Abstract
A few intriguing neuropsychologial studies report dissociations where agraphic patients are severely impaired for writing letters whereas they write digits nearly normally. Here, using functional magnetic resonance imaging (fMRI) together with graphic tablet recordings, we tested the hypothesis that the motor patterns for writing letters are coded in specific regions of the cortex. We found a set of three regions that were more strongly activated when participants wrote letters than when they wrote digits and whose response was not explained by low‐level kinematic features of the graphic movements. Two of these regions (left dorsal premotor cortex and supplementary motor complex) are part of a motor control network. The left premotor activation belongs to what is considered in the literature a key area for handwriting. Another significant activation, likely related to phoneme‐to‐grapheme conversion, was found in the right anterior insula. This constitutes the first neuroimaging evidence of functional specificity derived from experience in the cortical motor system. Hum Brain Mapp 35:6077–6087, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: domain‐specific functional response, writing, kinematic parameters, fMRI
INTRODUCTION
The visual brain can distinguish between arbitrary symbols such as letters and digits [Park et al., 2012 for review; Polk et al., 2002]. This is remarkable because letters and digits have quite similar low‐level visuospatial features, and they are only distinguished by cultural conventions and contexts of use. Most studies point toward a greater tuning of the visual system to letters. Letter recognition is more sensitive to brain damage, especially at the level of the left occipitotemporal cortex [Starrfelt and Behrmann, 2011], and leads to stronger and more focused activations than digits in the same region, along the fusiform gyrus [Flowers et al., 2004].
Preferential responses of a restricted region of the brain to visual perception of letters compared to digits correspond to what Kanwisher [2010] defines as the functional specificity. Functional specificity refers to “greater implication of a given brain region in one function than in other functions.” In its strong sense, functional specificity implies a nearly‐exclusive response of the brain region to the studied category of stimuli, as has been demonstrated in classical examples of “hardwired” functional specificity such as fusiform face area (FFA) or parahippocampal place area (PPA) [Kanwisher, 2010]. An important characteristic of specificity for letters is that it is experience‐dependent. It does not lead to exclusive responses in the visual system, but rather to preferential responses. For example, the left fusiform gyrus strongly responds to letters but also responds significantly to digits [Baker et al., 2007; Polk et al., 2002]. In such contrasts, then, functional specificity is not all or none, but rather a matter of degree. Here, we investigated whether a similar effect can be observed in the motor domain when handwriting movements are produced.
The cortical motor system, especially the premotor cortex, is organized as a function of the effectors it may engage, in combination with broad movement categories (such as pointing, reaching, grasping, or manipulating objects; [Kanwisher, 2010; Rizzolatti and Luppino, 2001; Schubotz et al., 2010]) and stimulus features processed in the course of action (timing, space, object; [Schubotz and von Cramon, 2001]). However, movement categories such as pointing and grasping differ in many respects such as for instance the preshaping of the hand as a function of the object. This organization thus does not correspond to the fine‐grained functionally specific responses of restricted subregions that are observed in the visual system.
Finer grained functional specificity has been proposed for a more complex motor skill, namely writing. The reliance of writing on relatively specialized neural substrates is suggested by the syndrome of pure apraxic agraphia, the acquired inability to produce writing movements in the absence of other motor impairments [Roeltgen, 1993]. Pure apraxic agraphia typically arises from lesions of the left superior parietal lobule [Sakurai et al., 2007] or the left premotor cortex at the “foot of the second frontal circonvolution” [Exner, 1881]. The latter, often referred to as Exner's area, is considered to be critical for coding graphic movements in memory, as its electrical stimulation in nonagraphic patients leads to writing disruptions [Roux et al., 2009]. The few available brain imaging studies of writing are generally consistent with these interpretations. They reliably show premotor and parietal activations in various tasks where actual handwriting movements are required, although the reported premotor activations tend to be more dorsal than the traditional Exner's area [Planton et al., 2013; Purcell et al., 2011]. Note, however, that because writing involves some of the most complex and most highly trained gestures humans master, its assessment by comparison with much simpler tasks typically used in brain imaging studies of writing (finger tapping, drawing of circles…) is debatable.
Motor regions that are as functionally specific as those described in the visual system may be revealed by more fine‐grained comparisons. For instance, some pure apraxic agraphics present with severely compromised letter writing in the face of close to normal digit writing ([Anderson et al., 1990; Delazer et al., 2002; Starrfelt, 2007; Zangwill, 1954]; to our knowledge the complementary dissociation has never been reported). This striking pattern clearly results from a central deficit of motor origin in generating graphemes. Indeed, when the patients attempt to write letters or words, the output consists of completely disorganized sequences of squiggles and strokes (see the illustrations from the patient in [Anderson et al., 1990], for the most striking example), and the movement kinematics are selectively impaired [Delazer et al., 2002]. In the patient reported by Anderson, the most prominent dissociation between letter and digit writing to date, the lesion involved Exner's area described above. Together, these neuropsychological observations rise the intriguing possibility that the retrieval of motor patterns relies on fine‐grained functional specificity is present in the cortical motor system, as it is the case in the visual system.
