Abstract
In male Caucasian subjects, learning is facilitated by receipt of social compared with non‐social feedback, and the neuropeptide oxytocin (OXT) facilitates this effect. In this study, we have first shown a cultural difference in that male Chinese subjects actually perform significantly worse in the same reinforcement associated learning task with social (emotional faces) compared with non‐social feedback. Nevertheless, in two independent double‐blind placebo (PLC) controlled between‐subject design experiments we found OXT still selectively facilitated learning with social feedback. Similar to Caucasian subjects this OXT effect was strongest with feedback using female rather than male faces. One experiment performed in conjunction with functional magnetic resonance imaging showed that during the response, but not feedback phase of the task, OXT selectively increased activity in the amygdala, hippocampus, parahippocampal gyrus and putamen during the social feedback condition, and functional connectivity between the amygdala and insula and caudate. Therefore, OXT may be increasing the salience and reward value of anticipated social feedback. In the PLC group, response times and state anxiety scores during social feedback were associated with signal changes in these same regions but not in the OXT group. OXT may therefore have also facilitated learning by reducing anxiety in the social feedback condition. Overall our results provide the first evidence for cultural differences in social facilitation of learning per se, but a similar selective enhancement of learning with social feedback under OXT. This effect of OXT may be associated with enhanced responses and functional connectivity in emotional memory and reward processing regions. Hum Brain Mapp 36:2132–2146, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: Oxytocin, fMRI, reinforcement learning, functional connectivity, emotional memory
Abbreviations
- BOLD
blood oxygenation level‐dependent
- FDR
false discovery rate
- fMRI
functional magnetic resonance imaging
- OXT
oxytocin
- PLC
placebo
- RALT
reinforcement association learning task
- ROI
region of interest
INTRODUCTION
There is increasing evidence for a key role of the hypothalamic neuropeptide oxytocin (OXT) in human social and emotional behaviors [Meyer‐Lindenberg et al., 2011; Striepens et al., 2011]. OXT has also been implicated in aspects of learning and memory, with studies claiming it to have both pro‐mnestic [Guastella et al., 2008; Hurlemann et al., 2010a; Rimmele et al., 2009; Savaskan et al., 2008] and amnestic [Bruins et al., 1992; Fehm‐Wolfsdorf et al., 1984; Ferrier et al., 1980; Heinrichs et al., 2004] effects. In a recent study in Caucasian subjects, we found social feedback (male or female neutral expression faces which switched to smiling or angry ones to indicate correct or error responses) facilitated subjects' learning compared to non‐social feedback (filled black circle changing to a red–error‐ or green–correct‐one) in the context of a reinforcement association learning task (RALT). We also found that intranasal OXT treatment selectively enhanced this facilitated learning only when social feedback was used [Hurlemann et al., 2010a]. These results provided experimental support both for pioneering psychological studies suggesting that social reinforcement is a potent facilitator of human learning [Allport, 1920; Dashiell, 1930; Gates and Rissland, 1923; Zajonc, 1965] and for a role of OXT in enhancing this effect.
There is increasing evidence for cross‐cultural differences in social cognition and associated brain processing [Han et al., 2013] and it is unclear whether the same facilitation of learning using social feedback observed in subjects from an independent culture will also occur in subjects from a collectivist one with different traditions in terms of social values and learning. Although several studies have reported effects of intranasal OXT in Chinese subjects in terms of increased ethnocentrism in relation to enhanced in‐group liking [Ma et al., 2014], and a reduced forgiveness of betrayal in the trust game [Yao et al., 2014], it is not clear whether this also extends into the domains of enhanced social learning and reward. In the present study we have therefore investigated whether Chinese male subjects show social facilitation of learning and how this would be affected by OXT as compared with that of the Caucasian subjects [Hurlemann et al., 2010a].
To investigate neural substrates and functional connections whereby OXT may affect learning with social feedback with Chinese subjects, we have also carried out a behavioral study combined with functional magnetic resonance imaging (fMRI). We have also shown previously that the social facilitation of learning effect we observed in the RALT was absent in two patients with bilateral amygdala damage due to Urbach‐Wiethe disease [Hurlemann et al., 2010a]. Furthermore, in healthy subjects the ß‐noradrenergic antagonist, propranolol, which selectively reduces amygdala responses to neutral and emotional faces [Hurlemann et al., 2010b] prevented the social facilitation of learning when used in the social feedback version of the RALT [Mihov et al., 2010]. Both animal and human studies have implicated the amygdala as a major target for OXT actions on social and emotional behaviors [Meyer‐Lindenberg et al., 2011; Striepens et al., 2011] and so a possible hypothesis is that both the social facilitation of learning and OXT's potentiation of this involve an amygdala‐associated network. On the other hand, our previous research has shown that learning in the RALT using non‐social feedback is associated with increased hippocampal activation and that this is sensitive to glutamate N‐methyl‐D‐aspartate (NMDA) receptor/cholinergic signaling [Becker et al., 2013; Onur et al., 2010]. Therefore, OXT might also facilitate learning via enhanced hippocampal activation.
Since social feedback also involves both emotional arousal and reward/punishment components we further hypothesized that other brain regions connected to the amygdala and involved in emotional facilitation of learning, such as the parahippocampal gyrus [LaBar and Cabeza, 2006; Phelps, 2004], and social reward such as the striatum and prefrontal cortex [Fareri and Delgado, 2014] might also show OXT effects associated with social feedback facilitation of learning. Furthermore since anticipation and receipt of reward/punishment can engage different neural systems [O'Doherty, 2004; Rademacher et al., 2010; Schultz 2006], we subdivided the RALT into response and feedback phases to determine whether OXT selectively influences neural responses to receipt of social feedback or to its anticipation.
METHODS
In Experiment 1, we first investigated whether 45 Chinese (Han) subjects (males = 25, mean±SD = 19.7 ± 1.9 years) exhibited social facilitation of learning in the RALT. In two further double‐blind between subject placebo (PLC) controlled studies (Experiments 2 and 3) we investigated the effect of intranasal OXT (24IU) on learning with either social or non‐social feedback in the RALT. In Experiment 2, there were 52 male Chinese (Han) subjects (mean ± SD 20.1±1.64 years) and in Experiment 3, 54 (19.8±1.49 years). In Experiment 2 subjects only performed the RALT whereas in Experiment 3 the RALT was performed in conjunction with fMRI. In Experiment 3 subjects were also asked to rate the attractiveness of all the feedback stimuli used in the RALT and also five neutral, happy and angry expression unfamiliar male and female Chinese faces. Subjects were all free of medical or psychiatric illness and drug or alcohol abuse.
In Experiments 2 and 3, subjects were either administered a single intranasal dose of 24IU OXT (Syntocinon Spray; Sichuan Meike Pharmacy Co. Ltd, Sichuan, China; three puffs of 4IU per nostril with 30 s between each puff) or PLC (also three puffs per nostril). The PLC treatment contained all of same ingredients other than the neuropeptide, and was provided in the same type of dispenser bottle by the pharmaceutical company supplying the OXT nasal spray. In line with many previous reports [Guastella et al., 2013; Striepens et al., 2011], the experimental paradigm started 45 min after OXT or PLC treatment which is estimated to allow maximum increased concentrations of the peptide to occur within the cerebrospinal fluid [Born et al., 2002; Chang et al., 2012; Striepens et al., 2014]. The RALT paradigm in Experiments 2 and 3 lasted around 30 min and valence ratings on the experimental and other social and non‐social stimuli in Experiment 3 lasted a further 15 min. Therefore, all experiments were completed within 1.5 h of OXT administration. This is similar to the majority of other studies reporting functional effects of OXT [Striepens et al., 2011, 2014]. In post‐experiment interviews subjects were unable to identify better than chance whether they had received the OXT or PLC treatment.
