Abstract
The role of the right hemisphere (RH) in metaphor comprehension is still controversial. Numerous neuroimaging studies have found that conventionality, sentential context, and task demand can influence the involvement of the RH in metaphor processing. The current meta‐analysis used foci from 17 original functional magnetic resonance imaging studies to identify what factors modulate the involvement of the RH in metaphor processing. Activation likelihood estimation was used for quantification. We focused on the contrast of metaphorical meaning processing versus literal meaning processing and calculated the meta‐analysis effects when (1) metaphorical meaning is conventional, (2) metaphorical meaning is novel, (3) metaphorical and literal meaning are presented in words, (4) metaphorical and literal meaning are presented in sentential context, (5) task is valence judgment, and (6) task is semantic relatedness judgment. The results indicated that the RH only showed significant effects in metaphor processing when the metaphorical meaning is novel, when metaphorical meaning is presented in sentential context, and when the task is semantic relatedness judgment. The effects were located in right fronto‐temporal regions, including inferior frontal gyrus, middle frontal gyrus, insula, superior temporal gyrus, and middle temporal gyrus. These results suggest that conventionality, contextual complexity, and task demand can modulate the effect of figurativeness and influence the involvement of RH in metaphor comprehension. The main role of the RH in metaphor processing is related with activating broad semantic fields and integrating concepts that may have distant semantic relations, and hence provide support for the view that the RH is responsible for processing coarse semantic information in language comprehension. Hum Brain Mapp 35:107–122, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: metaphor, right hemisphere, conventionality, sentential context, task demand, functional magnetic resonance imaging, activation likelihood estimation
INTRODUCTION
Metaphor is a literary device that can express abstract ideas vividly. Usually a metaphor uses conceptual knowledge from a concrete semantic domain (e.g., a tangible thing or an image) to represent conceptual knowledge in an abstract semantic domain [Lakoff and Johnson, 1980], such as He is the father of modern biology. The figurative meaning of a metaphor can diverge from its literal meaning that may make no sense at all. The brain mechanism of metaphor processing has attracted increasing attention because numerous evidence (though inconsistent) from behavioral, neuropsychological, and neuroimaging studies indicates that the right hemisphere (RH) may play a special role in metaphorical meaning processing.
Although the involvement of the left hemisphere (LH) in language processing has been well‐established, the role of the RH in language processing still needs further investigation. Recently, researchers put forward several hypotheses to interpret the hemispheric differences relevant to metaphor comprehension. One hypothesis is the fine versus coarse coding theory [Beeman, 1998; Beeman et al., 1994; Jung‐Beeman, 2005]. According to this theory, both hemispheres are involved in semantic access, integration, and selection. The LH is responsible for activating central word meanings and closely related semantic knowledge (fine coding), while the RH plays an important role in activating alternative aspects of word meanings or distantly related concepts (coarse coding). This difference implies different hemispheric mechanisms for metaphorical and literal language. When people comprehend literal language, the LH is strongly involved because the meaning is dominant, focal, and contextually relevant. When people comprehend metaphorical language, such as novel metaphors, the RH plays a more important role because the figurative meaning of metaphors requires activations of loosely related concepts in a broader semantic field.
Another important hypothesis relevant to metaphor processing is the graded salience hypothesis [GSH; Giora, 1997, 1999, 2002, 2003; Giora et al., 2000], which claims that the salience of the meaning determines the order by which the meaning to be retrieved. A meaning of a word or an expression is salient if it can be retrieved directly from the mental lexicon. By contrast, a meaning is non‐salient if it has to be accessed with integration and inferential process. Salient meaning is processed initially before non‐salient meanings, and the LH will be strongly involved. For non‐salient meaning processing, the RH plays a more important role [e.g., Bihrle et al., 1986; Brownell et al., 1990]. Although the GSH is a general linguistic theory, it provides predictions about the brain mechanism of metaphor processing. According to the GSH, it is the meaning salience rather than the figurativeness that determines the LH involvement and the RH involvement. In conventional metaphors, figurative meaning becomes part of the mental lexicon and it is the most salient meaning. Thus, the LH will be strongly involved in the comprehension of figurative meaning in conventional metaphors. In novel metaphors, however, the literal meaning is more salient and the access of figurative meaning requires contextual inferential mechanism. Thus the RH will be strongly involved in figurative meaning processing and the LH will be strongly activated by literal meaning.
So far, numerous studies have investigated the neural substrates of metaphor comprehension; however, the available evidence for a specific RH involvement is rather inconsistent. Some neuroimaging studies [e.g., Bottini et al., 1994; Eviatar and Just, 2006; Stringaris et al., 2006], neuropsychological studies [e.g., Brownell et al., 1990; MacKenzie et al., 1997; Winner and Gardner, 1977], and behavioral studies [e.g., Anaki et al., 1998; Faust and Mashal, 2007; Mashal and Faust, 2008] indicate a RH advantage for metaphor comprehension. In contrast, other studies failed to find a role of the RH in metaphor processing [e.g., Lee and Dapretto, 2006; Mashal et al., 2009; Rapp et al., 2004, 2007; Stringaris et al., 2007]. For example, Rapp et al. 2004 found that reading metaphors in contrast to literal sentences revealed stronger effects in the left inferior frontal gyrus (IFG), left inferior temporal gyrus (ITG), and posterior left middle temporal gyrus (MTG) but not in right brain areas. The authors suggest that RH involvement may neither be essential nor specific for understanding metaphors and that task instructions and complexity of language processing may play more important roles in involving the RH. Rapp et al. 2007 found that no significant differences in laterality across literal and metaphoric stimuli and suggest that both hemispheres have the ability to process metaphoric meaning and that other factors rather than figurativeness (i.e., a linguistic expression is metaphor or not) may trigger RH involvement. In a word, researchers have realized that RH involvement in metaphor comprehension may be influenced by other factors other than figurativeness.
One important factor is conventionality. Conventional metaphors can become instantiated in the semantic memory and the figurative meaning is more salient and easy to access. But in novel metaphors the literal meaning is salient and the access of figurative meaning needs contextual inferential mechanism [Giora and Fein, 1999]. The GSH predicts that the LH is more involved in processing conventional metaphors, and the RH is more involved in the comprehension of novel metaphors. This view is supported by some studies. For instance, using divided visual field technique, researchers found that when participants performed a semantic judgment on word pairs of literal, conventional metaphoric, and novel metaphoric expressions, responses to the left visual field (LVF)/RH presented target words were faster and more accurate than responses to the right visual field (RVF)/LH target words for novel metaphoric expressions, but not for conventional expressions [Faust and Mashal, 2007; Mashal and Faust, 2008]. In addition, when novel metaphoric stimuli were repeatedly presented (i.e., conventionalization), a shift from right to LH processing was indicated [Mashal and Faust, 2009]. Using functional magnetic resonance imaging (fMRI) technique, Lee and Dapretto 2006 showed that processing of conventional metaphors showed stronger effects only in the left IFG, the MTG, and the inferior parietal lobule (IPL) compared with literal comprehension, while Diaz et al. 2011 indicated that comprehending novel metaphors showed strong bilateral effects which included effects in the right posterior MTG, the temporal pole, and the IFG. Mashal et al. 2005 using principle components analysis found that the right posterior MTG had a special role in novel metaphor processing. The direct comparison between novel metaphors and conventional metaphors showed that novel metaphors elicited stronger bilateral activity that included effects in right frontal regions [Ahrens et al., 2007; Mashal et al., 2007] and temporal regions [Mashal et al., 2007]. Other studies show evidence inconsistent with the GSH, but they still reveal differences between novel metaphor comprehension and conventional metaphor comprehension. For example, Eviatar et al. 2006 found that conventional metaphors showed stronger activations than literal utterances not only in the left IFG, the left ITG, but also in the right ITG. Several studies indicate that processing novel metaphors only showed stronger effects in left brain regions compared with literal processing [e.g., Mashal et al., 2009; Rapp et al., 2004; Shibata et al., 2007]. In short, these studies demonstrate that novel and conventional metaphors have different brain mechanisms.