Investigating writing using brain imaging tools remains quite complex. To this date, graphic movements have never been monitored online during scanning, except in two methodological studies involving single participants [Hauptmann et al., 2009; Tam et al., 2011]. This, however, appears to be crucial to be able to contrast functional specificity from continuous variation along potentially confounded dimensions such as low‐level aspecific factors related to the execution and control of complex hand movements. Here, we report a study in which we recorded the fMRI BOLD signal while participants were instructed to write pairs of letters or pairs of digits under dictation. In addition, we recorded online writing kinematics. This allowed explicitly regressing out the variance related to low‐level kinematic features of the graphic movements at a trial by trial level when assessing the contrasts between letters and digits.
MATERIALS AND METHODS
Participants
Eighteen native French speakers (11 females), aged 18–35 years (mean 24), with normal audition and normal or corrected‐to‐normal vision participated in the experiment. All participants consistently used their right hand to write and to do most of the other daily manual activities (Edinburgh questionnaire ratios ranged between 75 and 100, mean 90). Prescan questionnaires ensured that all participants had normal writing practice. The study had a prior approval by the Ethics Committee of the Aix‐Marseille University and CNRS (N° RCB 2010‐A00155‐34), and the subjects signed a written informed consent after the procedure was fully explained.
Stimuli and Material
We used seven digits (1, 2, 3, 4, 6, 7, 9) and seven letter consonants (B, C, L, M, Q, R, S) matched for their visuospatial and motor complexity. This had been estimated on previous recordings of writing kinematics, performed for all digits and all uppercase letters of the alphabet on an independent group of volunteers. The items of each category were presented by pairs, leading to 24 letter stimuli and 24 digit stimuli. We chose to use pairs of items as stimuli to observe a larger variability in kinematic parameters than would have been obtained with single items. Vowels had been excluded from the pool of letters to avoid the grouping of letter pairs into syllables. The 14 individual items were recorded by a French male speaker in an anechoic room. During scanning, these audio stimuli were dictated, one per trial, through MRI compatible headphones (MR‐confon SILENTA stereo headphones using electrodynamic technology)
To record writing kinematics, we used an MRI compatible graphic tablet, developed locally on the basis of a resistive touch screen device (from Apex Material Technology Corp.) and a USB controller board (TSHARC‐10 from Hampshire Company). Touch activation force range was between 0.1 and 0.8 N. The USB Controller allowed 100 Hz sampling rate. After software calibration, the touch screen resolution was set to 1280 × 1024 pixels, resulting in a 0.3 mm spatial accuracy. The device was inserted into a rigid PVC panel and the controller was embedded in a shielded box. To ensure that no artifacts were detectable on the EPI images, the tablet was pretested prior to the experiment, first with phantoms and then with two pilot participants, The (x,y) coordinates of the pen tip were recorded as a function of time, and displayed online to the participant through a data video projector, a rear projection screen, and a mirror positioned in front of the participant's eyes. The tablet was positioned on top of a cushion over the participant's hips, and could be oriented in the scanner so that the writing posture was comfortable.
A specific stimulus presentation and response recording software was developed using the National Instruments LabVIEW® environment and digital hardware. These allowed triggering and precise synchronization of stimulus presentation and behavioral recordings with the MR scanner clock.
Procedure
Before the fMRI recordings, participants were familiarized with the procedure and trained to write single items and pairs of items on the tablet in a supine position, with the proper visual feedback. They were explicitly told which were the seven digits and seven letters that they would hear during the actual fMRI scanning.
The three experimental conditions (letters, digits, and control) were performed in blocks of four trials. The trials were grouped in blocks of four per condition to facilitate the perceptual processing of the stimuli and to avoid excessive switching between stimulus categories. One experimental trial is schematized in Figure 1a. In the course of one trial, participants first heard a 100 ms beep signaling them that they had to place the pen tip approximately at the center of the tablet. For the letters and digits pairs the beep was followed after 100 ms by the auditory target stimulus (duration range across stimulus: 300 to 600 ms). Participants were instructed to start writing at a natural speed once they had identified the whole pair. In the control condition, participants heard the initial beep and nothing else. They had to place the pen tip on the tablet, and to remain in that initial position throughout the trial. There were no constrains on the writing size. The trial ended 3.3 s after the onset of the auditory stimulus. After this delay, the screen was replaced by a fixation cross for a period randomly varying between 1 and 5.6 s (mean 1.94 s). There were three fMRI runs, each composed of six blocks of each condition. Overall there were 72 trials of each condition, each pair being repeated three times in the course of the experiment.
Figure 1.
