Abstract
This study investigated the effects of attentional load on neural responses to attended and irrelevant visual stimuli by recording high‐density event‐related potentials (ERPs) from the scalp in normal adult subjects. Peripheral (upper and lower visual field) and central stimuli were presented in random order at a rapid rate while subjects responded to targets among the central stimuli. Color detection and color‐orientation conjunction search tasks were used as the low‐ and high‐load tasks, respectively. Behavioral results showed significant load effects on both accuracy and reaction time for target detections. ERP results revealed no significant load effect on the initial C1 component (60–100 ms) evoked by either central‐relevant or peripheral‐irrelevant stimuli. Source analysis with dipole modeling confirmed previous reports that the C1 includes the initial evoked response in primary visual cortex. Source analyses indicated that high attentional load enhanced the early (70–140 ms) neural response to central‐relevant stimuli in ventral‐lateral extrastriate cortex, whereas load effects on peripheral‐irrelevant stimulus processing started at 110 ms and were localized to more dorsal and anterior extrastriate cortical areas. These results provide evidence that the earliest stages of visual cortical processing are not modified by attentional load and show that attentional load affects the processing of task relevant and irrelevant stimuli in different ways. Hum Brain Mapp 35:3008–3024, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: attention, event‐related potential (ERP), visual, C1, task relevant, task irrelevant, endogenous, exogenous
INTRODUCTION
Studies in both animals and humans are giving an increasingly detailed picture of the sites along the visual pathways where afferent information is modulated by selective attention [for reviews, see Desimone and Duncan, 1995; Hillyard and Anllo‐Vento, 1998; Hopf et al., 2009]. It is still debated, however, whether attention modulates visual inputs at the earliest levels of cortical processing. A pair of recent studies using endogenous spatial attention paradigms have provided evidence that attention can enhance the amplitude of the initial visual evoked response in the primary visual cortex (V1) starting at around 50–60 ms after stimulus onset [Kelly et al., 2008; Poghosyan and Ioannides, 2008]. These findings run counter to a larger body of evidence, however, which supports the view that the initial visual cortical processing in V1 is not affected by spatial attention [Aine et al., 1995; Clark and Hillyard, 1996; Di Russo et al., 2003, 2012; Fu et al., 2005; Gratton, 1997; Heinze and Mangun, 1995; Hopfinger and West, 2006; Johannes et al., 1995; Mangun et al., 2001; Martinez et al., 1999, 2001; Noesselt et al., 2002; Wijers et al., 1997; Woldorff et al., 1997, 2002; Yoshor et al., 2007]. These latter studies have found instead that the earliest modulation of visual processing by spatial attention takes place in extrastriate visual cortex starting at around 70–80 ms. There is evidence for a delayed activation of area V1 (i.e., after 100 ms) during endogenous visual attention, however, which has been attributed to delayed feedback from higher extrastriate areas [Aine et al., 1995; Di Russo et al., 2003, 2012; Martinez et al., 1999, 2001; Noesselt et al., 2002].
In recent years, mechanisms of selective attention have also been studied in humans using perceptual/attentional load paradigms. Numerous behavioral studies have found that the processing of peripheral, task‐irrelevant stimuli decreases as the perceptual demands of a central task are increased [e.g., Dark et al., 1985; Kahneman and Chajczyk, 1983; Lavie, 1995; Miller, 1991; Plainis et al., 2001; Williams, 1985; Yantis and Johnston, 1990]. According to the perceptual load theory proposed by Lavie and colleagues [Lavie and Tsal, 1994; Lavie, 1995, 2005], the more difficult a perceptual task is, the more attention will be allocated to the task‐relevant stimuli, with fewer resources being available to process the task‐irrelevant stimuli. Although endogenous attention paradigms typically compare the visual processing of task‐relevant versus irrelevant stimuli or cued versus uncued stimuli, the perceptual/attentional load paradigm investigates the effects of selective attention on the processing of task‐irrelevant stimuli by comparing conditions where the relevant stimuli require low‐load versus high‐load perceptual processing. Several studies using fMRI have demonstrated strong influences of perceptual/attentional load on the processing of both relevant and irrelevant stimuli in extrastriate visual cortical areas, including V2, V3, V4, TEO, V3A, and MT+/V5 [O'Connor et al., 2002; Pinsk et al., 2004; Rees et al., 1997; Schwartz et al., 2005; Yi et al., 2004]. Hemodynamic responses to peripheral irrelevant stimuli in primary visual cortex (area V1) may also be modulated by attentional load [Bahrami et al., 2007; O'Connor et al., 2002; Schwartz et al., 2005], but it is not clear whether the reported V1 modulations represented an effect on early feed‐forward or delayed feedback processing.
Using the event‐related potential (ERP) technique with its high temporal resolution, Handy and colleagues [Handy et al., 2001] found that increasing the perceptual load of a central task led to a significant decrease in the amplitude of the visual evoked P1 component (latency 100–150 ms) to peripheral task‐irrelevant stimuli. In a pair of recent studies, Rauss and colleagues [Rauss et al., 2009, 2012] have further reported a modulation of the amplitude of the earliest visual evoked component (C1, at 60–100 ms) elicited by peripheral task‐irrelevant stimuli as a function of the attentional load of the central visual task. In one study [Rauss et al., 2009] the C1 component itself was localized over midline occipital cortex and inverted in polarity for upper versus lower field stimuli, which is consistent with previous findings that C1 represents the initial evoked response in area V1 [e.g., Di Russo et al., 2003; Jeffreys and Axford, 1972]. The topography of the load‐induced modulation of C1 was not reported, however, and a statistical comparison of the C1 source activity between high and low load conditions showed that the early load effect (60–100 ms) was significant in prefrontal regions rather than in occipital cortex [Fig. 5b in Rauss et al., 2009]. In a further study [Rauss et al., 2012] where the task‐relevant and irrelevant stimuli were presented simultaneously rather than successively, the C1 amplitude to the irrelevant peripheral stimuli was actually increased in the high load condition, which contrasted with their previous results. This increased C1 amplitude was accompanied by a substantial pre‐stimulus baseline shift, however, and source analysis found this early load effect to be only marginally significant in occipital cortex as well as in several other cortical areas [Figs. 3 and 4b in Rauss et al., 2012]. These results suggest that the reported early load effects on C1 amplitude may not actually represent a modulation of the occipitally generated C1 itself, but rather an overlap with other ERP components elicited in these tasks.
Given the central role that area V1 plays in models of visual processing [e.g., Lamme and Roelfsema, 2000; Li et al., 2006; Olshausen and Field, 2005], it is important to determine the critical conditions under which the initial cortical evoked response in V1 may be modulated by selective attention. The present study aimed to clarify whether the earliest visual ERP component C1 can in fact be modulated by perceptual/attentional load. As in the attentional load studies of Rauss et al. [2009, 2012] and the perceptual load studies of Lavie [2005], we manipulated load by varying the attentional demand for processing for the same central‐relevant stimuli. We presented peripheral irrelevant stimuli similar to those used by Rauss et al. [2009, 2012] and a central grid of lines with colored targets as the relevant stimuli, all in random order. Both types of stimuli elicited large C1 components. ERPs were averaged over a large number of trials to increase the signal/noise ratios, and overlapping potentials from preceding stimuli were removed using the Adjacent Response (ADJAR) Technique [Woldorff, 1993]. Moreover, unlike the previous studies, we examined the effects of high versus low attentional load on the C1 components elicited by both the task‐relevant and irrelevant stimuli.
MATERIALS AND METHODS
Subjects
Nineteen right‐handed healthy adults (11 women, ages 18–34 years, mean age = 22 years) participated in this experiment. All participants had normal or corrected‐to‐normal vision. Subjects were recruited as volunteers, and informed consent was obtained before the beginning of each experiment. All procedures were approved by the University of California San Diego Institutional Review Board.