A second factor that may influence the brain mechanism of metaphor processing is whether metaphorical meaning is presented at word‐level or in sentential context. Behavioral studies using visual half field paradigm indicate stronger LH involvement when semantic processing is in context. In contrast, the RH is more sensitive to the comprehension at word‐level [e.g., Faust et al., 2003]. Federmeier 2007 suggested that the LH language processing seems to be expectancy driven and uses top‐down cues whereas the RH comprehension is more bottom‐up and toward the veridical maintenance of word information. Previous studies about metaphor comprehension at word or sentence‐level indicate different results of RH involvement. For instance, Lee and Dapretto 2006 investigated the neural substrates of conventional metaphor comprehension. Metaphorical meaning were presented in word pairs, e.g., cold‐unfriendly, and participants performed a semantic relatedness judgment task. Compared with literal word pairs, e.g., cold‐chilly, metaphorical meaning processing only activated stronger effects in left brain regions and no effect in the RH was revealed. In another study, Stringaris et al. 2006 also examined conventional metaphor comprehension with a semantic relatedness judgment task. In this study, metaphorical meaning was presented in sentential context, e.g., some answers are straight, and participants decided the semantic relatedness between each sentence and a probe word after the sentence. The results indicated that compared with literal sentences, metaphorical sentences elicited stronger bilateral effects which included effects in the right ventrolateral prefrontal areas. It suggests that processing metaphorical meaning at word‐level may involve the LH, while metaphor comprehension at sentence‐level can involve the RH. In fact, several studies about metaphorical sentence processing report RH activations [e.g., Ahrens et al., 2007; Bottini et al., 1994; Chen et al., 2008; Schmidt and Seger, 2009; Sotillo et al., 2005; Yang et al., 2009].
A third factor that may show influence on metaphor comprehension is task demand. Various tasks have been used in studies about metaphor processing, such as semantic relatedness judgment [e.g., Bambini et al., 2011; Diaz and Hogstrom, 2011; Lee and Dapretto, 2006; Mashal et al., 2007; Stringaris et al., 2006], valence judgment [Diaz et al., 2011; Kircher et al., 2007; Mashal et al., 2009; Rapp et al., 2004, 2007; Yang et al., 2009, 2010], plausibility judgment [e.g., Chen et al., 2008; Stringaris et al., 2007], comprehension task [e.g., Eviatar et al., 2006], coherence judgment [e.g., Uchiyama et al., 2012], and passive reading [e.g., Ahrens et al., 2007; Schmidt and Seger, 2009]. One recent study examines how different task demands influence metaphor processing [Yang et al., 2009]. In the study, participants read novel and conventional metaphorical sentences and performed a valence judgment task and an imageability judgment task. The results indicated that in the imageability judgment, novel metaphorical sentences elicited strong effects in the left IFG, left MTG, right fusiform gyrus (FG), bilateral superior temporal pole, and bilateral ITG, while in the valence task the left ITG, right FG, and bilateral IFG showed strong activity in the novel metaphor condition. In the imageability task, conventional metaphorical sentences elicited strong activations in the bilateral FG, bilateral IFG, left IPL, and right MFG, while in the valence judgment task the left middle occipital gyrus (MOG) showed strong effects. The authors concluded that RH involvement can be influenced by processing difficulty and that figurative comprehension depends on task demands.
Taken together, the brain mechanism of metaphor processing is still unclear, but it is evident that many factors do influence the mechanism. To address the issues, in the current study, we conducted an activation likelihood estimation (ALE) meta‐analysis to investigate the neural substrate of metaphor comprehension, especially the role of the RH. The meta‐analysis was conducted on studies that used fMRI to explore metaphor processing. We focused on the contrast between metaphor and literal processing, because this type of contrast could reveal a specific mechanism in metaphorical meaning processing (i.e., the effect of figurativeness). The influences of conventionality, sentential context, and task demand on the contrast of metaphor versus literal processing were examined. In this study, we chose conventionality but not the familiarity because familiarity has different meanings in different studies. In some studies, familiarity is considered to indicate conventionality and hence familiar metaphors are treated as conventional metaphors and unfamiliar metaphors as novel metaphors [e.g., Diaz et al., 2011]. In other studies, however, familiarity is different from conventionality. In the work by Schmidt and Seger 2009 and Bambini et al. 2011, the familiar/unfamiliar division was made within non‐conventional metaphors, thus both familiar and unfamiliar metaphors were novel metaphors and different from conventional metaphors. We agree that pure familiarity that is independent of conventionality has its own influence on metaphor processing, but in the present study we only concentrated on the influence of conventionality. As for the influence of task demand, we focused on the valence judgment and the semantic relatedness judgment because the two tasks have been employed most widely in the fMRI studies about metaphor comprehension.
To examine the influences of conventionality, sentential context, and task demand on the effect of figurativeness, we conducted a meta‐analysis of the contrast between metaphorical meaning processing and literal meaning processing when (1) metaphorical meaning is conventional, (2) metaphorical meaning is novel, (3) metaphorical and literal meaning are presented in words, (4) metaphorical and literal meaning are presented in sentential context, (5) task is valence judgment, and (6) task is semantic relatedness judgment. We predict that if RH involvement in metaphor processing is not modulated by the aforementioned factors, then effects in right brain regions should be observed in all six situations. However, if conventionality, contextual complexity, and task demand can modulate figurativeness and influence RH involvement, effects in the right brain regions should be observed selectively in specific situations. The meta‐analysis results for the contrast of metaphor processing versus a low‐level baseline and the contrast between different metaphors were also reported.
MATERIALS AND METHODS
Paper Selection
Papers were searched from the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) using the keywords “fMRI” and “metaphor.” Twenty‐seven papers were revealed and 19 of them are about metaphor processing. The inclusion criteria for the current meta‐analysis are listed as follows:
Studies that used fMRI technique and collected data from healthy adults were selected. We excluded studies that employed other techniques (positron emission tomography, transcranial magnetic stimulation, and electroencephalography) to make sure that all studies have approximately comparable spatial resolution. Studies that recruited a patient group and a control group were also selected if they reported the brain activity of the control group. All of the 19 papers met this criterion.
Studies that included a condition of metaphor comprehension were selected. All of the 19 remaining papers met this criterion.
Studies that reported the brain activity related to metaphor conditions and gave standard coordinates for activation foci in either Montreal Neurologic Institute (MNI) or Talairach [Talairach and Tournoux, 1988] spaces were selected. All the remaining 19 studies reported activations related to metaphor conditions, but three of them were excluded because they did not give standard coordinates for activation foci. Thus, 16 of the 19 papers met this criterion.
We performed an additional search in ScienceDirect (http://www.sciencedirect.com/) with the same keywords and found that one extra paper met the criteria [Lee and Dapretto, 2006]. The paper was also included. Thus, 17 studies were selected and the time span of all these studies is from 2004 to 2011. Table 1 indicates all 17 studies included in the study.
Table 1.