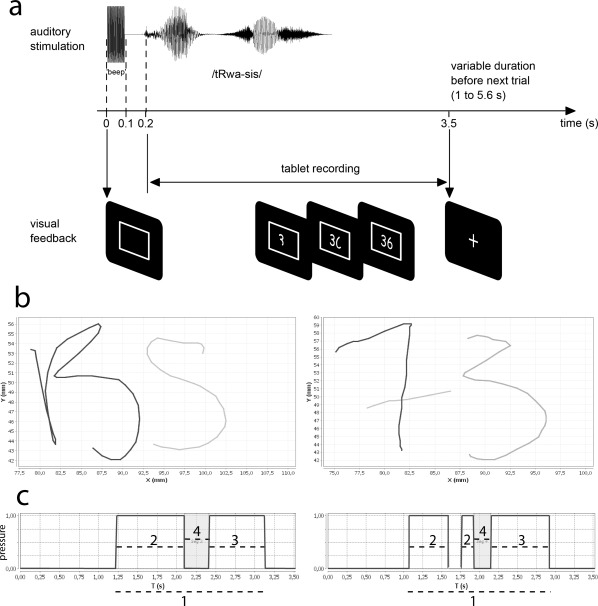
Experimental design and writing kinematics recordings. (a) Temporal structure of a trial. (b) Example of a trial recorded from the graphic tablet for a letter pair and a digit pair. XY coordinates used to calculate the trajectory length. (c) Pressure signal as a function of time, valued 0 when the pen is in the air, and 1 when it is in contact with the tablet. Segment 1 is the total writing duration. Segments 2 and 3 are the duration of the two individual items, used in the calculation of the average velocity. Segment 4 is the duration of the interval between items.
Analysis of Kinematic Data
Kinematic data were analyzed with homemade software providing a segmentation of the trace on the basis of the writing pressure. A segment was defined as a portion of the trajectory between two pen lifts (Fig. 1b). A pen lift was defined as a portion of a trajectory in air between the first character end and the onset of the following character.
For each trial, we extracted the following parameters From the recorded (x,y) trajectories (Fig. 1b):
Overall writing duration: Time between the first nonzero pressure point and last nonzero pressure point.
Mean length of the written trajectory: length of the path of all the segments where pressure was nonzero.
Mean writing velocity: ratio between the length of the written trajectory and the time spent in contact with the tablet.
Time interval between the two items (transition).
Trials with no response, those where only the first item of the pair was written, or where the written response was unrecognizable or unrelated to the stimulus were considered as errors (overall 1% of the trials, discarded from the kinematics analysis and modeled as errors in the fMRI data analysis). To estimate the relationship between kinematic parameters and the categorical contrast between letters and digits, the parameters averaged per participant were compared for letters and digits using paired Student t‐tests.
fMRI Data Recording and Analysis
Participants were scanned on a 3‐T MEDSPEC 30/80 AVANCE whole‐body imager (Bruker, Ettlingen, Germany), equipped with a circular polarized head coil. We first acquired a high‐resolution structural T1‐weighted image (3D sagittal volume using MPRAGE sequence). For functional imaging, we used a T2*‐weighted FID‐echo planar sequence (EPI), covering the whole brain with 36 interleaved 3‐mm‐thick axial slices with a 1 mm gap, parallel to the AC–PC plane (repetition time ‐ 2400 ms, echo time ‐ 30 ms, flip angle 82°, field of view 192 × 192 mm‐ matrix 64 × 64, voxel resolution of 3 × 3 × 4 mm). 167 functional images were acquired per session. A fieldmap acquisition was acquired to correct geometrical deformations on EPI images. A three‐dimensional gradient echo sequence with two echoes (echo times 3.7 and 8.252 ms) was used.
One participant was discarded from the analysis due to head movement artifacts in the fMRI data. Data were processed using the spm8 software, according to the general linear model [Friston et al., 1995]. The first four functional volumes of each session were removed to eliminate nonequilibrium effects of magnetization. The remaining 163 images were corrected for differences in slice acquisition time, and after this step we also discarded the first volume to prevent invalid temporal interpolation.
Fieldmaps were processed for each participant using the FieldMap toolbox implemented in SPM8 [Hutton et al., 2002]. The images were then realigned to the first image and corrected for interactions between movements and field inhomogeneities using the fielmap [Andersson et al., 2001], which allows the measured static distortions to be included in the estimation of distortion changes associated with head motion. Each participant's structural image was then coregistered to the mean of the motion‐corrected functional images using normalized mutual information, and segmented using affine registration to an international consortium for brain mapping (ICBM)/montreal neurologic institute (MNI) template space. The spatial normalization parameters resulting from the previous step were then applied to the functional images to allow for intersubject analysis, and finally the images were smoothed using a 9 mm FWHM Gaussian kernel.
fMRI Statistical Analysis
After preprocessing, individual functional images were entered in a first level general linear model with LETTERS, DIGITS, and CONTROL conditions modeled as events convolved with the hemodynamic response function (HRF), and a separate regressor for errors when necessary.
Another set of individual models was built where the kinematics parameters writing duration, velocity, and intervals between items were entered as additional parametric regressors over all the trials, independently from the stimulus category. One model was built for each parameter and each subject leading to three parametric models per participant. For each run, the values of the parameters for a given condition were centered to zero and only their relative variations were considered in the contrasts. This allowed explicitly regressing out the variance related to low‐level kinematic features of the graphic movements at a trial by trial level when assessing the contrasts between letters and digits. Importantly, the parametric regressors represent the trial by trial variations of the parameters for all writing trials, independent of the stimulus category. The structure of those regressors, therefore, encompasses both intra‐ and inter‐categories individual variations.