Immediately before Experiments 2 and 3 all subjects completed a range of questionnaires measuring IQ, personality types, state, and trait anxiety and shyness: Chinese versions of: Weschler‐C, Eysenck Personality Inventory (EPI), Positive and Negative Affect Schedule (PANAS), State‐Trait Anxiety Inventory (STAI) and Cheek and Buss Shyness Scale (CBSS). All subjects were also given a short presentation of a selection of neutral, happy and angry expression Chinese faces to confirm that they could discriminate between them accurately.
The study was approved by the ethical committee of the University of Electronic Science and Technology of China and all subjects gave informed and written consent to take part.
RALT Paradigm
For the RALT in all three experiments, subjects had to learn which of a set of 12 random three‐digit numbers was associated with either an arbitrary category A or B informed by either social or non‐social feedback stimuli (Fig. 1a). Learning performance was recorded across six cycles where the numbers were presented in a random order and the same type of feedback given. In all cases, the different feedback types in each experiment were given in a random order to control for possible order effects or decreases in elevated OXT concentrations over time. In the social feedback condition in Experiments 1 and 2, the subjects initially saw a neutral expression of Chinese male or female face and following their response this changed to a smiling (correct) or angry (incorrect) version of the same face. The Chinese male and female neutral and emotional faces were matched for valence and arousal (using scores from an independent group of 20 raters; n = 10 female). To control for a possible difference between faces used in the initial study by Hurlemann et al [2010a) on Caucasian subjects and the Chinese face stimuli, an initial experiment on 15 subjects (8 males, mean ± SD = 19.3 ± 1.2 years) established that learning performance was identical in the RALT using feedback with either the original male and female Caucasian face stimuli or the Chinese ones (see Supporting Information Fig. S1). In Experiment 3 only the female face was used for social feedback. In the non‐social condition a black circular light changed either to a green (correct) or to a red (incorrect) one (Experiments 1, 2 and 3) or a question mark changed to either a tick (correct) or cross (incorrect; Experiment 1), or a neutral expression yellow emoticon face changed to a smiling (correct) or angry (incorrect) one (Experiment 3). In Experiment 3 following the RALT task subjects were also presented with all the face and non‐face feedback images used individually and also face pictures of unfamiliar Chinese males and females with neutral, happy, and angry expressions (five of each). Pictures were shown in a random order for 3 s followed by a 3‐s period where subjects rated the picture on a 9‐point likeability scale (1 = dislike very much, 5 = neither like nor dislike, and 9 = like very much). This was to investigate whether OXT treatment had significant effects on the likability of the specific different social and non‐social feedback stimuli presented during the RALT or other unfamiliar neutral or happy/angry expression faces.
Figure 1.
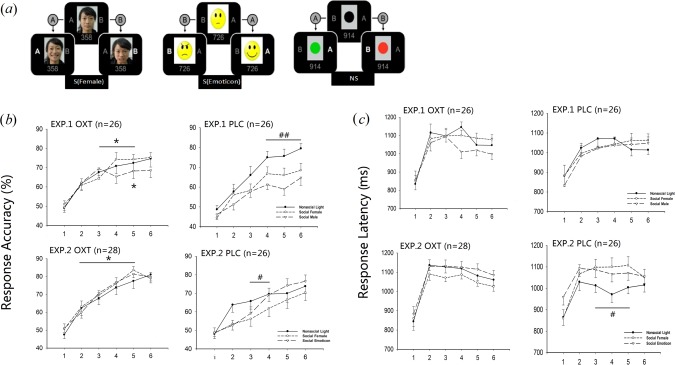
Reinforcement association learning task (RALT) behavioral results for oxytocin (OXT) and placebo (PLC) groups in Experiments 2 and 3. (a) shows feedback stimuli used in Experiment 3 (b) shows mean % correct responses across the six presentation cycles of the task for the three different feedback types in Experiments 2 and 3 (c) shows corresponding mean response times. **P < 0.01, *P < 0.05 for OXT versus PLC with female face feedback, ##P < 0.01, #P < 0.05 for light feedback versus social female feedback (in Experiment 2) and light feedback versus social emoticon and social female (Experiment 3) under PLC.
Acquisition and Analysis of fMRI Data
fMRI employing blood oxygenation level‐dependent (BOLD) contrast was carried out on a whole‐body 3.0 T MR scanner (Siemens Trio, Erlangen, Germany) with a 12‐channel head coil as signal receiver. Foam pads were used to restrict subjects' head motion. Three runs were conducted. For each run, 357 volumes of T2*‐weighted echo planar images were obtained with a gradient‐echo planar imaging sequence (repetition time, 2,000 ms; echo time, 30 ms; slices, 32; thickness, 4 mm; gap, 0 mm; field of view, 240 × 240 mm2; acquisition matrix, 64 × 64; flip angle, 90°; voxel size, 3.8 × 3.8 × 4 mm3). High‐resolution whole‐brain volume T1*‐weighted images were also acquired using a magnetization prepared gradient echo sequence (MPRAGE; repetition time, 1,900 ms; echo time, 2.26 ms; flip angle, 9°; sagittal field of view, 256 × 256 mm2; acquisition matrix, 256 × 256 × 176; thickness, 1 mm; voxel size, 1 × 1 × 1 mm3) to control for any anatomic abnormalities and increase normalization accuracy during pre‐processing.
Analysis of fMRI data was carried out using SPM8 software (Welcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 7 (The MathWorks Inc., Natick MA). For the RALT, the two phases of the paradigm were analyzed separately: the response phase (when subjects performed the category‐selection task) and the feedback phase (when subjects received feedback regarding their choice). In each phase, six conditions (“Social FemaleOXT,” “Social FemalePLC,” “EmoticonOXT,” “EmoticonPLC,” “Non‐social LightOXT,” and “Non‐social LightPLC”) were modeled by a stick function convolved with the hemodynamic response function. Head movements were included in the design matrix as confounds. Main effect of treatment (OXT vs. PLC) was analyzed using two‐sample t‐tests entering all conditions relative to the low‐level baseline. Parameter estimates for the contrast of main effect were subjected to two‐sample t‐tests on the second level for the whole‐brain with a significance threshold of P < 0.05 corrected for multiple comparisons (false discovery rate [FDR] corrected, Genovese et al., 2002]. A flexible factorial model was established to specifically examine the modulatory effects of OXT on feedback‐reinforced learning, including the between subjects factor “Treatment” (OXT vs. PLC) and the within subjects factor “Feedback” (Social Female, Emoticon, and Non‐social Light). The threshold for these specific contrasts was set at P < 0.05 (FDR corrected).
After the whole brain analysis, a hypotheses driven region of interest (ROI) analysis was performed using regions that have been associated with the neural effects of OXT and showed activity in comparable feedback guided learning tasks in previous fMRI studies, including the amygdala and the hippocampus [Becker et al., 2013; Meyer‐Lindenberg et al., 2011; Onur et al., 2010; Striepens et al., 2011, 2012]. The threshold of significance for the subsequent ROI analysis was set at P < 0.05, corrected for multiple comparisons within structurally defined regions.
RESULTS
Table 1 shows that subjects in the OXT and PLC groups in Experiments 2 and 3 did not differ significantly on their scores in the neuropsychological tests/questionnaires.
Table 1.