Overview of the 17 original fMRI studies included in the current meta‐analysis
| Study | Field strength | Space | Metaphor type | Word or sentence | Task | Comparison | Threshold | Voxel Size (mm3) | N |
|---|---|---|---|---|---|---|---|---|---|
| Ahrens et al. 2007 | 1.5 T | Talairach | Anomalous (novel), conventional | Sentence | Passive reading (press button when finished) | Anomalous (novel) metaphor > literal; conventional metaphor > literal; anomalous metaphor > conventional metaphor | P < 0.001, uncorrected | 3 × 3 × 5 | 8 |
| Bambini et al. 2011 | 1.5 T | Talairach | Novel | Sentence | Semantic relatedness judgments (passage‐ target word) | All metaphor > literal | P < 0.005, cluster size corrected for multiple comparison | 5 mm thickness | 9 |
| Chen et al. 2008 | 3.0 T | Talairach | Not reported | Sentence | Plausibility judgment | Metaphor > literal sentence | P < 0.01, voxel > 40 | 3 × 3 × 3 | 14 |
| Diaz and Hogstrom 2011 | 3.0 T | MNI | Novel, conventional | Sentence | Sentence judgment (related, unrelated, nonword) | Metaphor > literal | P < 0.01 corrected | 4 × 4 × 4 | 16 |
| Diaz et al. 2011 | 3.0 T | MNI | Novel, conventional | Sentence | Valence rating (positive, negative, neutral) | Novel metaphor > nonword baseline; conventional metaphor > nonword baseline; all metaphor > literal | P < 0.01, voxel > 7 | 4 × 4 × 4 | 16 |
| Eviatar and Just 2006 | 3.0 T | Talairach | Conventional | Sentence | Comprehension task | Metaphor > literal | P < 0.01 corrected | 3.125 × 3.125 × 3 | 16 |
| Kircher et al. 2007 | 1.5 T | Talairach | Novel | Sentence | Connotation judgment (positive or negative) | Metaphor > low‐level baseline; metaphor > literal | P < 0.00001 (uncorrected), voxel >10 | 3 × 3 × 5 | 12 |
| Lee and Dapretto 2006 | 3.0 T | Talairach | Conventional | Word | Semantic relatedness judgments | Metaphor > rest; metaphor > literal | P < 0.05, corrected | 4 mm thickness and 1 mm gap | 12 |
| Mashal et al. 2007 | 1.5 T | Talairach | Novel, conventional | Word | Semantic relatedness judgments | Novel metaphor > unrelated words; conventional metaphor > unrelated words; novel metaphor > literal word pairs; conventional metaphor > literal word pairs; novel metaphor > conventional metaphor | P < 0.05, uncorrected (compared with unrelated words); P < 0.01, uncorrected (compared with literal word pairs) | 4 mm thickness and 1 mm gap | 15 |
| Mashal et al. 2009 | 3.0 T | Talairach | Novel | Sentence | Connotation judgment (positive or negative) | Metaphoric sentence > non‐sense sentence; Metaphoric sentence > Literal sentence | P < 0.001, uncorrected | 3.5 mm thickness and 0 mm gap | 15 |
| Rapp et al. 2004 | 1.5 T | Talairach | Novel | Sentence | Connotation judgment (positive or negative) | Metaphor > low‐level baseline; metaphor > literal | P < 0.05, corrected, voxel > 10, (compared with low‐level baseline); P < 0.001, corrected or uncorrected (compared with literal) | 3 × 3 × 5 | 15 |
| Schmidt and Seger 2009 | 3.0 T | Talairach | Novel | Sentence | Passive reading (when understand press button) | All metaphor > non‐word sentence; all metaphor > literal; conventional metaphor > literal; familiar metaphor > unfamiliar metaphor; unfamiliar metaphor > familiar metaphor; easy metaphor > difficult metaphor; difficult metaphor > easy metaphor | P < 0.001, uncorrected | 5 mm thickness | 10 |
| Shibata et al. 2007 | 1.5 T | Talairach | Novel | Sentence | Comprehension task (understand or not) | Metaphor > non‐linguistic baseline; metaphor > literal | P < 0.001,uncorrected, voxel > 10 | 4 mm thickness and 0.8 mm gap | 13 |
| Stringaris et al. 2006 | 1.5 T | Talairach | Conventional | Sentence | Semantic relatedness judgments (sentence‐ target word) | Metaphor > literal | voxel level of P < 0.05 and cluster level of p < 0.0025 | 5 × 5 × 5 | 12 |
| Stringaris et al. 2007 | 1.5 T | Talairach | Conventional | Sentence | Semantic judgment (make sense or not) | Metaphor > literal | P < 0.007 uncorrected | 5 × 5 × 5 | 11 |
| Uchiyama et al. [2012] | 3.0 T | MNI | Not reported | Sentence | Coherence judgment | Metaphor > literal | Z > 3.09 (P < 0.05 corrected) | 3 × 3 × 4 | 20 |
| Yang et al. 2010 | 3.0 T | MNI | novel, conventional | sentence | valence task (positive or negative) | novel metaphor > low‐level baseline; conventional metaphor > low‐level baseline | P < 0.05, FDR corrected, voxel >10 | 3.44 × 3.44 × 4 | 12 |
Selection of Contrasts
We selected contrasts that were critical for metaphor comprehension. These contrasts could be divided into three types: (1) increasing activations in metaphor condition compared with a low‐level baseline (e.g., nonword, non‐linguistic condition, or rest) [Diaz et al., 2011; Kircher et al., 2007; Lee and Dapretto, 2006; Rapp et al., 2004; Schmidt and Seger, 2009; Shibata et al., 2007; Yang et al., 2010]; (2) increasing effects in metaphorical sentence/word condition compared with literal sentence/word condition [Ahrens et al., 2007; Bambini et al., 2011; Chen et al., 2008; Diaz and Hogstrom, 2011; Diaz et al., 2011; Eviatar and Just, 2006; Kircher et al., 2007; Lee and Dapretto, 2006; Mashal et al., 2009, 2007; Rapp et al., 2004; Schmidt and Seger, 2009; Shibata et al., 2007; Stringaris et al., 2006, 2007; Uchiyama et al., 2012]; (3) effects of contrasts between two different metaphor conditions (e.g., familiar metaphor versus unfamiliar metaphor, easy metaphor versus difficult metaphor) [Ahrens et al., 2007; Mashal et al., 2007; Schmidt and Seger, 2009].
Activation Likelihood Estimation Meta‐Analyses
In the current study, ALE meta‐analysis was performed based on the three types of contrasts with Ginger ALE 2.0.4 software (http://www.brainmap.org/ale/) [Laird et al., 2005].
The first ALE meta‐analysis included the foci from the contrast (1), i.e. increasing effects in metaphor condition compared with low‐level baselines. Seventy‐seven foci from seven studies were included in the meta‐analysis.
The second ALE meta‐analysis included the foci from the contrast (2), i.e. increasing effects in metaphorical sentence/word conditions compared with literal sentence/word conditions. One hundred and seventy‐two foci from 16 studies were included in the meta‐analysis. To investigate the influences of conventionality, sentential context, and task demand on metaphor comprehension, we additionally conducted ALE meta‐analysis for (a) increasing effects in conventional metaphor condition compared with literal condition, (b) increasing effects in novel metaphor condition compared with literal condition, (c) increasing effects in metaphorical word condition compared with literal word condition, (d) increasing effects in metaphorical sentence condition compared with literal sentence condition, (e) increasing effects in metaphor condition compared with literal condition in valence judgment task, and (f) increasing effects in metaphor condition compared with literal condition in semantic relatedness judgment task.
Finally, we conducted ALE meta‐analysis that included the foci from the contrast (3), i.e. effects of contrasts between two different metaphor conditions. Thirty‐five foci from three studies were included in the meta‐analysis.
All ALE meta‐analyses were conducted in the standard Talairach space. If coordinates of foci were reported in the standard MNI space, we transformed the coordinates into standard Talairach‐space with the icbm2tal algorithm [Lancaster et al., 2007] in the Ginger ALE software [Laird et al., 2005]. For each ALE meta‐analysis, all selected foci and subject numbers in the original studies were entered in the Ginger ALE as input. First, an ALE value was computed for each voxel in the brain with an empirically determined full‐width half‐maximum (FWHM) value [Eickhoff et al., 2009]. After that, an image containing the ALE values for all the foci and an image for the P value of each voxel were generated. Second, the threshold for the ALE map was calculated based on the generated P‐values with the algorithm from Tom Nichols's website (http://www.sph.umich.edu/~nichols/FDR/). A false discovery rate (FDR) was chosen for a significance level of P < 0.05 with a default cluster size in 200 mm3. Third, a cluster analysis was conducted on the thresholded ALE map and anatomical label of each cluster location was provided.
RESULTS
Meta‐Analysis of the Foci in Contrast (1) (Metaphor > Low‐Level Baseline)
The ALE meta‐analysis for all 77 foci from seven studies in the contrast (1) indicated 14 significant clusters in total (Fig. 1A).
Figure 1.
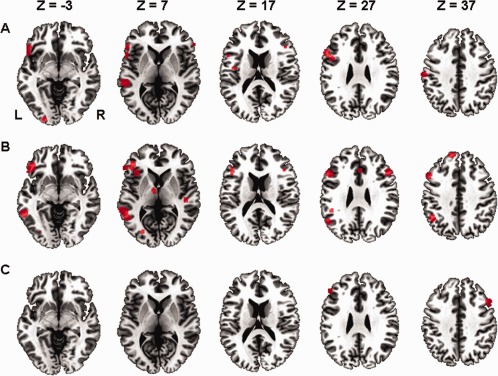
Results of the ALE meta‐analysis showing significant clusters (P < 0.05, FDR‐corrected for multiple comparisons, cluster size 200 mm3). A: ALE clusters for the contrast of metaphor processing versus low‐level baseline. B: ALE clusters for the contrast of metaphor processing versus literal language processing. C: ALE clusters for the contrast between different types of metaphors. L = left, R = right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In the LH, areas in the superior frontal gyrus (SFG, BA 6), the middle frontal gyrus (MFG, BA 9), the IFG (BA 47/45/9/44), the insula (BA 13), the precentral gyrus (BA 44), the postcentral gyrus (BA 3), the MTG (BA 22), and the inferior occipital gyrus (IOG, BA 17/18) showed significant effects.