The contrasts Letters vs. Digits and Digits vs. Letters were entered in second‐level one‐sample t‐tests. The effects of the three kinematic parameters were also tested at the group level using one‐sample t‐tests. Activations were considered significant if they reached a threshold of P < 0.001, uncorrected for multiple comparisons at the voxel level, and a FWE‐corrected threshold of P < 0.05 at the cluster level. Significant activations were localized using a brain atlas [Duvernoy, 1999]. In the figures, all the activations are displayed overlaid on the normalized structural MRI of one of the participants.
RESULTS
Analysis of Writing Kinematics
The writing kinematics were summarized by three parameters, extracted from the (x,y) coordinates of the pen tip as a function of time (Fig. 1c): overall writing duration (Fig. 1c, Value 1), average velocity (Fig. 1c, overall trajectory length divided by the sum of Values 2 and 3), and time interval between items (Fig. 1c Value 4). We did not consider the whole trajectory length separately because it was highly correlated to average velocity (a typical effect in graphic movements [Binet and Courtier, 1893]). Letter and digit writing performances differed significantly in terms of overall durations and average velocity. Participants produced faster movements for letters (mean 77.9 and 74.2 pixels/s for letters and digits, respectively, Student's t‐test P < 0.01), along with longer durations (mean 2.06 and 1.95 s for letters and digits resp., Student's t‐test P < 0.003). The time interval between items was not significantly different for letters and digits (mean 0.273 and 0.267 s for letters and digits, resp.). Together, these analyses indicate that letter and digit writing performance is substantially variable, and that these two categories of items do not have similar kinematics characteristics. These relatively small yet systematic variations need to be considered explicitly in the fMRI models, as described below.
fMRI Data Analysis : Contrast Between Letters and Digits and Influence of Kinematic Parameters
At the first level, we submitted the preprocessed fMRI data to a classical general linear model analysis where the three conditions, Letters, Digits, and Control were modeled as events. In addition, we built another set of three models per participant where the three kinematic parameters defined above were entered as additional parametric regressors over all the trials, independently from the stimulus category. Figure 2a shows the results of the group second‐level contrast between letters and digits when only the categorical difference is modeled. Figure 2b shows the results when both the categorical difference and the effects of writing kinematics are considered jointly at the first level. Specifically, Figure 2b represents the conjunction of the group contrasts between digits and letters resulting from the three models where the kinematics parameters were accounted for :{Letters vs. Digits controlled for writing duration, Letters vs. Digits controlled for velocity, Letters vs. digits controlled for interval}. This conjunction shows brain regions that are consistently more activated for Letters than Digits, but whose activation is not explained by trial by trial variations in how the graphic movement was performed, nor by a confounded effect of stimulus category on the kinematic parameters.
Figure 2.
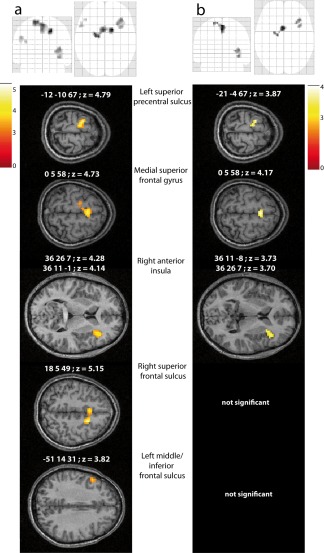
Results of the contrast between LETTERS and DIGITS viewed on glass brains, and on axial slices from an individual normalized MRI. (a) Model of the categorical difference only. (b) Conjunction {Letters vs. Digits controlled for duration, Letters vs. Digits controlled for velocity, Letters vs. digits controlled for interval}. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Significant activations were observed in three regions corresponding to the medial superior frontal gyrus, the left superior precentral sulcus, and the right anterior insula (the latter showing two distinct peaks). A cluster in the depth of the right superior frontal sulcus was only significant when the effects of kinematic variables were not considered (i.e., in Fig. 2a). Another region displayed a preferred response to letters in the simpler model, but its activation was only marginally significant (cluster FWE‐corrected P = 0.063, 49 voxels). We report it because it lies at the junction between the left inferior and middle frontal gyri, anatomically close to the area of damage of the patient reported by Anderson et al. [1990], although slightly more anterior and more ventral. This activation difference was completely washed out by the inclusion of the kinematic parameters in the models.
Because the three former activations survive the inclusion of the kinematic parameters in the statistical models, they are taken to represent performance‐invariant tuning to symbols from a specific domain of knowledge. As expected, two of the clusters were observed in frontal regions belonging to the cortical motor system and corresponding functionally to the supplementary motor complex [Nachev et al., 2008] and dorsal premotor cortex [Schubotz et al., 2010].
The opposite Digits vs. Letters contrast did not reveal any significant activation at the specified threshold. However, when considering smaller clusters (P < 0.001, uncorrected for multiple comparisons at the cluster level), we observed significant activations in the left insula (−36 −19 16) and the left angular gyrus (−42 −76 31). These two activations are presented in Figure 3.
Figure 3.
