuospatial skills or executive function. Relative to controls, gray matter (GM) volume within the right superior temporal gyrus (STG) was decreased, after controlling for total brain volume. Decreased volume in this region was significantly associated with social cognitive deficits in adults with NF1. We conclude that patients with NF1 are at high risk for a social cognitive deficit and provide evidence for a neuroanatomical basis for this deficit; GM volumetric reductions in the right STG. These findings improve our understanding of the nature of social interaction impairments in NF1 and add to the growing body of literature indicating the STG as a critical brain region for social cognition. Hum Brain Mapp 35:2372–2382, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: neurofibromatosis 1, social cognition, theory of mind, emotion perception, voxel‐based morphometry
INTRODUCTION
Neurofibromatosis type 1 (NF1) is a common, single gene disorder with a birth incidence of approximately 1 in 2,700 births (Evans et al., 2010). Common physical features include neurocutaneous stigmata, such as café‐au‐lait spots, neurofibromas, skinfold freckling, and Lisch nodules (National Institutes of Health, 1988). Although individuals with NF1 typically have full scale IQ scores in the normal range, specific neurocognitive impairments are common, including visuospatial, language, executive function, and attention deficits (Ozonoff, 1999, Hyman et al., 2005). Structural and functional brain abnormalities are a feature of NF1 and have been shown to contribute to cognitive dysfunction (Payne et al., 2010). The NF1 gene is expressed throughout the brain, both in neurons and glia (Daston and Ratner, 1992, Zhu et al., 2005). Neurofibromin, the protein product of NF1, plays a role in cell proliferation and differentiation (Lee et al., 2010) and is thought to impact on brain structure in patients with NF1. Indeed, the most consistent finding has been an increase in total brain volume, with increases in both gray (GM) and white matter (WM) reported (Moore et al., 2000, Greenwood et al., 2005, Payne et al., 2010). Anomalies in GM properties have been found to be particularly relevant to cognitive dysfunction in NF1 (Said et al., 1996, Moore et al., 2000, Steen et al., 2001).
In addition to cognitive impairment, social dysfunction is a prominent feature of NF1. Increased social problems (such as complaining of loneliness, does not get along with others, not liked by others) as well as poorer social skills and reduced social outcomes are reported in pediatric NF1 cohorts (Johnson et al., 1999, Barton and North, 2004, Noll et al., 2007). Although there is limited research on the social functioning of adults with NF1, qualitative studies suggest that social dysfunction persists into adulthood, with social isolation a common feature (Benjamin et al., 1993, Ablon, 1996). NF1 is also associated with a higher frequency of autism spectrum disorder (ASD) symptomatology, with two recent articles reporting 14–29% of children with NF1 are rated within the severe range on a screening tool of ASD; a range said to be associated with a clinical diagnosis of ASD (Garg et al., 2012, Walsh et al., 2012). Given ASD is a disorder characterized by impaired social interaction and deficits in social cognition (Baron‐Cohen, 2000) these findings support the growing evidence for an association between NF1 and features of ASD and highlight the importance of investigating the cognitive/psychological mechanism underlying the social difficulties in NF1.
The common occurrence of social dysfunction in a single gene disorder such as NF1 provides a valuable opportunity to explore its mechanistic and neuroanatomical basis. Recently, Huijbregts and colleagues examined the emotion and facial recognition skills of 32 children with NF1 and 32 controls using still photographs. They found that children with NF1 displayed a weakness with facial recognition and the identification of emotions, specifically fear and anger (Huijbregts et al., 2010). We propose that these deficits are part of a wider impairment of social cognition contributing to the social interaction difficulties experienced by individuals with NF1. Social cognition is a broad concept that includes a number of functions that allow an individual to recognize, understand, and behave with respect to socially relevant stimuli (Adolphs, 2001). The term encompasses not only basic emotion recognition, but also the ability to interpret higher order social inferences such as sarcasm and deception (Macdonald et al., 2003). These abilities require accurate processing and interpretation of paralinguistic cues (such as voice tone, facial expression, body language) (Macdonald et al., 2003) as well as “theory of mind”; the ability to attribute mental states to self and others in terms of beliefs, attitudes, thoughts and emotions (Premack and Woodruff, 1978, Leslie, 1987).
Although the impact of social interaction impairments can be profound, investigations into social cognition and the neurobiological basis of social behavior disruptions in NF1 have yet to occur. Advances in techniques for studying brain‐behavior relationships, such as functional MRI, voxel‐based morphometry and lesion‐based studies have led to increased focus on the link between specific brain structures and impairments in emotion recognition and theory of mind in several clinical disorders, particularly schizophrenia and ASD (Baron‐Cohen, 2000, Harrington et al., 2005, Pinkham et al., 2008). Such structure/function correlations, in addition to primate studies, have led to neurobiological models of social cognition that provide the foundation for understanding the neural network underlying this function in the wider population (Brothers, 1990, Adolphs, 2001). These models focus on regions within the occipital and temporal cortices such as the fusiform gyrus and superior temporal sulcus which underlie facial processing (Brothers, 1990, Haxby et al., 2000, Adolphs, 2001), and the amygdala, which is critical for recognizing emotions from facial expressions, particularly those that signal danger or threat (such as fear or anger) (Adolphs et al., 1994, Young et al., 1995, Morris et al., 1996). Higher‐order aspects of social cognition, such as theory of mind, have been found to be consistently associated with a network of frontotemporal structures. A review of 40 theory of mind studies reported that the medial prefrontal cortex and orbitofrontal cortex were implicated in over 88% of studies, the cingulate cortices in 55%, and the superior temporal sulcus in 45% of studies (Carrington and Bailey, 2009).
A recent study utilizing whole brain imaging methods in NF1 revealed structural anomalies in a number of key brain regions that form part of the network associated with social cognition. Duarte et al. (2012) compared GM and WM brain volume in 20 adults with NF1 and 35 controls and found increased WM in the anterior and posterior cingulate gyrus and parahippocampal gyrus in the NF1 group. Increases in GM were also documented in the fusiform gyrus and parahippocampal gyrus. Reductions in GM volume were reported in the posterior cingulate, and superior temporal gyrus.