Stimuli and Task
Both the central task‐relevant and peripheral irrelevant stimuli (Fig. 1) were presented as white or colored lines (average luminance= 30 cd/m2) on a uniform dark screen (0.4 cd/m2). The central relevant stimulus array (5.3° × 5.3°) was centered at fixation and consisted of a grid of twelve lines (six horizontal and six vertical), all but one of which were white. The one colored line could be either horizontal or vertical and was either red, green, yellow, blue, cyan or purple (i.e., R_H, G_H, Y_H, B_H, C_H, P_H, R_V, G_V, Y_V, B_V, C_V, P_V). Peripheral stimuli (34.7° × 8.0°) consisted of arrays of white horizontal line elements presented either in the upper visual field (UVF) or lower visual field (LVF) . The vertical distance between the fixation and the upper edge of the LVF stimuli ( or the lower edge of the UVF stimuli) was 3.1°. Peripheral (UVF/LVF) and central stimuli were presented in a pseudorandom sequence at a rapid rate. All stimuli were flashed for 100 ms durations, and SOAs were randomized between 350 and 633 ms.
Figure 1.
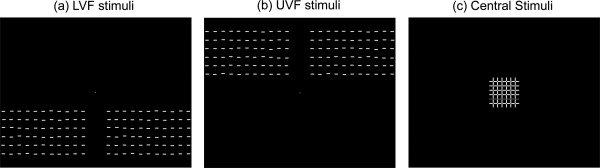
Experimental stimuli. Peripheral irrelevant stimuli were arrays of short lines that could occur at random in either the upper (UVF) or lower (LVF) visual field. Central stimuli consisted of twelve lines (six horizontal and six vertical), all of which were white except one (horizontal or vertical) that was either red, green, yellow, blue, cyan or purple (shown as the darker line in the figure). A central fixation dot was present throughout the experiment. In the low‐load condition, subjects detected a red line segment in the central stimuli. In the high‐load condition, they detected either a green‐horizontal or yellow‐vertical line.
Each subject performed a color‐orientation conjunction search task for the high‐load condition, and a color detection task for the low‐load condition. For both tasks, subjects were required to press a key using their dominant hand only when the central target was presented. In the high load condition the target was either a green vertical (G_V) or yellow horizontal line (Y_H) in the central array. In the low load condition, the target was a red vertical or horizontal line (R_V or R_H) in the central array. There were 12 blocks for each task, and the same physical stimuli were presented in both conditions. Each block contained 240 trials, including 10% UVF, 10% LVF, and 80% central stimuli. Among the central stimuli 10% were low‐load targets (i.e., R_V or R_H), 10% were high‐load targets (i.e., G_V or Y_H), 30% were confusable central nontargets (i.e., Y_V or G_H, which shared the same color with the high‐load target), and 30% were nonconfusable central nontargets (i.e., B_H, B_V, C_H, C_V, P_H, P_V). For each task condition, there were a total of 288 trials each for the UVF, LVF, and central target stimuli, and 864 trials each for the confusable central nontarget (Con) and the nonconfusable central nontarget (Ncon) stimuli. To avoid possible exogenous cueing effects between task‐irrelevant peripheral stimuli, UVF and LVF stimuli were never presented successively; half of the UVF/LVF stimuli were preceded by Con trials, and the other half were preceded by Ncon trials.
EEG Recording
The EEG was recorded from 62 scalp sites using the 10‐10 system montage (Nuwer et al, 1999). Standard 10–20 sites were FP1, FPz, FP2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1, Oz, O2, and M1. Additional intermediate sites were AF3, AFz, AF4, FC5, FC3, FC1, FCz, FC2, FC4, FC6, C5, C1, C2, C6, TP7, CP5, CP3, CP1, CPz, CP2, CP4, CP5, TP8, P5, P1, P2, P6, PO7, PO3, POz, PO4, PO8, I5, I3, Iz, I4, I6, SI3, SIz, and SI4. All scalp channels were referenced to the right mastoid (M2) during recording. Horizontal eye movements were monitored with a bipolar recording from electrodes at the left and right outer canthi. Blinks and vertical eye movements were recorded with an electrode below the left eye, which was also referenced to M2. Electrode impedances were kept below 5 kOhms. Scalp signals were amplified with a gain of 10,000 and band‐pass filtered from 0.1 to 80 Hz. Signals were digitized to disk at 500 Hz. Each recording session lasted 180–240 min, including cap/electrode preparation. Short breaks were given after each block of trials to help alleviate subject fatigue. Each block lasted approximately 2 min.
ERP Analyses
ERPs were time‐locked to stimulus onset, baseline corrected from −50 to 50 ms, and low‐pass filtered at 33 Hz. Trials contaminated by eye movements, eye blinks, or amplifier blocking were rejected. On average, 11% of trials were rejected due to these artifacts. ERPs from the scalp channels were re‐referenced off‐line to the average of left and right mastoids. The ADJAR algorithm [Woldorff, 1993] was used to remove overlapping ERPs from adjacent stimuli. Two‐tailed pair‐wise t‐tests were used to analyze load effects on the behavioral and ERP measures. For the central stimuli, early load effects are only reported for the nontarget stimuli, which produced better signal/noise ratios than did the less frequent targets.
RESULTS
Behavior
Pair‐wise t‐tests showed highly significant load effects on both accuracy and reaction time (RT). Target detection accuracy (hit rate) was higher in the low‐load (mean: 99.4%, s.e.: 0.2%) than in the high‐load condition (mean: 89.8%, s.e., 1.4%; t(18) = 7.5, P < 6×10−7), and RTs were faster in the low‐load (mean: 440 ms, s.e.: 8 ms) than in the high‐load condition (mean: 624 ms, s.e:12 ms; t(18) = 17.7, P < 8 × 10−13).
ERPs to Peripheral Stimuli
C1
As shown in Figures 2 and 3, both UVF and LVF stimuli evoked large C1 components over midline parieto‐occipital areas, with a maximum amplitude at POz and a peak latency of around 90 ms. However, there was no significant load effect on the C1 amplitude for either UVF or LVF, as revealed by paired t‐tests on the mean amplitude of C1 (80–100 ms) comparing low‐load and high‐load conditions at the midline parieto‐occipital sites (POz: t(18) = 0.05, P > 0.9 for UVF; t(18) = 0.89, P > 0.4 for LVF).
Figure 2.
