Abstract
The application of global signal regression (GSR) to resting‐state functional magnetic resonance imaging data and its usefulness is a widely discussed topic. In this article, we report an observation of segregated distribution of amygdala resting‐state functional connectivity (rs‐FC) within the fusiform gyrus (FFG) as an effect of GSR in a multi‐center‐sample of 276 healthy subjects. Specifically, we observed that amygdala rs‐FC was distributed within the FFG as distinct anterior versus posterior clusters delineated by positive versus negative rs‐FC polarity when GSR was performed. To characterize this effect in more detail, post hoc analyses revealed the following: first, direct overlays of task‐functional magnetic resonance imaging derived face sensitive areas and clusters of positive versus negative amygdala rs‐FC showed that the positive amygdala rs‐FC cluster corresponded best with the fusiform face area, whereas the occipital face area corresponded to the negative amygdala rs‐FC cluster. Second, as expected from a hierarchical face perception model, these amygdala rs‐FC defined clusters showed differential rs‐FC with other regions of the visual stream. Third, dynamic connectivity analyses revealed that these amygdala rs‐FC defined clusters also differed in their rs‐FC variance across time to the amygdala. Furthermore, subsample analyses of three independent research sites confirmed reliability of the effect of GSR, as revealed by similar patterns of distinct amygdala rs‐FC polarity within the FFG. In this article, we discuss the potential of GSR to segregate face sensitive areas within the FFG and furthermore discuss how our results may relate to the functional organization of the face‐perception circuit. Hum Brain Mapp 36:4089–4103, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: amygdala, fusiform gyrus, functional magnetic resonance imaging, resting‐state, global signal regression, face perception
INTRODUCTION
The application of global signal regression (GSR) to resting‐state functional magnetic resonance imaging (rs‐fMRI) data with the purpose of removal of non‐neuronal noise and its usefulness is an ongoing discussed topic. As regression against the global mean signal has been shown to shift correlation distributions toward a mean correlation value close to zero, a fundamental argument against application of GSR is the artificial introduction of negative correlations [Murphy et al., 2009, Weissenbacher et al., 2009]. Conversely, and in favor of GSR, it has been argued that GSR may also remove a true shared covariation in firing rate (i.e., a true global neuronal signal), thereby revealing relationships of neuronal populations otherwise masked by the dominant global signal [Fox et al., 2009; Keller et al., 2013; Scholvinck et al., 2010]. In this study, we offer support for another potential benefit of GSR, based on an unpublished observation that was done within the context of data analyses in a recent study targeting the impact of genetic risk variants on resting‐state functional connectivity (rs‐FC) between the amygdala and fusiform gyrus (FFG) [Kruschwitz et al., 2015]. During data analyses we observed that, when GSR was performed, amygdala rs‐FC delineated subregions of the FFG that spatially correspond to the commonly reported face sensitive areas, namely the occipital face area (OFA) and fusiform face area (FFA). Specifically, we observed that after applying GSR a cluster of positive amygdala rs‐FC approximately corresponded to the FFA while a cluster of negative rs‐FC corresponded best to the OFA.
In the current article, we describe this observation in detail, test for reliability of effects over different samples, and examine whether these amygdala rs‐FC defined clusters may indeed correspond to the face sensitive areas in the FFG. While doing so, we tested the following hypotheses, and applied the respective post hoc analyses to our data (which are described in detail in the methods section of this manuscript): first, if amygdala rs‐FC defined clusters do correspond to the commonly reported face sensitive areas in the FFG (i.e., FFA and OFA), then face sensitive areas as engaged by a face matching task should spatially map onto these rs‐FC clusters. To test this hypothesis, we used an emotional face matching task conducted in the same sample, and subsequently mapped task‐fMRI localized face sensitive areas onto the distinct amygdala rs‐FC clusters. Second, if the effect of distinct amygdala rs‐FC clusters is of neuronal origin, it should be possible to replicate this effect in independent subsamples of our data. Therefore, replication of distinct amygdala rs‐FC in the FFG was carried out in three independent subsamples (i.e., research sites) to confirm generalization. Third, if the amygdala rs‐FC defined clusters do correspond to distinct face sensitive areas, then they should, respectively, show differential rs‐FC connectivity patterns to other regions of the visual stream (cf., hierarchical face processing system as proposed by Haxby et al., [2000]) (see short summary at the end of the introduction1). Thus, additional rs‐FC analyses were conducted with the distinct FFG regions as seeds, probing whether the observed segregation of positive versus negative FFG clusters would also be reflected in differential rs‐FC of these clusters to other regions of the visual stream. Fourth, if amygdala defined rs‐FC clusters correspond to face sensitive areas, then, given a hierarchical coupling between regions of the visual stream [Haxby et al., 2000], we would expect the FFA cluster, as compared to the OFA cluster, to show decreases in rs‐FC variance with the amygdala (as a potential indicator of rs‐FC stability over time and thus closer coupling1) [Liao et al., 2014]. To test this hypothesis, we conducted dynamic rs‐FC analyses between these areas using a sliding‐window approach to access differences in rs‐FC variance of these regions over time. Taken together, in this article we provide results that may indicate the usefulness of GSR for unmasking “true” inter‐regional relationships [e.g., Fox et al., 2009].
METHODS
Participants
A group of 276 healthy volunteers (mean age: 33.8 years; range: 18 to 51 years; 143 female; 242 right‐handed; 6 both‐handed) from three different collaborating research centers (Bonn: n = 123, Berlin n = 77, Mannheim n = 76) participated in this study (Table 1). None of the participants reported a lifetime history of psychiatric disorder. Subjects with a history of major neurological disease or first degree relatives with schizophrenia or mood disorder were excluded from the study. Additional exclusion criteria included the presence of pregnancy, and general MRI contraindications. All subjects were of Central European descent. Participants were recruited as part of an ongoing study on neurogenetic risk mechanisms for major mood disorders and schizophrenia [Erk et al., 2010; Esslinger et al., 2009]. The study was approved by the local ethics committees and all subjects gave informed consent.
Table 1.
Distribution of age, years of education, membership of research site, gender, and handedness in the entire sample and among subsamples (age, gender, handedness, and research site were accounted as covariates of no interest in the analyses)
| Measures | Entire sample (n = 276) | Collaborating research sites | ANOVAa, or Chi‐squareb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1: Bonn | Site 2: Berlin | Site 3: Mannheim | |||||||||||
| n | Mean | S.D. | n | Mean | S.D. | n | Mean | S.D. | n | Mean | S.D. | P | |
| Age (years) | 276 | 33.77 | 9.96 | 123 | 33.07 | 10.3 | 77 | 35.47 | 9.24 | 76 | 33.2 | 10.12 | 0.212a |
| Years of education | 276 | 15.55 | 2.54 | 123 | 15.7 | 2.73 | 77 | 15.31 | 2.55 | 76 | 15.55 | 2.23 | 0.573a |
| Participants (n) | 276 | 123 | 77 | 76 | <0.001*, b | ||||||||
| Gender (n) | |||||||||||||
| Male | 133 | 59 | 39 | 35 | 0.848b | ||||||||
| Female | 143 | 64 | 38 | 41 | |||||||||
| Handedness (n) | |||||||||||||
| Right‐handed | 242 | 115 | 57 | 70 | 0.01*, b | ||||||||
| Left‐handed | 21 | 8 | 8 | 5 | |||||||||
| Both‐handed | 6 | 0 | 5 | 1 | |||||||||
ANOVA: between subjects factor: research site.
Chi‐squared test with all research sites.
*P < 0.05.
