Abstract
Sensory preconditioning (SPC; also known as behaviorally silent learning) consists of a combination of two neutral stimuli, none of which elicits an unconditional response. After one of them is later paired with an unconditional stimulus (US), the other neutral stimulus also yields a conditional response although it has never been paired with the US. In this study, an event‐related functional magnetic resonance imaging (fMRI) paradigm was used to specify brain regions involved in SPC. The results demonstrated that SPC was associated with significant changes in activity of several regions, notably, the left amygdala, the left hippocampus, the bilateral thalamus, the bilateral medial globus pallidus, the bilateral cerebellum, the bilateral premotor cortex, and the bilateral middle frontal gyrus. This is a first effort to use fMRI to examine the effects of SPC on brain activation. Our data suggest that there is a distributed network of structures involved in SPC including both cortical and subcortical regions, therefore add to our understanding of the neural mechanisms underlying the ability to associative learning. Hum Brain Mapp 35:1297–1304, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: sensory preconditioning, associative learning, fMRI, hippocampus, amygdala, thalamus, cerebellum, BA 10
INTRODUCTION
Pavlovian classical conditioning, the most basic form of learning, has been widely investigated for over 80 years. In 1939, Brodgen reported a novel experimental paradigm which is called sensory preconditioning (SPC) to demonstrate that a new association between events in the environment can be obtained even when the organism makes no overt behavioral and motivational response [Brodgen, 1939]. The classical procedure for SPC entails three phases: (1) subjects receive paired presentation of two neutral stimuli; (2) one of the pre‐exposed stimuli undergoes Pavlovian classical conditioning; (3) subjects are tested with the other stimulus. If the stimulus not trained in phase 2 is also capable of evoking the conditioned response, then a conclusion can be drawn about the successful formation of an association between the two neutral stimuli during the preconditioning phase 1.
SPC demonstrates a new evolutionary ability to record and save in memory biologically nonsignificant environmental regularities. This ability to perceive “the objective world,” i.e., the objectively existing associations of events regardless of their relationship to an animal's own needs and desires, may be speculated as a cornerstone of higher forms of mind.
After the early demonstrations in dogs [Brodgen, 1939], evidence that organisms can learn predictive associations between initially neutral stimuli (presented simultaneously or sequentially or even in a backward manner) has been found in many mammalian species, including human infants [Barr et al., 2003; Boller, 1997; Seidel, 1959; Ward‐Robinson and Hall, 1996; Ward‐Robinson et al., 1998; Wimmer and Shohamy, 2011]. The possibility of preconditioning in other species, particularly insects, has also been reported [Muller et al., 2000]. The neural mechanisms involved in SPC are not sufficiently clarified. Several studies stressed the essential role of the hippocampal system, given the importance of the hippocampus in processing behaviorally silent associative memory. The idea that the hippocampal system is the critical brain region for successful SPC was supported in animal lesion experiments indicating that lesions in the perirhinal cortex [Nicholson and Freeman, 2000], the hippocampus [Port et al., 1987; Talk et al., 2002] and the fimbrial [Port and Patterson, 1984] all can disrupt the establishment of SPC. However, results of other studies lead to the opposite view. Thus, the disruptive effect of hippocampal lesion on SPC was not replicated in a later experiment [Ward‐Robinson et al., 2001]. Further, preconditioning with very short interstimulus intervals is possible even in very young rats whose hippocampus is not yet ripe to perform its role [Chen et al., 1991; Cheslock et al., 2003]. Regardless of the hippocampus controversy, the data indicate that brain areas other than sensory structures may be necessary for the learning process in question. When we nevertheless use the term “sensory” preconditioning, we simply follow tradition but not claim that sensory processes are crucial for SPC.
Most of the findings cited above were obtained using neurotoxic lesion approach in prespecified regions, and thus, it is unclear whether other brain areas are also involved in SPC. Moreover, SPC is usually considered as a kind of “behaviorally silent learning” [Brodgen, 1939; Seidel, 1959] and thus a kind of learning in which neurophysiological measures can deliver additional information as compared with behavioral observation. In the present study, therefore, we attempted to characterize the neural response associated with the process of SPC. To this end, we performed a functional magnetic resonance imaging (fMRI) study composed of three stages. In the first stage, two neutral stimuli were presented in pairs (tone 1, light), whereas the third neutral stimulus (tone 2) was not combined with any other stimulus. As no meaningful, unconditional stimulus (US) was presented, no response was usually recorded. In the second stage, the light was paired with a nociceptive US, and thus a typical Pavlovian conditioning took place, i.e., the light became a conditional stimulus. In the third and final stage, only tone 1 and tone 2 were presented. We hypothesized that tone 1 indirectly associated with a nociceptive US (though never presented immediately with the latter) would result in stronger activations than tone 2. More specifically, the differential activation was expected in the hippocampal system, which has been suggested to play an important role in SPC, and in the brain, structures (e.g., amygdala) involved in aversive learning.