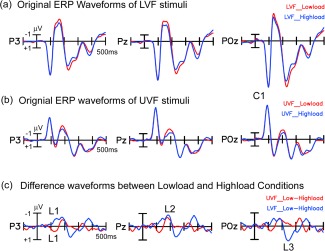
Grand averaged ERPs elicited by peripheral stimuli under high and low load conditions. For ERPs to both the UVF and LVF stimuli, the C1 component did not show a significant difference between load conditions. The first load effect (L1) started at about 110 ms and reversed in polarity for the UVF and LVF stimuli. The second load effect (L2) appeared around 170–200 ms, with the same polarity for UVF and LVF stimuli. The third load effect (L3, 220–270 ms) was present for LVF but not for UVF stimuli. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
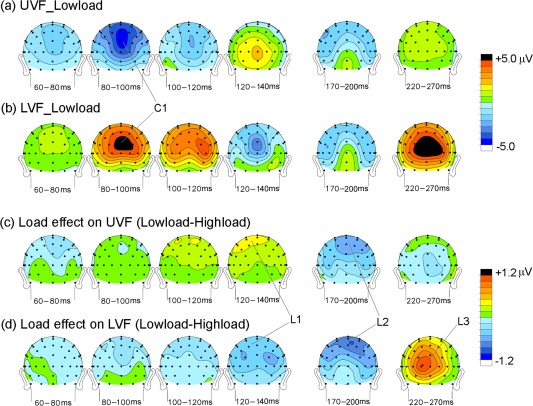
Voltage topographical maps of the grand averaged ERPs to the peripheral stimuli. (a) Maps of components in original waveforms elicited by UVF stimuli in the low‐load condition; (b) Same as (a) for LVF stimuli in the low‐load condition; (c) Maps of components in low‐load minus high‐load difference waves for UVF stimuli; (d) Same as (c) for LVF stimuli. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
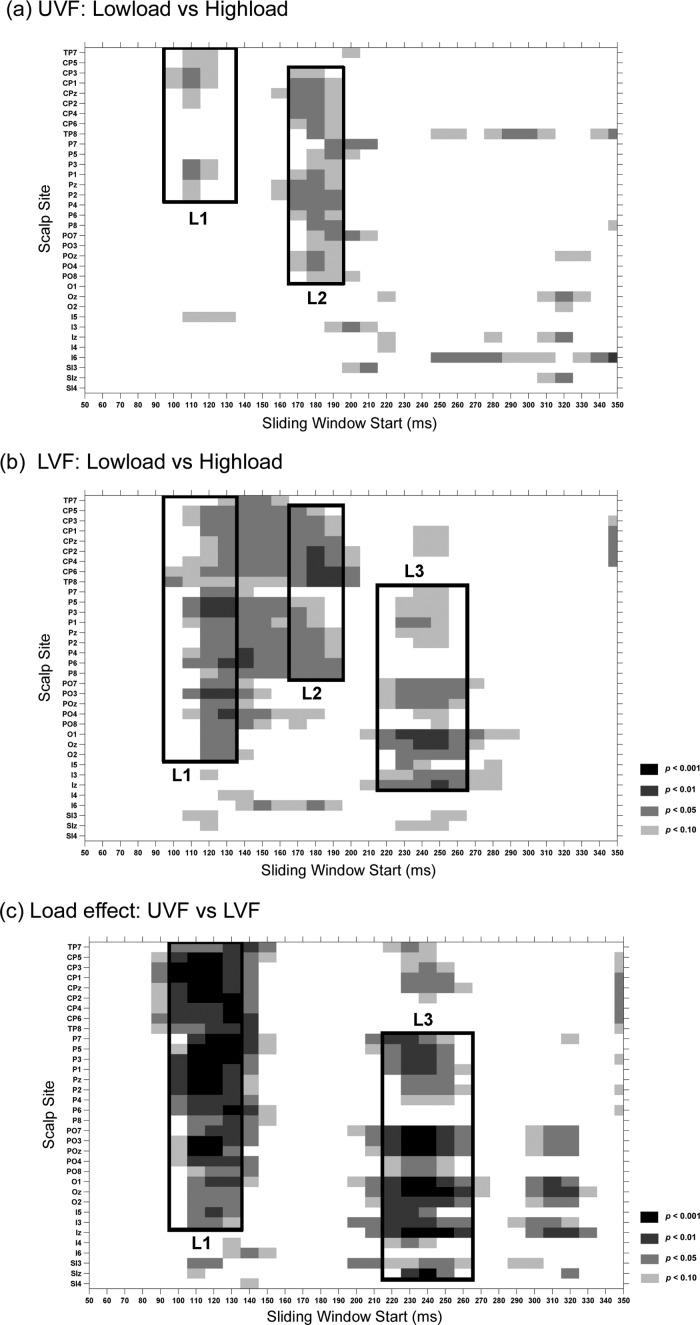
To examine the time course and scalp distribution of load effects on the visual ERP, pair‐wise t‐tests were used to compare the ERP mean amplitude between the low‐load and high‐load conditions for both UVF and LVF was tested. A sliding time window of 20 ms starting from 50 ms (e.g., 50–70, 60–80, and 70–90) was tested at each posterior scalp site (Fig. 4a,b). A similar analysis was also used to compare the load effects (measured in the low‐load minus high‐load difference waves) between UVF and LVF (Fig. 4c). These analyses revealed three major load effects (L1, L2, and L3), which are labelled in the grand‐average ERP waveforms (Fig. 2) and voltage topographies (Fig. 3).
Figure 4.
Statistical significance of ERP comparisons for peripheral stimuli. Pair‐wise t‐tests were carried out with a sliding time window of 20 ms and steps of 10 ms at each posterior scalp site. (a) Comparison between ERPs elicited under low‐load versus high‐load conditions for the UVF stimuli; (b) Same as (a) for LVF stimuli; (c) Comparison of the load effects between the UVF and LVF stimuli.
L1
The first load effect (L1) started at around 110 ms for both UVF and LVF, although its scalp distribution differed somewhat between UVF and LVF (Fig. 3c,d). For example, in the time window 110–130 ms, the load effect reached significance at sites P1, P3, CP1, and CP3 for UVF stimuli (t(18)'s > 2.11, P's < 0.05; Fig. 4a), and at P3, P5, P6, and PO3 for LVF stimuli (t(18)'s > 2.14, P's < 0.05; Fig. 4b). Interestingly, this load effect showed a polarity reversal between UVF and LVF stimuli; for UVF, the effect was more positive in the low‐load than high‐load condition, whereas for LVF the effect was more negative in the low‐load than high‐load condition (Fig. 2c). Further analysis confirmed that this early difference in the load effect between UVF and LVF stimuli was highly significant over posterior scalp sites (e.g., P3, P5, P1/2, Pz, CP3, CP5, CP1/2, CPz, PO3, POz) in the interval 110–130 ms (t(18)'s > 3.96, P's < 0.001; Fig. 4c).
L2
The second load effect (L2) appeared at around 170–200 ms for both UVF and LVF. In contrast with L1, L2 showed a similar scalp distribution and polarity for UVF and LVF stimuli, both with a maximum negativity over centro‐parietal areas (Figs. 2c and 3c,d). The L2 load effect was significant at sites CPz and Pz for both UVF (t(18)'s > 2.40, P's < 0.03; Fig. 4a) and LVF (t(18)'s > 2.39, P's < 0.03; Fig. 4b) over the time intervals of 170–190 ms and 180–200 ms. The L2 load effect did not differ significantly between UVF and LVF stimuli (170–190 ms and 180–200 ms, CPz and Pz, t(18)'s < 0.76, P's > 0.4, Fig. 4c).
L3
For the LVF stimuli, there was a third load effect (L3) during the interval 220–270 ms, with a maximum positivity over medial occipital sites, peaking at ∼250 ms. However, no such effect was seen for the UVF stimuli (Figs. 2 and 3c,d). The L3 effect was significant at POz, Oz and Iz for the LVF during the time intervals of 230–250, 240–260, and 250–270 ms (t(18)'s > 2.38, P's < 0.03; Fig. 4b), but not for UVF (t(18)'s < 1.54, P's > 0.1; Fig. 4a). Further analysis confirmed that this late L3 effect differed highly significantly between UVF and LVF (at POz, Oz and Iz sites; 230–250 and 240–260 ms: t(18)'s > 3.46, P's < 0.001, Fig. 4c).
ERPs to Central Nontarget Stimuli
Early load effect
As shown in Figures 5a,b and 6a, central stimuli elicited typical C1 and P1 components over posterior scalp areas (Ncon and Con stimuli elicited similar early ERPs in the low‐load condition, and only the topographical maps for the Ncon stimuli are shown in Fig. 6a). C1 started at about 60 ms and peaked at about 100 ms, with a maximum negative amplitude at IZ. P1 started at about 70 ms and peaked at about 120 ms, with a maximum amplitude at bilateral occipital sites (e.g., PO7/PO8). Both the C1 and P1 waveforms showed amplitude differences between the low‐load and high‐load conditions. Consistent with the load theory of selective attention (Lavie, 1995), the P1 to these task‐relevant stimuli was larger in the high‐load than low‐load condition. However, the C1 (at site Iz) was actually larger (more negative) in the low‐load than the high‐load condition, which is contrary to load theory. These results suggested that the early difference seen in the C1 waveform at the midline occipital site (Iz) was not actually a load effect on the C1 component. To confirm this supposition, we examined the spatio‐temporal properties of the difference wave between the high‐load and low‐load ERPs. As shown in Figures 5c and 6b, the high‐load minus low‐load difference wave started at about 70 ms and peaked at about 120 ms, with amplitude maxima over bilateral parietal‐occipital sites (because no reliable differences between Ncon and Con stimuli were found before 130 ms, only the topographical maps of the Ncon stimuli are shown in Fig. 6b). The significance of this load effect was confirmed by pair‐wise t‐tests on the ERP mean amplitude between the low‐load and high‐load conditions of the Ncon stimuli (60–80 ms, n.s. for all the posterior sites; 70–90 ms: t(18) = 2.94, P < 0.01 for PO8, t(18) = 1.85, P > 0.05 for Iz; 90–110 ms: t(18)'s > 3.65, P's < 0.002 for PO7 and PO8, t(18) = 2.39, P = 0.03 for Iz; Fig. 7a). Both the time course and the bilateral occipital scalp topography of this load effect indicated that it actually represented an amplitude modulation of the P1 component, which overlapped partially with the medially distributed C1.