Tasks
Participants completed a 5 min resting‐state scan in which they were instructed to relax, keep their eyes closed, not to focus on any specific thoughts and not to fall asleep. Additionally to the resting‐state scan, subjects completed a well‐established emotional face matching task. In short, the task had two conditions: in the face condition, subjects had to decide which of two simultaneously presented faces depicted the same emotion as a third face presented at the same time below the two faces. In the control condition, the task was to match geometrical figures. [For a detailed description compare: Esslinger et al., 2009; Hariri et al., 2002; Meyer‐Lindenberg et al., 2009; Tost et al., 2010]. For analysis of amygdala rs‐FC within the FFG, the main effect (face > control condition) was used to construct a sample specific spatially defined mask covering the FFG (one subject included in the resting‐state analysis did not complete the face matching task). Post hoc, this task also served as a localizer to spatially localize the commonly reported face sensitive areas within the FFG (i.e., FFA and OFA).
fMRI Data Acquisition
Resting‐state and task‐fMRI measurements were performed using a standardized protocol at all three sites. For each subject, gradient echoplanar imaging (EPI) volumes (resting‐state: 150 volumes; task‐fMRI: 130 volumes) depicting BOLD contrast were acquired at three identical 3 T Siemens Trio scanners in Berlin, Bonn, and Mannheim. For the resting‐state and task‐fMRI scans, the following scanning parameters were used: number of axial slices = 28, slice thickness = 3 mm, interslice gap = 1 mm, voxel size = 3 × 3 × 3, flip angle = 80°, TR = 2 s, TE = 30 ms. Quality control measurements were conducted at all sites on every day of data collection according to a multicenter quality assurance protocol [Friedman and Glover, 2006], revealing stable signals over time and comparable quality between sites.
fMRI Data Analysis Software
For resting‐state fMRI, single subject data preprocessing was carried out using the Data Processing Assistant for Resting‐State fMRI [Yan, 2010]. Single subject data processing for task‐fMRI was carried out in SPM8 [please refer to Esslinger et al., 2009; Meyer‐Lindenberg et al., 2009 for more detail]. Second‐level group analyses for both, resting‐state as well as task‐fMRI data, were performed using the statistical parametric‐mapping software package SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Sliding‐window analysis of the resting‐state data was carried out using the DynamicBC toolbox [Liao et al., 2014]. All three software packages were implemented under Matlab R2011b (The Math Works Inc., http://www.mathworks.com).
fMRI Data Preprocessing
Resting‐state fMRI data preprocessing
In favor of signal equilibrium and to minimize artifacts triggered by adaption to scanner noise, the first 10 volumes of data for each participant were removed from the analysis. The remaining volumes underwent slice timing correction, realignment, normalizing (standard EPI template, 3 × 3 × 3 mm voxels), and smoothing procedures (8 mm full width at half maximum [FWHM] Gaussian kernel: main analysis; 3 mm FWHM kernel: reanalysis of the data to rule out spurious results due to blurred time signals from neighboring voxels). Subsequently, the data were detrended to remove systematic signal changes induced by long‐term physiological shifts, movement related noise or instrumental instability. Moreover, a temporal filter (0.01–0.08 Hz) was applied to minimize effects of high‐frequency noise and low‐frequency drift. Finally, multiple‐regression was performed on the data to remove possible sources of artifacts (six estimated movement parameters, global mean signal, cerebrospinal fluid signals, white matter signals) interfering with the low frequency signal fluctuations of interest. Additionally, to test whether observed effects would be replicated when no global mean signal would be removed, we also performed the described multiple‐regression method without removal of the global mean signal.
Task fMRI data preprocessing
The acquired volumes underwent slice timing correction (to correct for time differences in slice acquisition), realignment (to spatially correct the confounding effects of subject movement), normalizing (spatial normalization to the standard EPI template and resampled at 3 × 3 × 3 mm voxels) procedures and were smoothed with a 8 mm FWHM kernel to account for individual variations of the anatomical landmarks.
FFG Mask
First, to obtain a sample specific region of interest (ROI) mask for analysis of amygdala rs‐FC within the FFG, a functional mask was created based on the subject's task responsive activity (one subject included in the resting‐state analysis did not complete the face matching task). For this purpose, a one‐sample t‐test was computed to derive whole‐brain activation for the faces task (contrast: faces > shapes), which was subsequently masked with a template of the FFG using the WFU_pickatlas (atlas = “human‐atlas AAL; Version 2.5, Wake Forest University, School of Medicine, Winston‐Salem, North Carolina; http://www.ansir.wfubmc. edu”). The left and right FFG were combined in one mask. To increase spatial specificity of the mask, the activation corresponding T‐values were set to T ≥ 15, such that only the most significant voxels contributed to the final functional FFG mask (volume of the mask: 1062 voxels). The mask for the FFG is shown in Supporting Information Fig. 1.
fMRI Data Analysis Pathway
The a priori defined analyses (Resting‐state amygdala seed definition section – Analysis of amygdala rs‐FC with the FFG section) were conducted within the context of a recent study about the impact of genetic risk variants and personality on rs‐FC between the amygdala and FFG [Kruschwitz et al., 2015]. Based on the observations in the a priori analyses, effects were target to post hoc follow‐up analyses (Localization and definition of face sensitive areas using task‐fMRI section to Analysis of rs‐FC of amygdala rs‐FC defined FFA and OFA clusters back to the amygdala and among themselves section) to determine their reliability and nature in more detail.
A priori defined analyses
Resting‐state amygdala seed definition
To determine amygdala rs‐FC, the right and left amygdala were separately defined as AAL Atlas volume‐based seed regions (left amygdala seed: 62 voxel; right amygdala seed: 70 voxel; Supporting Information Fig 1).
Analysis of amygdala rs‐FC with the FFG
After computing separate voxel‐wise correlations with the signal extracted from the left and right amygdala (i.e., seed based functional connectivity analysis), the obtained correlation coefficients (Pearson correlation) for each participant underwent Fisheŕs r‐to‐z conversion to obtain z‐values that fulfilled the requirements of a normal distribution.
To determine significant amygdala rs‐FC within the FFG, the individual z‐scaled amygdala rs‐FC maps (respectively, left and right amygdala seed) were entered into one‐sample t‐tests (to determine both positive and negative rs‐FC). For each seed‐based t‐test, small‐volume corrections (FFG mask; P ≤ 0.05, FWE corrected) were applied. This analysis was carried out using the entire sample. Moreover, we also reanalyzed the data with a voxel‐size smoothing kernel of 3‐mm FWHM to rule out spurious results due to blurring of time signals of neighboring voxels (especially signals of amygdala and anterior temporal cortex) in the initial coincidental finding (i.e., segregation of amygdala defined rs‐FC clusters). The initial analysis was also carried out for the resting‐state data without global mean signal regression. Results obtained from this latter analysis underwent a stepwise increased thresholding, to test if an anterior–posterior distribution (similar to the finding with GSR) of clusters in the FFG could be observed.
Post hoc follow‐up analyses
Localization and definition of face sensitive areas using task‐fMRI
To delineate the commonly reported [e.g., Gschwind et al., 2012; Rossion et al., 2003] distinct face sensitive areas (i.e., FFA and OFA) within the FFG, the face matching task‐activation corresponding T‐values were increased in a step‐wise manner (starting from T ≥ 15) within the previously defined FFG mask until separate contiguous clusters of activation emerged within the FFG mask (i.e., respectively, in the anterior FFG (FFA) and in the posterior FFG (OFA) in each hemisphere). At a T‐value threshold of T ≥ 34 cluster‐wise separation into the two face sensitive areas was accomplished.