Subjects' scores on neuropsychology questionnaires
| Experiment 1 | Experiment 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Scale | PLC | OXT | T | P‐value | PLC | OXT | T | P‐value |
| EPI | ||||||||
| Extraversion | 5.04 ± 0.52 | 5.50 ± 0.44 | 0.66 | 0.51 | 4.43 ± 0.44 | 4.89 ± 0.46 | 0.74 | 0.46 |
| Neuroticism | 10.96 ± 0.85 | 10.59 ± 1.05 | 0.27 | 0.79 | 14.12 ± 0.86 | 12.00 ± 0.87 | 1.72 | 0.09 |
| Psychoticism | 10.96 ± 0.88 | 11.68 ± 1.05 | 0.53 | 0.6 | 9.19 ± 0.98 | 10.93 ± 1.09 | 1.18 | 0.24 |
| Liability to all | 9.13 ± 0.66 | 8.41 ± 0.51 | 0.86 | 0.4 | 11.61 ± 0.64 | 11.25 ± 0.65 | 0.4 | 0.69 |
| Weschler‐C | 66.13 ± 2.66 | 65.55 ± 2.62 | 0.16 | 0.88 | 68.89 ± 2.11 | 66.68 ± 2.11 | 0.8 | 0.43 |
| PANAS | ||||||||
| Positive | 28.35 ± 1.06 | 29.50 ± 1.35 | 0.68 | 0.5 | 31.23 ± 1.07 | 30.50 ± 0.91 | 0.52 | 0.6 |
| Negative | 16.22 ± 0.92 | 16.82 ± 1.17 | 0.41 | 0.69 | 16.73 ± 1.22 | 16.93 ± 1.04 | 0.2 | 0.9 |
| STAI | ||||||||
| State | 45.43 ± 1.16 | 44.05 ± 1.08 | 0.87 | 0.39 | 45.50 ± 1.08 | 44.75 ± 0.92 | 0.53 | 0.6 |
| Trait | 44.38 ± 1.01 | 43.50 ± 0.98 | 0.63 | 0.53 | 43.65 ± 0.98 | 43.79 ± 0.94 | 0.1 | 0.92 |
| CBSS | 35.91 ± 1.82 | 36.73 ± 1.58 | 0.34 | 0.74 | 31.27 ± 1.46 | 35.18 ± 1.55 | 1.83 | 0.07 |
CBSS, Cheek and Bus Shyness Scale; EPI, Eysenck's Personality Inventory; OXT, oxytocin; PANAS, Positive and Negative Affect Schedule; PLC, placebo; STAI, State and Trait Anxiety Inventory.;
Behavioral Results
In all the experiments, behavioral data were analyzed using a three‐way ANOVA (using SPSS version 17.0) with presentation cycle and feedback type as within subject factors and treatment as a between subject factor. Significant interactions were investigated within SPSS using the simple effects test and Fishers Least Significant Difference (LSD). Results in Experiment 1 for accuracy (see Supporting Information Fig. S2) revealed significant main effects of presentation cycle (F5,44 = 60.09, P < 0.001), feedback type (F3,44 = 3.351, P = 0.021) and a feedback x presentation cycle interaction (F15,44 = 1.052, P = 0.025). Post‐hoc tests revealed that accuracy during the non‐social light feedback condition was significantly higher than in the social female face feedback one for cycles 4 (P = 0.011) and 5 (P = 0.007) and marginally higher than for the social male feedback condition for cycles 4 (P = 0.061) and 5 (P = 0.082). Accuracy during the non‐social feedback condition with a tick or cross feedback was also higher than that in the social female feedback one in cycles 4 (P = 0.061), 5 (P = 0.003), and 6 (P = 0.015) and the social male feedback in cycles 5 (P = 0.014) and 6 (P = 0.064). There were no significant differences between the two individual non‐social or social feedback conditions. We also ran an additional ANOVA with the two social and non‐social feedback conditions combined and with social versus non‐social feedback as a factor. This revealed both a main effect of type of feedback (P = 0.005) and an interaction between feedback and presentation cycle (P = 0.02; see Supplementary section S2 for details). Thus in direct contrast to the previous study on Caucasian subjects [Hurlemann et al., 2010a], Chinese subjects perform significantly worse in the RALT when given social as opposed to non‐social feedback. We also ran a further ANOVA including subject gender as a factor and this revealed no significant effects (P > 0.1).
Results in the first OXT experiment (Experiment 2) for accuracy revealed significant main effects of presentation cycle (F 5,50 = 80.59, P < 0.001), feedback type (F 2,50 = 3.397, P = 0.037), and a trend for treatment (F 1,50 = 3.777, P = 0.058). There was also a significant feedback × treatment interaction (F 2,50 = 3.397, P = 0.037). Post‐hoc tests revealed that, as in Experiment 1, accuracy was significantly better in the PLC group with non‐social (light) compared with both types of social feedback: female face (on presentation cycles 4, 5, and 6, P < 0.001) and male face (on presentation cycle 6, P = 0.018). Once again there was no significant difference between learning with feedback from male versus female faces (P > 0.05 for all presentation cycles). By contrast, in the OXT group there were no significant differences in accuracy between the three non‐social and social feedback conditions across the six individual cycles indicating that OXT treatment had rendered social feedback as effective as non‐social feedback. This also showed that there was no significant difference between male vs female feedback despite female faces appearing to be more effective than male ones under OXT. Indeed, a between group comparison revealed that accuracy was significantly increased in the OXT versus PLC group in the social female feedback condition on cycles 3 (P = 0.0037), 4 (P = 0.0003), and 5 (P = 0.0262) although for the male feedback only on cycle 5 (P = 0.0236; Fig. 1b). There were no significant differences across presentation cycles for OXT versus PLC in the non‐social (light) feedback condition. For response times there was only a significant main effect of presentation cycle (F 5,50 = 40.58, P < 0.001) although this was mainly due to the first presentation cycle having shorter response times than the others. Although there was no main effect of treatment there was a significant presentation × treatment interaction (F 5,50 = 2.768, P = 0.019) due to response times under OXT being generally faster on cycle 1 and slower on the remaining cycles, although significance was not achieved for any individual cycle across the three types of feedback (Fig. 1c).
In the second OXT experiment (Experiment 3—performed in conjunction with fMRI and where feedback with a male face was substituted with an emoticon face) there were again significant main effects of both presentation cycle (F 5,52 = 86.803, P < 0.0001) and treatment (F 1,52 = 5.227, P = 0.026) and trends towards significant feedback type × treatment (F 2,52 = 2.807, P = 0.065) and presentation cycle × treatment (F 5,52 = 2.135, P = 0.062) interactions. Post‐hoc tests revealed that as in Experiment 2 accuracy was significantly better in the PLC group with non‐social (light) compared to the social (female face) feedback (presentation cycles 3, P = 0.0075 and 4, P = 0.0198). In the OXT group there were no significant differences in accuracy across the three feedback types replicating the finding in Experiment 2 that OXT rendered social feedback as effective as non‐social feedback in this task. Between group post‐hoc tests also revealed that as in Experiment 2 accuracy was significantly increased in the OXT vs PLC group in the social female feedback condition in presentation cycles 2, (P = 0.0361), 3 (P = 0.0046), 4 (P = 0.0144), and 5 (P = 0.0013; Fig. 1b) but not in either the non‐social (light) or emoticon conditions (P > 0.05 across all individual presentation cycles). For response times there were significant main effects of feedback (F 2,52 = 3.916, P = 0.023) and presentation cycle (F 5,52 = 53.22, P < 0.001). Although there was no main effect of treatment, there were significant interactions of feedback × treatment (F 5,52 = 4.492, P = 0.013) and presentation cycle × treatment (F 5,52 = 2.463, P = 0.033). Post‐hoc tests revealed that these interactions were mainly due to longer response times under PLC during the emoticon and social female feedback (in both cases in presentation cycles 3, 4, and 5; P < 0.05) compared with the non‐social light feedback. This did not occur in the OXT treatment condition. Response times were also significantly increased in the non‐social light feedback condition under OXT in presentation cycles 2, 3, 4, and 5 (P < 0.05 in all cases; Fig. 1c).