Figure 5.
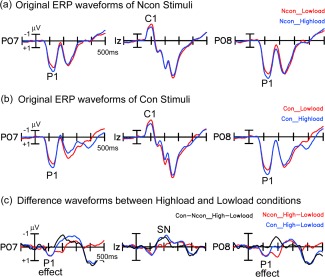
Grand averaged ERP waveforms elicited by the central stimuli. For both the Con and Ncon stimuli, the P1 amplitude (starting at 70 ms) was significantly larger in the high‐load than the low‐load condition. The difference in load effect between Con and Ncon stimuli did not reach significance until 140 ms, at which time a feature‐based Selective Negativity (SN) effect was elicited. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 6.
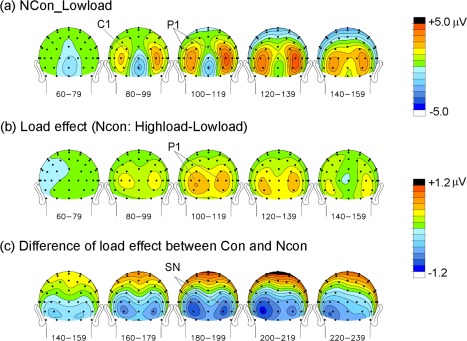
Voltage topographical maps of the grand averaged ERPs to the central stimuli. (a) Maps of ERPs elicited by Ncon stimuli in the low‐load condition; (b) Maps of difference wave components between the low‐load and high‐load conditions (high‐load minus low‐load ERPs) for Ncon stimuli; (c) Maps of difference in load effects between Con and Ncon stimuli. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 7.
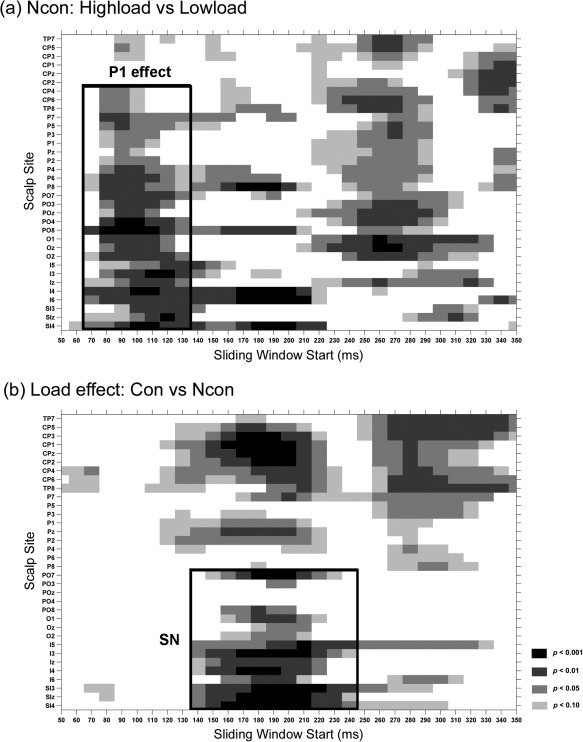
Statistical significance of ERP comparisons for central stimuli. Pair‐wise t‐tests were carried out with a sliding time window of 20 ms and steps of 10 ms at each posterior scalp site. (a) Comparison between the high‐load and low‐load conditions of the Ncon stimuli; (b) comparison of the load effect between the Con and Ncon stimuli.
Comparison between confusable (Con) and nonconfusable (Ncon) nontargets
As shown in Figure 5c, ERPs to Con and Ncon stimuli showed a similar load effect for the early P1 component. The earliest difference in load effect between Con and Ncon started at 140–160 ms, with a maximum negative amplitude at bilateral occipital electrodes that peaked at around 200 ms (e.g., at I3 and SI3/4 sites, 140–160 ms, t(18)'s > 2.43, P's < 0.03; 190–210 ms, t(18)'s > 5.01, P's < 0.0001; Fig. 7b). This effect was considered to be a selection negativity (SN) component, because it showed a similar time course and scalp distribution to that of the typical SN induced by feature‐selective attention [Hillyard and Anllo‐Vento, 1998].
Source Analyses of the Original ERPs and Load Effects
The neural sources of early visual components and load effects were estimated by dipole modeling using the Brain Electrical Source Analysis (BESA) algorithm based on the grand average voltage topographical data and the 4‐shell ellipsoidal head model. A single pair of dipoles having both symmetrical position and symmetrical orientation was fit to each individual component or load effect (Figs. 8 and 9).
Figure 8.
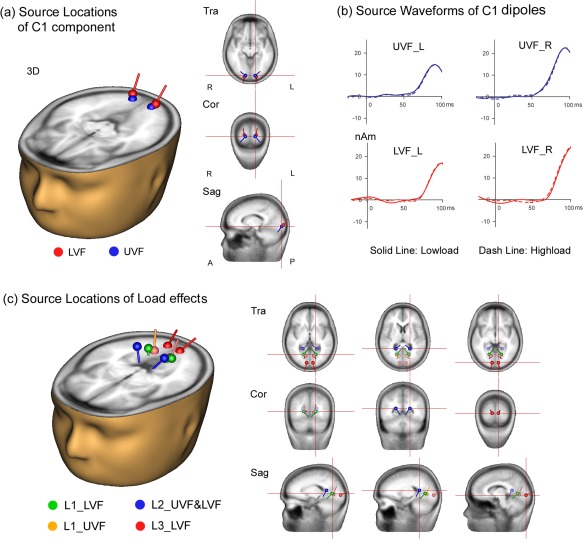
Dipole models of cortical sources of ERPs to peripheral stimuli. (a,b) Dipole model of intracranial sources of the C1 component. The C1 dipoles were located in or near area V1 for both the UVF and LVF stimuli. Dipoles were projected on to the standard head model as shown in (a). Source waveforms in (b) show time course of modeled activity for UVF and LVF dipoles (L: left hemisphere, R: right hemisphere). Note that the source waveforms did not differ between low‐load and high‐load conditions. (c) Dipole models of intracranial sources of the load effects (L1, L2 and L3). The L1 dipoles were located in anterior lingual gyrus but with high residual variance. The L2 dipoles were located in parieto‐occipital cortex. The L3 dipoles were situated in the posterior lingual gyrus in or near area V1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 9.