On the basis of the structural anomalies recently identified in the frontotemporal network (Duarte et al., 2012), as well as the high frequency of social impairment documented in NF1, the current study aimed to determine whether deficits in social cognition are present in adults with NF1 and to understand the neuroanatomical correlates of these deficits. The following hypotheses were proposed: (i) NF1 is associated with impairments in higher‐order social cognition and emotion recognition; (ii) higher‐order social cognitive impairments correlate with structural abnormalities in the ventromedial prefrontal cortex, orbitofrontal cortex, the superior temporal gyrus and the cingulate cortices, regions typically found to be associated with social cognitive dysfunction; and (iii) greater emotion recognition deficits correlate with structural abnormalities in regions associated with facial and emotion recognition, specifically the fusiform gyrus and the amygdala.
METHODS
Participants
Thirty‐four adults with NF1 were recruited from a specialist Neurogenetic Clinic for patients with neurofibromatosis and related disorders. NF1 participants fulfilled diagnostic criteria specified by the National Institutes of Health (1988) statement. Patients were excluded if any of the following criteria were met (1) under 18 or over 60 years of age; (2) diagnosed with intracranial pathology such as brain tumors (including symptomatic optic gliomas) or epilepsy (n = 2); (3) insufficient knowledge of English (4) abnormal vision or hearing that could not be corrected to normal; (5) IQ < 70; (6) history of a diagnosed psychiatric disorder (n = 1); or (7) presence of a genetic or neurological condition (other than NF1) that could affect test performance. This resulted in a final sample of 29 who completed neuropsychological testing, 16 of which underwent volumetric MRI scans. Thirty controls aged 18–60 years were recruited from various sources including parents of unaffected children who were controls in a concurrent study examining the cognitive phenotype of toddlers with NF1, unaffected parents of children with sporadic NF1 (nonfamilial) who were seen at the hospital's Neurogenetic Clinic and individuals from the wider community that responded to advertisements. This group of 30 control participants completed neuropsychological testing but did not take part in neuroimaging. An additional 16 gender‐ and age‐matched (±3 years) normal control participants were recruited as part of a concurrent study examining brain structure in adults with multiple sclerosis. These 16 participants formed the MRI control group and completed the same MRI protocol as the NF1 patients. All control participants spoke English as their first language, had normal or corrected‐to‐normal vision and hearing, and had no record of psychiatric, genetic, or neurological disorders. Participants were tested at the Children's Hospital at Westmead. Written informed consent was obtained from all participants according to the Declaration of Helsinki and the study was approved by The Children's Hospital at Westmead Human Research Ethics Committee.
Measures
The Awareness of Social Inference Test‐Form A (TASIT) was used to examine social cognition. This measure assesses both basic emotion recognition (EET: Emotion Evaluation Test) and higher‐order social cognition (SI‐M: Social Inference Minimal) (Macdonald et al., 2003). The EET uses 28 professionally enacted video vignettes lasting 20–30 s in which an actor portrays one of seven basic emotions (happiness, sadness, anger, disgust, fear, surprise, and neutral). Participants were required to state the emotion portrayed based on response cards which contained each of the emotions in a random order. To exclude a relevant language deficit, each participant's understanding of each emotion was clarified before testing. SI‐M assesses the ability to infer the mental state of others who are in conversational exchange through cues such as facial expression, tone of voice, and gesture (paralinguistic cues). It comprises 15 video vignettes depicting sincere, sarcastic or paradoxically sarcastic interactions between two actors (Appendix 1). In the sincere exchanges, the speaker means what he/she is saying and non‐verbal cues are consistent with this. In the simple sarcastic exchanges the actors use exaggerated facial, vocal and body language indicating sarcasm which diverges from what they are actually saying. Paradoxical sarcastic exchanges consists of scenes in which the dialogue does not make sense unless it is understood that the speaker is being sarcastic. For example, in one scene one actor asks another whether he has his ticket and passport. In response the actor rolls his eyes and sarcastically states that he has “torn it up and thrown it away” to which the first actor replies “Good, that's OK then”. After viewing each video clip participants are asked to either endorse or reject (by responding either “yes” or “no”) four statements about the speaker's emotional state, what he/she is saying, what the speaker may be thinking (theory of mind judgment) and intending their conversational partner to think (intent: second order theory of mind). The TASIT was individually administered on a 15″ computer screen and had a total playing time of ∼20 min.
The Wechsler Adult Intelligence Scale‐ Third Edition (WAIS‐III) was administered to all patients to assess general cognitive abilities (Wechsler, 1997). Participants with NF1 who underwent neuroimaging (n = 16) were also administered a measure of visual spatial skills [the Judgment of Line Orientation (JLO); (Benton et al., 1976)], divided, selective, and switching attention [Test of Everyday Attention (TEA) Telephone Search Dual Task, Telephone Search and Visual Elevator; (Robertson et al., 1994)] and a questionnaire assessing executive functions [The Behavior Rating Inventory of Executive Function‐ Adult Version (BRIEF‐A)] (Roth et al., 2005). This self‐report scale provides nine clinical scales which combine to form one composite (GEC, global executive composite).
Behavioral Data Analysis
Statistical analysis of behavioral data was conducted using SPSS v19. Assumptions of normality and homoscedasticity were tested. Significantly skewed data were transformed using arcsin transformations. Cognitive scores were converted to standardized scores based on normative data. Parametric data for patients and controls were compared with independent t‐tests and analysis of variance (ANOVA), with post hoc t‐tests as necessary. One‐tailed t‐tests were used in instances where a directional effect was hypothesized (e.g., group TASIT performance). Categorical data were investigated with χ2 tests. Holm‐Bonferroni correction was used for multiple comparisons. Pearson's correlations were used to examine the relationship between variables. Total intracranial volume was controlled for when investigating the relationship between region of interest volume values and performance on the TASIT. Since multiple correlations were conducted, a threshold of α < 0.01 was used.