There was no significant effect of OXT on likeability for the different feedback stimuli used in Experiment 3 (25 subjects in each treatment group completed this task: PLC vs, OXT – female face: neutral – 4.60 ± 0.17 vs. 4.48 ± 0.19, t 49 = 0.46, P = 0.65; happy – 6.68 ± 0.13 vs. 6.28 ± 0.22, t 48 = 1.58, P = 0.12; angry – 1.36 ± 0.14 vs. 1.48 ± 0.22, t 48 = 0.47, P = 0.64: emoticon: neutral – 4.28 ± 0.22 vs. 3.80 ± 0.16, t 48 = 1.75, P = 0.09; happy – 6.32 ± 0.23 vs. 6.40 ± 0.23, t 48 = 0.25, P = 0.81; angry – 2.40 ± 0.28 vs. 2.28 ± 0.19, t 48 = 0.35, P = 0.73: light: black – 3.24 ± 0.31 vs. 2.72 ± 0.29, t 48 = 1.23, P = 0.23; green – 5.08 ± 0.31 vs. 5.40 ± 0.33, t 48 = 0.71, P = 0.48; red – 3.04 ± 0.40 vs. 2.56 ± 0.40, t 48 = 0.86, P = 0.39). There was also no significant OXT effect on likeability of unfamiliar female faces (neutral – 3.81 ± 0.12 vs. 3.69 ± 0.14, t 48 = 0.69, P = 0.50; happy – 5.96 ± 0.12 vs. 5.74 ± 0.19, t 48 = 0.95, P = 0.35; angry – 2.45 ± 0.16 vs. 2.43 ± 0.13, t 48 = 0.11, P = 0.64). There were no significant OXT effects on unfamiliar male face stimuli or non‐social stimuli such as ticks and crosses (P > 0.13 in all cases).
fMRI Results
Response phase
In the response phase, a whole‐brain analysis revealed a main effect of task (all responses relative to the implicit baseline) on BOLD activations in left caudate (t 52 = 8.74, P < 0.05, FDR corrected, located in Montreal Neurological Institute (MNI) space at −16/3/24), left fusiform (t 52 = 11.33, P < 0.05, FDR corrected, located in MNI space at −44/−54/−16], right fusiform (t 52 = 10.52, P < 0.05, FDR corrected, located in MNI space at 43/–46/–20], left mid occipital gyrus (t 52 = 9.66, P < 0.05, FDR corrected, located in MNI space at −25/−58/40), left inferior parietal gyrus (t 52 = 9.07, P < 0.05, FDR corrected, located in MNI space at −41/−46/36), right superior temporal gyrus (t 52 = 5.53, P < 0.05, FDR corrected, located in MNI space at 54/–39/12).
Next we analyzed the main effect of OXT at the whole brain level and this revealed an increased BOLD signal in the left fusiform (t 52 = 8.05, P < 0.05, FDR‐corrected, located in MNI space at −40/−69/−16), right fusiform (t 52 = 6.95, P < 0.05, FDR‐corrected, located in MNI space at 24/–80/–12) and mid occipital gyrus (t 52 = 6.26, P < 0.05, FDR‐corrected, located in MNI space at −25/–61/–32) for the OXT vs the PLC group contrast. Further, comparison between feedback types across the two groups ([Social FemaleOXT+PLC > EmoticonOXT+PLC], [Social FemaleOXT+PLC > Non‐social LightOXT+PLC]) revealed an increased BOLD signal in right amygdala (t 52 = 4.10, P < 0.001, uncorrected, located in MNI space at 24/–8/–12), right hippocampus (t 52 = 4.02, P < 0.001, uncorrected, located in MNI space at 32/–8/–16) and right superior temporal gyrus (t 52 = 4.67, P < 0.001, uncorrected, located in MNI space at 54/–12/–8).
We also examined the OXT effects on BOLD responses at the whole brain level for each of the different feedback types by computing the contrasts (Social FemaleOXT > Social FemalePLC), (EmoticonOXT > EmoticonPLC), and)Non‐social LightOXT > Non‐social LightPLC). This analysis revealed social‐female‐specific activations in left putamen, left thalamus and right parahippocampal gyrus regions. An overview of the findings is provided in Table 2.
Table 2.
Activation table for the whole brain general linear model (GLM) analysis in response phase
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain region | Right/left | Cluster size | t‐Score | x | y | z |
| OXT > PLC (non‐social light) | ‐ | |||||
| Middle occipital gyrus | L | 278 | 4.69 | −29 | −80 | 4 |
| Fusiform gyrus | L | 185 | 5.67 | −33 | −77 | −16 |
| Fusiform gyrus | R | 156 | 4.40 | 28 | −80 | −8 |
| Cerebellum | R | 98 | 4.29 | 32 | −73 | −20 |
| Inferior parietal lobule | L | 26 | 3.67 | −48 | −39 | 28 |
| Precuneus | L | 20 | 5.50 | −22 | −65 | 36 |
| Middle frontal gyrus | L | 16 | 3.88 | −33 | 45 | −16 |
| Cingulate gyrus | R | 13 | 3.21 | 5 | −35 | 44 |
| OXT > PLC (social emoticon) | ||||||
| Superior temporal gyrus | R | 49 | 4.73 | 39 | 7 | −24 |
| Fusiform gyrus | L | 25 | 4.29 | −41 | −54 | −12 |
| Medial frontal gyrus | R | 23 | 4.02 | 5 | 53 | 0 |
| Lingual gyrus | R | 21 | 4.07 | 28 | −77 | −16 |
| OXT > PLC (social female) | ||||||
| Fusiform gyrus | R | 74 | 5.12 | 24 | −77 | −8 |
| Cerebellum | L | 65 | 4.32 | −32 | −73 | −20 |
| Fusiform gyrus | L | 193 | 4.72 | −41 | −54 | −12 |
| Precuneus | L | 128 | 4.55 | −18 | −62 | 36 |
| Posterior cingulate gyrus | L | 56 | 4.35 | −12 | −46 | 10 |
| Lingual gyrus | L | 103 | 4.46 | −29 | −80 | 8 |
| Middle occipital gyrus | L | 72 | 4.11 | −29 | −77 | −24 |
| Parahippocampal gyrus | R | 38 | 3.45 | 20 | −35 | −4 |
| Putamen | L | 28 | 3.90 | −22 | 7 | 8 |
| Thalamus | L | 18 | 4.40 | −6 | −16 | 4 |
MNI,; OXT, oxytocin; PLC, placebo.
P < 0.05, FDR‐corrected, cluster size>=10, coordinates correspond to MNI space.
Next, BOLD responses were analyzed in the hypothesis‐driven predefined ROIs (left and right hippocampus, left and right amygdala, left and right parahippocampal gyrus), that have previously been associated with emotional memory [LaBar and Cabeza, 2006; Phelps 2004]. This revealed increased activity for OXT compared with PLC groups for the contrast (Social FemaleOXT > Social FemalePLC) in right amygdala [t 52 = 4.00, P (FWE) = 0.001, peak MNI coordinates at 24/–8/–12] and right hippocampus [t 52 = 3.54, P (FWE) = 0.013, peak MNI coordinates at 20/–35/–4]. No significant differences were found for the emoticon (EmoticonOXT > EmoticonPLC) and the non‐social conditions (Non‐social LightOXT > Non‐social LightPLC), indicating a specific effect of OXT on the social face condition.
To further disentangle the interaction effect, percent signal changes were extracted from those predefined ROIs. We also extracted signal change data from the peak coordinates of regions activated in the whole brain analysis (left precuneus, left posterior cingulate gyrus, left thalamus and left putamen) to increase the sensitivity of the analysis. Inspection of extracted individual percent signal changes confirmed that this effect was due to a differential response as a result of OXT treatment: Two‐way ANOVAs (treatment × feedback type) showed only a main effect of treatment in the left precuneus (F 1,52 = 12.107, P = 0.001), right parahippocampal gyrus (F 1,52 = 6.289, P = 0.015) and right posterior cingulate gyrus (F 1,52 = 11.060, P = 0.002; Fig. 2). A treatment × feedback type interaction was found in right hippocampus (F 2,104 = 4.296, P = 0.016), right amygdala (F 2,104 = 3.505, P = 0.034), left thalamus (F 2,104 = 3.164, P = 0.046) and left putamen (F 2,104 = 3.386, P = 0.038; Fig. 3).