MATERIALS AND METHODS
Subjects
Sixteen subjects (aged 20–33 years, mean = 25.63, standard deviation (SD) = 4.41; 9 females) took part in the experiment. Participants were medical students at the University of Tuebingen who received class credit for their involvement. All subjects had normal or corrected‐to‐normal visual acuity. All reported no history of psychiatric or neurological disorders, and no current use of any psychoactive medications. The study was approved by the local Ethics Committees (University of Tuebingen) and informed consent was obtained from all participants.
Stimuli and Experimental Design
Phase 1
In the first phase, participants were presented with two neutral tones which served as the conditional stimuli (CS). CS1 (700 Hz, 75 dB, 1,000 ms) was immediately (i.e., 0 interval) followed by a 500‐ms yellow flash, while CS2 (1,400 Hz, 75 dB, 1,000 ms) was never paired with the flash. The compound (CS1 → flash) was presented 30 times as well as the CS2. The intertrial interval (ITI) was 4 s. The order of trials was pseudorandomized, and no more than two trials of the same sort were presented consecutively.
The first phase had the duration of 315 s and was intended to initiate the preconditioning learning of the CS1 → flash association.
Phase 2
In the next stage, subjects received a conditioning trial consisting of a 500‐ms presentation of flash followed by a 2‐ms electric shock (the unconditional stimulus (US) details in Apparatus below) after a trace period of 500 ms. The flash → US combination was delivered 15 times with an ITI of 4 s.
The second phase lasted ∼ 75 s and was a standard Pavlovian aversive conditioning procedure with flash as a conditional stimulus.
Phase 3
Following phase 2, the tones CS1 and CS2 were presented to the subjects 20 times each with an ITI of 10 s. To prevent possible extinction, five additional flash → US pairings were presented randomly in this phase. These combinations did not immediately follow any of the tones, but were separated from them with the same 10‐s interval. The preceding tone was either CS1 (two times) or CS2 (three times).
The third phase had duration of 440 s and was intended to test the associative link formed between CS1 and US.
The three phases followed each other without breaks.
Apparatus
Subjects heard auditory stimuli via MRI‐compatible headphones with efficient gradient noise suppression (up to 45 dB) and a filter system with more than 90‐dB radio frequency suppression (MR Confon System, Leibniz‐Institute for Neurobiology at Magdeburg, Germany). The two tones were computer generated and selected on the basis that they differed from each other in fundamental frequency but were perceived as neutral and evoked no emotional responses. To test for this, 10 healthy subjects (who did not participate in the fMRI experiment) scored their own emotions using the Self‐Assessment Mannequin [SAM; Bradley and Lang, 1994]. The method requested each participant to indicate their emotional reaction on a 9‐point scale that is represented as nine different images on a dimension of affective valence (from 1 = very pleasant to 9 = very unpleasant) as well as on a dimension of arousal (from 1 = very calm to 9 = very arousing). There was no significant difference in the emotional responses to the two tones (valence: t = −0.56, P = 0.59; arousal: t = 1.40, P = 0.19). Furthermore, an additional fMRI experiment was performed in another group of 10 healthy volunteers to determine whether there are inherent differences in the hemodynamic response to the passive listening of the two tones. The direct statistical comparisons between the two tones (CS1 – CS2, and vice versa) did not result in any significant cluster.
Visual stimulus (i.e., yellow flash) was rear projected onto the screen by an liquid crystal display video projector and delivered through a 45°‐angled mirror. During the ITI, the screen was dark.