Voxel‐Based Morphometry
Image acquisition
Anatomical MRI data was acquired for 16 NF1 and 16 control subjects using a 1.5 Tesla GE Signa HDx scanner (GE Healthcare, Milwaukee, Wisconsin) at Westmead Hospital, Sydney. Acquisition was performed using an 8‐channel head coil. 3D T1‐weighted MR images were acquired in the sagittal plane using a 3D SPGR sequence (TR = 13.7 ms; TE = 6.3 ms; Flip Angle = 20°; NEX = 1). A total of 165 contiguous 1 mm slices were acquired with a 256 x 256 matrix with an in‐plane resolution of 1 mm × 1 mm resulting in isotropic voxels.
Whole Brain Voxel‐Based Morphometry Analysis
T1 image data were preprocessed and analyzed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) and the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm). Images were corrected for bias‐field inhomogeneities, tissue‐classified into GM, WM, and CSF and registered to standard space using high‐dimensional DARTEL normalization (Ashburner, 2007). The segmentation approach is based on an adaptive maximum, a posterior technique which does not need a priori information about tissue probabilities (Rajapakse et al., 1997). The segmentation procedure is further refined by accounting for partial volume effects (Tohka et al., 2004) and by applying a hidden Markov random field model which incorporates spatial prior information of the adjacent voxels into the segmentation estimation. The warped tissue type images were modulated to preserve the volume of a particular tissue within a voxel by multiplying voxel values in the segmented images by the Jacobian determinants derived from the spatial normalization step. The analysis of these modulated images allows testing for regional differences in absolute volume of tissue class. Finally, images were smoothed with a full‐width half‐maximum kernel of 8mm. Statistical analyses were performed on these images for whole brain comparison of volume between the NF1 and control group. Data were submitted for analysis of covariance (ANCOVA) using group membership as a factor (two levels) and total intracranial volume as a nuisance covariate. Total intracranial volume was calculated by summing the number of voxels in each segmented tissue class and multiplying the total by the voxel volume. The statistical threshold of significance was set at a false discovery rate (FDR) <0.05 with a cluster threshold (K) > 10 voxels.
Regions of Interest Analysis
On the basis of prior studies of social cognition as well as structural neuroanatomical studies of NF1 patients, we hypothesized six regions of interest with abnormal GM volume in NF1 and also their volume to be related to behavioral performance. The regions were: fusiform gyrus, the amygdala, the ventromedial prefrontal cortex (which included the anterior cingulate), the posterior cingulate, the orbitofrontal cortex, and the superior temporal gyrus. We extracted the mean GM adjusted volumes for these regions bilaterally using the Automated Anatomical Labelling atlas regional templates and marsbar software (http://marsbar.sourceforge.net) (Brett et al., 2002) and entered each as a dependent variable into an ANCOVA. Total intracranial volume was entered as a covariate to adjust for total brain size. Statistical threshold was at P < 0.05 corrected for multiple comparisons using the Holm‐Bonferroni method.
RESULTS
Demographic Data
Demographic data for the cognitive and imaging groups are shown in Tables 1 and 2. There was no difference between NF1 participants and controls in gender (χ2 < 0.01, df = 1, P = 0.91), age [t(57) = −0.04, P = 0.97], years of education [t(57) = −0.9, P = 0.37], or full scale IQ [t(57) = −1.9, P = 0.06]. There was also no difference in age and gender between the two imaging groups (Table 2).
Table 1.
Demographic and cognitive data of participants who completed the TASIT
| NF1 (n = 29) | Control (n = 30) | |
|---|---|---|
| Age (years) | 34.38 (9.91) | 34.48 (8.06) |
| Sex (male:female) | 12:17 | 12:18 |
| Years of education | 12.82 (2.05) | 13.37 (2.45) |
| FSIQ | 100.72 (14.45) | 106.90 (9.91) |
| TASIT EET | 1.19 (0.95)a | 1.25 (0.12) |
| TASIT SI‐M | ||
| Sincere | 1.06 (0.28)a | 1.26 (0.18) |
| Simple Sarcasm | 1.36 (0.22) | 1.37 (0.18) |
| Paradoxical Sarcasm | 1.30 (0.21)a | 1.41 (0.18) |
| JLO | −0.63 (0.82)b | — |
| GEC | 56.50 (11.60)b | — |
| TEA‐Divided | 8.58 (3.81)b | — |
| TEA‐Selective | 10.21 (4.20)b | — |
| TEA‐Switching | 9.38 (2.88)b | — |
Mean values are given for age, education, and cognitive variables with standard deviation (SD) in parentheses.
P < 0.05.
= subset of NF1 participants completed this test (n = 16).
M, male; F, females; FSIQ, Full Scale Intelligence (Mean = 100, SD =15); TASIT, The Awareness of Social Inference Test; EET, Emotion Evaluation Test; SI‐M, Social Inference Minimal; JLO, Judgement of Line Orientation (Mean = 0, SD =1); GEC, Global Executive Composite (Mean = 50, SD = 10; Higher GEC score indicates more severe ratings); TEA, Test of Everyday Attention (Mean = 10, SD = 3). Standardized scores are given for the JLO, GEC, FSIQ, and TEA. Transformed scores are given for the TASIT.
Table 2.
Demographic data and volumes of brain tissue for imaging groups
| NF1 | Control | t(df=30) | |
|---|---|---|---|
| Age (years) | 29.8 (3.5) | 33.1 (7.2) | −1.67 |
| Sex (male:female) | 6:10 | 6:10 | – |
| Grey matter | 661.7 (69.4) | 576.4 (41.0) | 4.23b |
| White matter | 648.8 (86.5) | 616.9 (68.1) | 1.16 |
| CSF | 204.4 (40.1) | 203.8 (34.2) | 0.05 |
| Whole brain volume | 1515.0 (181.5) | 1397.0 (120.3) | 2.17a |
Mean values are given for age, education, and brain tissue volumes with standard deviation in parentheses. CSF, cerebrospinal fluid.
P < 0.05
P < 0.001.