Figure 2.
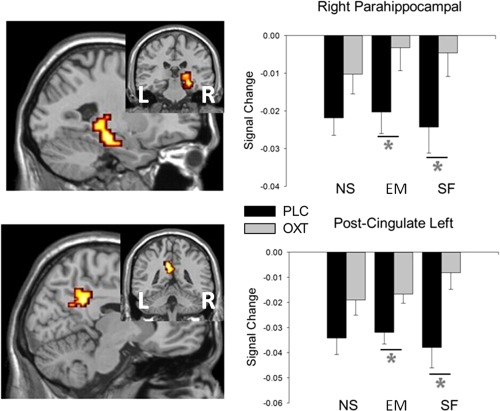
Extracted Signal change data from peak coordinates activated for (Social FemaleOXT > Social FemalePLC) during the whole brain analysis. Only a main effect of treatment was detected, indicating that OXT is non‐selectively reducing the magnitude of signal change in these regions for all three feedback types. Right parahippocampal gyrus: F(1,52) = 6.289, P = 0.015 Left posterior cingulate gyrus: F(1,52) = 11.060, P = 0.002. EM, emoticon feedback; NS, non‐social light feedback; . *P < 0.05 OXT versus PLC
Figure 3.
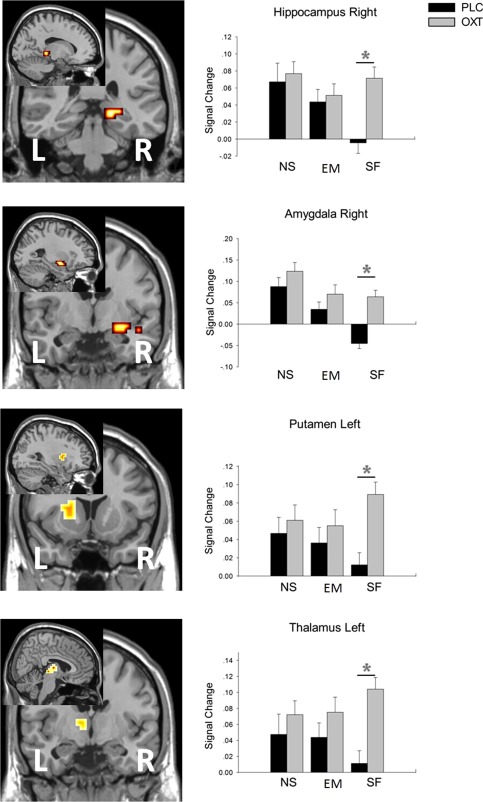
Extracted signal change data from pre‐defined ROIs. Both a treatment main effect and an interaction treatment × feedback were found, showing OXT mediated increases occur only for SF in those regions. EM, emoticon feedback; NS, non‐social light feedback; SF, social female feedback. Main effect: right hippocampus: F(1,52) = 4.267, P = 0.044; right amygdala: F(1,52) = 10.766, P = 0.002; left putamen: F(1,52) = 4.930, P = 0.031; left thalamus: F(1,52) = 6.251, P = 0.016. Interaction: right hippocampus: F(2,104) = 4.296, P = 0.016; right amygdala: F(2,104) = 3.505, P = 0.034; left putamen: F(2,104) = 3.386, P = 0.038; left thalamus: F(2,104) = 3.164, P = 0.046. *P < 0.05 OXT versus PLC.
Feedback phase
In the feedback phase analysis only was performed for correct responses due to their much higher frequency than incorrect ones. When the OXT group was compared with the PLC group (main effect of treatment), whole‐brain analysis revealed activations in left fusiform (t 52 = 3.89, P < 0.001, uncorrected, located in MNI space at −30/−75/−14), and right fusiform (t 52 = 3.57, P < 0.001, uncorrected, located in MNI space at 31/–77/–12).
We also computed OXT versus PLC contrasts for each feedback condition (Social FemaleOXT > Social FemalePLC), (EmoticonOXT > EmoticonPLC), and (Non‐social LightOXT > Non‐social LightPLC). The activated regions for correct responses are shown in Table 3. No significant differences were found in the hypothesis‐driven predefined ROIs.
Table 3.
Activation table for the whole brain GLM analysis in feedback phase
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain region | Right/left | Cluster size | t‐Score | x | y | z |
| OXT > PLC (non‐social light), correct responses | ‐ | |||||
| Superior occipital gyrus | L | 18 | 3.63 | −33 | −87 | 30 |
| Lingual gyrus | L | 23 | 4.23 | −18 | −78 | −6 |
| Lingual gyrus | R | 17 | 3.83 | 27 | −81 | −9 |
| OXT > PLC (social emoticon), correct responses | ||||||
| Medial frontal gyrus | R | 13 | 3.76 | 6 | 63 | 6 |
| Precuneus | L | 13 | 3.92 | −24 | −72 | 42 |
| OXT > PLC (social female), correct responses | ||||||
| Medial frontal gyrus | R | 16 | 3.81 | 3 | 60 | 3 |
| Precuneus | L | 87 | 4.88 | −6 | −45 | 30 |
| Precuneus | R | 104 | 4.79 | 3 | −33 | 45 |
MNI,; OXT, oxytocin; PLC, placebo.
P <= 0.0001, uncorrected, cluster size >= 10, coordinates correspond to MNI space.
Connectivity analysis
A functional connectivity analysis was performed for the response phase using a generalized psychophysiological interactions (PPI) approach [gPPI; https://www.nitrc.org/projects/gppi; McLaren et al., 2012]. Two different categories of seed regions were used, firstly those showing altered activation during in the whole brain analysis (left precuneus, left posterior cingulate gyrus, left thalamus, left putamen) and using a 6 mm radius sphere centered at the peak activation co‐ordinates, and secondly the predefined ROIs based on their involvement in learning and emotional processing (left and right hippocampus, amygdala, and parahippocampal gyrus) where structural ROIs were obtained using the WFU Pickatlas.
To test OXT's effect under different feedback types, three PPI contrasts were set (based on the response phase): (Social FemaleOXT > Social FemalePLC), (EmoticonOXT > EmoticonPLC), and (Non‐social LightOXT > Non‐social LightPLC). Although no functional connectivity changes were detected under social emoticon and non‐social light conditions, the OXT group showed increased connectivity between right amygdala and left caudate (MNI coordinates: −6,17,7, P(uncorrected) = 0.01, cluster size = 34, t = 2.97) and between right amygdala and left insula (MNI coordinates: −39/−13/−11, P(uncorrected) = 0.01, cluster size = 32, t = 3.42) only during the social (female face) feedback condition. Extracted parameter estimates are given in Figure 4.
Figure 4.
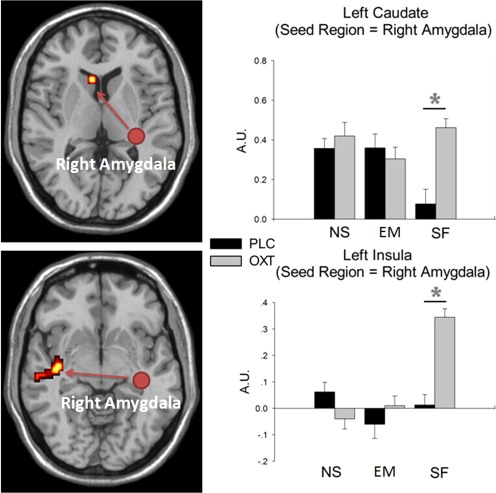
Top panel: Extracted parameter estimates from left caudate using right amygdala as the seed region. A main effect of treatment was shown: F(1,52) = 7.285, P = 0.009. An interaction between treatment and feedback type was also shown: F(2,104) = 7.374, P < 0.001. Bottom panel: Extracted parameter estimates from left insula using right amygdala as the seed region. A main effect of treatment was shown: F(1,52) = 15.081, P < 0.001. An interaction between treatment and feedback type was also shown: F(2,104) = 15.242, P < 0.001. EM, emoticon feedback; NS, non‐social light feedback; SF, social female feedback. *P < 0.05 OXT versus PLC.