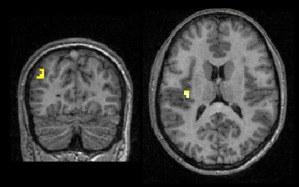
Activations uncorrected for multiple comparisons in the left angular gyrus (−39 −76 31; z‐score = 3.46; 10 voxels) and left posterior insula (−36 −19 13; z‐score = 4.01; 17 voxels) in the contrast Digits vs. Letters. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Preferential vs. Exclusive Responses to Letters
The three regions evidenced in the contrast between letters and digits may result from a selective recruitment for letters or, alternatively, from an increased response to letters compared to digits. To clarify this alternative, we computed the conjunction between the contrasts Letters vs. Control and Digits vs. Control, which assessed the broad sensorimotor network recruited by handwriting compared to holding the pen still (Fig. 4 and Table 1).
Figure 4.
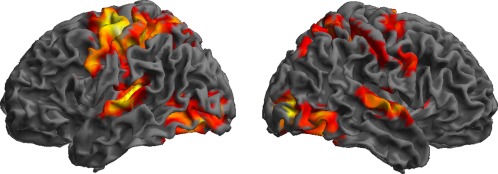
Surface overlay of the network involved in controlling graphic movements compared to holding the pen still (conjunction of Letters vs. Control and Digits vs. Control contrasts; see also Table 1). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Regions activated in the conjunction between Letters vs. Control and Digits vs. Control, showing a main effect of writing vs. holding the pen still on the tablet
| Lobe | Region | Hemisphere | Peak z‐value | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Cb | Cerebellar Hemisphere | R | 6.71 | 27 | −46 | −26 |
| Cb | Cerebellar Hemisphere | R | 6.10 | 15 | −64 | −41 |
| Cb | Cerebellar vermis | M | 5.97 | 6 | −52 | −8 |
| Cb | Cerebellar Hemisphere | L | 5.69 | −27 | −55 | −26 |
| Cb | Cerebellar Hemisphere | L | 5.51 | −15 | −64 | −44 |
| O | Middle occipital gyrus, superior part | R | 5.41 | 41 | −61 | −8 |
| O | Superior occipital gyrus | R | 6.20 | 30 | −88 | −2 |
| F/P | Central sulcus | L | 6.17 | −36 | −22 | 52 |
| F | Superior frontal sulcus | L | 6.02 | −27 | −7 | 58 |
| SC | Thalamus | L | 5.97 | −15 | −22 | −2 |
| SC | Putamen | L | 5.25 | −18 | −7 | 4 |
| T | Superior temporal gyrus | L | 5.73 | −42 | −28 | 10 |
| T | Superior temporal gyrus | R | 5.60 | 60 | −10 | 4 |
Only the activations reaching a threshold of P < 0.05 FWE‐corrected at the voxel level are shown for the sake of space. For a global overview of the activations, see Supporting Information Figure 1.
The result of this conjunction was used as an inclusive mask for the network reported in Figure 2b. As shown in Figure 5a, the left superior precentral cluster was embedded in a wide precentral and postcentral activation. Similarly, the medial frontal cluster constitutes the most anterior part of a broader cluster spreading over medial frontal and cingulate cortices. Those two regions, therefore, show preferential responses to letters but also significant responses to digits (Fig. 5b), and belong to a broad network sustaining graphic movements. Conversely, the anterior insula was not activated in the main Writing vs. Control contrast. Figure 5b indicates that neither the Letter vs. Control nor the Digit vs. Control contrasts were individually significant in that region, which responded exclusively when Letters and Digits were compared. This was also further confirmed by checking that the activations in the Letters vs. Control contrast taken separately did not include the anterior insular cluster. This exclusive response of the anterior insula, therefore, represents a clear domain‐selective activation, but most likely not specific to graphic movements.
Figure 5.
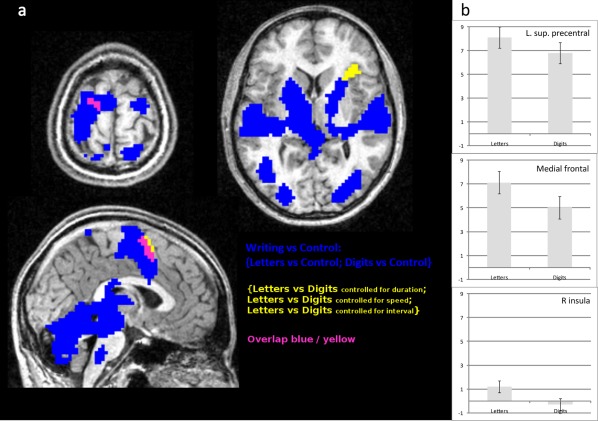
Preferential vs. exclusive responses to letters. (a) Broad sensorimotor network sustaining writing relative to holding the pen still on the tablet (blue, see Supporting Information Table 1 for quantitative results) superimposed on the results reported on Figure 2b. (b) Values of the contrasts Letters vs. Control and Digits vs. Control in the left superior precentral gyrus, the medial superior frontal gyrus, and the right anterior insula.