Social Cognition
For emotion evaluation test (EET) transformed scores, a one tailed t test revealed a significant difference between groups. Overall, adults with NF1 were poorer at recognizing emotions than controls (t(57) = −1.97, P = 0.026). Given previous findings of impairment in the recognition of anger and fear in children with NF1, we performed post hoc analyses to compare recognition of each type of emotion. Post hoc contrasts showed a significant deficit in recognizing anger when compared to controls [t(57) = −3.1, P = 0.003; Fig. 1).
Figure 1.
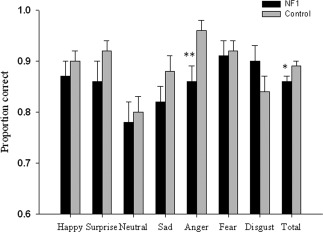
Emotion evaluation test, shown separately for each type of emotion and total score. Significant difference indicated by asterisk. * P < 0.05 **P < 0.01. Significant difference using Holm‐Bonferroni correction. Error bars show standard error.
For SI‐M transformed scores, participants with NF1 had impaired higher‐order social cognition [main effect for group, F(1,57) = 11.1, P = 0.002]. Post hoc analyses showed NF1 patients demonstrated an impaired ability to correctly interpret sincere [t(57) = −3.2, P = 0.001] and paradoxical sarcastic scenarios [t(57) = −2.3, P = 0.014] but were no worse at understanding simple sarcasm [t(57) = −0.2, P = 0.425; Fig. 2]. There was no significant relationship between EET performance and SI‐M conditions (sincere, simple sarcasm, paradoxical sarcasm) in either group suggesting that poor performance by the NF1 group on SI‐M was not associated with impaired emotion evaluation (all, P > 0.1). The relationship between SI‐M conditions, EET, JLO, TEA subsets, and BRIEF‐A was also examined to determine if specific cognitive deficits contribute to TASIT performance in NF1 subjects. No relationship between cognitive variables and TASIT variables was observed (all, P > 0.1).
Figure 2.
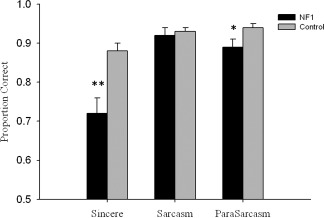
Social cognition group results. Sincere, sarcastic and paradoxical sarcasm (ParaSarcasm) categories from TASIT test. Holm‐Bonferroni correction used. *P ≤ 0.02; **P ≤ 0.01. Error bars show standard error.
Voxel‐Based Morphometry
Whole brain comparison between NF1 participants and controls
Results are summarized in Tables 2 and 3. Patients with NF1 demonstrated significantly higher total intracranial (P = 0.04) and global GM volumes (P < 0.001) than controls. In contrast, global WM volume and CSF were not significantly different from controls (both P > 0.05). We observed significantly higher GM volume relative to total intracranial volume in participants with NF1 in a number of brain regions. Increases were predominately found within the frontal and temporal lobes including the left medial frontal gyrus (BA10), bilateral superior frontal gyrus (BA8), left precentral gyrus (BA6), right middle frontal gyrus (BA10), right inferior frontal gyrus (BA47), left inferior temporal gyrus (BA20), left transverse temporal gyrus (BA42), and left middle temporal gyrus (BA22). Increases were also found in a number of other cortical regions such as the right cingulate gyrus (BA23), bilateral lingual gyrus (BA18), left postcentral gyrus (BA40), and bilateral inferior and superior parietal lobule (BA40/7). NF1 was also associated with GM decreases in the right superior temporal gyrus (BA38), bilateral superior frontal gyrus (BA11), and orbital gyrus (BA11) (Table 3; Fig. 3).
Table 3.
Brain regions in which voxels showed significant GM changes in participants with NF1 compared with controls
| Region | Side | Cluster size | FDR‐ corr | Z | x | y | z |
|---|---|---|---|---|---|---|---|
| NF1 > Controls | |||||||
| Inferior temporal gyrus | L | 91777 | <0.001 | 6.78 | −63 | −37 | −21 |
| Lingual gyrus | R | <0.001 | 5.68 | 8 | −69 | −5 | |
| L | <0.001 | 5.43 | −18 | −91 | −18 | ||
| Medial frontal gyrus | L | 7227a | <0.001 | 4.82 | −2 | 64 | 7 |
| L | 3797 | 0.025 | 4.82 | −2 | 64 | 7 | |
| Superior frontal gyrus | R | 7227a | 0.001 | 4.72 | 2 | 26 | 60 |
| L | 82 | 0.034 | 2.88 | −20 | 53 | 27 | |
| Transverse temporal gyrus | L | 6116 | 0.001 | 4.5 | −63 | −10 | 13 |
| Postcentral gyrus | L | 0.002 | 4.12 | −66 | −25 | 21 | |
| Precentral gyrus | L | 0.001 | 4.33 | −63 | −1 | 19 | |
| L | 278 | 0.009 | 3.49 | −33 | −19 | 39 | |
| Cerebellar tonsil | R | 941 | 0.002 | 4.27 | 21 | −52 | −59 |
| L | 540 | 0.002 | 4.11 | −51 | −52 | −45 | |
| Caudate | R | 704 | 0.004 | 3.93 | 2 | 18 | 16 |
| L | 0.015 | 3.27 | −6 | 9 | 19 | ||
| R | 573a | 0.006 | 3.71 | 14 | −12 | 27 | |
| L | 76 | 0.019 | 3.15 | −18 | −30 | 24 | |
| Cingulate gyrus | R | 573a | 0.017 | 3.54 | 4 | −25 | 24 |
| Inferior parietal lobule | L | 361a | 0.010 | 3.45 | −52 | −55 | 49 |
| R | 209 | 0.016 | 3.24 | 51 | −64 | 43 | |
| Superior parietal lobule | L | 361a | 0.041 | 2.78 | −39 | −60 | 55 |
| R | 22 | 0.030 | 2.93 | 32 | −61 | 62 | |
| Middle frontal gyrus | R | 233 | 0.016 | 3.24 | 48 | 56 | −3 |
| Pyramis | R | 320 | 0.016 | 3.22 | 52 | −67 | −42 |
| Middle temporal gyrus | L | 100 | 0.028 | 2.96 | −56 | −40 | 3 |
| L | 12 | 0.040 | 2.8 | −66 | −16 | −8 | |
| Inferior semi‐lunar lobule | L | 101 | 0.031 | 2.93 | 42 | −64 | −56 |
| Culmen | R | 15 | 0.031 | 2.92 | 0 | −51 | −30 |
| Inferior frontal gyrus | R | 52 | 0.032 | 2.91 | 20 | 28 | −3 |
| L | 10 | 0.042 | 2.77 | −52 | 23 | 25 | |
| Uvula | R | 43 | 0.034 | 2.88 | 8 | −66 | −41 |
| Insula | R | 10 | 0.039 | 2.81 | 42 | 3 | −3 |
| NF1 < Controls | |||||||
| Superior temporal gyrus | R | 112 | 0.037 | 4.91 | 36 | 18 | −47 |
| Superior frontal gyrus | L | 251 | 0.037 | 4.82 | −22 | 44 | −23 |
| R | 85 | 0.037 | 4.68 | 26 | 44 | −23 | |
| Orbital gyrus | R | 17 | 0.040 | 4.31 | 4 | 44 | −32 |
The table reports cluster size and peak voxel location and Z score. Only voxels surviving FDR corrected P < 0.05 threshold are reported. Coordinates (x, y, z) are in standard Montreal Neurological Institute (MNI) anatomical space.