The US was an electrical stimulus applied on the left index finger by a finger electrode (Schuler Medizintechnik, Freiburg, Germany), delivered by an electrical stimulus generator (Digitimer, DS7A, UK). The level of shock was set by the participants via a work up procedure that ensured the shocks were “painful,” but not harmful. Within this procedure, participants were first given a mild shock (2 ms, 5 mA) and gradually increased until the participant indicated it as “distinctly painful” (level ranged from 6 to 18 mA, mean = 10.56, SD = 3.03). The task sequence in an event‐related mode was controlled by a PC running “Presentations” software (Neurobehavioral Systems, Albany, CA).
After the scanning sessions, participants were asked to rate the emotional valence and arousal of the CS1, CS2, flash, and the US using the SAM procedure described above.
Data acquisition and analyses
Participants were scanned in a 3‐T Siemens Trio scanner (Siemens, Erlangen, Germany). High‐resolution anatomical images were acquired using a T 1‐weighted Magnetization Prepared Rapid Gradient Echo sequence with the following scanning parameters: repetition time = 2,300 ms, echo time = 2.98 ms, 160 slices, slice thickness = 1 mm, voxel size 1.0 × 1.0 × 1.1 mm. The functional volume acquisitions utilized a T 2*‐weighted gradient‐echo pulse sequence (repetition time = 2,380 ms, echo time = 25 ms, field of view = 210 mm, flip angle = 90°, 64 × 64 matrix, 40 slices covering the whole brain, slice thickness 3 mm, no gap, voxel size 3.3 × 3.3 × 3.0 mm).
Image processing was carried out using SPM8 software package (Wellcome Department of Cognitive Neurology, London, England, UK) running under a Matlab R2010a environment (Mathworks, Sherborn, MA). Preprocessing included slice timing, realignment, coregistration of the high‐resolution scans with the functional images, segmentation into gray and white matter, normalization, and spatial smoothing with an 8‐mm full‐width half‐maximum isotropic Gaussian kernel. A first‐level fixed effects analysis was computed subjectwise using the general linear model in SPM. Event‐related brain responses were modeled as delta functions and time‐locked to the onset of the presentation of CS1 and CS2. Regressors of interest modeling the different event types were set up, and then convolved with a canonical hemodynamic response function and their temporal and dispersion derivatives. The contrast used in the main analysis tested for greater responses evoked by CS1 stimuli relative to CS2. First‐level contrasts were submitted to a second‐level random‐effect analysis. Main effects were computed using one‐sample t‐tests, including all subjects for the contrast of interest (CS1 versus CS2, and vice versa). The analysis was performed covering the whole brain. All the statistical maps were thresholded at P < 0.001 uncorrected to identify differential activations between conditions, and only multiple comparison‐corrected P values less than 0.05 on cluster level were considered significant.
RESULTS
Behavioral Results
The average valence scores (1 = very pleasant, 5 = neutral, 9 = very unpleasant) as rated by the participants after fMRI data acquisition were 6.63 (SD = 0.72) for CS1, 4.62 (SD = 0.96) for CS2, 5.75 (SD = 0.68) for flash, and 7.81 (SD = 0.91) for US. The mean values of arousal (1 = not aroused, 9 = highly aroused) were 3.50 (SD = 1.63) for CS1, 2.50 (SD = 1.83) for CS2, 4.69 (SD = 1.85) for flash, and 7.31 (SD = 1.54) for US. The critical contrasts between the responses to the two tones revealed that CS1 was rated as significantly more arousing (t = 3.04, P = 0.008) and less pleasant than CS2 (t = 10.95, P < 0.001). The difference in ratings between the different stimuli is summarized in Figure 1.
Figure 1.
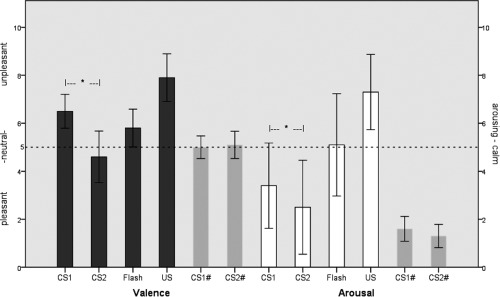
Subjective ratings of valence (black columns: 1 = very pleasant, 9 = very unpleasant) and arousal (white columns: 1 = not aroused, 9 = highly aroused) for different stimuli. Gray columns (marked with the number sign) indicate ratings of the tones by individuals who did not participate in the preconditioning experiment. The asterisks indicate significant differences between CS1 and CS2.
fMRI Results
To examine differences in neural circuitry underlying preconditioning with two neutral stimuli, we compared responses evoked by CS1 and CS2 during phase 3. This comparison revealed differential activation of the left amygdala, the left hippocampus, the bilateral thalamus, and the medial globus pallidus. Further differential responses were detected at the cortical level bilaterally in the premotor cortex and in the Brodmann area 10. We also observed bilateral cerebellar activation. The areas are listed in Table 1 according to anatomical regions, Montreal Neurological Institute coordinates, cluster sizes, Z‐score, and significance levels of activations. Figure 2 shows the average differential activations across all participants, projected on a standard anatomical template.