Figure 5.
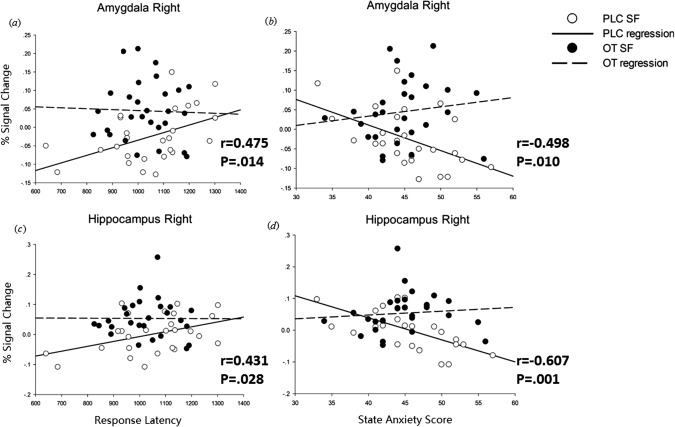
Under PLC, but not OXT, %blood oxygenation level‐dependent signal changes in amygdala and hippocampus regions of interest are positively correlated with response time in the social feedback condition in the RALT but negatively correlated with state anxiety scores.
Correlations between behavior and BOLD signal changes
Correlations (Pearson test) between percent BOLD signal changes and accuracy and response latency across the six presentation cycles of the RALT and with scores on neuropsychological questionnaires were calculated in the PLC and OXT groups in Experiment 3. This revealed no significant correlations within individual presentation cycles, after appropriate correction, and so instead we used mean values across all six presentation cycles. There were no significant correlations between ROI BOLD signal changes and response accuracy, however, there were a number with response latency. BOLD signal changes in the right amygdala, left hippocampus, parahippocampal gyrus and putamen were positively correlated with response times only in the PLC group and only in the social feedback condition. Furthermore, the BOLD signal changes in these same regions were all negatively correlated with state anxiety scores only in the PLC group and only in the female social feedback condition (right amygdala: PLC = −0.498, P = 0.0097; OXT = 0.064 P = 0.47; left hippocampus: PLC = −0.607, P = 0.001; OXT = 0.09, P = 0.64; left parahippocampal gyrus: PLC = −0.481, P = 0.013; OXT = 0.119, P = 0.55; left putamen: PLC = −0.411, P = 0.0371; OXT = −0.119, P = 0.55) (Fig. 5). Thus overall under PLC the BOLD signal changes in these ROIs are positively correlated with response time, but negatively correlated with state anxiety. However, these associations both become uncoupled under OXT.
DISCUSSION
Overall our behavioral results from three independent experiments have shown that, contrary to our previous findings in male Caucasian subjects [Hurlemann et al., 2010a], learning in the RALT task in male Chinese subjects is inferior with social compared with non‐social feedback. Nevertheless, in agreement with our previous study on Caucasian subjects OXT compared with PLC significantly and selectively enhanced learning with social feedback using emotional faces, particularly female ones, although interestingly not using simple facial emoticons. An fMRI analysis carried out in the final experiment revealed that with social feedback OXT facilitated activation during the response component of the RALT. Activity changes were found primarily in hippocampus, parahippocampal gyrus, amygdala, thalamus and putamen, as well as increased functional connectivity between the amygdala and the insula and caudate. Thus OXT may selectively facilitate learning with social feedback via an action on limbic regions associated with emotional modulation of memory and their connectivity with regions controlling salience and reward.
All the experiments in the current study demonstrated that, in contrast to Caucasian (German) subjects [Hurlemann et al., 2010a; Mihov et al., 2010] under control conditions learning with social feedback provided by happy and angry male or female faces is inferior in Chinese subjects to that following both non‐social (red and green lights or ticks and crosses) or emoticon based (smiling and angry face emoticons) feedback. We confirmed that this difference in Chinese subjects was not due to the Chinese face stimuli used since in an initial study we found that learning performance with the face feedback used was identical to that with the same male and female Caucasian faces used in the original studies [Hurlemann et al 2010a; Mihov et al., 2010]. The cultural difference could also not be explained by difficulty in discriminating emotional faces since all the subjects were able to discriminate accurately between happy, angry, and neutral expression faces. A further possibility might be that Chinese subjects found real faces more complex than the non‐social stimuli. This motivated us to use simple smiling and angry emoticons in Experiment 3. However, while learning performance was enhanced using these emoticon stimuli it still was not significantly better than non‐social stimuli. Furthermore, the fact that OXT did not have any facilitatory effect on learning with emoticon feedback suggests that they may be considered more as non‐social than social stimuli. Indeed, a study has reported that emoticons convey emotions without cognition of faces [Yuasa et al., 2006]. Also, while autistic patients are impaired on processing emotion using real faces they show no such impairment with emoticons [Grelotti et al., 2005; Rosset et al., 2008].
How then might we explain this apparent cultural difference in social facilitation of learning using emotional faces? One possible explanation may reside in differential experiences in educational learning environments, with Chinese students being more familiar with simple non‐social feedback on performance than with overt socio‐emotional feedback from teachers. Indeed, a number of subjects reported in post‐experiment interviews that they found the social feedback condition more challenging and also unusual in this learning context. However, a second related possibility is that collectivist cultures, such as Chinese, promote social harmony as being of great importance in every aspect of life. As such, anger in all its various expressions is generally avoided and shunned in collectivist compared with independent cultures [Han et al., 2013; Kövecses, 2000]. A recent study has for example shown that in negotiation contexts Asian subjects, in complete contrast to Caucasian ones, do not offer large concessions in response to anger from the person they are negotiating with when they think the expression of anger is inappropriate [Adam et al., 2010]. Therefore emotional stimuli conveying anger may not normally be as potent in promoting feedback learning in Chinese subjects. Another study has reported differences between Chinese and German subjects during empathic responses to anger which suggest greater emotional control in the Chinese subjects [de Greck et al., 2012]. Thus, the extent to which social facilitation of learning occurs in different cultures using emotional faces may be strongly dependent upon educational experience and whether anger is used in the context of feedback. Clearly further experiments are required to allow us to identify the precise factors which are influencing cultural differences in social facilitation of learning in the kinds of general feedback learning contexts used in the current study.
In both the between subjects design experiments conducted on Caucasian [Hurlemann et al., 2010a] and Chinese subjects OXT was found to facilitate learning performance compared to PLC treatment only with social feedback. However, while there was no overall significant face gender effect in Chinese subjects our results showed that the female face feedback exhibited more robust effects of OXT than male ones. Since this might possibly reflect a further cultural difference we reanalyzed the data from the original Hurlemann et al 2010a study where findings from male and female face feedback were presented combined. While, as in the current study, there was no overall significant effect of face gender it was also clear from this reanalysis that performance under OXT was best when subjects received female rather than male face feedback (see Supporting Information and Fig. S3 for details). Thus OXT may promote learning best in both Caucasian and Chinese men when the emotional feedback faces used are female. This could reflect previous observations that OXT treatment makes men rate female faces as more attractive [Striepens et al., 2014b; Theodoridou et al., 2009], although we did not observe any significant changes in valence ratings under OXT.
Previous studies in Caucasian subjects have reported that OXT can decrease aversion to angry faces [Evans et al., 2010] which suggests that it may have increased the salience of angry faces, thereby promoting learning in Chinese subjects by rendering them less aversive. Similarly, OXT may also have increased the salience of happy faces in this respect. While, a recent paper on Caucasian subjects has reported that OXT can decrease social reward learning using happy faces [Clark‐Elford et al., 2013], in their paradigm it was face identity rather than emotion that guided learning and so OXT may have increased the salience of happy emotional expressions which then distracted subjects from discriminating face identity.