= same cluster L, left; R, right.
Figure 3.
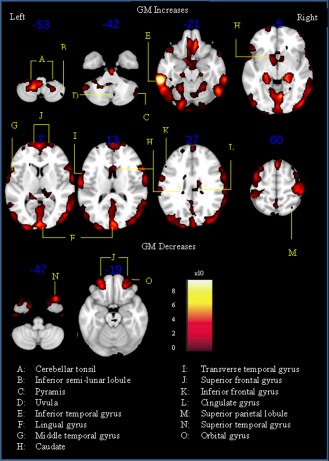
SPM(t) of gray matter increases and decreases in patients with neurofibromatosis type 1 versus controls. Only voxels surviving FDR corrected P < 0.05 threshold are shown overlaid on representative T 1 brain in standard space using Montreal Neurological Institute (MNI) template. The z‐coordinate for each axial slice in the standard MNI space is given. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Region of Interest Analysis and Correlations with the TASIT
Region of interest (ROI) analysis revealed that the NF1 group had increased GM volume in the ventromedial prefrontal cortex [F(1,30) = 9.8, P = 0.004], and the posterior cingulum [F(1,30) = 10.3, P = 0.003] compared with controls. No group difference in volume was evident in the amygdala, fusiform gyrus, orbitofrontal cortex, or superior temporal gyrus. To determine the relationship between regional GM volumes and TASIT variables, correlations were performed in the NF1 group. While no significant relationship was evident between amygdala GM volume and EET performance (r = −0.3, P = 0.40), a trend was present between the latter variable and fusiform gyrus GM volume whereby larger left fusiform gyrus volume was associated with poorer total emotion recognition (r = −0.55, P = 0.05). We also examined the relationship between higher‐order social cognition (TASIT SI‐M) and GM volume of the posterior cingulate, ventromedial prefrontal cortex, orbitofrontal cortex, and superior temporal gyrus. Decreased GM volume in the right superior temporal gyrus was strongly associated with poorer understanding of scenarios involving paradoxical sarcasm (r = 0.71, P = 0.007) in NF1. Understanding of this type of scenario was not correlated with GM volume in the ventromedial prefrontal cortex (r = 0.44, P = 0.13), posterior cingulate (r = 0.33, P = 0.28) or orbitofrontal cortex (r = 0.33, P = 0.27). There was also no association between the ROIs and performance on the sincere scenarios (all P > 0.1).
DISCUSSSION
The objective of this study was to define social cognitive impairments in adults with NF1 and to understand their neuroanatomical correlates. Compared to demographically analogous controls, we have demonstrated that adults with NF1 display social cognitive deficits—specifically with their ability to infer the thoughts and feelings of another person during social interactions involving paradoxical sarcasm or sincere exchanges. Impairment in emotion recognition was also identified, manifesting as a reduced ability to identify anger from facial expressions and voice tone.
Analysis of MRI data confirmed previously described effects of NF1 on brain structure, including increases in gray matter (GM) volume in the frontal, temporal, parietal and limbic lobes. NF1 was also associated with a decrease in GM in the right superior temporal gyrus, superior frontal gyrus and orbital gyrus. We extended the voxel‐based morphometry approach to look specifically for structural regional abnormalities that were associated with deficits in social cognition—both emotion recognition and sarcasm detection. We found that poor understanding of sarcasm was significantly associated with decreased GM volume in the right superior temporal gyrus. A trend was also present between impaired emotion recognition and larger GM volume in the left fusiform gyrus.
Higher‐Order Social Cognition in NF1 and its Neural Correlates
This study demonstrated that adults with NF1 have impaired social cognitive abilities. Specifically, when inferring the mental state of others, adults with NF1 are able to integrate and understand paralinguistic information when the discrepancy between statement and intention is straightforward (simple sarcasm), but show deficiency when attempting to understand such information when the discrepancy is especially convoluted and contradictory (paradoxical sarcasm) or when the social cues are subtle (paradoxical sarcasm and sincere). We found no association between these impairments and visuospatial abilities, attention, executive function or basic emotion recognition. Based on the current study design, it is not possible to determine the level at which the failure in social cognition occurs—NF1 patients may have simple perceptual deficits in decoding or detecting paralinguistic cues displayed by others (social processing) or more general deficits in theory of mind.