Replication of observed amygdala rs‐FC distribution in subsamples
To test for the reliability of observed effects of distinct spatially distributed amygdala rs‐FC within the FFG, the analysis described under Analysis of amygdala rs‐FC with the FFG section (amygdala rs‐FC with the FFG) was carried out for the three independent scanner sites separately.
Resting‐state FFA and OFA seed definition
To probe whether the observed segregation of positive versus negative FFG clusters would also be reflected in differential rs‐FC of these clusters to other regions of the visual stream, four separate seed regions were defined for the distinct FFG regions (i.e., the positive FFA and negative OFA clusters in each hemisphere, which were identified in the combined left and right amygdala connectivity results based on 8 mm FWHM smoothing).
Analysis of functional segregation of amygdala rs‐FC defined FFA and OFA clusters
After computing voxel‐wise correlations for the FFA and OFA clusters (as defined under Resting‐state FFA and OFA seed definition section), for each cluster and hemisphere separately, the obtained correlation coefficients (Pearson correlation) for each participant underwent Fisheŕs r‐to‐z conversion to obtain z‐values that fulfilled the requirements of a normal distribution.
To determine whether the FFA and OFA clusters would show differential rs‐FC patterns, the individual z‐scaled FFA and OFA rs‐FC maps were entered into paired‐sample t‐tests (for each hemisphere separately). First, to examine whether the FFA and OFA clusters would show differential connectivity patterns with regions of the face processing system (with special focus on the posterior superior temporal sulcus [pSTS] as the third region of the core face processing system) [Haxby et al., 2000], a mask (volume: 1669 voxels) was created based on regions involved in face processing (as defined by an image of the http://Neurosynth.org reverse inference tool with keyword “face”), which provided the search space for the analyses (small‐volume FWE correction, P ≤ 0.05. Due to the high power in this study, we restricted us to only report peak voxel activity in clusters with k ≥ 10 contiguous voxels). To test for differences in rs‐FC between the FFA and OFA seeds, the following contrasts were estimated: FFA > OFA and OFA > FFA. Subsequently, rs‐FC between FFA/OFA and the paired‐sample t‐test resulting peak voxel (3‐mm sphere) within the pSTS (i.e., one paired‐sample t‐test with FFA and OFA for each hemisphere) were extracted to explore how the FFA and OFA would distinctly contribute to the observed difference in rs‐FC with the pSTS. As the paired‐sample t‐tests also showed a significant difference in FFA versus OFA rs‐FC pattern in the left parahippocampal gyrus and the left middle occipital gyrus (please refer to the results section), we also extracted the respective rs‐FC values for these regions (peak voxel with 3‐mm sphere). To probe, whether FFA and OFA clusters would also show differential connectivity patterns with other regions of the face processing system when not removing the global signal, the same analysis was carried out with individual z‐scaled FFA and OFA rs‐FC maps, for which no prior GSR was performed. As these analyses target the relative difference in rs‐FC between FFA and OFA to other regions, reliability of results would be indicated by similar outcomes when removing versus not removing the global signal (in contrast to the analysis of amygdala rs‐FC with the FFG (Analysis of amygdala rs‐FC with the FFG section), where GSR may help by pulling apart neighboring, but functionally distinct, brain regions based on the FC distribution). The variables age, gender, handedness, and (if applicable) site were included in the models as covariates of no interest in all analyses [He et al., 2006; Kilpatrick et al., 2006; Li et al., 2012; Song et al., 2012].
Analysis of rs‐FC of amygdala rs‐FC defined FFA and OFA clusters back to the amygdala and among themselves
To probe whether amygdala rs‐FC defined FFA and OFA clusters would show connectivity to different parts of the amygdala, but with reversed polarity, z‐scaled FFA and OFA rs‐FC maps were entered into one‐sample t‐tests (for each hemisphere separately), whereby the AAL atlas derived amygdala seed from analysis Analysis of amygdala rs‐FC with the FFG section was used as a volume of interest. Lastly, to further illustrate the direction of rs‐FC between the amygdala rs‐FC defined FFA and OFA clusters, functional connectivity analyses were calculated between the left FFA and OFA, and between the right FFA and OFA (i.e., FFA cluster, respectively, defined as seed, whereas significant individual rs‐FC values for the OFA cluster (average rs‐FC with P < 0.001 FWE correction) were extracted.
Analysis of variance of amygdala rs‐FC defined FFA and OFA clusters to the amygdala across time
To determine whether the amygdala rs‐FC defined FFA and OFA clusters would not only show spatial segregation but would also differ with respect to the temporal domain (i.e., variance of rs‐FC to the amygdala across time as a potential indicator of differences in rs‐FC stability in a hierarchical face processing system), we performed a sliding‐window approach for each subject (with window‐size of 20 time points and a window overlap of 90% as well as 75%) using the extracted time series signal of the ROIs. To rule out spurious results due to blurring of time signals of neighboring voxels the sliding‐window analysis was also performed with extracted time series of data with a voxel‐size smoothing kernel of 3‐mm FWHM (additionally to regular 8 mm FWHM smoothing kernel). A window overlap of 75% resulted in 26 windows and a 90% overlap results in 65 windows per subject. Subsequently, rs‐FC between amygdala rs‐FC defined FFA and OFA (bilateral) and amygdala (bilateral) was computed for each subject for each window. Finally, the variance of rs‐FC between FFA and amygdala (bilateral), as well as OFA and amygdala (bilateral) was computed for each participant and was subject to group analyses (i.e., paired t‐tests between variance of amygdala‐FFA and amygdala‐OFA rs‐FC). We chose a window size of 20 time points as Allen et al. [2014] found that a sliding window size of about 22 TRs (44 s) provided a good trade‐off between the ability to resolve dynamics and the quality of connectivity estimation. The two TR difference can be neglected as Li et al. [2014] showed that changes of brain connectivity are not sensitive to the specific time‐window length (in the range of 10–20 TRs, 20–40 s).
RESULTS
A Priori Defined Analyses
Analysis of amygdala rs‐FC with the FFG
First, as listed in Table 2, the one‐sample t‐tests of left and right amygdala rs‐FC within the FFG mask revealed that amygdala rs‐FC was significantly distributed as distinct anterior versus posterior FFG clusters according to rs‐FC polarity. Specifically, we observed positive amygdala rs‐FC in the anterior/medial FFG, whereas the posterior FFG was characterized by negative rs‐FC with the amygdala seeds. Bilateral FFG connectivity was found for both the left and right amygdala (Fig. 1a). (It is important to note that both amygdala seeds obtained high correlations to the time course of the respective contralateral amygdala seed. Thus, as we did not partial out the respective contralateral amygdala time course when performing the amygdala rs‐FC analyses, bilateral amygdala‐FFG projections may be explained by the seed to seed correlation.) The same analyses on the preprocessed data with a voxel‐size smoothing kernel of 3‐mm FWHM resulted in comparable results (Supporting Information Fig. 2). When we performed the same analysis on the resting‐state data without GSR, we did not observe the distinct patterns of different rs‐FC polarity, only positive amygdala rs‐FC within the entire FFG (Fig. 1b,c).
Table 2.