Table 1.
Regions with a stronger activation to CS1 than CS2 during the third phase of the experiment
| Regions | L/R | p | Cluster size (voxels) | Peak in MNI | Z‐score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Amygdala | L | 0.003 | 120 | −27 | −7 | −17 | 3.95 |
| Hippocampus | L | −35 | −10 | −20 | 3.76 | ||
| Thalamus | L | 0.005 | 111 | −12 | −4 | 7 | 5.50 |
| Medial globus pallidus | L | −12 | 2 | −5 | 3.99 | ||
| Thalamus | R | 0.01 | 94 | 12 | −4 | 7 | 4.70 |
| Medial globus pallidus | R | 12 | 2 | −2 | 4.06 | ||
| Middle frontal gyrus (BA 10) | L | 0.016 | 85 | −30 | 59 | −5 | 4.23 |
| Middle frontal gyrus (BA 10) | R | 0.013 | 89 | 33 | 53 | 25 | 4.19 |
| Premotor cortex (BA 6) | L+R | <0.001 | 292 | 6 | 26 | 61 | 4.24 |
| Cerebellum | L+R | <0.001 | 977 | 9 | −55 | −20 | 5.20 |
Clusters identified with a threshold of P < 0.05. Familywise error corrected for multiple comparisons. MNI, Montreal Neurological Institute coordinates. BA, Brodmann area.
Figure 2.
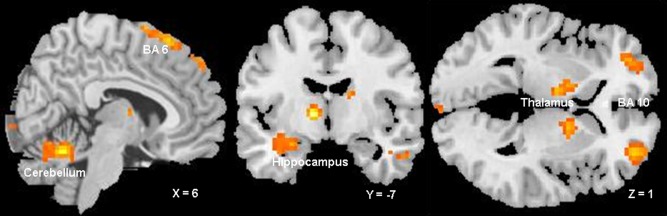
Significant clusters from the random‐effects contrast of CS1 versus CS2. The statistical threshold used was an uncorrected P value of 0.001 for illustrative purposes. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The reverse comparison (CS2 − CS1) produced no differential activity at the standard threshold of P < 0.05 familywise error corrected.
DISCUSSION
Behavior
The goal of this study was to investigate the neural substrate underlying SPC. To this end, subjects were presented with pairings of “neutral” sensory stimuli (tone 700 Hz → flash in phase 1) and then received a revaluation procedure in which presentations of flash were followed by an aversive US (flash → electric shock in phase 2). Using an fMRI paradigm, we were able to demonstrate behavioral and neural responses that are in good agreement with both empirical results and theoretical perspectives concerning the neural response associated with the process of preconditioning. Our participants were passively exposed to the stimuli without any explicit task and without being aware of the aim of the study. After the scanning sessions, all of them reported that they anticipated the US after CS1, although CS1 was never presented in temporal connection with a pain stimulus. This pain anticipation was not reported for CS2. This indicates that subjects formed an indirect association between CS1 and the US, supposedly mediated by the flash stimulus.
Moreover, affective rating revealed that CS1, which had been indirectly linked to pain, was perceived as significantly less pleasant and more arousing than a very similar tone CS2. Note that this rating was also performed by a different group of volunteers who did not participate in the preconditioning experiment, and no difference between CS1 and CS2 in terms of emotional valence or arousal was obtained. This indicates that our experimental design was effective in generating SPC effect.