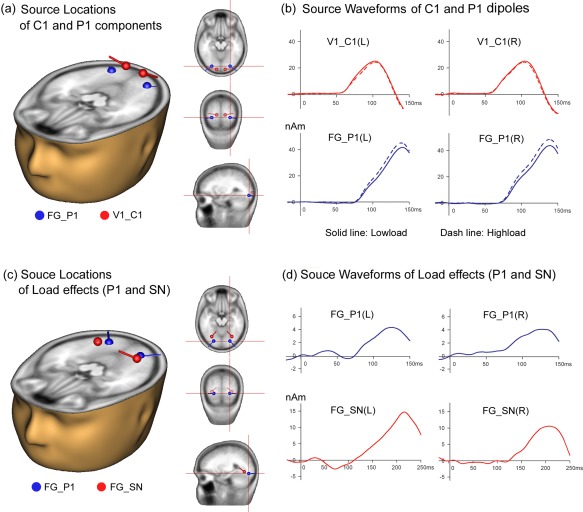
Dipole models of cortical sources of ERPs to central task‐relevant stimuli. (a,b) Dipole modeling of intracranial sources of the C1 and P1 components elicited by Ncon stimuli. (a) The C1 dipoles were located in or near area V1 and the P1 dipoles were localized to the fusiform gyrus (FG), as shown in the projections of dipoles onto the standard head model. (b) Source waveforms show time courses of modeled activity for C1 and P1 dipoles (L: left hemisphere, R: right hemisphere). Note that the source waveforms show an obvious difference between low‐load and high‐load conditions for the P1 but not for the C1 dipoles. (c,d) Dipole modeling of intracranial sources of the load effects. (c) The dipole pairs for the P1 effect and for the SN effect were both located in the fusiform gyrus (FG). (d) Source waveforms show time course of modeled activity for the dipoles of P1 and SN load effects. Note that both the location and the time course of activity of the dipoles of the P1 load effect (derived from difference waveforms, as shown in (c,d)) were similar to those of the P1 component (from original waveforms, as shown in (a,b)). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
ERPs to peripheral stimuli
As in previous reports, a symmetrical pair of dipoles located in or near primary visual cortex, V1 (BA17; Talairach coordinates: ±14, −90, −7 for UVF stimuli and ±17, −94, −3 for LVF stimuli) could account for the C1 scalp voltage distribution in the original waveforms with low residual variance (RV) over the time interval of 60–100 ms for both the UVF and LVF stimuli (RV's < 8%; Fig. 8a). The dipole source waveforms further confirmed that these early C1 components were not modulated by attentional load (Fig. 8b).
As shown in Figure 8c, a pair of dipoles located in the inferior parietal‐occipital region near posterior cingulate cortex (PCC, Talairach coordinates: ±20, −48, 11) could account for more than 85% of the variance in the L2 scalp voltage distribution over the time interval of 170–190 ms for both the UVF and LVF stimuli (RV's < 15%; Fig. 8b). A pair of dipoles located in posterior lingual gyrus (LG) in or near area V1 (BA17; Talairach coordinates: ±9, −88, −3) gave a good fit to the L3 load effect (224–254 ms) for LVF stimuli (RV = 7%).
A satisfactory dipole model of the L1 load effect could not be achieved, however, due to its low amplitude and diffuse scalp distribution. As shown in Figure 8c, the best fitting symmetrical pair of dipoles for the L1 effect (110–140 ms) for UVF stimuli was situated in the anterior lingual gyrus (LG, Talairach coordinates: ±12, −68, −1), but this model had a high RV = 35%. Another pair of dipoles located in the anterior LG (Talairach coordinates: ±17, −64, 2) was fit to the L1 effect (120–150 ms) for LVF stimuli, but also with a high RV = 19%. Note that these dipoles fit to L1 for UVF and LVF stimuli showed opposing orientations, consistent with the observed scalp polarity reversal of the L1 effect between UVF and LVF. Given the high RV of these models for the L1 load effect, however, we regard their localizations as only crude approximations.
ERPs to central stimuli
Two symmetrical pairs of dipoles, fitting the C1 (58–78 ms) and P1 (72–122 ms) components respectively, could account for more than 95% of the variance of the voltage topography in the original waveforms over the time interval of 50–130 ms. The C1 dipoles were located in or near area V1 (BA17; Talairach coordinates: ±13, −93, −4), whereas the P1 dipoles were situated in the fusiform gyrus (FG, Talairach coordinates: ±27, −83, −12) (RV's < 5% for both high‐load and low‐load conditions; Fig. 9a). The source waveforms of these dipoles further confirmed that attentional load modulated the P1 rather than the C1 component elicited by the central task‐relevant stimuli (Fig. 9b). A symmetrical pair of dipoles located in FG (Fig. 9c, Talairach coordinates: ±24, −80, −13) provided a good fit to the scalp distribution of the P1 load effect seen in the high‐load minus low‐load difference wave over the interval 70–130 ms (RV = 5.7%). Another symmetrical pair of dipoles located in FG (Talairach coordinates: ±30, −67, −8) could account for the difference in load effect between Con and Ncon stimuli over the interval of 140–240 ms (SN effect; RV = 2.8%). Source waveforms showed that the P1 effect occurred about 70 ms earlier than the SN effect (Fig. 9d).
DISCUSSION
The present study used high‐density ERP recordings to investigate the effects of attentional load on the cortical processing of central task‐relevant and peripheral irrelevant stimuli. Two important findings emerged from the ERP data: first, the initial visual evoked component C1 was not affected by attentional load for either central‐relevant or peripheral irrelevant stimuli, and second, attentional load modulated the subsequent cortical processing of central relevant and peripheral irrelevant stimuli in different spatial‐temporal configurations. Although the effects of load on the processing of irrelevant stimuli (L1, L2, and L3) started at around 110 ms and involved multiple cortical regions, load effects on ERPs to central relevant stimuli (including the P1 and SN effect) began at around 70 ms and were localized to ventral‐lateral extrastriate cortex in or near the fusiform gyrus.
C1 Component Did Not Differ Between High‐Load and Low‐Load Conditions
In the present study, color‐orientation conjunction search and color detection tasks served as the high‐load and low‐load conditions, respectively. Similar tasks have been used in many previous studies of perceptual/attentional load [e.g., Handy et al., 2001; Lavie, 1995; Rauss et al., 2009, 2012]. Consistent with previous findings, the present study showed that the behavioral performance of the central task was much better in the low‐load than the high‐load condition. Because we manipulated attentional load by varying the task requirements while keeping the stimulus set unchanged, the present load effects cannot be ascribed to the “distractor dilution” mechanism proposed by Tsal and Benoni [Benoni and Tsal, 2010; Tsal and Benoni, 2010]. In the present study, the earliest visual‐evoked C1 component did not show a significant load modulation for either the peripheral task‐irrelevant stimuli or the central task‐relevant stimuli (although for the central stimuli the measured C1 amplitude was modulated by overlap with the concurrent P1 component arising from extrastriate cortex). This negative result vis a vis the C1 component differed from those of two recent studies by Rauss et al. [2009, 2012], which used peripheral irrelevant stimuli and central tasks similar to those of the present study and reported that the C1 elicited by the peripheral stimuli was modulated in amplitude as a function of the load of the central task. As mentioned in the Introduction, however, the early load effects on measured C1 amplitude reported in these two studies might not actually represent modulations of the C1 component generators in area V1, but rather overlapping ERPs elicited by the preceding stimuli. In the present study, the ADJAR Technique [Woldorff, 1993] was used to remove overlapping ERP activity, and ERPs were averaged over a larger number of stimuli (at least 250 for each ERP condition) than in the studies of Rauss et al (at most 60 or 100 trials for each condition). Source analyses confirmed that the present C1 components originated in or near visual area V1, and that the source waveforms of the initial C1 dipole activity (60–100 ms) were unchanged by attentional load. Taken together, the present results provide strong ERP evidence in support of the proposition that the initial visual cortical processing of both task‐relevant and task‐irrelevant stimuli in area V1 is not substantially modulated by attentional load, at least in the typical attentional load paradigm that was employed in the present study. The present results also concur with the studies of Fu and colleagues, who used a different experimental manipulation and also concluded that attentional load is not a critical factor that modulates early visual processing at the level of primary visual cortex [Fu et al., 2010b, 2012]. Nonetheless, we cannot rule out the possibility that a more extreme or more competitive load manipulation might modulate early visual processing more extensively.