Results of the one‐sample t‐tests with left and right amygdala rs‐FC within the FFG mask (entire sample: n = 276; all P: FWE, small‐volume corrected; covariates of no interest: age, gender, handedness, scanner site)
| Amygdala seed | rs‐FC polarity | Cluster size | Cluster center of mass | P – cluster level | L/R | Area | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Left amygdala | POS | 166 | −33 | −49 | −16 | <0.001 | L | Anterior FFG (FFA) |
| POS | 161 | 27 | −43 | −16 | <0.001 | R | Anterior FFG (FFA) | |
| NEG | 85 | −31 | −76 | −16 | 0.001 | L | Posterior FFG (OFA) | |
| NEG | 84 | 33 | −74 | −14 | 0.001 | R | Posterior FFG (OFA) | |
| Right amygdala | POS | 157 | −33 | −49 | −16 | <0.001 | L | Anterior FFG (FFA) |
| POS | 218 | 32 | −46 | −16 | <0.001 | R | Anterior FFG (FFA) | |
| NEG | 60 | −32 | −76 | −16 | 0.004 | L | Posterior FFG (OFA) | |
| NEG | 76 | 34 | −74 | −15 | 0.002 | R | Posterior FFG (OFA) | |
L: left, R: right, POS: positive, NEG: negative, FFG: fusiform gyrus, FFA: fusiform face area, OFA: occipital face area.
Figure 1.
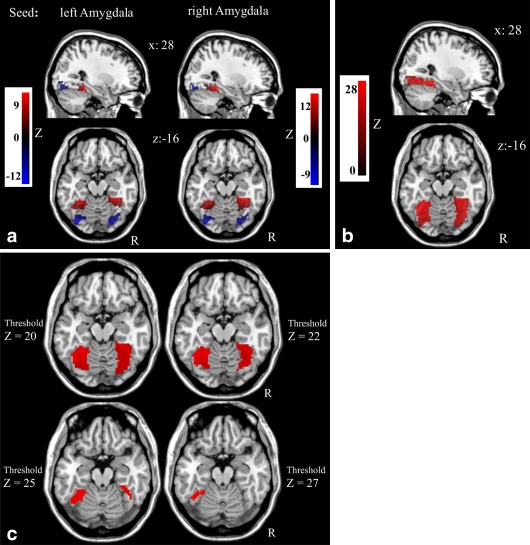
(a) Polarity dependent distinct amydala rs‐FC distribution within the fusiform gyrus (i.e., anterior vs. posterior clusters according to rs‐FC polarity with global signal regression). All P‐cluster level ≤ 0.01 FWE small‐volume corrected with bilateral fusiform gyrus mask; Table 2. Red: positive rs‐FC; Blue: negative rs‐FC. (b) Results of the amygdala rs‐FC analysis without removal of the global mean signal in the entire sample (rs‐FC overlays of left and right amydala). (c) Thresholding of the results of the amygdala rs‐FC analysis without removal of the global mean signal in the entire sample (rs‐FC overlays of left and right amydala) does not emerge in anterior–posterior distributed clusters. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Post Hoc Follow‐Up Analyses
Localization and definition of face sensitive areas using task‐fMRI
After thresholding of task‐based activity within the functionally defined FFG mask, an overlay of the task‐derived face sensitive areas (Table 3 and Fig. 2) and the resting‐state connectivity results (entire sample) revealed that positive rs‐FC in the anterior/medial FFG corresponded to the FFA, while the negative rs‐FC in the posterior FFG corresponded to the OFA (Fig. 3). Table 4 displays the percentage of overlapping voxels of amygdala rs‐FC defined FFA and OFA (Table 2) clusters with the task‐based activity thresholded defined activations in FFA and OFA (Table 3).
Table 3.
Results of task‐based activity thresholding (T ≥ 34) to delineate the face sensitive areas within the FFG (entire sample: n = 276; all P: FWE, small‐volume corrected; covariates of no interest: age, gender, handedness, scanner site)
| Hemisphere | Cluster size | Peak‐voxel activity | P – cluster level | Area | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right FFG | 65 | 27 | −88 | −6 | <0.001 | Posterior FFG (OFA) |
| 16 | 39 | −52 | −21 | <0.001 | Anterior FFG (FFA) | |
| Left FFG | 19 | −21 | −85 | −12 | <0.001 | Posterior FFG (OFA) |
| 8 | −39 | −76 | −15 | <0.001 | Posterior FFG (OFA) | |
| 2 | −18 | −85 | −18 | <0.001 | Posterior FFG (OFA) | |
| 6 | −36 | −52 | −21 | <0.001 | Anterior FFG (FFA) | |
FFG: fusiform gyrus, FFA: fusiform face area, OFA: occipital face area.
Figure 2.
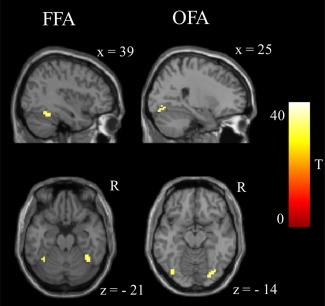
Face sensitive areas within the fusiform gyrus that were identified by thresholding (T ≥ 34) of task‐evoked activity. All P‐cluster level ≤ 0.01 FWE small‐volume corrected with bilateral fusiform gyrus mask; Table 3). FFA: fusiform face area; OFA: occipital face area. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
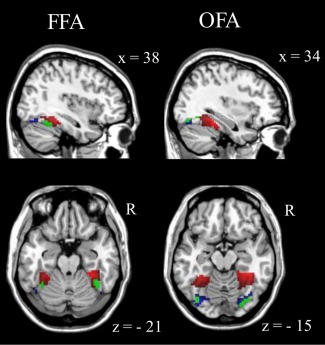
Overlays of the task‐based functionally defined face sensitive areas (green; according to Fig. 2) with the resting‐state derived polarity distributions (according to Fig. 1a) of bilateral amygdala rs‐FC (entire sample) revealed that positive rs‐FC (red) in the anterior/medial FFG, respectively, corresponded to the fusiform face area and that negative rs‐FC (blue) in the posterior FFG, respectively, corresponded to the occipital face area (Table 4). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 4.
Percentage of overlapping voxels of amygdala rs‐FC defined FFA and OFA (Table 2) clusters with the task‐based activity thresholded defined activations in FFA and OFA (Table 3)
| Amygdala seed | L/R | rs‐FC polarity/area | Cluster size rs‐FC cluster | Face sensitive areas (task‐based activity thresholding) | |||
|---|---|---|---|---|---|---|---|
| Left FFA (6 voxel) | Right FFA (16 voxel) | Left OFA (19 voxel) | Right OFA (65 voxel) | ||||
| Left amygdala | L | POS (FFA) | 166 | 6 (100%) | |||
| R | POS (FFA) | 161 | 6 (37%) | ||||
| L | NEG (OFA) | 85 | 11 (57%) | ||||
| R | NEG (OFA) | 84 | 37 (56%) | ||||
| Right amygdala | L | POS (FFA) | 157 | 5 (83%) | |||
| R | POS (FFA) | 218 | 8 (50%) | ||||
| L | NEG (OFA) | 60 | 11 (57%) | ||||
| R | NEG (OFA) | 76 | 25 (38%) | ||||
Replication of observed amygdala rs‐FC distribution in subsamples
Analyses of the three independent scanner sites (Table 5 and Fig. 4) revealed similar patterns of positive and negative amygdala rs‐FC within the FFG in all three sites, although in site three the right amygdala negative connectivity with the right sided posterior FFG cluster did not survive cluster correction with P < 0.05 (FWE, small‐volume).
Table 5.