Brain Imaging Data
Compared with CS2, CS1 elicited differential activation in the left hippocampus during the third phase of SPC. Additionally, differential brain activations were observed in the left amygdala, the bilateral Brodmann area 10, the bilateral premotor cortex, the bilateral thalamus, and the medial globus pallidus, and in the bilateral cerebellum. This difference in neural response between CS1 and CS2 was found in the context of similar behavioral performance (i.e., arousal/valence ratings). Hippocampus is hypothesized to be a key structure important for successful transfer of newly learned value to stimuli that have a pre‐established relational representation [Wimmer and Shohamy, 2011]. It is also assumed that context information is encoded by the hippocampus and converges with information about the US [Alvarez et al., 2008; Kalisch et al., 2006; Lang et al., 2009]. Further, the hippocampus is involved in trace conditioning paradigms in which a trace between the CS+ and the US have to be kept in memory to enable associative learning [Buechel et al., 1999]. Our findings of hippocampal activity in SPC are convergent with Port et al. 1987, who demonstrated that neurotoxic lesions of the hippocampus disrupt the ability of the rabbits for SPC, and with Nicholson and Freeman 2000, who showed that lesions of the perirhinal cortex prevent the establishment of SPC in rats. Likewise, Talk et al. 2002 found that the capacity of hippocampal‐lesioned rats to perform a SPC task is impaired. On the basis of these consistent effects of hippocampal damage on associative learning, the hippocampus has been proposed to play an essential role in SPC through its unique support to develop associations between sensory stimuli. Although an SPC paradigm offers possibilities to distinguish between encoding and retrieval processes (i.e., phases 1 and 3) compared with their equivocal relationship in traditional classical conditioning, surprisingly few studies of SPC in humans have examined the involvement of hippocampus in the different stages of encoding and retrieval. Our results can therefore be viewed to provide some evidence that the hippocampus is involved in retrieving associative information between sensory stimuli in SPC (i.e., phase 3). This is in line with a previous study showing that the hippocampus plays a critical role in detecting retrieval‐generated mismatches after sensory association is established [Honey et al., 1998].
Despite many years of study, a critical question of which other structures of the limbic system (e.g., thalamus and amygdala), additionally to the hippocampus, play important roles in SPC has not been definitively answered. A few animal studies using a SPC procedure have shown that rats with lesions of the basolateral amygdala can represent the sensory aspects of neutral events and thus the lesions have no effect on SPC [Blundell et al., 2003; Dwyer and Killcross, 2006], which is not congruent with our results. Also, the effects of selective lesions of the thalamus have been investigated in SPC procedures although they do not appear to prevent the basic Pavlovian conditioning. For example, rats with lesions of the anterior thalamic nuclei were first exposed to two stimuli compound (AX and BY) and then to a signaling relationship in which X was paired with an aversive US but Y was not, and this procedure resulted in more conditioned fear to A than B, which means that the thalamic lesions did not impair the preconditioning performance [Ward‐Robinson et al., 2002].
However, the differences in terms of experimental subjects, stimuli, and designs among different studies should be kept in mind. Specifically, the majority of studies on SPC investigated this issue in different species (animal versus human) using different stimuli (thermal or flavor versus auditory) and at the level of lesions, whereas our noninvasive imaging method allowed us to identify the intact neural network involved in SPC without the disconnection effects produced by the neurotoxic lesions [Blundell et al., 2003; Dwyer and Killcross, 2006; Ward‐Robinson et al., 2002]. We thus speculate that the coordinated processes in the limbic system play an important role in preconditioning procedures based on the formation of associations between the sensory aspects of neutral events. This interpretation is in good agreement with the theoretical assumption of an “extended hippocampal system” based on the extensive neural connections between the thalamus, amygdala, and hippocampus [Vann and Aggleton, 2004]. Further support for the role of neural connections in the extended hippocampal system comes from a lesion study that demonstrated the abolished effect of SPC in the rabbit eyeblink preparation due to the fimbrial lesion that disrupts the essential input and output pathway for the communications between subcortical structures and the hippocampus [Port and Patterson, 1984].
In addition to this extended hippocampal system, the cerebellum is also involved in SPC, in particular, in the retrieval of associative information. Already Berger et al. 1986 demonstrated some potential interactions between the hippocampal and cerebellar brain systems. Neurophysiological evidence suggests that the cerebellum and the hippocampus can be functionally connected during eyeblink conditioning [Hoffmann and Berry, 2009; Kirsch et al., 2003]. Another explanation may be that the cerebellum appears to have a role in processing anticipated sensory input and thus in preparation to impending pain [Moulton et al., 2010]. Our finding that all participants reported a general effect of pain anticipation elicited by CS1 in the test phase is consistent with this view. Moreover, such a finding is in good agreement with a previous fMRI study demonstrating that the expectation of pain activates the cerebellum [Ploghaus et al., 1999].