Parametric Effects of Writing Performance
When assessed separately, the parametric regressors Writing velocity and Interval between items had no significant effect on the BOLD signal at the specified FWE clusterwise corrected threshold. Conversely, as can be seen in Figure 6, writing duration had a strong effect on the signal, the longer the movement duration the greater the activation in a broad network composed mainly of precentral, postcentral and superior parietal regions, subcortical and cerebellar structures, and parts of the visual system (fusiform and middle occipital gyri) and superior temporal sulcus, strongly lateralized on the right hemisphere (see Table 2). The three regions evidenced in the Letters vs. Digits parameter contrast are not part of this network, as expected by the design of the statistical models. This further confirms that the observed differences between Letters and Digits are not related to low‐level kinematic features.
Figure 6.
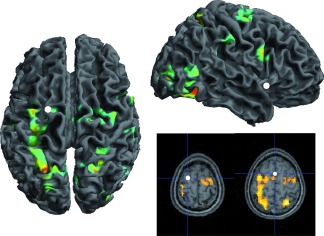
Network of regions parametrically modulated by writing duration, for both letters and digits. The statistical values and position of the activations can be found in Supporting Information Table 2. The white patches indicate the position of the three regions evidenced in the Letters vs. Digits parameter contrast. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Regions displaying a parametric effect of writing duration
| Lobe | Region | Hemisphere | Peak z‐value | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| O | Fusiform gyrus | R | 5.28 | 36 | −58 | −20 |
| O | Superior occipital gyrus | R | 4.02 | 33 | −88 | 1 |
| O | Fusiform gyrus | L | 3.70 | −30 | −52 | −23 |
| F | Precentral gyrus | L | 4.60 | −33 | −4 | 61 |
| F | Inferior frontal gyrus | R | 4.43 | 60 | 8 | 25 |
| F | Precentral gyrus | R | 3.94 | 21 | −16 | 64 |
| F/P | Central sulcus | L | 4.38 | −33 | −22 | 64 |
| P | Superior parietal gyrus | L | 4.92 | −24 | −61 | 61 |
| P | Postcentral gyrus | L | 4.38 | −36 | −37 | 55 |
| P | Angular gyrus | L | 4.37 | −21 | −76 | 37 |
| P | Superior parietal gyrus | R | 4.89 | 27 | −58 | 58 |
| P | Supramarginal gyrus | R | 3.70 | 33 | −40 | 46 |
| P | Postcentral gyrus | L | 4.16 | −54 | −22 | 31 |
| P | Supramarginal gyrus | R | 3.79 | 54 | −28 | 34 |
| PO | Precuneus | M | 4.20 | 0 | −58 | 37 |
| SC | Caudate nucleus | R | 4.40 | 6 | −7 | 19 |
| SC | Thalamus | R | 4.19 | −9 | −28 | −2 |
| T | Middle temporal gyrus | R | 4.25 | 45 | −64 | −2 |
| Cb | Cerebellar hemisphere | R | 4.53 | 33 | −49 | −47 |
| Cb | Cerebellum (vermis) | R | 4.34 | 12 | −61 | −17 |
| Cb | Cerebellar hemisphere | L | 3.97 | −27 | −52 | −35 |
| Cingulate gyrus | M | 4.05 | −3 | −28 | 34 | |
DISCUSSION
The activations observed for letter writing compared to digit writing in the left superior precentral gyrus and medial frontal cortex are preferential, rather than exclusive to the letter domain because they are also significant, although smaller, for writing digits. In addition, they are independent from low‐level kinematic parameters and, therefore, motor execution processes. Because of their spatial localization, in brain regions having a pivotal role in motor processes, they likely compute higher‐level motor information related to the selection and/or implementation of highly trained and practiced graphic motor patterns. Building on previous studies that investigated the contrast between letters and digits in the visual domain [Baker et al., 2007; Flowers et al., 2004; Polk et al., 2002], our results indicate very fine‐grained functional specificity derived from experience in the human cortical motor system. Further investigations, where letters writing would be contrasted with other types of graphic shapes, are necessary to confirm this first evidence.
Conversely, the insula is a typical lesion site in phonological agraphia, a disorder where patients have difficulties with phoneme‐grapheme conversion rules [Roeltgen, 1993] which could explain why its activation is exclusive for letters in this study (see also [Joseph et al., 2006; Kersey and James, 2013], for selective insular activation in relation to phonological processing of letters).
How can functional specificity emerge with experience? A possible explanation of why some neural populations might get more finely tuned to the graphic production of letters relative to numbers can be found in earlier work by Polk and Farah [Polk and Farah, 1998] in the visual domain. These authors developed the “co‐occurence” hypothesis, building on the fact that letters tend to co‐occur more often and in a more correlated spatial fashion than digits or symbols. This strong statistical spatiotemporal organization would promote the segregation of neural tissue underlying letter visual recognition through Hebbian mechanisms. In addition, Polk and Farah [Polk and Farah, 1995] hypothesized that “the co‐occurrence hypothesis could explain other examples of environmental influences such as, for example, the neural segregation of handwriting compared with manual control tasks” (p 649). In favor of this view is the repeated evidence from brain imaging studies that the left superior frontal/superior precentral sulcus holds a major importance in handwriting [Longcamp et al., 2003; Planton et al., 2013; Purcell et al., 2011; Roux et al., 2009; Sugihara et al., 2006] and the idea that Exner's area is “especially trained from childhood through the formation of engrams to function as a writing center” [Nielsen, 1946]. Because the processing of digits in the course of learning is less correlated in space and time [Polk and Farah, 1995], the representations built in superior precentral cortex for digits might be less stable than those constructed for letters. Converging results motor learning research support the idea that both the dorsal premotor cortex and supplementary motor cortex have a pivotal role in motor learning, through, respectively, visuomotor integration and sequential processing [Hardwick et al., 2013]. It is noteworthy that the opposite contrast between Digits and Letters only yielded activations visible at a threshold uncorrected for multiple comparisons, in the left angular gyrus and posterior insula. In the visual domain, only two recent studies reported a region of the visual ventral stream as being more responsive to digits than letters [Park et al., 2012; Shum et al., 2013]. Clearly, specificity of the neural responses to digits is less reliable than specificity for letters. This might extend to the motor domain.