Results of the subsample specific one‐sample t‐tests with left and right amygdala rs‐FC within the FFG mask (all P: FWE, small‐volume corrected; covariates of no interest: age, gender, handedness)
| Collaborating research site | Amygdala seed | rs‐FC polarity | Cluster size | Cluster center of mass | P – cluster level | L/R | Area | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Site 1 (Bonn; n = 123) | Left amygdala | POS | 152 | −33 | −49 | −16 | <0.001* | L | Anterior FFG (FFA) |
| POS | 179 | 33 | −44 | −16 | <0.001* | R | Anterior FFG (FFA) | ||
| NEG | 73 | −32 | −76 | −16 | 0.002* | L | Posterior FFG (OFA) | ||
| NEG | 58 | 35 | −73 | −16 | 0.003* | R | Posterior FFG (OFA) | ||
| Right amygdala | POS | 119 | −32 | −47 | −16 | <0.001* | L | Anterior FFG (FFA) | |
| POS | 201 | 32 | −45 | −16 | <0.001* | R | Anterior FFG (FFA) | ||
| NEG | 76 | −32 | −76 | −16 | 0.002* | L | Posterior FFG (OFA) | ||
| NEG | 45 | 34 | −75 | −16 | 0.007* | R | Posterior FFG (OFA) | ||
| Site 2 (Berlin; n = 77) | Left amygdala | POS | 84 | −31 | −46 | −16 | 0.001* | L | Anterior FFG (FFA) |
| POS | 71 | 29 | −40 | −17 | 0.001* | R | Anterior FFG (FFA) | ||
| NEG | 44 | −33 | −78 | −17 | 0.005* | L | Posterior FFG (OFA) | ||
| NEG | 13 | 42 | −78 | −18 | 0.039* | R | Posterior FFG (OFA) | ||
| Right amygdala | POS | 81 | −30 | −46 | −15 | 0.001* | L | Anterior FFG (FFA) | |
| POS | 109 | 30 | −43 | −15 | <0.001* | R | Anterior FFG (FFA) | ||
| NEG | 41 | −34 | −77 | −17 | 0.007* | L | Posterior FFG (OFA) | ||
| NEG | 22 | 36 | −75 | −17 | 0.021* | R | Posterior FFG (OFA) | ||
| Site 3 (Mannheim; n = 76) | Left amygdala | POS | 38 | −33 | −43 | −21 | 0.008* | L | Anterior FFG (FFA) |
| POS | 34 | 34 | −37 | −21 | 0.01* | R | Anterior FFG (FFA) | ||
| NEG | 48 | −30 | −79 | −16 | 0.005* | L | Posterior FFG (OFA) | ||
| NEG | 50 | 28 | −74 | −13 | 0.004* | R | Posterior FFG (OFA) | ||
| Right amygdala | POS | 84 | −34 | −46 | −19 | 0.001* | L | Anterior FFG (FFA) | |
| POS | 97 | 35 | −41 | −19 | 0.001* | R | Anterior FFG (FFA) | ||
| NEG | 12 | −31 | −82 | −17 | 0.043* | L | Posterior FFG (OFA) | ||
| NEG | 8 | 35 | −76 | −17 | 0.061 | R | Posterior FFG (OFA) | ||
For facility of inspection detached clusters with a voxel size k < 10 voxels are not included in the table (no clusters with POS rs‐FC polarity were located within the posterior FFG and no NEG rs‐FC clusters were observed in the anterior FFG). See Supporting Information Table 2 for the same analysis without global signal regression, where results are similar. L: left, R: right, POS: positive, NEG: negative, FFG: fusiform gyrus, FFA: fusiform face area, OFA: occipital face area; *P < 0.05.
Figure 4.
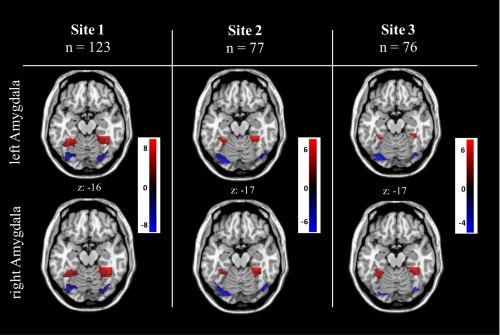
Analyses in the independent scanner sites confirmed reliability as revealed by similar patterns of amygdala rs‐FC polarity distribution within the FFG in all three sites, whereas in site three the right sided cluster based on negative right amygdala rs‐FC in the posterior FFG did not survive (P = 0.061) the cluster correction with P < 0.05 (FWE, small‐volume). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Analysis of functional segregation of amygdala rs‐FC defined FFA and OFA clusters
As listed in Table 6, the paired‐sample t‐tests (analyses of differential FFA and OFA connectivity) for the contrast FFA > OFA (for the left and right hemisphere separately) revealed a significant difference in rs‐FC patterns within a cluster stretching from right FFA to the right pSTS (no difference was observed in the left pSTS for both the left and right hemispheric paired t‐tests). Extracted FFA and OFA connectivity values to the peak voxel in the pSTS revealed that the difference in rs‐FC between FFA and OFA to the pSTS was driven by relatively more rs‐FC of the bilateral FFA to the pSTS (Fig. 5a). In addition, we observed greater FFA than OFA rs‐FC to large bilateral clusters in the limbic lobe surrounding the bilateral amygdala (including hippocampal and parahippocampal gyri), to the bilateral FFG itself, and to a separate left parahippocampal gyrus cluster (Fig. 5b). The reverse contrast (OFA > FFA) did not reveal rs‐FC differences to the pSTS, but showed differences in rs‐FC to the bilateral OFA itself, and to the left middle occipital gyrus (Table 6 and Fig. 5c). As listed in Supporting Information Table 2, the same analysis without prior removal of the global signal resulted in comparable results.
Table 6.
Results of the paired sample t‐tests with amygdala rs‐FC defined FFA and OFA clusters as seeds (entire sample: n = 276; all P: FWE, small‐volume corrected; covariates of no interest: age, gender, handedness)
| Hemisphere for t‐test | Contrast paired sample t‐test | Cluster size | Peak‐voxel coordinates | P – peak voxel | L/R | Area | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Right | FFA > OFA | 301 | 30 | −45 | −15 | <0.001 | R | Anterior FFG (FFA) |
| 48 | −51 | −12 | <0.001 | R | Posterior STS | |||
| 259 | 24 | −12 | −27 | <0.001 | R | Limbic lobe (hippocampus/parahippocampus) and amygdala | ||
| 257 | −24 | −9 | −24 | <0.001 | L | Limbic lobe (hippocampus/parahippocampus) and amygdala | ||
| 150 | −27 | −45 | −12 | <0.001 | L | Anterior FFG (FFA) | ||
| 14 | −21 | −33 | −12 | <0.001 | L | Parahippocampal gyrus | ||
| OFA > FFA | 244 | 36 | −72 | −18 | <0.001 | R | Posterior FFG (OFA) | |
| 189 | −24 | −78 | −18 | <0.001 | L | Posterior FFG (OFA) | ||
| 20 | −15 | −102 | −9 | <0.001 | L | Middle occipital gyrus | ||
| Left | FFA > OFA | 287 | 30 | −39 | −15 | <0.001 | R | Anterior FFG (FFA) |
| 48 | −54 | 9 | <0.001 | R | Posterior STS | |||
| 267 | −24 | −9 | −27 | <0.001 | L | Limbic lobe (hippocampus/parahippocampus) and amygdala | ||
| 259 | 24 | −12 | −27 | <0.001 | R | Limbic lobe (hippocampus/parahippocampus) and amygdala | ||
| 204 | −30 | −48 | −15 | <0.001 | L | Anterior FFG (FFA) | ||
| 14 | −21 | −33 | −12 | <0.001 | L | Parahippocampal gyrus | ||
| OFA > FFA | 199 | 30 | −81 | −18 | <0.001 | R | Posterior FFG (OFA) | |
| 161 | −30 | −78 | −15 | <0.001 | L | Posterior FFG (OFA) | ||
| 20 | −21 | −99 | 3 | <0.001 | L | Middle occipital gyrus | ||
For facility of inspection clusters with a voxel size k < 10 voxels are not included in the table. L: left, R: right, FFG: fusiform gyrus, FFA: fusiform face area, OFA: occipital face area, STS: superior temporal sulcus.