Prior Studies of Attention Effects on the C1 Component
As noted in the Introduction, the majority of ERP/MEG studies that used endogenous cueing or sustained attention paradigms found no effect of spatial‐selective attention on the amplitude or latency of the C1 component. Two recent studies, however, reported that C1 was increased in amplitude when attention was directed endogenously to the location of visual stimuli [Kelly et al., 2008; Poghosyan and Ioannides, 2008]. It may be questioned, however, whether these findings actually represent modulation of the initial feed‐forward response in area V1. In the experiment of Kelly et al. [2008], where a central cue indicated the location of the to‐be‐attended stimulus, the upper and lower field stimuli were always aligned along a diagonal, so that the well‐known upper versus lower field polarity reversal of the C1 could not be demonstrated unambiguously. Thus, the observed amplitude modulations could have originated from a neural source outside of area V1 having a laterally oriented dipole; this hypothesis is consistent with Kelly et al.'s source analysis (using the LAURA algorithm), which showed that the attention‐related modulations of C1 were localized to a source 23–24 mm lateral to the midline, at the margin of the calcarine cortex. In an MEG study, Poghosyan and Ioannides [2008] also reported that spatial attention enhanced an early visual‐evoked response at 55–90 ms that was localized to area V1, but this source localization was based on waveforms averaged over only 18 presentations of each visual stimulus type in each visual field per subject and thus had low signal/noise ratios. Finally, as mentioned previously, the C1 modulations produced by endogenous manipulations of attentional load described by Rauss et al. [2009, 2012] were not convincingly localized to the region of primary visual cortex. When taken together with results of the present study, there is scant evidence that endogenous manipulations of attention or attentional load modulates the initial evoked response in visual area V1.
In contrast with the paucity of reports that endogenous attention modulates the C1 component, numerous studies using fMRI have found increased neural activity in visual area V1 contralateral to attended visual stimuli. Such a retinotopic increase has been observed in tasks where attended and unattended stimuli were presented concurrently [Di Russo et al., 2003, 2012; Martinez et al., 1999, 2001; Noesselt et al., 2002] and in cueing paradigms during the interval between an endogenous attention‐directing cue and the visual target [Kastner et al., 1999; O'Connor et al., 2002; Sylvester et al., 2007]. Given the evidence that a substantial portion of the C1 component is generated in area V1, this contrast between the ERP and fMRI findings in attention paradigms requires further consideration. As noted in the Introduction, several studies that combined ERP recordings with fMRI [Di Russo et al., 2003, 2012; Martinez et al., 2001; Noesselt et al., 2002] found that attended stimuli elicited enhanced neural activity localized to V1 but after a delay beyond the latency of the C1; this suggested that attended stimuli triggered a delayed feedback into area V1 from higher extrastriate areas, an idea consistent with findings in non‐human primates [Lamme and Roelfsema, 2000; Super et al., 2001]. It is also conceivable that attention might modulate neural activity in V1 without affecting the C1 amplitude if the neural activity was not well time‐locked to the attended stimuli (and thus would not be registered in the averaged ERP) or if the activity took place in neurons with “closed field” dendritic arbors that would not produce a far‐field ERP [Martinez et al., 2001]. As for the neural activity observed in area V1 in the interval following an attention‐directing cue, there is evidence that such anticipatory activity may not necessarily result in selectively enhanced processing of the attended target stimulus [Kastner et al., 1999]. This suggests that anticipatory activation in area V1 may reflect general processes of preparation and expectation that do not result in the selective modulation of attended versus unattended target stimuli at the level of V1.
Several studies have investigated the effects of nonpredictive, exogenous cueing of attention on the C1 [Fu et al., 2009, 2010a; Hopfinger and West, 2006; Khoe et al., 2005]. These studies have produced inconsistent results. Although some studies did not find any attention effects on C1 [e.g., Hopfinger and West, 2006], others reported that the C1 amplitude was modified by exogenous cueing [e.g., Fu et al., 2009, 2010a; Khoe et al., 2005]. In some cases, however, the reported C1 amplitude modulation might have resulted from an overlap with the P1 component of extrastriate origin, as occurred for the central stimuli in the present study. Moreover, even if a reported C1 effect actually originated from area V1, it is not clear whether such an early effect could be attributed to selective attention or to a sensory interaction between the exogenous cue and target. Note that even if a valid exogenous cue and the target are not presented at exactly the same location [e.g., Fu et al., 2009], the lateral interconnections among V1 neurons could mediate an early sensory interaction between the cue and the target. Accordingly, caution should be exercised when interpreting early ERP modulations in studies using exogenous, noninformative cue paradigms. Such caution also applies to the studies reporting C1 effects induced by physical stimulus differences [e.g., Zhang et al., 2012].
Effect of Load on Processing Peripheral, Task‐Irrelevant Stimuli
Spatiotemporal patterns of brain activities
The earliest load effect (L1) started at about 110 ms for both the UVF and LVF stimuli, well after the initial visual‐evoked C1 component at 60–90 ms. Interestingly, this load effect showed opposite scalp polarity between the UVF and the LVF stimuli. Consistent with the study of Handy et al. [2001], which presented task‐irrelevant stimuli only in the UVF and reported a larger P1 (100–150 ms) component at midline parietal‐occipital sites in the low‐load than in the high‐load condition, the present L1 load effect included an increased positivity to UVF stimuli at parietal sites over the interval 110–140 ms when the load of the central task was decreased. The polarity reversal of L1 between the UVF and the LVF stimuli strongly suggests that this early load effect arises from retinotopically organized visual cortex, such as areas V1–V3, which reportedly give rise to polarity reversals [Ales et al., 2010] and/or polarity shifts [Schroeder et al., 1995; Simpson et al., 1995] in response to UVF versus LVF stimuli. This is consistent with recent fMRI studies that found attentional load modulated neural activity elicited by peripheral task‐irrelevant stimuli in the low‐level visual cortical areas, including V1–V4 [Bahrami et al., 2007; O'Connor et al., 2002; Schwartz et al., 2005]. The relatively long latency of this load effect (110 ms), however, indicates that it does not represent modulation of the initial feed‐forward activity in the early retinotopic areas but rather a delayed feedback into these areas. Source analysis of the L1 load effect suggested neural generators in inferior occipito‐parietal regions near the anterior lingual gyrus, but the dipole model for this effect had a high residual variance and could only be considered an approximation.
In contrast with the earliest load effect, the second effect (L2, at 170–200 ms) showed a consistent polarity and scalp distribution for the UVF and LVF stimuli, which suggests it did not originate from retinotopically organized visual cortical areas but rather from higher brain regions along the visual pathway. As suggested by source analysis, the L2 effect appears to originate from inferior occipital‐parietal regions near the posterior cingulate cortex (PCC). The engagement of occipital‐parietal regions around PCC might reflect modulation of the “default mode” network by attentional load. The PCC is considered to be a central node in the default mode network of the brain, which is important for task‐independent evaluation of the environment [Raichle et al., 2001]. Previous studies have shown that large, bright, textured stimuli can elicit neural responses in PCC, even if they are totally irrelevant to the task being performed [Vogt et al., 1992]. The present proposal that the activity of the default mode network evoked by irrelevant stimuli could be modulated by attentional load of the task is consistent with the view that default activity of the brain is curtailed when task performance demands focused attention [Raichle et al., 2001].
The third load effect (L3, 220–270 ms), like the first, showed significant differences between UVF and LVF stimuli. L3 was observed only for the LVF and not for the UVF stimuli, suggesting that this later effect might arise from lower‐level retinotopically organized visual cortex, which is consistent with its source localization in posterior lingual gyrus in or near V1. The finding that attentional load might modulate activity in the earliest retinotopic areas is consistent with previous fMRI studies [Bahrami et al., 2007; O'Connor et al., 2002; Schwartz et al., 2005]. The present results reinforce the view that such modulation of activity in low‐level visual areas takes place at a late, re‐entrant rather than an early feed‐forward processing stage.
To summarize, increases in attentional load first affects the processing of peripheral task‐irrelevant stimuli in retinotopically organized extrastriate cortical areas, followed by a modulation in the inferior occipital‐parietal regions near the posterior cingulate cortex, and then a modulation of re‐entrant feedback into the low‐level occipital cortex (lingual gyrus in or near V1). This sequence of load effects is most likely a consequence of the top‐down modulation (induced by task‐load) of bottom‐up attentional capture driven by the peripheral irrelevant stimuli, or, in other words, an interaction between exogenous and endogenous attention. The exogenous attentional capture may trigger activity in inferior occipital‐parietal regions near PCC (the “default mode network”), which interacts with re‐entrant processing in early retinotopic areas. The modulation of re‐entrant processing in low‐level visual cortex may produce perceptual effects such that the task‐irrelevant stimuli reach different levels of consciousness under different task load conditions [Boehler et al., 2008; Lamme, 2006; Lavie, 2006].