Interestingly, the CS1 versus CS2 contrast in our study revealed activations not only in subcortical structures but also in cortical regions, particularly in the Brodmann area (BA) 10. BA 10 is probably the largest cytoarchitectonic area of the human prefrontal cortex [Christoff et al., 2001]. However, little is known about its functions in complex aspects of human cognition. It is likely that BA 10 plays a role in aversive conditioning because of its efferent inputs to the ventral and dorsal striatum [Ikemoto, 2007] as well as its extensive bidirectional connections to the amygdala [Amaral and Insausti, 1992]. Another possible role of the BA 10 in SPC may be considered in light of the “metacognition” hypothesis. This hypothesis posits that BA10 is involved in the processing of one's own thoughts, or thinking in a very controlled, goal‐directed mode, or even reflecting the states of consciousness [Burgess et al., 2007; Johnson et al., 2002]. However, there is no universally accepted definition of metacognition and the explicit role of BA 10 in “metacontrol” processes remains unclear. Our study gives the first direct indication for a role of BA10 in SPC. Therefore, it appears that the functionality of BA 10 is not domain specific, but may rather be related to complex cognitive processes of memory retrieval or decision making.
Future Research
Being the first investigation of the neurophysiological basis of SPC, the present study contained several important limitations that should be overcome in future experiments. First, we only compared the hemodynamic responses to CS1 and CS2 during phase 3, thus only the result of preconditioning was studied. A different design is required in a next experiment in order to obtain meaningful brain data during phases 1 and 2 to investigate the encoding process of preconditioning. Second, we assessed the emotional responses using the SAM procedure only after the scanning sessions, and therefore cannot be sure whether the observed introspective self‐reports of emotional experience are specifically associated with SPC. In the following studies, autonomic measures (skin conductance and heart rate) would be useful, because these measures would deliver information about emotional arousal simultaneously with fMRI, and without requiring a volitional response from the subject. Third, all emotional responses (i.e., subjective, autonomic, and central neurophysiological responses) may be specific for the kind of US used (i.e., pain), and other US (e.g., affectively positive stimuli) might result in a different pattern of brain activity. Last but not least, since CS1 was followed by light in phase 1 but not in phase 3, one might suggest that the differences between CS1‐ and CS2‐responses were related to the orienting response to the “decomposition” of the CS1‐light complex. On the other hand, this view can hardly accommodate the fact of emotional responses to CS1, because it remains unclear why the disappearance of light should elicit negative emotions. However, this additional factor should be controlled in a future experiment, in which both CS1 and CS2 will be paired in phase 1 with two different visual stimuli, whereas in phase 2, only one of the visual stimuli will be paired with a US.
CONCLUSIONS
The results of the present experiment provide the first neuroimaging evidence for the neural responses associated with the process of SPC. Consistent with previous literature, we found hippocampal activity during the test phase. Furthermore, the present results suggest that there is a distributed network of structures involved in aversive SPC including both cortical and subcortical regions, therefore adding some evidence on the neural mechanisms implicated in our ability to learn associations.