A whole brain fMRI analysis revealed an overall main effect of the response phase in the RALT on a number of parietal (left inferior parietal gyrus) and temporal (left and right fusiform gyri and right superior temporal gyrus) cortical regions involved in attention and memory function as well in the reward system (left caudate) and in the visual cortex. At the whole brain level there was a main effect of OXT in the right and left fusiform gyrus and the right middle occipital gyrus, superior temporal gyrus, and hippocampus suggesting some general effects of the neuropeptide on many of the same regions engaged during the response phase of the RALT. We have shown previously that the NMDA receptor agonist, cycloserine, facilitates hippocampal activation and learning with non‐social feedback in the RALT [Onur et al., 2010] and so possibly there may be some interaction between OXT and glutamate signaling. However, since there were no consistent overall behavioral effects of OXT across all of the three feedback conditions it is unclear what the functional consequences of changes in activity in these regions might be.
Specific contrasts investigating regions where OXT effects were significantly greater during the social feedback condition revealed enhanced activation in right hippocampus, parahippocampal gyrus, amygdala and left putamen and thalamus. These regions comprise a network commonly associated with learning and reward [LaBar and Cabeza, 2006; Li et al., 2012; Phelps 2004], incentive anticipation [Cho et al., 2013] and also prediction error for rewards and punishments [Metereau and Dreher, 2013]. Both the dorsal and the ventral striatum have been shown to exhibit equivalent increased activation when subjects have the prospect of earning positive feedback, either in terms of a smiling face or money [Spreckelmeyer et al., 2009]. Therefore, OXT may be particularly facilitating learning by enhancing anticipatory aspects of social reward.
The BOLD signal changes in the right amygdala and hippocampus and left putamen and thalamus were also positively associated with response time and negatively associated with state anxiety scores only in the social feedback condition in the PLC group. These correlations were however absent in the OXT group. Therefore, the OXT treatment effectively uncoupled the association between the BOLD response and response time and state anxiety in the social feedback condition and made it equivalent to the other two feedback conditions where there was no such association in either PLC or OXT groups. This suggests that under PLC more anxious subjects may have found the social feedback stimuli more anxiety provoking and less rewarding, resulting in reduced BOLD signal changes in amygdala, hippocampus, putamen and thalamus and reduced response times, although not in increased errors. Under OXT on the other hand there is increased activity in these regions due to anticipated social feedback stimuli becoming both more salient and rewarding which effectively negates the effects of anxiety. There is evidence that OXT can produce anxiolytic effects in some contexts [Striepens et al., 2011], although in others it appears to have the opposite effect [Striepens et al., 2012].
Functional connectivity was increased between the right amygdala and both the left caudate and left insula in the OXT group during the social feedback condition. This suggests that OXT may have enhanced both the anticipation of reward (amygdala – caudate) and salience (amygdala‐insula) of the face feedback stimuli to promote learning [Gasquoine, 2014; LaBar and Cabeza, 2006]. The strength of functional connectivity between the amygdala and striatum has previously been shown to be associated with learning during feedback‐guided decision making [Cohen et al., 2008]. The effect of OXT on increasing functional connectivity between the amygdala and insula during the social feedback condition was the only situation where it produced a change which was markedly different from that observed in either the PLC or OXT groups in the two other feedback conditions. This suggests a potential unique contribution of amygdala‐insula interactions in relation to learning with social feedback. Interestingly, this finding is in agreement with our previous study showing that OXT strengthened functional connectivity between the right amygdala and left insula during successful encoding of neutral emotional stimuli but weakened it for negative emotional stimuli [Striepens et al., 2012]. Although resting‐state functional connectivity between the left amygdala and insula has been shown to be linked to state anxiety [Baur et al., 2013] in the current task‐dependent study, no associations were found with either PANAS or state or trait anxiety scores.
Since the salience/reward network impacted by OXT is involved in learning motivated by both social and non‐social cues, and indeed there were some main effects of OXT independent of feedback type, this raises the question as to why it primarily influences learning, neural activity and functional connectivity changes in the social feedback condition. One possibility is that OXT shows a selective facilitation in the social feedback condition in the current experiment because under PLC treatment it is the least effective form of feedback for promoting learning and associated neural changes and may therefore be easier to improve. However, if this was the case then one might have expected OXT to have similar strong facilitatory effects on learning with social feedback using male faces as with female ones, since the former were also less effective than non‐social feedback. Another possibility is that it is the amygdala which is playing a key role in OXT's selective effect in relation to social feedback. Although there is substantial overlap between neural substrates engaged during social and monetary reward learning [Lin et al., 2012], anticipation of social, as opposed to monetary rewards particularly engages the amygdala and also insula [Rademacher et al., 2010]. We have shown previously that the facilitation of learning with social feedback in the RALT task in Caucasian subjects is absent in patients with bilateral calcification lesions of the amygdala [Hurlemann et al., 2010a] and also in healthy subjects treated with the ß‐noradrenergic antagonist, propranolol [Mihov et al., 2010]. In our current study we found increased activation in the amygdala and hippocampus across treatment groups for the contrasts social>non‐social feedback and social>emoticon feedback suggesting that both regions are more strongly activated during social than non‐social feedback conditions. It is also clear that OXT effects related to facilitation of learning with social feedback particularly involve the amygdala and its functional connections with the insula and striatum and so it is possible that this is how OXT achieves specificity. Both animal and human‐based research has emphasized the amygdala as a key target for OXT's functional effects [Meyer‐Lindenberg et al., 2011; Striepens et al., 2011].
Importantly, we did not find any significant effects of OXT on activity during the feedback phase of the RALT in any of the same ROIs exhibiting changes during the response phase. Thus although OXT has been shown to influence amygdala, insula and fusiform and superior temporal gyri responses to emotional faces [Domes et al., 2007, 2010; Gamer et al., 2010; Kirsch et al., 2005] there was no evidence for this, at least in the context of the neutral expression face switching to a happy expression one in the current experiment. We also found no evidence for an OXT effect on the likeability of any of the social or non‐social feedback stimuli used in Experiment 3 or on that for neutral, happy or angry expression faces of unfamiliar females or males. Thus it seems unlikely that the behavioral and neural effects of OXT associated with the facilitation of learning during social feedback are due to it influencing the likability of neutral and happy faces or dislike of angry ones used during feedback. Instead, this finding lends further support to the view that in the context of the RALT OXT may be selectively facilitating learning in association with social feedback by increasing their salience and anticipation of their associated reward/punishment as feedback in the RALT, but not their valence per se. As discussed above in relation to Chinese subjects this might have particular relevance to the angry face stimuli where an increase in salience could render them more effective in being a positive influence on learning behavior by reducing their perceived inappropriateness.
In summary we have demonstrated a potential cultural difference in the impact of social as opposed to non‐social feedback on general learning with Chinese subjects showing impaired performance compared to an improvement in Caucasian subjects. However, similar to findings in Caucasian subjects, OXT selectively facilitates of learning with social feedback in Chinese subjects and this is associated with enhanced responses in both salience and reward networks in the brain and strengthened functional connections between the amygdala and the striatum and insula. This provides support for the potential use of OXT in therapeutic contexts involving social interactions between patient and therapist such as cognitive behavioral therapy where the patient is encouraged to learn appropriate strategies to help cope with their symptoms.
Supporting information
Supporting Information
Supporting Information
Author Contributions: J.H. and K.M.K designed the experiments; J.H., L.L. and S.G. conducted the experiments; J.H., S.Q., B.B and K.M.K. analyzed the data; J.H., S.Q., B.B, L.L., S.G, Q.G., R.H. and K.M.K wrote the paper.