Figure 5.
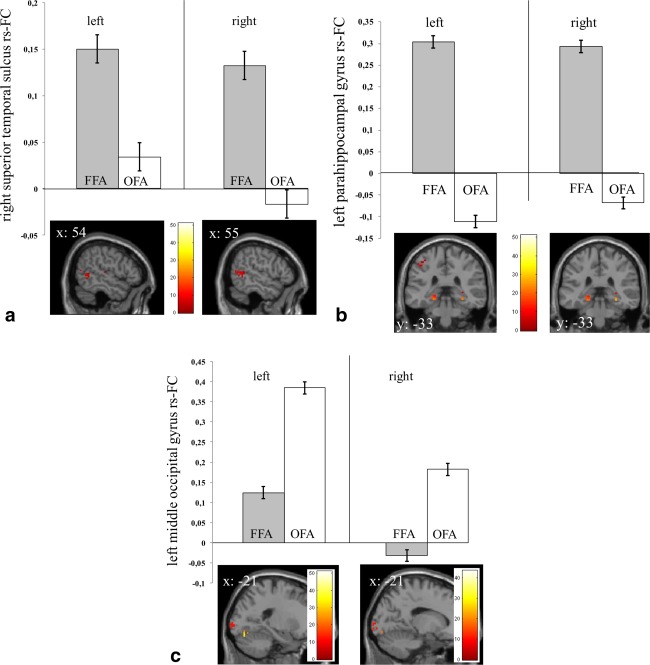
(a–c) Differential resting‐state connectivity pattern of amygdala seed rs‐FC defined FFG clusters (i.e., FFA and OFA) to (a) the right superior temporal sulcus, (b) the left parahippocampal gyrus, (c) the left middle occipital gyrus as revealed by paired sample‐t‐tests per hemisphere (left/right: extractions of rs‐FC for peak voxel according to Table 6 with 3‐mm sphere). All P‐peak voxel ≤ 0.05 FWE small‐volume corrected with a mask (1669 voxels) defined by the http://Neurosynth.org reverse inference tool with keyword “face”). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Analysis of rs‐FC of amygdala rs‐FC defined FFA and OFA clusters back to the amygdala and among themselves
One‐sample t‐tests with the amygdala rs‐FC defined FFA and OFA clusters revealed the same pattern as observed in the reversed FC‐analysis (i.e., rs‐FC with amygdala seeds): FFA clusters showed positive rs‐FC to the entire amygdala, whereas OFA clusters showed negative rs‐FC to the amygdala (P < 0.001, FWE corrected). Rs‐FC analyses between FFA and OFA revealed positive rs‐FC between the two clusters (left FFA‐OFA mean rs‐FC: r = 0.2967; right FFA‐OFA mean rs‐FC: r = 0.4966).
Analysis of variance of amygdala rs‐FC defined FFA and OFA clusters to the amygdala across time
As listed in Table 7, sliding‐window analyses with a window overlap of 75% and 90% on 8 mm FWHM smoothed data (as well as on 3 mm FWHM smoothed data; Supporting Information Table 1) revealed the following results: right amygdala rs‐FC defined FFA and OFA differed significantly with respect of their rs‐FC variance to the amygdala across time. Specifically, the FFA as compared to OFA showed significantly reduced rs‐FC variance with the right amygdala. Besides a trend in the same direction, for the left amygdala no significant differences in rs‐FC variance for FFA and OFA were observed.
Table 7.
Sliding‐window analyses with the amygdala rs‐FC defined FFA and OFA clusters with respect to their variance of rs‐FC to the amygdala, respectively, in the entire sample (extracted time courses from 8 mm FWHM smoothed data; comparable results were obtained with 3 mm FWHM smoothed data: see Supporting Information Table 1)
| Window overlap seed hemisphere | Window overlap: 75% (26 windows) | Window overlap: 90% (65 windows) | ||||||
|---|---|---|---|---|---|---|---|---|
| Seed: Left amygdala | Right amygdala | Left amygdala | Right amygdala | |||||
| Hemisphere: Left | Right | Left | Right | Left | Right | Left | Right | |
| FFA rs‐FC variance | 0.1524 | 0.1523 | 0.1443 | 0.1464 | 0.1501 | 0.1503 | 0.1414 | 0.1438 |
| OFA rs‐FC variance | 0.1613 | 0.1550 | 0.1571 | 0.1564 | 0.1589 | 0.1525 | 0.1544 | 0.1532 |
| Paired t‐test P | 0.120 | 0.591 | 0.014** | 0.031* | 0.109 | 0.648 | 0.009** | 0.036* |
| Bootstrapping P | 0.137 | 0.591 | 0.018** | 0.034* | 0.108 | 0.683 | 0.014** | 0.033* |
*P ≤ 0.05; **P ≤ 0.01; bootstrapping with 1000 iterations.
DISCUSSION
Here, we report the observation that, when applying GSR, distinct amygdala rs‐FC clusters in the FFG spatially correspond to the commonly reported face sensitive areas: the OFA and FFA. Specifically, when regressing out the global signal, we observed that amygdala rs‐FC was distributed within the FFG as distinct anterior versus posterior clusters, delineated by positive versus negative rs‐FC polarity. Direct overlays of post hoc task‐fMRI derived face sensitive areas and clusters of positive versus negative amygdala rs‐FC revealed that the positive amygdala rs‐FC clusters showed good spatial overlap with the FFA, whereas the OFA spatially corresponded to negative amygdala rs‐FC clusters. Both the left and right amygdala demonstrated similar rs‐FC with the bilateral FFG. Importantly, subsample specific analyses in three independent research sites confirmed reliability of this coincidental finding, as revealed by similar patterns of distinct amygdala rs‐FC based on polarity within the FFG in each of the three sites. Additional to this spatial segregation, both FFG clusters showed differential connectivity with other regions of the visual stream: the FFA showed greater rs‐FC to the pSTS (the third region in the “core face processing system”) [Haxby et al., 2000] than the OFA, as well as to the limbic lobe, including the hippocampal and parahippocampal gyri. In contrast, the OFA showed greater rs‐FC to regions associated with early visual processing (middle occipital gyrus) than the FFA. Moreover, when applying a sliding‐window analysis with focus on the rs‐FC variance of FFA versus OFA to the amygdala, we observed that bilateral FFA clusters showed significantly reduced rs‐FC variance with the right amygdala as compared to the OFA clusters. Taken together, we interpret the findings of this study as providing evidence for the potential of amygdala rs‐FC to segregate face sensitive areas within the FFG, when applying GSR. More generally, this opens up the possibility that rs‐fMRI data with applied GSR may serve also in other domains as a method to segregate functionally distinct areas.