In the RH, areas in the IFG (BA 45), STG (BA 38), and MOG (BA 18) showed strong effects.
Meta‐Analysis of the Foci in the Contrast (2) (Metaphor > Literal)
The ALE meta‐analysis for all 172 foci from 16 studies in the contrast (2) indicated 13 significant clusters in total (Fig. 1B).
In the LH, areas in the SFG (BA 8), MFG (BA 46/9), IFG (BA 45/47), insula (BA 13), IPL (BA 40), ITG (BA 20), MTG (BA 21/22/37/39), lingual gyrus, and thalamus had significant effects.
In the RH, areas in the MFG (BA 46), IFG (45/13), STG (BA 22), and cingulate gyrus (BA 32) showed strong effects.
Increasing effects in conventional metaphors compared with literal condition
The ALE meta‐analysis for all 61 foci from seven studies in the contrast between conventional metaphor processing and literal processing indicated five significant clusters in total (Fig. 2A and Table 2). In the LH, areas in the MFG (BA 46), IFG (BA 47/44), IPL (BA 40), MTG (BA 37), and lingual gyrus showed significant effects.
Figure 2.
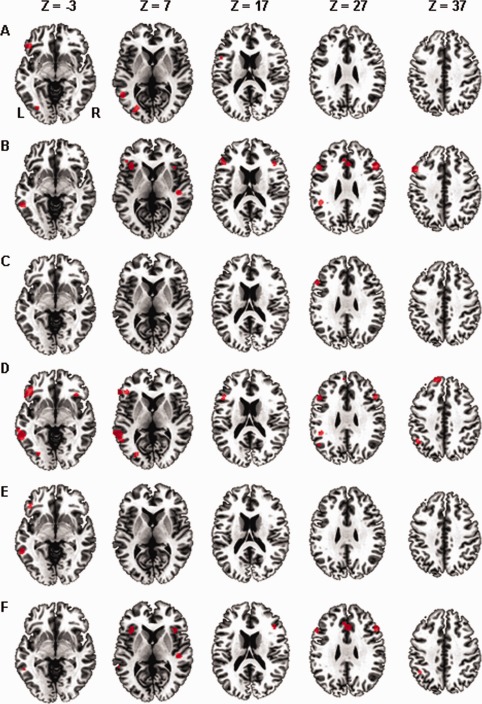
Results of the ALE meta‐analysis showing significant clusters (P < 0.05, FDR‐corrected for multiple comparisons, cluster size 200 mm3) for the contrast of metaphor processing versus literal language processing. A: ALE clusters for the contrast of conventional metaphor processing versus literal processing. B: ALE clusters for the contrast of novel metaphor processing versus literal processing. C: ALE clusters for the contrast of metaphorical word processing versus literal word processing. D: ALE clusters for the contrast of metaphorical sentence processing versus literal sentence processing. E: ALE clusters for the contrast of metaphorical processing versus literal processing in valence judgment task. F: ALE clusters for the contrast of metaphorical processing versus literal processing in semantic relatedness judgment task. L = left, R = right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Peak activation for the contrast of conventional metaphor processing versus literal processing and the contrast of novel metaphor processing versus literal processing
| Cluster | Volume (mm3) | Region | Hemisphere | BA | x | y | z | ALE value |
|---|---|---|---|---|---|---|---|---|
| Conventional metaphor > literal | ||||||||
| 1 | 200 | Middle frontal gyrus | L | 46 | −44 | 16 | 18 | 0.0081 |
| Inferior frontal gyrus | L | 44 | −46 | 8 | 18 | 0.0077 | ||
| 2 | 496 | Inferior frontal gyrus | L | 47 | −44 | 28 | −4 | 0.0124 |
| 3 | 560 | Inferior parietal lobule | L | 40 | −32 | −54 | 42 | 0.0093 |
| L | 40 | −40 | −50 | 40 | 0.0082 | |||
| 4 | 512 | Middle temporal gyrus | L | 37 | −50 | −52 | 4 | 0.0126 |
| 5 | 912 | Lingual gyrus | L | −26 | −74 | 2 | 0.0130 | |
| Novel metaphor > literal | ||||||||
| 1 | 1464 | Insula | L | 13 | −34 | 20 | 8 | 0.0106 |
| Middle frontal gyrus | L | 46 | −44 | 28 | 18 | 0.0091 | ||
| Middle frontal gyrus | L | 46 | −46 | 32 | 14 | 0.0085 | ||
| Inferior frontal gyrus | L | 47 | −40 | 34 | 2 | 0.0086 | ||
| 2 | 1312 | Middle frontal gyrus | L | 9 | −48 | 18 | 32 | 0.0161 |
| Cingulate gyrus | L | 32 | −4 | 30 | 28 | 0.0085 | ||
| 3 | 352 | Middle frontal gyrus | L | 47 | −44 | 38 | −10 | 0.0099 |
| 4 | 344 | Insula | L | 13 | −44 | −40 | 26 | 0.0101 |
| 5 | 232 | Superior frontal gyrus | L | 8 | 0 | 30 | 50 | 0.0089 |
| 6 | 384 | Inferior parietal lobule | L | 40 | −42 | −48 | 42 | 0.0097 |
| 7 | 904 | Middle temporal gyrus | L | 22 | −54 | −44 | 0 | 0.0148 |
| 8 | 720 | Cingulate gyrus | R | 32 | 2 | 24 | 30 | 0.0134 |
| 9 | 920 | Middle frontal gyrus | R | 46 | 48 | 22 | 26 | 0.0156 |
| 10 | 616 | Inferior frontal gyrus | R | 13 | 40 | 26 | 14 | 0.0103 |
| Insula | R | 13 | 38 | 18 | 8 | 0.0087 | ||
| 11 | 520 | Superior temporal gyrus | R | 22 | 44 | −24 | 4 | 0.0126 |
No effect was found in the RH.
Increasing effects in novel metaphors compared with literal condition
The ALE meta‐analysis for all 92 foci from eight studies in the contrast between novel metaphor processing and literal processing indicated 11 significant clusters in total (Fig. 2B and Table 2). In the LH, areas in the MFG (BA 46/9/47), IFG (BA 47), SFG (BA 8), IPL (BA 40), MTG (BA 22), insula (BA 13), and cingulate gyrus (BA 32) showed significant effects.
In the RH, area in the IFG/insula (BA 13), MFG (BA 46), STG (BA 22), and cingulate gyrus (BA 32) had significant activations.
Increasing effects in metaphorical word condition compared with literal word condition
The ALE meta‐analysis for all 40 foci from two studies in this contrast indicated only one significant cluster in the left MFG (BA 9) (Fig. 2C and Table 3).
Table 3.