Effect of Load on Processing Central Task‐Relevant Stimuli
The earliest load effect on the task‐relevant ERP started at 70 ms, with a bilateral occipital scalp distribution for both confusable (Con) and nonconfusable (Ncon) stimuli. Both its time course and scalp distribution indicated that this effect was a modulation of the early P1 component, which was increased in amplitude when the task became more difficult. This early modulation was localized by dipole modeling to the ventral extrastriate cortex (region of the fusiform gyrus), suggesting that attentional load first modulates feed‐forward processing at extrastriate rather than striate levels of processing. This result is consistent with our recent finding that task difficulty modulated the amplitude of P1 component and its training effect [Wang et al., 2010]. Because Con and Ncon stimuli differed in color but shared the same location, the finding of no significant difference between Con and Ncon stimuli in the P1 modulation indicated that this effect was not due to color‐selective attention. Instead, the P1 effect most likely reflects a modulation of spatial‐selective attention induced by task load, with higher loads resulting in an increased allocation of spatial attention to the location of the central task‐relevant stimuli and a corresponding increase in P1 amplitude. This finding is consistent with previous ERP studies showing that spatial‐selective attention first modulates early visual processing in the extrastriate cortex as reflected in the P1 component [e.g., Di Russo et al., 2003; Martinez et al., 1999; Noesselt et al., 2002], even for central‐foveal stimuli [Frey et al., 2010].
The difference in load effect between ERPs to the Con and Ncon stimuli started at around 140 ms, with greater negativity for the Con stimuli. This difference was clearly a modulation produced by feature‐selective rather than spatial‐selective attention, because that the Con and Ncon stimuli differed only in color. In the high‐load condition, the Con stimuli shared the same colors (yellow or green) with the target stimuli, whereas the Ncon stimuli did not; thus, more feature selective attention was allocated to the Con stimuli to determine whether or not they were targets. The proposal that this difference in load effect between Con and Ncon stimuli might reflect feature‐selective attention is consistent with the fact that this negative ERP difference has a similar bilateral occipital scalp distribution and time course as the well‐known SN (selective negativity) that has been observed in numerous feature‐selective attention paradigms [e.g., Harter and Aine, 1984; Hillyard and Anllo‐Vento, 1998].
In sum, the present results suggest that high‐load tasks require subjects to allocate more endogenous attention to the relevant stimuli than do low‐load tasks. In other words, the load effects on the relevant stimuli reflect the engagement of goal‐directed endogenous attention by the task demands. Consistent with previous studies [e.g., Hopfinger et al., 2000; Hopf et al., 2009; Schoenfeld et al., 2007], the present results add to the evidence that endogenous selective attention results in enhanced processing of attended stimuli in ventral extrastriate cortex in the region of the fusiform gyrus, both for spatial‐selective and feature‐selective attention.
CONCLUSION
Endogenous vs. Exogenous Allocations of Attention
By using high‐density ERP recordings, the present study investigated the brain mechanisms of attentional load effects on both the central task‐relevant and peripheral task‐irrelevant stimuli. The results showed that the C1 component was not modified by attention load for either central relevant or peripheral irrelevant stimuli, further supporting the view that the initial visual cortical processing is not sensitive to selective attention. In addition, the present load effects showed different spatio‐temporal patterns of brain activity between central‐relevant and peripheral‐irrelevant stimuli, suggesting that selective attention may modulate the cortical processing of task‐relevant and task‐irrelevant stimuli through different brain systems. Whereas the load‐related modulations of ERPs to central relevant stimuli began as early as 70 ms and were localized to ventral extrastriate cortex (vicinity of fusiform gyrus), the modulations of ERPs to peripheral irrelevant stimuli did not reach significance until 110 ms and were localized to more dorsal‐medial cortical regions including retinotopic areas in or near the lingual gyrus and inferior occipital‐parietal regions near posterior cingulate cortex. These differential effects for relevant and irrelevant stimuli suggest (not surprisingly) that dissociable neural systems are involved in endogenous and exogenous attentional mechanisms. Specifically, when increased endogenous attention is allocated to the central‐relevant stimuli (in the high‐load condition), those stimuli are processed more intensively in the ventral lateral visual pathways that include the fusiform gyrus. In contrast, when more attention is attracted exogenously by the peripheral‐irrelevant stimuli (in the low‐load condition), those stimuli are processed more extensively by recurrent mechanisms in early retinotopic areas and in more dorsal occipito‐parietal cortex. It must be acknowledged, however, that the physical differences between the central‐relevant and peripheral‐irrelevant stimuli in the present study might have contributed to the different patterns of ERP modulations that were observed. Further studies are needed to test the generality of the exogenous/endogenous processing differences that were observed here.
REFERENCES
- Aine CJ, Supek S, George JS (1995): Temporal dynamics of visual‐evoked neuromagnetic sources: Effects of stimulus parameters and selective attention. Int J Neurosci 80:79–104. [DOI] [PubMed] [Google Scholar]
- Ales JM, Yates JL, Norcia AM (2010): V1 is not uniquely identified by polarity reversals of responses to upper and lower visual field stimuli. Neuroimage 52:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami B, Lavie N, Rees G (2007): Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr Biol 17:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoni H, Tsal Y (2010): Where have we gone wrong? Perceptual load does not affect selective attention. Vision Res 50:1292–1298. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Schoenfeld MA, Heinze HJ, Hopf JM (2008): Rapid recurrent processing gates awareness in primary visual cortex. Proc Natl Acad Sci U S A 105:8742–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V, Hillyard SA (1996): Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J Cogn Neurosci 8:387–402. [DOI] [PubMed] [Google Scholar]
- Dark VJ, Johnston WA, Myles‐Worsley M, Farah MJ (1985): Levels of selection and capacity limits. J Exp Psychol Gen 114:472–497. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J (1995): Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA (2003): Source analysis of event‐related cortical activity during visuo‐spatial attention. Cereb Cortex 13:486–499. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Stella A, Spitoni G, Strappini F, Sdoia S, Galati G, Hillyard SA, Spinelli D, Pitzalis S (2012): Spatiotemporal brain mapping of spatial attention effects on pattern‐reversal ERPs. Hum Brain Mapp 33:1334–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey HP, Kelly SP, Lalor EC, Foxe JJ (2010): Early spatial attentional modulation of inputs to the fovea. J Neurosci 30:4547–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Caggiano DM, Greenwood PM, Parasuraman R (2005): Event‐related potentials reveal dissociable mechanisms for orienting and focusing visuospatial attention. Brain Res Cogn Brain Res 23:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Huang Y, Luo Y, Wang Y, Fedota J, Greenwood PM, Parasuraman R (2009): Perceptual load interacts with involuntary attention at early processing stages: Event‐related potential studies. Neuroimage 48:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Fedota J, Greenwood PM, Parasuraman R (2010a): Early interaction between perceptual load and involuntary attention: An event‐related potential study. Neurosci Lett 468:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Fedota JR, Greenwood PM, Parasuraman R (2010b): Dissociation of visual C1 and P1 components as a function of attentional load: An event‐related potential study. Biol Psychol 85:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Fedota JR, Greenwood PM, Parasuraman R (2012): Attentional load is not a critical factor for eliciting C1 attentional effect – A reply to Rauss, Pourtois, Vuilleumier, and Schwartz. Biol Psychol 91:321–324. [Google Scholar]
- Gratton G (1997): Attention and probability effects in the human occipital cortex: An optical imaging study. NeuroReport 8:1749–1753. [DOI] [PubMed] [Google Scholar]