Effects observed in this current study corroborate the findings of Roy et al. [2009], who examined whole‐brain rs‐FC of the amygdala while using GSR and reported positive rs‐FC in the FFG, as well as negative rs‐FC in the occipital cortex. Most importantly, our study extends these general findings by Roy et al. [2009] by providing several arguments (i.e., overlap with task‐fMRI derived face sensitive areas, differential rs‐FC to areas of the visual stream, temporal distinction with respect to the rs‐FC variance over time) that the borders of these correlation‐defined clusters may map on FFA and OFA. Thus, our findings contribute to the current literature as we show that the spatial distribution of amygdala rs‐FC polarity closely corresponds to the commonly reported distinct face sensitive areas within the FFG. Although it remains debatable if anti‐correlations seen in human data following GSR are artificial or not [e.g. Chai et al., 2012 and Wong et al., 2012 provided evidence for the existence of anti‐correlations without GSR], previous methodological studies [Fox et al., 2009; Murphy et al., 2009; Weissenbacher et al., 2009] discussed the question how to interpret negative rs‐FC as a byproduct of GSR (i.e., negative rs‐FC as a marker of anti‐correlated behavior between two regions vs. an artificially introduced result of GSR). Specifically, regression against the global mean signal has been shown to obtain the potential to shift correlation distributions toward a mean correlation value close to zero, thereby artificially introducing negative correlations [Murphy et al., 2009, Weissenbacher et al., 2009]. Conversely, this artificial “zero‐centering” method may also help by pulling apart neighboring, but functionally distinct, brain regions based on the FC distribution. In line with this, these anti‐correlations appear to be spatially specific, and, most importantly, are reproducible while potentially resembling neurophysiologically relevant relationships between regions and networks—for example, the default‐mode network [Fox et al., 2009; Shehzad et al., 2009]. Furthermore, it has been argued that GSR may also remove a true shared covariation in firing rate (i.e., a true global neuronal signal), thereby revealing relationships of neuronal populations otherwise masked by the dominant global signal [Fox et al., 2009; Keller et al., 2013; Scholvinck et al., 2010]. Consistent with this idea, Keller et al [2013] demonstrated that both positive and negative BOLD correlations have neurophysiological correlates reflected in fluctuations of spontaneous neuronal activity, which led the authors to conclude that GSR likely reveals more than it obscures. Although these arguments make it difficult to attach a functional significance to these anticorrelations, our results do suggest that GSR may delineate regions in a functionally meaningful way, indicating that splitting the correlation distribution (of, in this case, the amygdala) into positive (FFA) and negative (OFA) correlation values may correspond to an underlying difference in function. This conclusion is further substantiated by the distinct connectivity patterns of the two FFG clusters with other regions of the visual stream, as well as by the observed differences in rs‐FC variance over time.
With respect to the biological meaning of the observed results (taking the above stated caveats about the nature of negative connectivities into account) the anterior–posterior distinction of connectivity within the FFG could correspond to subregions differing in functional association with the amygdala. This difference may be state‐related, as recent findings of temporal dynamics of resting‐state connectivities [Chang et al., 2013] imply that during a single resting‐state scan a region normally changes its connected counterpart(s) as a function of ongoing brain states [Chang et al., 2013]. Therefore, different levels of functional associations between the OFA and FFA and amygdala could be interpreted as a consequence of differences in occurrences of states, where OFA and FFA are differentially recruited in the same network as the amygdala. This idea is supported by our data, as we observed a significant reduction in rs‐FC variance of FFA to the (right) amygdala as compared to the OFA over time. As the variance of rs‐FC time series may be interpreted as a proxy of how “stable” a connection between two areas is [Liao et al., 2014], it could be speculated that increases in rs‐FC variance in the OFA (accompanied by decreases in rs‐FC), as compared to the FFA, suggest a more independent functioning of this subregion to amygdala activations. Decreases in rs‐FC variance in the FFA (accompanied by increases in rs‐FC) could, conversely, indicate a closer coupling of FFA and amygdala during rest. This assumption is consistent with the idea that the neural system of face perception is hierarchically organized, comprising, among other regions, the amygdala, FFA, and the OFA as core components within this hierarchical system [e.g., Fairhall and Ishai 2007; Haxby et al., 2000; Liu et al., 2010]. In contrast to the FFA, the OFA is suggested to be the first stage in this hierarchical face perception network [Pitcher et al., 2011], providing inputs to the FFA [Fairhall and Ishai 2007; Haxby et al., 2000, 2002] and decoding basic facial components prior to subsequent processing of more complex features in higher face‐selective cortical regions, such as the FFA [e.g., Pitcher et al., 2011]. This proposed coupling of OFA and FFA is consistent with our resting‐state results as we observed significant (positive) correlations between these regions, independent of amygdala rs‐FC. In turn, as is assumed in recent models of face perception, the FFA is thought to be involved in a later stage of more complex information processing, directly sending and receiving signals to and from the extended face processing system, including the amygdala [Fairhall and Ishai 2007; Herrington et al., 2011; Vuilleumier et al., 2001, 2003, 2004]. Taking this idea into account it may be speculated that rs‐FC increases in the FFA, as compared to rs‐FC decreases in the OFA, and decreases in rs‐FC variance in FFA with the amygdala (as compared to the OFA), could indeed represent a closer coupling of the FFA to amygdala activations at rest, whereas activity of the OFA may be more independent of amygdala activity at rest.
Consistent with the finding of a spatial segregation within the FFG (i.e., FFA and OFA) based on amygdala rs‐FC, we also observed differential resting‐state connectivity patterns of both FFG regions with other regions of the visual stream. The OFA showed greater rs‐FC to the early visual cortex (middle occipital gyrus; V1/V2) than the FFA. This observation is consistent with the hypothesis that the OFA receives input from early visual cortex and is positioned between early visual areas and the FFA in the visual cortical hierarchy [Hemond et al., 2007; Pitcher et al., 2011]. In contrast, the FFA showed rs‐FC to the pSTS, whereas rs‐FC between the OFA and pSTS was close to zero. The pSTS is considered the third region of the “core face processing system” and is originally thought to receive input from both the OFA and FFA [Haxby et al., 2000]. However, whereas in line with recent findings demonstrating rs‐FC between FFA and the pSTS [Turk‐Browne et al., 2010], the current findings do not support evidence for direct communication (at least during rest) between the OFA and pSTS, as is proposed in the model of Haxby et al. [2000]. Interestingly, a similar observation was reported by Garrido et al. [2013], who found a preferential coupling (rs‐FC) of the STS with the FFA relative to the OFA as well. We also observed increased FFA rs‐FC with the bilateral amygdala (as expected from the rs‐FC analyses with bilateral amygdala seeds), compared with the OFA, which extended into the limbic lobe, including the hippocampal and parahippocampal gyri. As parts of the parahippocampal formation have been shown to correspond specifically to encoding of scenes (rather than faces or objects) [e.g., Epstein et al., 1999], these functional connectivity patterns may suggest that higher face‐selective cortical areas, such as the FFA, are coupled to other higher‐order regions involved in visual processing that are not restricted to face perception. Thus, based on the present findings and future research designed to address this question specifically, models proposing subcortical input from the amygdala to the FFA [e.g., Fairhall and Ishai, 2007; Vuilleumier et al., 2001, 2003, 2004] could be extended by assuming information transfer between the FFA and other subcortical structures, such as the hippocampus and parahippocampal gyrus.
At this moment, we cannot make detailed inferences about the possible meaning of the difference in amygdala rs‐FC polarity between the two face sensitive areas, as well as its underlying mechanism and role in the perception of faces, since current approaches to investigate effective connectivities during rest, such as granger causality, have been highly criticized for their vulnerability to hemodynamic differences [Deshpande and Hu, 2012]. Also, the observed connectivity profiles could correspond to regional specificity not just in cortex but also in amygdala subregions, which our study could not resolve due to the spatial resolution of the scans (i.e., the reverse rs‐FC analyses with FFA and OFA clusters as seed regions that showed the initially observed connectivity profile with the entire amygdala may suffer from the spatial resolution). However, based on these resting‐state findings several testable hypotheses (with an appropriate task) may be generated: that is, does the ratio of FFA to OFA activation across subjects predict the degree of amygdala activation, and is this, in turn, related to the amount of differences in FFA‐amygdala versus OFA‐amygdala rs‐FC? Does the FFA activate more than the OFA in response to fearful faces, thus activating the amygdala, while the OFA activates more (or equally) than FFA in response to neutral faces, thus suppressing or perhaps disengaging the amygdala? As the face matching task used in this study to delineate the face sensitive areas, is not elaborate enough for drawing conclusions about specific differences in amygdala‐OFA versus amygdala‐FFA functional connectivity during face processing, these speculations remain to be clarified in future connectivity studies that combine both task and rs‐fMRI.