Peak activation for the contrast of metaphorical word processing versus literal word processing and the contrast of metaphorical sentence processing versus literal sentence processing
| Cluster | Volume (mm3) | Region | Hemisphere | BA | x | y | z | ALE value |
|---|---|---|---|---|---|---|---|---|
| Metaphorical word > literal word | ||||||||
| 1 | 432 | Middle frontal gyrus | L | 9 | −50 | 18 | 30 | 0.0102 |
| Metaphorical sentence > literal sentence | ||||||||
| 1 | 2400 | Inferior frontal gyrus | L | 47 | −42 | 30 | 0 | 0.0186 |
| Inferior frontal gyrus | L | 45 | −54 | 28 | 4 | 0.0121 | ||
| Middle frontal gyrus | L | 47 | −44 | 38 | −10 | 0.0100 | ||
| Inferior frontal gyrus | L | 47 | −52 | 20 | −6 | 0.0079 | ||
| 2 | 856 | Middle frontal gyrus | L | 9 | −46 | 18 | 28 | 0.0109 |
| Middle frontal gyrus | L | 46 | −44 | 18 | 20 | 0.0097 | ||
| 3 | 640 | Superior frontal gyrus | L | 8 | −12 | 48 | 36 | 0.0119 |
| 4 | 224 | Insula | L | 13 | −44 | −40 | 26 | 0.0101 |
| 5 | 200 | Inferior parietal Lobule | L | 7 | −30 | −56 | 42 | 0.0092 |
| 6 | 344 | Inferior parietal Lobule | L | 40 | −44 | −52 | 38 | 0.0093 |
| Superior temporal gyrus | L | 39 | −46 | −58 | 26 | 0.0081 | ||
| 7 | 2560 | Middle temporal gyrus | L | 21 | −56 | −42 | 0 | 0.0209 |
| Superior temporal gyrus | L | 22 | −52 | −50 | 8 | 0.0091 | ||
| 8 | 432 | Middle temporal gyrus | L | 21 | −54 | −10 | −14 | 0.0089 |
| Middle temporal gyrus | L | 21 | −56 | −16 | −12 | 0.0085 | ||
| Sub‐gyral | L | 21 | −46 | −6 | −10 | 0.0084 | ||
| Inferior temporal gyrus | L | 20 | −58 | −8 | −20 | 0.0082 | ||
| 9 | 584 | Lingual gyrus | L | −26 | −74 | 2 | 0.0130 | |
| 10 | 432 | Inferior frontal gyrus | R | 47 | 42 | 32 | −8 | 0.0103 |
| Inferior frontal gyrus | R | 47 | 36 | 24 | −6 | 0.0094 | ||
No effect was revealed in the RH.
Increasing effects in metaphorical sentence condition compared with literal sentence condition
The ALE meta‐analysis for all 132 foci from 14 studies in this contrast indicated 10 significant clusters in total (Fig. 2D and Table 3).
In the LH, areas in the IFG (BA 45/47), MFG (BA 46/9/47), SFG (BA 8), IPL (BA 40/7), STG (BA 22/39), MTG (BA 21), and ITG (BA 20) showed strong effects.
In the RH, areas in the IFG (BA 47) had strong activation.
Increasing effects in metaphor condition compared with literal condition in valence judgment task
The ALE meta‐analysis for all 16 foci from four studies in this contrast indicated two significant clusters in the LH (Fig. 2E and Table 4). The first one was located in the IFG (BA 47) and the second was located in the MTG (BA 21).
Table 4.
Peak activation for the contrast of metaphor processing versus literal processing in valence judgment task and in semantic relatedness judgment task
| Cluster | Volume (mm3) | Region | Hemisphere | BA | x | y | z | ALE value |
|---|---|---|---|---|---|---|---|---|
| Valence judgment: metaphor > literal | ||||||||
| 1 | 448 | Middle temporal gyrus | L | 21 | −56 | −44 | −2 | 0.0120 |
| 2 | 320 | Inferior frontal gyrus | L | 47 | −42 | 30 | 0 | 0.0096 |
| Semantic relatedness judgment: metaphor > literal | ||||||||
| 1 | 424 | Insula | L | 13 | −34 | 20 | 6 | 0.0103 |
| 2 | 376 | Middle frontal gyrus | L | 9 | −50 | 18 | 30 | 0.0102 |
| 3 | 320 | Inferior parietal Lobule | L | 40 | −40 | −48 | 42 | 0.0082 |
| 4 | 304 | Middle temporal gyrus | L | 22 | −54 | −42 | 2 | 0.0089 |
| 5 | 432 | Middle frontal gyrus | R | 46 | 48 | 22 | 26 | 0.0121 |
| 6 | 656 | Inferior frontal gyrus | R | 13 | 40 | 26 | 14 | 0.0103 |
| 7 | 624 | Cingulate gyrus | R | 32 | 2 | 24 | 28 | 0.0134 |
| Cingulate gyrus | R | 32 | 2 | 24 | 30 | 0.0134 | ||
| Cingulate gyrus | L | 32 | −4 | 30 | 28 | 0.0085 | ||
| 8 | 464 | Superior temporal gyrus | R | 22 | 44 | −24 | 4 | 0.0126 |
No effect was revealed in the RH.
Increasing effects in metaphor condition compared with literal condition in semantic relatedness judgment task
The ALE meta‐analysis for all 66 foci from five studies in this contrast indicated eight significant clusters in total (Fig. 2F and Table 4).
In the LH, areas in the MFG (BA 9), IPL (BA 40), MTG (BA 22), insular (BA 13), and cingulate gyrus (BA 32) showed strong effects.
In the RH, areas in the IFG (BA 13), MFG (BA 46), STG (BA 22), and cingulate gyrus (BA 32) showed strong effects.
Meta‐Analysis of the Foci in Contrast (3) (Contrasts Between Two Different Metaphor Conditions)
The ALE meta‐analysis for all 35 foci from three studies in contrast (3) indicated two significant clusters in total (Fig. 1C).
The first cluster was located in the left MFG (cluster size = 760 mm3, centered at x = −45.6, y = 33.1, z = 22.2). Label for this cluster was BA 46. Cluster 2 was located in the right MFG (cluster size = 680 mm3, centered at x = 47.8, y = 15.8, z = 36.6). Label for this cluster was BA 9.
DISCUSSION
The aim of the current study was to investigate the brain mechanism of metaphor comprehension, especially the role of the RH in metaphor processing. To achieve this goal we conducted a quantitative meta‐analysis of fMRI studies about metaphor comprehension. We calculated the meta‐analysis results for the contrast of metaphor processing versus low‐level baseline, the contrast of metaphor processing versus literal processing, and the contrast between different metaphor conditions. Results for all three contrasts indicated significant bilateral activations which included activation in right frontal areas. In addition, results in the contrast between metaphor processing and a low‐level baseline and the contrast between metaphor processing and literal processing reveal significant effect in the right STG. These findings suggest that the RH is involved during metaphor comprehension.
However, previous studies have found that many factors can influence the neural substrate of metaphor processing and RH involvement, and the critical work is not investigating whether the RH is involved in metaphor processing or not, but investigating under what circumstance the RH is involved. To achieve this goal, we focused on the contrast between metaphor processing and literal processing and examined the influence of conventionality, sentential context, and task demand on the contrast effects. We found that when compared with literal comprehension, RH involvement in metaphor processing is modulated by these factors.
Influence of Conventionality
Conventional metaphors have figurative meaning that is more salient than literal meaning, while novel metaphors have figurative meaning that is less salient than literal meaning. According to the GSH, the LH is strongly involved in processing the salient meaning while the RH plays an important role in processing the non‐salient meaning. Thus, the LH should be activated during the comprehension of figurative meaning in conventional metaphors, while the RH will be involved in the processing of figurative meaning in novel metaphors.
In the current study, the meta‐analysis for contrast between metaphor processing and literal processing indicated that comprehension of conventional metaphor showed stronger activity than literal processing only in the left fronto‐parietal‐temporal regions, and that no significant effect in the RH was found. Comprehension of novel metaphor, however, showed stronger effects than literal processing in bilateral brain regions which included the right IFG/insula, MFG, and STG. The results support the GSH and suggest that salience can modulate figurativeness and influence RH involvement in metaphor comprehension. In novel metaphors, figurative meaning cannot be retrieved directly from the mental lexicon and has to be accessed based on inferential mechanism. And because the figurative meaning is non‐salient in novel metaphors, the concepts within the figurative meaning can be distantly and loosely related with contextual information. Thus, the specific RH involvement in the processing of novel metaphors indicates that RH is strongly involved in activating broader semantic fields and integrating coarse semantic information [Beeman, 1998; Jung‐Beeman, 2005]. This view is supported by a study about story comprehension [e.g., Nichelli et al., 1995], which found stronger right fronto‐temporal activity when participants comprehended the moral of a story that is opposite with its literal meaning. Other studies about generating causal inference [e.g., Mason and Just, 2004], processing unusual semantic relations [Seger et al., 2000], comprehending the literal meaning of idioms [e.g., Mashal et al., 2008], and processing subordinate meanings of words [e.g., Brownell et al., 1984; Grindrod and Baum, 2003] also indicate that the RH contributes to coding coarse semantic information.