- Handy TC, Soltani M, Mangun GR (2001): Perceptual load and visuocortical processing: Event‐related potentials reveal sensory‐level selection. Psychol Sci 12:213–218. [DOI] [PubMed] [Google Scholar]
- Harter MR, Aine C (1984): Brain mechanisms of visual selective attention In: Parasuraman R, Davies DR, editors. Varieties of Attention. New York: Academic Press; pp 293–321. [Google Scholar]
- Heinze HJ, Mangun GR (1995): Electrophysiological signs of sustained and transient attention to spatial locations. Neuropsychologia 33:889–908. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo‐Vento L (1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Heinze HJ, Schoenfeld MA, Hillyard SA (2009): Spatio‐temporal analysis of visual attention In: Gazzaniga MS, editor. The Cognitive Neurosciences IV. Cambridge, MA: MIT Press; pp 235–250. [Google Scholar]
- Hopfinger JB, West VM (2006): Interactions between endogenous and exogenous attention on cortical visual processing. Neuroimage 31:774–789. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3:284–291. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA, Axford JG (1972): Source locations of pattern‐specific components of human visual evoked potentials. I. Component of striate cortical origin. Exp Brain Res 16:1–21. [DOI] [PubMed] [Google Scholar]
- Johannes S, Munte TF, Heinze HJ, Mangun GR (1995): Luminance and spatial attention effects on early visual processing. Brain Res Cogn Brain Res 2:189–205. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Chajczyk D (1983): Tests of the automaticity of reading: Dilution of Stroop effects by color‐irrelevant stimuli. J Exp Psychol Hum Percept Perform 9:497–509. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–761. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez‐Ramirez M, Foxe JJ (2008): Spatial attention modulates initial afferent activity in human primary visual cortex. Cereb Cortex 18:2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoe W, Mitchell JF, Reynolds JH, Hillyard SA (2005): Exogenous attentional selection of transparent superimposed surfaces modulates early event‐related potentials. Vision Res 45:3004–3014. [DOI] [PubMed] [Google Scholar]
- Lamme VA (2006): Towards a true neural stance on consciousness. Trends Cogn Sci 10:494–501. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR (2000): The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci 23:571–579. [DOI] [PubMed] [Google Scholar]
- Lavie N (1995): Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform 21:451–468. [DOI] [PubMed] [Google Scholar]
- Lavie N (2005): Distracted and confused?: Selective attention under load. Trends Cogn Sci 9:75–82. [DOI] [PubMed] [Google Scholar]
- Lavie N (2006): The role of perceptual load in visual awareness. Brain Res 1080:91–100. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y (1994): Perceptual load as a major determinant of the locus of selection in visual attention. Percept Psychophys 56:183–197. [DOI] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD (2006): Contour saliency in primary visual cortex. Neuron 50:951–962. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hinrichs H, Scholz M, Mueller‐Gaertner HW, Herzog H, Krause BJ, Tellman L, Kemna L, Heinze HJ (2001): Integrating electrophysiology and neuroimaging of spatial selective attention to simple isolated visual stimuli. Vision Res 41:1423–1435. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo‐Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA (1999): Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2:364–369. [DOI] [PubMed] [Google Scholar]
- Martinez A, Di Russo F, Anllo‐Vento L, Sereno MI, Buxton RB, Hillyard SA (2001): Putting spatial attention on the map: Timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Res 41:1437–1457. [DOI] [PubMed] [Google Scholar]
- Miller J (1991): The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: A search for boundary conditions. Percept Psychophys 49:270–288. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ (2002): Delayed striate cortical activation during spatial attention. Neuron 35:575–587. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Fukui MM, Pinsk MA, Kastner S (2002): Attentional modulates response in the human lateral geniculate nucleus. Nat Neurosci 5:1203–1209. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ (2005): How close are we to understanding v1? Neural Comput 17:1665–1699. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S (2004): Push‐pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol 92:622–629. [DOI] [PubMed] [Google Scholar]
- Plainis S, Murray IJ, Chauhan K (2001): Raised visual detection thresholds depend on the level of complexity of cognitive foveal loading. Perception 30:1203–1212. [DOI] [PubMed] [Google Scholar]
- Poghosyan V, Ioannides AA (2008): Attention modulates earliest responses in the primary auditory and visual cortices. Neuron 58:802–813. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauss KS, Pourtois G, Vuilleumier P, Schwartz S (2009): Attentional load modifies early activity in human primary visual cortex. Hum Brain Mapp 30:1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauss K, Pourtois G, Vuilleumier P, Schwartz S (2012): Effects of attentional load on early visual processing depend on stimulus timing. Hum Brain Mapp 33:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N (1997): Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science 278:1616–1619. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, Heinze HJ, Hillyard SA (2007): Spatio‐temporal analysis of feature‐based attention. Cereb Cortex 17:2468–2477. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Steinschneider M, Javitt DC, Tenke CE, Givre SJ, Mehta AD, Simpson GV, Arezzo JC, Vaughan HG Jr (1995): Localization of ERP generators and identification of underlying neural processes. Electroencephalogr Clin Neurophysiol Suppl 44:55–75. [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J (2005): Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task‐irrelevant stimulation in the peripheral visual field. Cereb Cortex 15:770–786. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Pflieger ME, Foxe JJ, Ahlfors SP, Vaughan HG Jr, Hrabe J, Ilmoniemi RJ, Lantos G (1995): Dynamic neuroimaging of brain function. J Clin Neurophysiol 12:432–449. [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA (2001): Two distinct modes of sensory processing observed in monkey primary visual cortex (V1). Nat Neurosci 4:304–310. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, Corbetta M (2007): Asymmetry of anticipatory activity in visual cortex predicts the locus of attention and perception. J Neurosci 27:14424–14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsal Y, Benoni H (2010): Diluting the burden of load: Perceptual load effects are simply dilution effects. J Exp Psychol Hum Percept Perform 36:1645–1656. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb Cortex 2:435–443. [DOI] [PubMed] [Google Scholar]
- Wang Y, Song Y, Qu Z, Ding Y (2010): Task difficulty modulates electrophysiological correlates of perceptual learning. Int J Psychophysiol 75:234–240. [DOI] [PubMed] [Google Scholar]
- Wijers AA, Lange JJ, Mulder G, Mulder LJ (1997): An ERP study of visual spatial attention and letter target detection for isoluminant and nonisoluminant stimuli. Psychophysiology 34:553–565. [DOI] [PubMed] [Google Scholar]
- Williams LJ (1985): Tunnel vision induced by a foveal load manipulation. Hum Factors 27:221–227. [DOI] [PubMed] [Google Scholar]
- Woldorff MG (1993): Distortion of ERP averages due to overlap from temporally adjacent ERPs: Analysis and correction. Psychophysiology 30:98–119. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, Seabolt M, Glass T, Gao JH, Martin CC, Jerabek P (1997): Retinotopic organization of early visual spatial attention effects as revealed by PET and ERPs. Hum Brain Mapp 5:280–286. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Liotti M, Seabolt M, Busse L, Lancaster JL, Fox PT (2002): The temporal dynamics of the effects in occipital cortex of visual‐spatial selective attention. Brain Res Cogn Brain Res 15:1–15. [DOI] [PubMed] [Google Scholar]
- Yantis S, Johnston JC (1990): On the locus of visual selection: Evidence from focused attention tasks. J Exp Psychol Hum Percept Perform 16:135–149. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Woodman GF, Widders D, Marois R, Chun MM (2004): Neural fate of ignored stimuli: Dissociable effects of perceptual and working memory load. Nat Neurosci 7:992–996. [DOI] [PubMed] [Google Scholar]
- Yoshor D, Ghose GM, Bosking WH, Sun P, Maunsell JH (2007): Spatial attention does not strongly modulate neuronal responses in early human visual cortex. J Neurosci 27:13205–13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhaoping L, Zhou T, Fang F (2012): Neural activities in v1 create a bottom‐up saliency map. Neuron 73:183–192. [DOI] [PubMed] [Google Scholar]