REFERENCES
- Adam H, Shirako A, Maddux WW (2010): Cultural variance in the interpersonal effects of anger in negotiations. Psychol Sci 21:882–889. [DOI] [PubMed] [Google Scholar]
- Allport FH (1920): The influence of the group upon association and thought. J Exp Psychol 3:159–182. [Google Scholar]
- Baur V, Hänggi J, Langer N, Jäncke L (2013): Resting‐state functional and structural connectivity within an insula‐amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92. [DOI] [PubMed] [Google Scholar]
- Becker B, Klein EM, Striepens N, Mihov Y, Schlaepfer TE, Reul J, Goossens L, Schruers K, Kendrick KM, Hurlemann R (2013): Nicotinic acetylcholine receptors contribute to learning‐induced metaplasticity in the hippocampus. J Cog Neurosci 25: 986–997. doi: 10.1162/jocn_a_00383. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, Mcgregor G P, Bickel U, Fehm H (2002): Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5: 514–516. doi: 10.1038/nn0602-849. [DOI] [PubMed] [Google Scholar]
- Bruins J, Hijman R, Van Ree JM (1992): Effect of a single dose of desglycinamide‐[Arg8] vasopressin or oxytocin on cognitive processes in young healthy subjects. Peptides 13: 461–468. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Becket Ebitz R, Watson KK, Platt ML (2012): Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus monkeys (Macaca mulatta). Proc Natl Acad Sci USA 109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M (2013): Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage 66:508–521. doi: 10.1016/j.neuroimage2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark‐Elford R, Nathan PJ, Auyeung B, Voon V, Sule A, Müller, Dudas R, Sahakian BJ, Luan Phan K, Baron‐Cohen S (2013): The effects of oxytocin on social reward learning in humans. Int J Neuropsychopharmacol 17:199–209. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. (2008): Amygdala tractography predicts functional connectivity and learning during feedback‐guided decision making. Neuroimage 39:1396–1407. [DOI] [PubMed] [Google Scholar]
- Dashiell JF (1930): An experimental analysis of some group effects. J Abnorm Soc Psych 25:190–199. [Google Scholar]
- de Greck M, Shi ZH, Wang G, Zuo XY, Yang XD, Wang XY, Northoff G, Han SH (2012): Culture modulates brain activity during empathy with anger. NeuroImage 59:2871–2882. doi:10.1016/j.neuroimage.2011.09.052. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007): Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 62:1187–1190. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossman A, Hauenstein K, Heinrichs M, Herpertz SC (2010): Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35:83–93. [DOI] [PubMed] [Google Scholar]
- Evans S, Shergill SS, Averbeck BB (2010): Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacology 35:2502–2509. doi:10.1038/npp.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Delgado MR (2014): The importance of social rewards and social networks in the human brain. Neuroscientist. doi: 10.1177/10738. [DOI] [PubMed] [Google Scholar]
- Fehm‐Wolfsdorf G, Born J, Voigt KH, Fehm HL (1984): Human memory and neurohypophyseal hormones: opposite effects of vasopressin and oxytocin. Psychoneuroendocrinology 9:285–292. [DOI] [PubMed] [Google Scholar]
- Ferrier BM, Kennett DJ, Devlin MC (1980): Influence of oxytocin on human memory processes. Life Sci 27:2311–2317. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C (2010): Different amygdala subregions mediate valence‐related attentional effects of oxytocin in humans. Proc Natl Acad Sci USA 107: 9400–9405. doi/ 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasquoine PG (2014): Contributions of the insula to cognition and emotion. Neuropsych Rev 24: 77–87. [DOI] [PubMed] [Google Scholar]
- Gates GS, Rissland LQ (1923): The effect of encouragement and discouragement upon performance. J EducPsychol 14: 21–26. [Google Scholar]
- Genovese CR., Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Grelotti D, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, Volkmar FR, Schultz RT (2005): fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia 43:373–385. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F (2008): Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry 64:256–258. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G, Vogeley K, Wexler BE, Kitayama S, Varnum MEW (2013): A cultural neuroscience approach to the biosocial nature of the human brain. Annu Rev Psychol 64:335–359. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH (2004): Selective amnesic effects of oxytocin on human memory. Physiol Behav 83:31–38. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuiness MM, Otis M, Woods EA, Disinger HM, Chane HK, Chene TF, Banatia RB (2013): Recommendations for the standardization of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38:612–625. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM (2010a): Oxytocin enhances amygdala‐dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30:4999–5007. doi: 10.1523/jneurosci.5538-09-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, Schnell K, Musshoff F, Keysers C, Maier W, Kendrick KM, Onur OA (2010b): Human amygdala reactivity is diminished by the beta‐noradrenergic antagonist propranolol. Psychol Med 27:1–10. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. (2005): Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25:11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kövecses Z (2000): The concept of anger: universal or culture specific? Psychopathology 33:159–170. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R (2006): Cognitive neuroscience of emotional memory. Nature Neurosci Rev 7:54–64. [DOI] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND (2012): Differential roles of striatum and amygdala in associative learning. Nature Neurosci 14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. (2012): Social and monetary reward learning engage overlapping neural substrates. Soc Cog Affect Neurosci 7:274–281. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XL, Luo LZ, Geng YY, Zhao WH, Zhang Q, Kendrick KM (2014): Oxytocin increases liking for a country's people and national flag but not for other cultural symbols or consumer products. Front Behav Neurosci 8:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison of standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metereau E, Dreher J‐C (2013): Cerebral correlates of salient prediction error for different rewards and punishments. Cereb Cortex 23:477–487. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011): Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–538. [DOI] [PubMed] [Google Scholar]
- Mihov Y, Mayer S, Musshoff F, Maier W, Kendrick KM, Hurlemann R (2010): Socially reinforced learning in humans is beta‐noradrenergic‐dependent. Neuropsychologia 48:3168–3172. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP (2004): Reward representations and reward‐related learning in the human brain: insights from neuroimaging. Curr Op Neurobiol 14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A, Otte D‐M, Shah NJ, Maier W, Kendrick KM, Fink GR, Hurlemann R (2010): The NMDA receptor c‐agonist D‐cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry 67:1205–1211. [DOI] [PubMed] [Google Scholar]
- Phelps EA (2004): Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Op Neurobiol 14:198–202. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN (2010): Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49:3276–3285. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P (2009): Oxytocin makes a face in memory familiar. J Neurosci 29:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset DB, Rondan C, Da Fonseca D, Santos A, Assouline B., Deruelle C (2008): Typical emotion processing for cartoon but not real faces in children with autism spectrum disorders. J Autism Dev Disord 38:919–925. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H (2008): Post learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 33:368–374. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2006): Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57:87–115. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Gründer G (2009): Anticipation of monetary and social reward differently activates mesolimbic brain structure in men and women. Soc Cog Affect Neurosci 4:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R (2011): Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Striepens N, Scheele D, Kendrick KM, Becker B, Mihov Y, Schaefer L, Schwalba K, Reul J, Maier W, Hurlemann R (2012): Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA 109:16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Hurlemann R (2014): Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports 3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B, Lang M, Coenen HH, Maier W, Hurlemann R, Bauer A (2014b): Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology 39:74–87. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton‐Voak IS, Rogers PJ (2009): Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav 56:128–132. [DOI] [PubMed] [Google Scholar]
- Yao S, Zhao W, Cheng R, Geng Y, Luo L, Kendrick, KM (2014): Oxytocin makes females, but not males, less forgiving following betrayal of trust. Int J Neuropsychopharmacol 17:1785–1792. [DOI] [PubMed] [Google Scholar]
- Yuasa M, Saito K, Mukawa N (2006): Emoticons convey emotions without cognition of faces: an fMRI study. Proc CHI EA 6:1565–1570. doi: 10.1145/125451.1125737. [DOI] [Google Scholar]
- Zajonc RB (1965): Social facilitation. Science 149:269–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