We hypothesized that the cingulate cortices, orbitofrontal cortex superior temporal gyrus and ventromedial prefrontal cortex would be abnormal in NF1 and would relate to higher‐level social cognitive abilities. This was based on previous neuroimaging studies that suggest these regions play a key role in theory of mind abilities and sarcasm interpretation (Carrington and Bailey, 2009). A structural difference was not found for the orbitofrontal cortex. While the cingulate cortices and ventromedial prefrontal cortex were structurally larger in adults with NF1, there was no association between these anomalies and TASIT performance. Intriguingly, we found a strong link between right superior temporal gyrus volume, and TASIT performance in NF1, whereby smaller volume was associated with deficits in understanding paradoxical sarcasm. There is now an a extensive body of neuroimaging literature demonstrating the superior temporal gyrus' role in the processing of visual and auditory social cues (social processing) (Pelphrey et al., 20042004b, 2004c, Ruby and Decety, 2004, Schultz et al., 2004, Zilbovicius et al., 2006, Bigler et al., 2007, Jou et al., 2010). Furthermore, GM volume anomalies in this structure found in individuals with schizophrenia and autism spectrum disorder (ASD) have been implicated in the social processing deficits present in these populations (Zilbovicius et al., 2006, Williams, 2008, Jou et al., 2010). Duarte et al. (2012) recently reported that the superior temporal gyrus is structurally abnormal (smaller) in children and adults with NF1 (Duarte et al., 2012). Our whole brain analysis confirmed this finding, showing decreased GM in the right superior temporal gyrus in adults with NF1 compared with demographically matched controls. Our findings are consistent with a potential disruption in the superior temporal gyrus in NF1 and suggest a neural basis for the deficits in social cognition found in this study. The superior temporal gyrus is highly connected with other regions of the social brain including the medial prefrontal cortex, fusiform gyrus, and the cingulate cortices; regions shown by us and others to be structurally abnormal in NF1 (Duarte et al., 2012). It is feasible that functional connectivity impairments between this structure and other regions critical to social understanding underlie social cognitive deficits in NF1.
Emotion Recognition in NF1 and its Neural Correlates
While NF1 participants were impaired in their recognition of anger, recognition of other types of emotions was preserved, suggesting that the patients understood the task and were able to respond appropriately. This finding is consistent with a previous report on emotion recognition in a sample of children with NF1 (Huijbregts et al., 2010). In that study, participants viewed still photographs of emotional facial expressions and demonstrated deficits in facial recognition and identification of fear and anger, thus providing convergent evidence for a deficit in the processing of anger. It is unclear why individuals with NF1 experience difficulty with emotion recognition. Previously identified impairments in facial recognition abilities (Huijbregts et al., 2010) suggest that poor anger perception in NF1 may not be specific to the emotional component per se, but rather a result of reduced facial processing abilities. In agreement with this, the present study found a trend between poor emotion recognition and increased GM volume in the left fusiform gyrus, a region previously found to be structurally larger in NF1 adults (Duarte et al., 2012). There is a wealth of research suggesting a key role for the fusiform gyrus in face detection and recognition (Puce et al., 1996, Kanwisher et al., 1997) with structural and functional abnormalities in this region consistently linked to face processing deficits in a number of disorders characterized by poor emotion recognition such as ASD (Kwon et al., 2004, Pelphrey et al., 2004a, Williams, 2008). Although speculative, these findings collectively suggest that abnormal facial processing contributes to the emotion recognition impairments in NF1. An alternative possibility is that individuals with NF1 do not visually process faces in the same way as neurologically normal individuals. For example, they may spend less time visually scanning core features of the face, such as the eyes and mouth which provide information on emotion. Eye tracking and functional MRI studies examining face and object processing in NF1 may help to distinguish between these two possibilities.
It has been hypothesized that structural brain changes in patients with NF1 reflect pathological patterns in the normal neurodevelopmental process of neuronal cell death (apoptosis) (Moore et al., 2000). It is possible that aberrant RAS‐related GTPase, known to occur in NF1 (Cui et al., 2008) impacts on this neuronal “pruning” process during development resulting in the abnormal brain volumes observed in this study. Alternatively, disruption to biochemical pathways known to play a role in brain growth which occur downstream of NF1‐related Ras signaling may be involved in the pathogenesis of these structural abnormalities and associated social cognitive deficits. One such pathway is the P13K signaling pathway which activates mTOR and is thought to be affected in NF1 (Rosner et al., 2008). Mutations in the negative regulator of this pathway, PTEN, have been found to cause macrocephaly in humans and are present in a small proportion of ASD patients (Hoeffer and Klann, 2010). Interestingly, mice with PTEN loss in postmitotic neurons in the cortex and hippocampus have been shown to develop macrocephaly and behaviors reminiscent of ASD (Kwon et al., 2006). Collectively, these findings not only suggest a possible pathway underlying the social cognitive deficits in NF1, but highlight the potential of single gene disorders, such as NF1, in providing insights into the molecular and neurobiology of social behaviors. Although anatomical and behavioral parallels have been demonstrated between Nf1 mouse models and human NF1 phenotypes, including an enlarged corpus callosum (Wang et al., 2012) and dysfunction in attention and visual spatial memory function (Brown et al., 2010; Silva et al., 1997), there are currently no studies of social behavior phenotypes in the Nf1 mouse model. Although some higher order aspects of human social cognition do not seem amendable to modeling in the mouse, social memory and social recognition can be assessed (Ferguson et al., 2000, McNaughton et al., 2008). The current findings should stimulate increased interest in studies of social behavioral phenotypes in the NF1 mouse which will be important in elucidating the specific neural and molecular pathways by which NF1 expression affects clinically important aspects of social behavior.
As a limitation of this study, we acknowledge that, VBM has potential confounds, including mis‐registration to templates of tissue classification. Besides pure volumetric differences, our results could also reflect differences in tissue content that may produce differences in signal T1 properties. To reduce these confounds and minimize mis‐registration we utilized DARTEL, a sophisticated registration model developed to counter these criticisms (Ashburner, 2007). It is also worth noting that while this study's whole brain analysis found significant structural differences in the superior temporal gyrus, our region of interest analysis did not. It is likely that different statistical approaches to control for type 1 error (i.e., FDR versus Bonferroni‐Holm) contributed to this discrepancy.