Although our fMRI results were controlled for low‐level kinematic effects, it remains that letters and digits also differed on average on basic execution parameters. Letters were associated to longer durations and were written faster than digits. Qualitative or quantitative differences in the movements performed to write letters and digits or symbols are already detectable in very young children aged around two [Yamagata, 2007], undergo a strong dissociation around age six [Adi‐Japha and Freeman, 2001] and are still measurable in adults [Delazer et al., 2002] as in this study. This is another possible consequence of different contextual handwriting training effects for letters and digits leading to more stable cortical representations of letters. Indeed, more stable central representations are likely to impact execution parameters such as velocity or duration, as shown in the course of handwriting acquisition and practice [Zesiger et al., 1993].
The contrast between letters and digits also revealed two regions whose activations were no longer significant when the kinematic parameters were included in the analysis. This means that their activation in the Letter vs. Digit contrast is driven by low‐level sensori‐motor processes that tend to differ between letters and digits (in terms of input and output parameters, see above).
These results shed new light on the brain correlates of handwriting movements, which have remained poorly understood up to now partly because of the inaccurate matching between the writing task and its control condition, and the lack of behavioral control in existing studies. As shown by both the results of the Writing vs. Control contrast and the parametric effect of writing duration, writing engages an extended motor‐perceptual network. Only two very restricted areas discriminate between letters and digits. The dorsal premotor cortex in particular seems to be a fundamental node of the brain network sustaining handwriting, as a counterpart of Exner's area, originally defined in brain‐damaged agraphic patients [Exner, 1881]. Altogether, our results support the idea first developed by Anderson et al.[Anderson et al., 1990] that the motor patterns for producing letters compared to digits are implemented in specific precentral regions. Notably, contrary to the strong dissociation evidenced in brain‐damaged patients by several authors [Anderson et al., 1990; Delazer et al., 2002; Starrfelt, 2007; Zangwill, 1954], our results suggest a difference of degree of preference rather than a strong categorical difference between letters and digits in those regions. In fact such extreme cases of dissociations between letters and digits remain rare, and clinicians report more a tendency of stronger disturbance of writing movements for letters than for digits in apraxic agraphic patients, even if this tendency is not systematically evaluated, especially not with tools such as graphic tablets that would allow an accurate quantification of the performance (Michel Habib, personal communication). Finally, the supplementary motor and dorsal premotor cortex have been reported as strongly activated during visual perception of handwritten compared to printed letters [Longcamp et al., 2011; Nakamura et al., 2012], also consistent with the idea that they compute an important information for online simulation of handwriting movements. Anderson's patient was also left unable to correctly recognize visually presented letters, pointing to a possible important functional role of those writing centers for reading letters and words.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
This work was supported by IFR Sciences du Cerveau et de la Cognition. We are grateful to Jean Claude Gilhodes, Jasmin Sadat, and Michel Habib for support on various aspects of the study. The work was performed at Lab. Neurosciences Cognitives and Centre IRMf.