Influence of Sentential Context
The evidence for the influence of sentential context on the LH and RH involvement in language processing is inconsistent. Some researchers suggest that the LH is primarily sensitive to sentence‐level semantic processing, and that the RH is more involved in processing at word‐level and less sensitive to context or message processing [e.g., Faust et al., 1995, 2003]. For instance, Faust et al. 2003 found stronger priming effects in the RVF (LH) than in the LVF (RH) when meaningful sentences preceded target words. By contrast, when words associated with target words were embedded in meaningless sentence primes, the LVF (RH) showed strong priming effect. This result suggests that the RH is sensitive to semantic association at word‐level, while the LH is sensitive to semantic association at sentence‐level. However, findings from studies of metaphor comprehension are inconsistent with this view. Some studies show that metaphor processing at word‐level does not necessarily involve the RH [e.g., Lee and Dapretto, 2006] and that metaphor processing at sentence‐level does not necessarily exclude the RH [e.g., Ahrens et al., 2007; Chen et al., 2008; Schmidt and Seger, 2009].
In the current meta‐analysis, the contrast of metaphorical word processing versus literal word processing indicated significant effects only in the left MFG and no effect was found in the RH. On the contrary, the contrast of metaphorical sentence processing versus literal sentence processing indicated significant activity in areas of bilateral brain regions which included an area in the right IFG (BA 47). This result pattern seems to suggest that the RH is involved in metaphor processing at sentence‐level and that contextual complexity can modulate figurativeness and influence RH involvement in metaphor comprehension. Previous studies have found that right brain regions are involved in semantic processing in complex contexts, such as in sentential contexts [e.g., Ben Shachar et al., 2004; Constable et al., 2004; Kircher et al., 2001] and narrative contexts [e.g., Faust et al., 2003; St. George et al., 1999]. For instance, an fMRI study found that when participants read word lists, unrelated sentences, and coherent narratives, the RH was increasingly active as contextual complexity increased, maximal at the narrative level [Xu et al., 2005]. Another study found that processing untitled story activates stronger effects in right brain regions compared with processing titled story [St George et al., 1999]. One possible reason for the RH involvement in context processing might be that complex contexts contain semantic information in broad semantic fields and thus require integration of concepts that are closely or distantly related with each other to achieve high‐level semantic representation.
However, one needs to be cautious about the conclusion that contextual complexity can modulate figurativeness and influence RH involvement in metaphor comprehension. This is because in the current study irrelevant factors might also cause different RH involvements in metaphorical sentence and word processing. One such irrelevant factor is the number of foci included in the meta‐analysis. Only two fMRI studies conducted contrast between metaphor and literal expressions at word‐level (total foci N = 27), whereas 14 studies conducted such contrasts at sentence‐level (total foci N = 132). The statistical power difference may have some influence on the results. One possibility is that RH involvement might be too weak to be found in the meta‐analysis of metaphorical word comprehension due to the small number of foci. The small foci number issue is true for complex cognitive processes that can involve wide‐spread brain regions. Because metaphor comprehension is quite complex and can engage many brain regions, larger number of foci is more ideal to show substantial convergence. Thus, if more studies about metaphorical word comprehension can be added into meta‐analysis in the future, significant effects of RH involvement may be revealed.
Influence of Task Demand
Many researchers suggest that task demands can influence RH involvement in metaphor processing [Ahrens et al., 2007; Mashal et al., 2009; Rapp et al., 2004; Yang et al., 2009]. In the present study, we chose valence judgment task and semantic relatedness judgment task to examine the influence of task demand on metaphor comprehension. Both tasks are widely used in the fMRI studies about metaphor processing, but they require different types of semantic processing. In valence judgment task, participants decide whether sentences have positive, neutral, or negative connotation. This task may require “deep semantic processing and an assessment of the ‘ground’ of the metaphor” [Rapp et al., 2004, p. 396]. It is suggested that deciding whether a sentence has positive or negative meaning is like performing semantic categorization which mainly relies on the left brain functions [Ahrens et al., 2007; Rapp et al., 2004]. It should be noted that semantic relations among words is important for valence decision, because figurative meaning has to be integrated based on the analysis of the semantic relations of words.
Semantic relatedness judgment task emphasizes the semantic relations between two words [Lee and Dapretto, 2006; Mashal et al., 2007], between words and sentences [e.g., Stringaris et al., 2006], between words and passages [e.g., Bambini et al., 2011], or between two sentences [e.g., Diaz and Hogstrom, 2011]. To fulfill the task, participants need to access the figurative meaning of metaphors and detect possible semantic associations between two different concepts, even though the semantic relations between two concepts is distant and loose.
Thus, both valence judgment and semantic relatedness judgment in metaphor processing require the processing of figurative meaning and semantic relations. However, the complexity or difficulty of processing of semantic relations can be different. Firstly, in valence judgment the analysis of semantic relations is implicit, and participants do not need to make decisions about the relations. On the contrary, in semantic relatedness judgment, the analysis of semantic relations is explicit, and participants need to retrieve multiple semantic associations at the same time, compare different semantic information, and make selection/decision between different possibilities (related or unrelated). Secondly, the stimuli used in semantic relatedness judgment may make the task complex. In valence judgment task, semantic relations among words in metaphoric sentence stimuli can be loose or tight, but it is rare that words within a metaphoric sentence are unrelated with each other. In semantic relatedness judgment task, however, the semantic relations between metaphoric stimuli and probe words or sentences can be loose, tight, or unrelated. Thus, the semantic relations in semantic relatedness decision task are more various. Moreover, in valence judgment task, participants comprehend one sentence in each trial to make a decision, while in semantic relatedness judgment participants have to read one word or sentence or paragraph and a probe word or sentence in each trial to make a decision. Therefore, the semantic processing load may be heavier in semantic relatedness judgment task.
In summary, in the semantic relatedness judgment tasks the processing of semantic relations is explicit and more complex than that in valence judgment tasks. In the current study, the meta‐analysis result indicated that in the valence judgment tasks metaphor comprehension elicited stronger activity than literal comprehension only in the left brain regions including the left IFG and MTG. However, in the semantic relatedness judgment tasks, metaphor processing elicited stronger activity in bilateral brain regions, including the right IFG, MFG, and STG. This result pattern suggests that tasks demands can modulate figurativeness and influence RH involvement in metaphor comprehension. Performing valence judgment can involve the left brain regions probably because it is like performing a categorization task [Ahrens et al., 2007; Rapp et al., 2004]. Performing semantic relation judgment can involve right brain regions because this task requires activation of multiple semantic associations, comparisons between these associations, and decision of the semantic relations.
The effects of the right brain regions in semantic relatedness judgment task in the current study are consistent with studies that report RH activity in semantic association tasks in literal language compression [e.g., Adams and Janata, 2002; Booth et al., 2002; Gurd et al., 2002; Kosslyn et al., 1994; McDermott et al., 2003; Petersen et al., 1989; Warburton et al., 1996]. These results support the fine coding versus coarse coding theory [Beeman, 1998; Jung‐Beeman, 2005] which suggests that the RH plays an important role in activating loosely related concepts. The results are also consistent with recent findings that indicate right frontal regions are sensitive to the requirement of task‐relevant executive control functions (see section “Possible Roles of RH Regions in Metaphor Processing” for a more detailed discussion).
Possible Roles of RH Regions in Metaphor Processing
The current meta‐analysis indicates that the RH is involved in metaphor comprehension compared with literal comprehension when (1) metaphorical meaning is novel, (2) metaphorical meaning is presented in sentential context, and (3) task requires processing of semantic associations. The result pattern suggests that during metaphor comprehension, the effect of figurativeness can be modulated by conventionality, sentential context and task demand, and that the RH involvement is influenced by these factors. However, it is hard to conclude that figurativeness does not play a role in engaging the RH in metaphor processing based on the current results. The influences of conventionality, sentential context, and task demand on RH involvement are found based on the effect of figurativeness (the contrast effect between metaphor and literal comprehension). Without the effect of figurativeness, whether these factors can influence RH involvement in language processing is unknown from the present study.