CONCLUSIONS
Our data provide novel insights into the basis of social dysfunction in NF1, demonstrating a deficit in emotion recognition and higher‐order social cognition. This allows better characterization of the nature of social dysfunction in NF1 and may facilitate the development of new therapeutic approaches, with a focus on the innate processing and perception of social signals and voice stimuli. Without effective treatment, social skill deficits present a major barrier to successful integration and impact on social participation, interpersonal relationships, self‐worth, and ultimately quality of life. In addition, this study identified a structural correlate of social cognitive performance; right superior temporal gyrus gray matter volume correlated positively with the ability to understand paradoxical sarcasm, suggesting that this is a critical brain region for social cognition.
ACKNOWLEDGMENTS
The authors acknowledge Sheryl Foster, Tania Pickering, and Skye McDonald for their contribution to this research.
Appendix 1
Sample Scripts and Probe Questions Used in Social Inference ‐ Minimal Test
Sincere or Simple Sarcastic Items ‐ (Enacted either sarcastically or sincerely)
Ruth: Great movie, wasn't it?
Michael: Oh, yeah, great.
Ruth: I thought it was terrific. I was on the edge of my seat.
Michael: Oh, me too, on the edge of my seat.
Ruth: Well, weren't you surprised by the ending?
Michael: Oh yeah, the ending was a huge surprise.
Ruth: I thought the actors were really good. I really like that main girl.
Michael: She was unbelievable, and the guy opposite her‐what a performance!
Ruth: It's a shame its closing. I'd like to see it.
Michael: Yeah, what a shame. I feel I could see it another dozen times.
Probe questions
Act: Is Michael agreeing with Ruth about the movie?
Say: Does he mean the actors were good?
Think: Does he think the movie was bad?
Feel: Did he enjoy the movie?
Paradoxical Sarcasm ‐ Script is nonsensical except if one speaker is assumed to be insincere
Gary: Have you got your ticket?
Keith: Nope. I tore it up and threw it away.
Gary: Good. And your passport's safe?
Keith: Sure, I threw that in the bin along with my ticket.
Gary: So, you've got everything.
Probe questions
Do: Is Keith seriously trying to make Gary think he's lost his ticket?
Say: Does Keith mean he has got his ticket and passport?
Think: By the end of the scene, does Gary think Keith has his ticket?
Feel: Is Keith grateful that Gary checked about his ticket?
REFERENCES
- Ablon J (1996): Gender response to neurofibromatosis 1. Soc Sci Med 42:99‐109. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2001): The neurobiology of social cognition. Curr Opin Neurobiol 11:231‐239. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669‐672. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95‐113. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S (2000): Theory of mind and autism: A fifteen year review In: Baron‐Cohen S., et al., editors. Understanding Other Minds, 2nd ed. New York: Oxford University Press. [Google Scholar]
- Barton B, North K (2004): Social skills of children with neurofibromatosis type 1. Dev Med Child Neurol 46:553‐563. [DOI] [PubMed] [Google Scholar]
- Benjamin CM, Colley A, Donnai D, Kingston H, Harris R, Kerzin‐Storrar L (1993): Neurofibromatosis type 1 (NF1): Knowledge, experience, and reproductive decisions of affected patients and families. J Med Genet 30:567‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A, Varney N, Hamsher K (1976): Judgment of line orientation. Iowa City: Department of Neurology, University of Iowa. [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE (2007): Superior temporal gyrus, language function, and autism. Dev Neuropsychol 31:217‐238. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J (2002): Region of interest analysis using an SPM toolbox In: 8th International Conference on Functional Mapping of the Human Brain Sendai, Japan. [Google Scholar]
- Brothers L (1990): The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci 1:27‐51. [Google Scholar]
- Brown JA, Emnett RJ, White CR, Yuede CM, Conyers SB, O'Malley KL, Wozniak DF, Gutmann DH (2010): Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis‐1 mutant mice. Hum Mol Genet 19:4515‐4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ (2009): Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp 30:2313‐2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ (2008): Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135:549‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston MN, Ratner N (1992): Neurofibromin, a predominately neuronal GTPase activating protein in the adult, is ubiquitously expressed during development. Dev Dyn 195:216‐226. [DOI] [PubMed] [Google Scholar]
- Duarte JV, Ribeiro MJ, Violante IR, Cunha G, Silva E, Castelo‐Branco M (2012): Multivariate pattern analysis reveals subtle brain anomalies relevant to the cognitive phenotype in neurofibromatosis type 1. Hum Brain Mapp. Doi: 10.1002/hbm.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F (2010): Birth incidence and prevalence of tumour‐prone syndromes: Estimates from a UK family register service. Am J Med Genet A 152:327‐332. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, JHearn EF, Matzuk MM, Insel TR, Winslow JT (2000): Social amnesia in mice lacking the oxytocin gene. Nat Genet 25:284‐288. [DOI] [PubMed] [Google Scholar]
- Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, Green J (2012): Autism and other psychiatric comorbidity in neurofibromatosis type 1: Evidence from a population‐based study. Dev Med Child Neurol 55:139‐145. [DOI] [PubMed] [Google Scholar]
- Greenwood RS, Tupler LA, Whitt JK, Buu A, Dombeck CB, Harp AG, Payne ME, Eastwood JE, Krishnan KR, MacFall JR (2005): Brain morphometry, T2‐weighted hyperintensities, and IQ in children with neurofibromatosis type 1. Arch Neurol 62:1904‐1908. [DOI] [PubMed] [Google Scholar]
- Harrington L, Siegert RJ, McClure J (2005): Theory of mind in schizophrenia: A critical review. Cognit Neuropsychiatry 10:249‐286. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cognit Sci 4:223‐233. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E (2010): mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci 33:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts S, Jahja R, De Sonneville L, De Breij S, Swaab‐Barneveld H (2010): Social information processing in children and adolescents with neurofibromatosis type 1. Dev Med Child Neurol 52:620‐625. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North K (2005): The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurol 65:1037‐1044. [DOI] [PubMed] [Google Scholar]