REFERENCES
- Adi‐Japha E, Freeman NH (2001): Development of differentiation between writing and drawing systems. Dev Psychol 37:101–114. [PubMed] [Google Scholar]
- Anderson SW, Damasio AR, Damasio H (1990): Troubled letters but not numbers: Domain specific cognitive impairments following focal damage in frontal cortex. Brain 113:749–766. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K (2001): Modeling geometric deformations in EPI time series. NeuroImage 13:903–919. [DOI] [PubMed] [Google Scholar]
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N (2007): Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci USA 104:9087–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet A, Courtier J (1893): Sur la vitesse des mouvements graphiques. Rev Philos France Let 35:664–671. [Google Scholar]
- Delazer M, Lochy A, Jenner C, Domahs F, Benke T (2002): When writing 0 (zero) is easier than writing O (o): A neuropsychological case study of agraphia. Neuropsychologia 40:2167–2177. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (1999): The Human Brain: Surface, Blood Supply, and Three‐Dimensional Sectional Antomy, Vol. 2 Wien, New York: Springer. [Google Scholar]
- Exner S (1881): Untersuchungen uber die localisation der functionen in der groáhirnrinde des menschen. Wien: Wilhelm BraumÂller. [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, Eden GF (2004): Attention to single letters activates left extrastriate cortex. NeuroImage 21:829–839. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SRC, Frackowiak RSJ, Turner R (1995): Analysis of fMRI time‐series revisited. NeuroImage 2:45–53. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013): A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage 67:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann B, Sosnik R, Smikt O, Okon E, Manor D, Kushnir T, Flash T, Karni A (2009): A new method to record and control for 2D‐movement kinematics during functional magnetic resonance imaging (fMRI). Cortex 45:407–417. [DOI] [PubMed] [Google Scholar]
- Hutton C, Bork A, Josephs O, Deichmann R, Ashburner J, Turner R (2002): Image distortion correction in fMRI: A quantitative evaluation. NeuroImage 16:217–240. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Cerullo MA, Farley AB, Steinmetz NA, Mier CR (2006): fMRI correlates of cortical specialization and generalization for letter processing. NeuroImage 32:806–820. [DOI] [PubMed] [Google Scholar]
- Kanwisher N (2010): Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA 107:11163–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey AJ, James KH (2013): Brain activation patterns resulting from learning letter forms through active self‐production and passive observation in young children. Front Psychol 4:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcamp M, Anton JL, Roth M, Velay JL (2003): Visual presentation of single letters activates a premotor area involved in writing. NeuroImage 19:1492–1500. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Hlushchuk Y, Hari R (2011): What differs in visual recognition of handwritten vs. printed letters? An fMRI study. Hum Brain Mapp 32:1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008): Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9:856–869. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kuo W‐J, Pegado F, Cohen L, Tzeng OJL, Dehaene S (2012): Universal brain systems for recognizing word shapes and handwriting gestures during reading. Proc Natl Acad Sci USA 109:20762–20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JM (1946): Agnosia, apraxia, aphasia: Their value in cerebral localization (2nd ed.). New York: Paul B. Hoeber. [Google Scholar]
- Park J, Hebrank A, Polk TA, Park DC (2012): Neural dissociation of number from letter recognition and its relationship to parietal numerical processing. J Cogn Neurosci 24:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planton S, Jucla M, Roux F‐E, Démonet J‐F (2013): The “handwriting brain”: A meta‐analysis of neuroimaging studies of motor versus orthographic processes. Cortex 49:2772–2787. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ (1995): Brain localization for arbitrary stimulus categories: A simple account based on Hebbian learning. Proc Natl Acad Sci USA 92:12370–12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Farah MJ (1998): The neural development and organization of letter recognition: Evidence from functional neuroimaging, computational modeling, and behavioral studies. Proc Natl Acad Sci USA 95:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D'Esposito M, Detre DA, Farah MJ (2002): Neural specialization for letter recognition. J Cogn Neurosci 14:145–159. [DOI] [PubMed] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, Rapp B (2011): Examining the central and peripheral processes of written word production through meta‐analysis. Front Psychol 2:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001): The cortical motor system. Neuron 31:889–901. [DOI] [PubMed] [Google Scholar]
- Roeltgen D (1993): Agraphia In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology, 3rd ed New York: Oxford University Press; Vol. 2, pp 63–89. [Google Scholar]
- Roux F‐E, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, Démonet J‐F (2009): The graphemic/motor frontal area Exner's area revisited. Ann Neurol 66:537–545. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Onuma Y, Nakazawa G, Ugawa Y, Momose T, Tsuji S, Mannen T (2007): Parietal dysgraphia: Characterization of abnormal writing stroke sequences, character formation and character recall. Behav Neurol 18:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY (2001): Functional organisation of the lateral premotor cortex: fMRI reveals different regions activated by anticipation of object properties, location and speed. Cogn Brain Res 11:97–112. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Anwander A, Knösche TR, von Cramon DY, Tittgemeyer M (2010): Anatomical and functional parcellation of the human lateral premotor cortex. NeuroImage 50:396–408. [DOI] [PubMed] [Google Scholar]
- Shum J, Hermes D, Foster BL, Dastjerdi M, Rangarajan V, Winawer J, Miller KJ, Parvizi J (2013): A brain area for visual numerals. J Neurosci 33:6709–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrfelt R (2007): Selective alexia and agraphia sparing numbers‐a case study. Brain Lang 102:52–63. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Behrmann M (2011): Number reading in pure alexia—A review. Neuropsychologia 49:2283–2298. [DOI] [PubMed] [Google Scholar]
- Sugihara G, Kaminaga T, Sugishita M (2006): Interindividual uniformity and variety of the “Writing center”: A functional MRI study. NeuroImage 32:1837–1849. [DOI] [PubMed] [Google Scholar]
- Tam F, Churchill NW, Strother SC, Graham SJ (2011): A new tablet for writing and drawing during functional MRI. Hum Brain Mapp 32:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K (2007): Differential emergence of representational systems: Drawings, letters, and numerals. Cogn Dev 22:244–257. [Google Scholar]
- Zangwill OL (1954): Agraphia due to a left parietal glioma in a left handed man. Brain 77:510–520. [DOI] [PubMed] [Google Scholar]
- Zesiger P, Mounoud P, Hauert CA (1993): Effects of lexicality and trigram frequency on handwriting production in children and adults. Acta Psychol (Amst) 82:353–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