In this study, RH activations are located in areas of fronto‐temporal regions, such as the MFG, IFG, insula, MTG, and STG. Previous studies find that the right temporal lobe is involved in semantic processing of context, and that patients with right temporal lobe damage have difficulties in semantic integration based on contextual information [e.g., Beeman, 1993; Beeman et al., 2000; Kaplan et al., 1990]. The role of the right temporal lobe in semantic processing may be maintaining weak and secondary semantic features in broad semantic fields and helping to detect diffuse semantic relations [Jung‐Beeman, 2005]. Previous studies find that right IFG is important for task requiring selection [Milham et al., 2001] and that right insula is involved during auditory selective attention tasks [e.g., Jancke and Shah, 2002; Poeppel et al., 2004]. For instance, Milham et al. 2001 found that the cingulate cortex and right prefrontal cortex were involved when participants confronted with response conflict in the Stroop task. Seger et al. 2000 found that the right IFG is involved in selecting unusual semantic relations. A recent meta‐analysis of the RH role in semantic, phonological, and sentence processing suggests that these right frontal areas may be not specific for a given language processing but related to executive control function [Vigneau et al., 2011]. These right frontal areas are involved when participants perform tasks that require manipulating information in working memory.
In a word, the current study suggests that the right frontal and temporal regions work together to support coarse semantic coding during metaphor processing. This is consistent with Jung‐Beeman 2005, which proposed bilateral brain processes for natural language comprehension. According to his view, semantic processing includes three components: activation, integration, and selection. The temporal lobe (MTG/STG) is mainly responsible for semantic activation and integration, and the frontal lobe (IFG) is responsible for semantic selection. Each component involves bilateral brain regions, and RH regions perform coarser computations than LH regions for the same process.
Limitations and Future Work
Although the current results indicate that RH involvement in metaphor comprehension is modulated by conventionality, sentential context, and task demand, there are several limitations and unanswered questions that require future investigation. The first limitation comes from the original article selection. In the present study, we only included studies using fMRI and excluded studies that employ other techniques (PET, TMS, and EEG) to make sure that all original studies have approximate spatial resolution. However, fMRI scanner could also deliver heterogeneous data. In different studies, field strength, voxel size, and field of view are different, and this may make it hard to get comparable spatial resolution. And because all these factors are not taken into account in the ALE meta‐analysis, it is hard to know how they influence the final results. Given that the number of original papers on this field of research is small (17 studies), it is difficult to conduct a meta‐analysis based on the studies that have similar scan parameters. But this should be easier in the future when more fMRI evidence about metaphor processing is reported.
The second limitation is that while we examined how conventionality, sentential context, and task demand influence RH involvement in metaphor processing separately, we did not examine the interactions among the three factors systematically. Previous findings suggest that one factor might modulate another factor in metaphor comprehension. For instance, Mashal and coworkers found that novel metaphor processing involves the RH, even when metaphors are presented at word‐level [Faust and Mashal, 2007; Mashal and Faust, 2008, 2009; Mashal et al., 2005, 2007; Pobric et al., 2008]. Thus, it is possible that two or more factors may interact with each other to influence RH involvement in metaphor comprehension, but the clear effects of the interactions of these factors are hard to know from the current meta‐analysis. One reason that we did not examine the interaction effects is that the number of original studies is too small. For example, it is impossible to examine the interaction between conventionality and sentential context, because only two fMRI studies used words to present metaphorical meaning and one is about novel metaphor processing while the other is about conventional metaphor processing. Another reason that we did not examine the interaction effect is that the findings from individual studies about metaphor comprehension suggest that factors like conventionality may show their influence on figurativeness across different experimental paradigms [Schmidt and Seger, 2009], and hence it is important for the current meta‐analysis to investigate how a single factor modulates figurativeness and influences RH involvement in metaphor comprehension. Future work should be conducted to see how two or more factors interact and what information the interactions reveal about the role of the RH in metaphor comprehension.
The third limitation is that in the current study the influences of conventionality, context complexity and task demand are examined on the basis of the contrasts between metaphoric and literal condition. Although the contrasts between metaphor and literal processing can help us focus on RH involvement specific in metaphor processing, it may make us overlook the RH role in general language processing. Previous findings suggest that RH involvement may neither be essential nor specific for understanding metaphors [Rapp et al., 2004] and that both hemispheres have the ability to process metaphoric meaning [Rapp et al., 2007]. It is possible that the RH is involved in both literal and metaphor processing in some situations and that coarse semantic coding can be important for both type of language processing in these situations. Thus, we suggest that the RH functions revealed in the current study might be unique to metaphoric stimuli. Whether the RH has similar functions, such as coarser computation of semantic activation, integration and selection, in other types of language processing (e.g., literal language processing) still need further investigations.
CONCLUSION
In conclusion, the present meta‐analysis investigated how conventionality, sentential context, and task demand influence RH involvement in metaphor processing. The results showed that these factors can modulate figurativeness and that the right fronto‐temporal regions are involved in the comprehension of metaphorical meaning when coarser semantic activation, integration, and selection are required. Our findings suggest that coarse semantic coding plays an important role in involving the RH during metaphor comprehension. Future research should further investigate effects of interactions between different factors and exclude the interference from irrelevant variables.
ACKNOWLEDGMENT
The author thanks Manali Khadilkar for editing the manuscript.
REFERENCES
- Adams RB, Janata P (2002): A comparison of neural circuits underlying auditory and visual object categorization. Neuroimage 16:361–377. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Liu HL, Lee CY, Gong SP, Fang SY, Hsu YY (2007): Functional MRI of conventional and anomalous metaphors in Mandarin Chinese. Brain Lang 100:163–171. [DOI] [PubMed] [Google Scholar]
- Anaki D, Faust M, Kravetz S (1998): Cerebral hemispheric asymmetries in processing lexical metaphors. Neuropsychologia 36:353–362. [DOI] [PubMed] [Google Scholar]
- Bambini V, Gentili C, Ricciardi E, Bertinetto PM, Pietrini P (2011): Decomposing metaphor processing at the cognitive and neural level through functional magnetic resonance imaging. Brain Res Bull 86:203–216. [DOI] [PubMed] [Google Scholar]
- Beeman M (1993): Semantic processing in the right hemisphere may contribute to drawing inferences from discourse. Brain Lang 44:80–120. [DOI] [PubMed] [Google Scholar]
- Beeman M (1998): Coarse semantic coding and discourse comprehension In: Beeman M, Chiarello C, editors. Right Hemisphere Language Comprehension: Perspectives from Cognitive Neuroscience.Mahwah, NJ:Lawrence Erlbaum; pp255–284. [Google Scholar]
- Beeman M, Friedman RB, Grafman J, Perez E, Diamond S, Lindsay MB (1994): Summation priming and coarse semantic coding in the right hemisphere. J Cogn Neurosci 6:26–45. [DOI] [PubMed] [Google Scholar]
- Beeman M, Bowden EM, Gernsbacher MA (2000): Right and left hemisphere cooperation for drawing predictive and coherence inferences during normal story comprehension. Brain Lang 71:310–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Shachar M, Palti D, Grodzinsky Y (2004): Neural correlates of syntactic movement: converging evidence from two fMRI experiments. Neuroimage 21:1320–1336. [DOI] [PubMed] [Google Scholar]
- Bihrle AM, Brownell HH, Powelson JA, Gardner H (1986): Comprehension of humorous and nonhumorous materials by left and right brain‐damaged patients. Brain Cogn 5:399–311. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2002): Modality independence of word comprehension. Hum Brain Mapp 16:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD (1994): The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain 117:1241–1253. [DOI] [PubMed] [Google Scholar]
- Brownell HH, Potter HH, Michelow D, Gardner H (1984): Sensitivity to lexical denotation and connotation in brain‐damaged patients: a double dissociation. Brain Lang 22:253–265. [DOI] [PubMed] [Google Scholar]
- Brownell HH, Simpson TL, Bihrle AM, Potter HH, Gardner H (1990): Appreciation of metaphoric alternative word meanings by left and right brain damaged patients. Neuropsychologia 28:375–393. [DOI] [PubMed] [Google Scholar]
- Chen E, Widick P, Chatterjee A (2008): Functional–anatomical organization of predicate metaphor processing. Brain Lang 107:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni W, Shankweiler D (2004): Sentence complexity and input modality effects in sentence comprehension: An fMRI study. Neuroimage 22:11–21. [DOI] [PubMed] [Google Scholar]