REFERENCES
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C (2008): Contextual fear conditioning in humans: Cortical‐hippocampal and amygdala contributions. J Neurosci 28:6211–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Insausti R (1992): Retrograde transport of D‐[3H]‐aspartate injected into the monkey amygdaloid complex. Exp Brain Res 88:375–388. [DOI] [PubMed] [Google Scholar]
- Barr R, Marrott H, Rovee‐Collier C (2003): The role of sensory preconditioning in memory retrieval by preverbal infants. Learn Behav 31:111–123. [DOI] [PubMed] [Google Scholar]
- Berger TW, Weikart CL, Bassett JL, Orr WB (1986): Lesions of the retrosplenial cortex produce deficits in reversal learning of the rabbit nictitating membrane response: Implications for potential interactions between hippocampal and cerebellar brain systems. Behav Neurosci 100:802–809. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S (2003): Preserved sensitivity to outcome value after lesions of the basolateral amygdala. J Neurosci 23:7702–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller K (1997): Preexposure effects on infant learning and memory. Dev Psychobiol 31:93–105. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ (1994): Measuring emotion: The Self‐Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry 25:49–59. [DOI] [PubMed] [Google Scholar]
- Brogden WJ (1939): Sensory pre‐conditioning. J Exp Psychol:323–332. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ (1999): Amygdala‐hippocampal involvement in human aversive trace conditioning revealed through event‐related functional magnetic resonance imaging. J Neurosci 19:10869–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ (2007): The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci 11:290–298. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Lariviere NA, Heyser CJ, Spear LP, Spear NE (1991): Age‐related differences in sensory conditioning in rats. Dev Psychobiol 24:307–326. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, High JM, Spear NE (2003): Higher order conditioning in the newborn rat: Effects of temporal disparity imply infantile encoding of simultaneous events. Infancy 4:157–176. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD (2001): Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage 14:1136–1149. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Killcross S (2006): Lesions of the basolateral amygdala disrupt conditioning based on the retrieved representations of motivationally significant events. J Neurosci 26:8305–8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LC, Berry SD (2009): Cerebellar theta oscillations are synchronized during hippocampal theta‐contingent trace conditioning. Proc Natl Acad Sci USA 106:21371–21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RC, Watt A, Good M (1998): Hippocampal lesions disrupt an associative mismatch process. J Neurosci 18:2226–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S (2007): Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens‐olfactory tubercle complex. Brain Res Rev 56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP (2002): Neural correlates of self‐reflection. Brain 125(Pt 8):1808–1814. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ (2006): Context‐dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J. Neurosci. 26:9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Achenbach C, Kirsch M, Heinzmann M, Schienle A, Vaitl D (2003): Cerebellar and hippocampal activation during eyeblink conditioning depends on the experimental paradigm: A MEG study. Neural Plast 10:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H (2009): Context conditioning and extinction in humans: Differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci 29:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Schmahmann JD, Becerra L, Borsook D (2010): The cerebellum and pain: Passive integrator or active participator? Brain Res Rev 65:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Gerber B, Hellstern F, Hammer M, Menzel R (2000): Sensory preconditioning in honeybees. J Exp Biol 203(Pt 8):1351–1364. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH Jr (2000): Lesions of the perirhinal cortex impair sensory preconditioning in rats. Behav Brain Res 112:69–75. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN (1999): Dissociating pain from its anticipation in the human brain. Science 284:1979–1981. [DOI] [PubMed] [Google Scholar]
- Port RL, Beggs AL, Patterson MM (1987): Hippocampal substrate of sensory associations. Physiol Behav 39:643–647. [DOI] [PubMed] [Google Scholar]
- Port RL, Patterson MM (1984): Fimbrial lesions and sensory preconditioning. Behav Neurosci 98:584–589. [DOI] [PubMed] [Google Scholar]
- Seidel RJ (1959): A review of sensory preconditioning. Psychol Bull 56:58–73. [DOI] [PubMed] [Google Scholar]
- Talk AC, Gandhi CC, Matzel LD (2002): Hippocampal function during behaviorally silent associative learning: Dissociation of memory storage and expression. Hippocampus 12:648–656. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP (2004): The mammillary bodies: Two memory systems in one? Nat Rev Neurosci 5:35–44. [DOI] [PubMed] [Google Scholar]
- WardRobinson J, Hall G (1996): Backward sensory preconditioning. J Exp Psychol Anim B 22:395–404. [Google Scholar]
- Ward‐Robinson J, Symonds M, Hall G (1998): Context specificity of sensory preconditioning: Implications for processes of within‐event learning. Anim Learn Behav 26:225–232. [Google Scholar]
- Ward‐Robinson J, Coutureau E, Good M, Honey RC, Killcross AS, Oswald CJP (2001): Excitotoxic lesions of the hippocampus leave sensory preconditioning intact: Implications for models of hippocampal functioning. Behav Neurosci 115:1357–1362. [DOI] [PubMed] [Google Scholar]
- Ward‐Robinson J, Wilton LAK, Muir JL, Honey RC, Vann SD, Aggleton JP (2002): Sensory preconditioning in rats with lesions of the anterior thalamic nuclei: Evidence for intact nonspatial ‘relational’ processing. Behav Brain Res 133:125–133. [DOI] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D (2011): The striatum and beyond: Hippocampal contributions to decision making In: Delgado M, Phelps EA, Robbins TW, editors. In Attention & Performance. Oxford:Oxford University Press; pp281–309. [Google Scholar]