- Johnson NS, Saal HM, Lovell AM, Schorry EK (1999): Social and emotional problems in children with neurofibromatosis type 1: evidence and proposed interventions. J Pediatr 134:767‐772. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Minshew NJ, Keshaven MS, Vitale MP, Harden AY (2010): Enlarged right superior temporal gyrus in children and adolescents with autism. Brain Res Dev Brain Res 1360:205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception J Neurosci 17:4302‐4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF (2006): Pten regulates neuronal aborization and social interaction in mice. Neuron 50:126‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Ow AW, Pedatella KE, Lotspeich LJ, Reiss AL (2004): Voxel‐based morphometry elucidates structural neuroanatomy of high‐functioning autism and Asperger syndrome. Dev Med Child Neurol 46:760‐764. [DOI] [PubMed] [Google Scholar]
- Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH (2010): Neurofibromatosis‐1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region‐specific manner Genes Dev 24:2317‐2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A (1987): Pretence and representation: the origins of ‘theory of mind’. Psychological Review 94:412‐426. [Google Scholar]
- Macdonald S, Flanagan S, Rollins J, Kinch J (2003): A new clinical tool for assessing social perception after traumatic brain injury J Head Trauma Rehabil 18:219‐239. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ (2008): Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Neuroscience 122. [DOI] [PubMed] [Google Scholar]
- Moore BD III, Slopis JM, Jackson EF, De Winter AE, Leeds NE (2000): Brain volume in children with neurofibromatosis type 1: Relation to neuropsychological status. Neurol 54:914‐920. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383:812‐815. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (NIH) (1988): Neurofibromatosis Conference Statement. National Institute of Health Consensus Development Conference. Arch Neurol 45:576‐578. [PubMed] [Google Scholar]
- Noll RB, Reiter‐Purtill J, Moore BD, Schorry EK, Lovell AM, Vannatta K, Gerhardt CA (2007): Social, emotional, and behavioural functioning of children with NF1. Am J Med Genet Part A. 143A:2261‐2273. [DOI] [PubMed] [Google Scholar]
- Ozonoff S (1999): Cognitive impairment in neurofibromatosis type 1. Am J Med Genet 89:45‐52. [PubMed] [Google Scholar]
- Payne JM, Moharir MD, Webster R, North K (2010): Brain structure and function in neurofibromatosis type 1: Current concepts and future directions J Neurol Neurosurg Psychiatry 81:304‐309. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Adolphs R, Morris JP (2004a): Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil 10:259‐271. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G (2004b): Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cognit Neurosci 16:1706‐1716. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G (2004c): When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psycholog Sci 15:598‐603. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL (2008): Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders Schizophr Res 99:164‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Woodruff G (1978): Does the chimpanzee have a ‘theory of mind’? Behav Brain Sci 4:515‐526. [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G (1996): Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional MRI study J Neurosci 16:5205‐5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL (1997): Statistical approach to segmentation of single‐channel cerebral MR images. IEEE Trans Med Imaging 16:176‐186. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo‐Smith I (1994): The Test of Everyday Attention Bury St. Edmunds: Thames Valley Test Company. [Google Scholar]
- Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschlager M (2008): The mTOR pathway and its role in human genetic diseases. Mutat Res 659:284‐292. [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia GA (2005): Behavior Rating Inventory of Executive Function‐ Adult Version (BRIEF‐A) professional manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Ruby P, Decety J (2004): How would you feel versus how do you think she would feel? A neuroimaging study of perspective taking with social emotions J Cognit Neurosi 16:988‐999. [DOI] [PubMed] [Google Scholar]
- Said SM, Yeh TL, Greenwood RS, Whitt JK, Tupler LA, Krishnan KR (1996): MRI morphometric analysis and neuropsychological function in patients with neurofibromatosis Neuroreport 7. [DOI] [PubMed] [Google Scholar]
- Schultz J, Imamizu H, Kawato M, Frith CD (2004): Activation of the human superior temporal gyrus during observation of goal directed attribution by intentional objects J Cognit Neurosci 16:1695‐1705. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R (1997): A mouse model for the learning and memory deficits associated with neurofibromatosis type I Nat Genet 15:281‐284. [DOI] [PubMed] [Google Scholar]
- Steen RG, Taylor JS, Langston JW, Glass JO, Brewer VR, Reddick WE, Mages R, Pivnick EK (2001): Prospective evaluation of the brain in asymtomatic children with neurofibromatosis type 1: Relationship of macrocephaly to T1 relaxation changes and structural brain abnormalities Am J Neuroradiol 22:810‐817. [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A (2004): Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage 23:84‐97. [DOI] [PubMed] [Google Scholar]
- Walsh K, Valez J, Kardel P, Imas D, Muenke M, Packer R, Castellanos FX, Acosta MT (2012): Autism spectrum disorder (ASD) symptomatology in a neurofibromatosis type 1 (NF1) population. Dev Med Child Neurol 55:131‐138. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim E, Wang X, Novitch BG, Yoshikawa K, Chang L, Zhu Y (2012): ERK inhibition rescues defects in fate specification of NFL‐deficient neural progenitors and brain abnormalities. Cell 150:816‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997): Wechsler Adult Intelligence Scale, 3rd ed. (WAIS‐III). San Antonio TX: Harcourt Assessment. [Google Scholar]
- Williams LM (2008): Voxel‐based morphometry in schizophrenia: Implications for neurodevelopmental connectivity models,cognition and affect. Exp Rev 8:1049‐1065. [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson M, Broks P, Hanley JR (1995): Face processing impairments after amygdalotomy. Brain 118:15‐24. [DOI] [PubMed] [Google Scholar]
- Zhu A, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF (2005): Inactivation of NF1 in CNS causes glial progenitor proliferation and optic glioma formation. Development 132:5577‐5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N (2006): Autism, the superior temporal sulcus and social perception. Trends Neurosci 29:359‐366. [DOI] [PubMed] [Google Scholar]