- Diaz MT, Hogstrom LJ (2011): The influence of context on hemispheric recruitment during metaphor processing. J Cogn Neurosci 23:3586–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Barrett KT, Hogstrom LJ (2011): The influence of sentence novelty and figurativeness on brain activity. Neuropsychologia 49:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 309:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eviatar Z, Just MA (2006): Brain correlates of discourse processing: An fMRI investigation of irony and conventional metaphor comprehension. Neuropsychologia 44:2348–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Mashal N (2007): The role of the right cerebral hemisphere in processing novel metaphoric expressions taken from poetry: a divided visual field study. Neuropsychologia 45:860–870. [DOI] [PubMed] [Google Scholar]
- Faust M, Babkoff H, Kravetz S (1995): Linguistic processes in the two cerebral hemispheres: Implications for modularity vs interactionism. J Clin Exp Neuropsychol 17:171–192. [DOI] [PubMed] [Google Scholar]
- Faust M, Bar‐lev A, Chiarell C (2003): Sentence priming effects in the two cerebral hemispheres: Influences of lexical relatedness, word order, and sentence anomaly. Neuropsychologia 41:480–492. [DOI] [PubMed] [Google Scholar]
- Federmeier KD (2007): Thinking ahead: The role and roots of prediction in language comprehension. Psychophysiology 44:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giora R (1997): Understanding figurative and literal language: The graded salience hypothesis. Cogn Linguist 7:183–206. [Google Scholar]
- Giora R (1999): On the priority of salient meanings: Studies of literal and figurative language. J Pragm 31:919–929. [Google Scholar]
- Giora R (2002): Literal vs. figurative language: Different or equal? J Pragm 34:487–506. [Google Scholar]
- Giora R (2003): On Our Mind: Salience, Context and Figurative Language.New York:Oxford University Press. [Google Scholar]
- Giora R, Fein O (1999): On understanding familiar and less familiar figurative language. J Pragm 31:1601–1618. [Google Scholar]
- Giora R, Zaidel E, Soroker N, Batori G, Kasher A (2000): Differential effects of right‐ and left‐hemisphere damage on understanding sarcasm and metaphor. Metaphor Symb 15:63–83. [Google Scholar]
- Grindrod CM, Baum SR (2003): Sensitivity to local sentence context information in lexical ambiguity resolution: evidence from left‐ and right‐hemisphere‐damaged individuals. Brain Lang 85:503–523. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR (2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: An fMRI study with clinical implications. Brain 125:1024–1038. [DOI] [PubMed] [Google Scholar]
- Jancke L, Shah NJ (2002): Does dichotic listening probe temporal lobe functions? Neurology 58:736–743. [DOI] [PubMed] [Google Scholar]
- Jung‐Beeman M (2005): Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9:712–718. [DOI] [PubMed] [Google Scholar]
- Kaplan JA, Brownell HH, Jacobs JR, Gardner H (1990): The effects of right hemisphere damage on the pragmatic interpretation of conversational remarks. Brain Lang 38:315–333. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Tous AN, Williams SC, McGuire PK (2001): Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia 39:798–809. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Leube DT, Erb M, Grodd W, Rapp AM (2007): Neural correlates of metaphor processing in schizophrenia. Neuroimage 34:281–289. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK (1994): Identifying objects from different viewpoints. A PET investigation. Brain 117:1055–1071. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox M, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoff G, Johnson M (1980): Metaphors We Live By.Chicago:Univ. Chicago Press. [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Dapretto M (2006): Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. Neuroimage 292:536–544. [DOI] [PubMed] [Google Scholar]
- MacKenzie C, Begg T, Brady M, Lees KR (1997): The effects on verbal communication skills of right hemisphere stroke in middle age. Aphasiology 11:929–945. [Google Scholar]
- Mashal N, Faust M (2008): Right hemisphere sensitivity to novel metaphoric relations: Application of the signal detection theory. Brain Lang 104:103–112. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M (2009): Conventionalization of novel metaphors: A shift in hemispheric asymmetry. Laterality 14:573–589. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T (2005): The role of the right hemisphere in processing nonsalient metaphorical meanings: Application of principal components analysis to fMRI data. Neuropsychologia 43:2084–2100. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung‐Beeman M (2007): An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Lang 100:115–126. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung‐Beeman M (2008): Hemispheric differences in processing the literal interpretation of idioms: Converging evidence from behavioral and fMRI studies. Cortex 44:848–860. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung‐Beeman M (2009): An fMRI study of processing novel metaphoric sentences. Laterality 14:30–54. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA (2004): How the brain processes causal inferences in text: A theoretical account of generation and integration component processes utilizing both cerebral hemispheres. Psychol Sci 15:1–7. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG (2003): A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia 41:293–303. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF (2001): The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res 12:467–473. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R (1995): Where the brain appreciates the moral of a story. Neuroreport 6:2309–2313. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Rauchle ME (1989): Positron emission tomography studies of the processing of single words. J Cogn Neurosci 1:153–170. [DOI] [PubMed] [Google Scholar]
- Pobric G, Mashal N, Faust M, Lavidor M (2008): The role of the right cerebral hemisphere in processing novel metaphoric expressions: A transcranial magnetic stimulation study. J Cogn Neurosci 20:170–181. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR (2004): Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia 42:183–200. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TT (2004): Neural correlates of metaphor processing. Brain Res Cogn Brain Res 203:395–402. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TT (2007): Laterality in metaphor processing: Lack of evidence from functional magnetic resonance imaging for the right hemisphere theory. Brain Lang 100:142–149. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, Seger CA (2009): Neural correlates of metaphor processing: The roles of figurativeness, familiarity and difficulty. Brain Cogn 71:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Desmond JE, Glover GH, Gabrieli JD (2000): Functional magnetic resonance imaging evidence for right‐hemisphere involvement in processing unusual semantic relationships. Neuropsychology 14:361–369. [DOI] [PubMed] [Google Scholar]
- Shibata M, Abe J, Terao A, Miyamoto T (2007): Neural mechanisms involved in the comprehension of metaphoric and literal sentences: An fMRI study. Brain Res 1166:92–102. [DOI] [PubMed] [Google Scholar]
- Sotillo M, Carreti L, Hinojosa JA, Tapia M, Mercado F, Lopez‐Martin S, Albert J (2005): Neural activity associated with metaphor comprehension: Spatial analysis. Neurosci Lett 373:5–9. [DOI] [PubMed] [Google Scholar]
- St George M, Kutas M, Martinez A, Sereno MI (1999): Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain 122:1317–1325. [DOI] [PubMed] [Google Scholar]
- Stringaris AK, Medford NC, Giora R, Giampietro V, Brammer MJ, David AS (2006): How metaphors influence semantic relatedness judgments: The role of the right frontal cortex. Neuroimage 33:784–793. [DOI] [PubMed] [Google Scholar]
- Stringaris AK, Medford NC, Giampietro V, Brammer MJ, David AS (2007): Deriving meaning: Distinct neural mechanisms for metaphoric, literal, and non‐meaningful sentences. Brain Lang 100:150–162. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐Planar Stereotaxic Atlas of the Human Brain.New York:Thieme Medical. [Google Scholar]
- Uchiyama HT, Saito DN, Tanabe HC, Harada T, Seki A, Ohno K, Koeda T, Sadato N (2012): Distinction between the literal and intended meanings of sentences: A functional magnetic resonance imaging study of metaphor and sarcasm. Cortex 48:563–583. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Jobard G, Petit L, Crivello F, Mellet E, Zago L, Mazoyer B, Tzourio‐Mazoyer N (2011): What is right‐hemisphere contribution to phonological, lexico‐semantic, and sentence processing? Insights from a meta‐analysis. Neuroimage 54:577–593. [DOI] [PubMed] [Google Scholar]
- Warburton EA, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RSJ, (1996): Noun and verb retrieval by normal subjects. Studies with PET. Brain 119:159–179. [DOI] [PubMed] [Google Scholar]
- Winner E, Gardner H (1977): The comprehension of metaphor in brain‐damaged patients. Brain 100:717–729. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A (2005): Language in context: Emergent features of word, sentence, and narrative comprehension. Neuroimage 25:1002–1015. [DOI] [PubMed] [Google Scholar]
- Yang FG, Edens J, Simpson C, Krawczyk DC (2009): Differences in task demands influence the hemispheric lateralization and neural correlates of metaphor. Brain Lang 111:114–124. [DOI] [PubMed] [Google Scholar]
- Yang FG, Fuller J, Khodaparast N, Krawczyk DC (2010): Figurative language processing after traumatic brain injury in adults: A preliminary study. Neuropsychologia 48:1923–1929. [DOI] [PubMed] [Google Scholar]