Findings presented in this article have to be interpreted within the methodological limitations of this investigation. First, main effects observed in the current study (i.e., segregation of amygdala rs‐FC defined anterior vs. posterior cluster in the FFG) were not the original focus of this experiment (which concerned genetic effects), and we had thus no a priori hypothesis about a differential association of amygdala rs‐FC to the FFA and OFA. Therefore, future hypothesis driven studies are necessary to confirm and extend described effects. Second, although we accounted for potential differences in image acquisition among the three research sites (all sites had equal scanning protocols, while quality control measurements revealed comparable hardware performance between sites) by including additional site‐specific regressors in the second level analysis, we cannot exclude influences of research site on the results (e.g., biased due to differences in sample sizes). However, reliability of described effects is supported by similar rs‐FC polarity patterns within the FFG in all three independent subsamples, as well as very low and acceptable fractional displacement (FD) values for the entire sample and for each side separately (entire sample mean FD: 0.23; Site 1: mean FD: 0.24; Site 2: mean FD: 0.19; Site 3: mean FD: 0.27; with standard deviations of around 0.1).
In conclusion, findings of this study provide initial evidence for the potential of amygdala rs‐FC to segregate face‐selective areas within the FFG when regressing out the global signal. Specifically, the two commonly reported face sensitive areas within the FFG appear to map on distinct patterns of amygdala rs‐FC polarity when GSR is performed. These resting‐state findings are in line with recent task‐fMRI derived theories suggesting that face perception is under direct influence of the amygdala. Lastly, the results may be interpreted as further evidence for the efficacy of GSR in unmasking “true” inter‐regional relationships [e.g., Fox et al., 2009].
Supporting information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Tables
Conflict of interest: The authors declare no conflict of interest.
Footnotes
In contrast to the FFA, the OFA is suggested to be an earlier stage in a hierarchical face perception network (Pitcher et al., 2011), where basic facial components are decoded. The OFA provides input to higher face selective cortical regions, such as the FFA (Haxby et al., 2000), in which more complex features are processed (Haxby et al., 2000; Pitcher et al., 2011). In turn, the FFA is thought to be involved in a later stage of more complex information processing and is assumed to exert the dominant influence (as compared to the OFA) to the extended face processing system, which, among others, includes the amygdala (Fairhall and Ishai, 2007; Herrington et al., 2011; Vuilleumier et al., 2003, 2004; Vuilleumier and Pourtois, 2007).
REFERENCES
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole‐brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Öngür D, Whitfield‐Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M (2013): Association between heart rate variability and fluctuations in resting‐state functional connectivity. Neuroimage 68:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N (1999): The parahippocampal place area: Recognition, navigation, or encoding? Neuron 23:115–125. [DOI] [PubMed] [Google Scholar]
- Erk S, Meyer‐Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P, Grimm O, Arnold C, Haddad L, Witt S, Cichon S, Nöthen M, Rietschel M, Walter H (2010): Brain function in carriers of a genome‐wide supported bipolar disorder variant. Arch Gen Psychiatry 67:803–811. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt S, Rietschel M, Cichon S, Meyer‐Lindenberg A (2009): Neural mechanisms of a genome‐wide supported psychosis variant. Science 324:605. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Hu X (2012): Investigating effective brain connectivity from FMRI data: Past findings and current issues with reference to granger causality analysis. Brain Connect 2:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A (2007): Effective connectivity within the distributed cortical network for face perception. Cereb Cortex 17:2400–2406. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Glover GH (2006): Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging 23:827–839. [DOI] [PubMed] [Google Scholar]
- Garrido L, Holmes A, Hollinshead M, Buckner R, Nakayama K (2013): The face network estimated by intrinsic functional connectivity employing a large sample (N = 296). J Vis 13:1108. [Google Scholar]
- Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P (2012): White‐Matter Connectivity between Face‐Responsive Regions in the Human Brain. Cerebral Cortex 22:1564–1576. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al (2002): Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cogn Sci 4:223–233. [DOI] [PubMed] [Google Scholar]
- He Y, Zang Y, Jiang T, Gong G, Xie S, Xiao J (2006): Handedness‐related functional connectivity using low‐frequency blood oxygenation level‐dependent fluctuations. Neuroreport 17:5–8. [DOI] [PubMed] [Google Scholar]
- Hemond CC, Kanwisher N, Op de Beeck HP (2007): A preference for contralateral stimuli in human object‐ and face‐selective cortex. PLoS One 2:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Taylor JM, Grupe DW, Curby KM, Schultz RT (2011): Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage 56:2348–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD (2013): Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci 33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF (2006): Sex‐related differences in amygdala functional connectivity during resting conditions. Neuroimage 30:452–461. [DOI] [PubMed] [Google Scholar]
- Kruschwitz JD, Walter M, Varikuti D, Jensen J, Plichta MM, Haddad L, Grimm O, Mohnke S, Pöhland L, Schott B, Wold A, Mühleisen TW, Heinz A, Erk S, Romanczuk‐Seiferth N, Witt SH, Nöthen MM, Rietschel M, Meyer‐Lindenberg A, Walter H (2015): 5‐HTTLPR/rs25531 polymorphism and neuroticism are linked by resting state functional connectivity of amygdala and fusiform gyrus. Brain Struct Funct 220:2373–2385. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin W, Jiang T, Zhang Y, Yu C (2012): Sex‐dependent correlations between the personality dimension of harm avoidance and the resting‐state functional connectivity of amygdala subregions. PLoS One 7:e35925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu D, Jiang X, Jin C, Zhang X, Guo L, Zhang J, Hu X, Li L, Liu T (2014): Dynamic functional connectomics signatures for characterization and differentiation of PTSD patients. Hum Brain Mapp 35:1761–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Wu GR, Xu Q, Ji GJ, Zhang Z, Zang YF, Lu G (2014): DynamicBC: A MATLAB toolbox for dynamic brain connectome analysis. Brain Connect 4:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N (2010): Perception of face parts and face configurations: An FMRI study. J Cogn Neurosci 22:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR (2009): Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry 14:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anticorrelated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B (2011): The role of the occipital face area in the cortical face perception network. Exp Brain Res 209:481–493. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M (2003): The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. Neuroimage 19:877–883. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA (2010): Neural basis of global resting‐state fMRI activity. Proc Natl Acad Sci USA 107:10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP (2009): The resting brain: Unconstrained yet reliable. Cereb Cortex 19:2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Desphande AS, Meier TB, Tudorascu DL, Vergun S, Nair VA, Biswal BB, Meyerand ME, Birn RM, Bellec P, Prabhakaran V (2012): Age‐related differences in test‐retest reliability in resting‐state brain functional connectivity. PLoS One 7:e49847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer‐Lindenberg A (2010): A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic–limbic structure and function. Proc Natl Acad Sci USA 107:13936–13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk‐Browne NB, Norman‐Haignere SV, McCarthy G (2010): Face‐specific resting functional connectivity between the fusiform gyrus and posterior superior temporal sulcus. Front Hum Neurosci 4:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G (2007): Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia 45:174–194. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony J, Driver J, Dolan RJ (2001): Effects of attention and emotion on face processing in the human brain: An event‐related fMRI study. Neuron 30:829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ (2003): Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 6:624–631. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ (2004): Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci 7:1271–1278. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009): Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O Liu TT (2012): Anti‐correlated networks, global signal regression, and the effects of caffeine in resting‐state functional MRI. NeuroImage 63:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan (2010): DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Frontiers in System Neuroscience. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Tables


