Abstract
Eight decades after Penfield's discovery of the homunculus only sparse evidence exists on the cortical representation of the lumbar spine. The aim of our investigation was the description of the lumbar spine's cortical representation in healthy subjects during the application of measured manual pressure. Twenty participants in the prone position were investigated during functional magnetic resonance imaging (fMRI). An experienced manual therapist applied non‐painful, posterior‐to‐anterior (PA) pressure on three lumbar spinous processes (L1, L3, and L5). The pressure (30 N) was monitored and controlled by sensors. The randomized stimulation protocol consisted of 68 pressure stimuli of 5 s duration. Blood oxygenation level dependent (BOLD) responses were analyzed in relation to the lumbar stimulations. The results demonstrate that controlled PA pressure on the lumbar spine induced significant activation patterns. The major new finding was a strong and consistent activation bilaterally in the somatosensory cortices (S1 and S2). In addition, bilateral activation was located medially in the anterior cerebellum. The activation pattern also included other cortical areas probably related to anticipatory postural adjustments. These revealed stable somatosensory maps of the lumbar spine in healthy subjects can subsequently be used as a baseline to investigate cortical and subcortical reorganization in low back pain patients. Hum Brain Mapp 35:3962–3971, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: fMRI, functional spinal units, spinal stimulation, somatosensory cortex, postural control
INTRODUCTION
The illustration of the so‐called homunculus provides a topography of the body surface related to the stimulation of discrete parts of the brain [Penfield and Boldrey, 1937; Penfield and Rasmussen, 1950]. That seminal work was one of the first to localize the “back” in the primary somatosensory cortex (S1) on the convexity of the postcentral gyrus between the hip and the shoulder. Eight decades later, only sparse evidence exists on the cortical representation of the back. To our knowledge, only three neuroimaging studies have investigated the cortical organization of the lower back in healthy subjects and cortical reorganization in low back pain (LBP) patients [Flor et al., 1997; Kobayashi et al., 2009; Lloyd et al., 2008]. The first study used magnetoencephalography (MEG) to measure brain activity induced by intracutaneous electric stimuli to the left side on the lower back at nonpainful and painful intensities in a control group and LBP patients [Flor et al., 1997]. The stimulation revealed contralateral cortical activity in S1 in both groups, but in patients the activity was stronger and localized more medially compared to the controls. The second study, using functional magnetic resonance imaging (fMRI), described the cortical representation of low back stimulation in healthy subjects and LBP patients [Lloyd et al., 2008]. They used unpleasant stimulation by mechanical plate vibration located bilaterally on the lower back. In the control group, activation was revealed in S1, the secondary somatosensory area (S2) and the inferior parietal, temporal and insular cortices, but only in the right hemisphere, even though the stimulation was bilateral. In the third study, an unpleasant paravertebral mechanical compression was applied on the left side of healthy subjects and chronic LBP patients while brain activation patterns were recorded using fMRI. For the healthy subjects, activation was only found in the premotor cortex. This investigation did not show any activation in S1 for neither healthy subjects nor LBP patients. However, the activation pattern was stronger in LBP patients [Kobayashi et al., 2009]. The question remains whether directly tackling functional spinal units (FSUs), the smallest physiological motion entity of the lumbar spine, reveals strong and consistent brain activation patterns. The objective of this explorative study was to describe the cortical representation of the lumbar spine in healthy participants during the manual application of nonpainful posterior‐to‐anterior pressure onto lumbar vertebrae directly by using fMRI with a whole brain approach.
MATERIALS AND METHODS
Participants
Twenty right‐handed healthy subjects (14 females, mean age of 35 ± 13.4 years) participated in this fMRI study. Exclusion criteria were low back pain within the last 6 months, history of neurological, psychiatric and presence of indwelling metal or medical devices incompatible with fMRI. All participants signed a consent form after the study and the investigative procedures had been explained. The study was approved by the Canton of Zurich's Ethics Committee and was conducted in compliance with the declaration of Helsinki. Participants were compensated for travel expenses and the burden of participation.
Experimental Procedures
Prior to the fMRI experiment, the participant's first, third and fifth spinous processes of the lumbar vertebrae (L1, L3, L5) were palpated and the skin subsequently marked with a dermatograph. These particular spinous processes were selected based on the segmental overlapping innervations of neighboring vertebrae [Bogduk, 1983; Bogduk et al., 1982]. Anatomically, two adjacent vertebrae are forming an FSU, a complex functional entity including disc, ligaments and zygapophysial joints. The entire spine is functionally divided in FSUs, each consisting of similar biomechanical characteristics [Panjabi, 2003]. Furthermore, mechanosensory afferents from FSUs are important in postural control and movement [Izzo et al., 2013; Sjolander et al., 2002].
The participants were scanned in prone position [Kobayashi et al., 2009], lying face down on a special pillow (adapted Posifix Prone Headrest pillow) that was designed to allow normal breathing, prevent head movements and provide maximal comfort. Foam padding was placed between the subject's head and the MRI head coil and straps were attached around the shoulder girdle (Fig. 1a).
Figure 1.
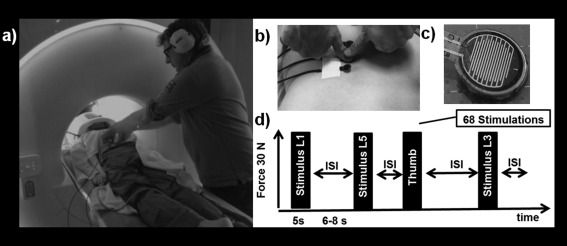
Experimental set‐up and fMRI paradigm. (a) Participant in prone position with the face lying on a special pillow. Lumbar stimulation applied on L1, L3, and L5. (b) Thumb grip for pressure on sensors. (c) Sensors from the lower surface. (d) Protocol of the fMRI experiment with timing and random order of stimulation sites.
The stimulus consisted of a nonpainful pressure exerted with a thumb grip over a small flat circular plate placed perpendicularly to the spinous processes of either L1, L3, and L5 (Fig. 1b). The stimulation induced a posterior‐to‐anterior (i.e., dorso‐ventral) intervertebral movement (PA). This technique is one of the most commonly applied manual techniques for assessment of spinal movement (joint‐play) and spinal treatment [Snodgrass et al., 2006]. To minimize the variability of the stimulation, all experiments were performed by the same experienced manual therapist (BB). In addition, pressure stimulation onto the carpometacarpal joint of the right thumb was performed as a control for the cortical representation.
To control for equal pressure, four force sensors (Fig. 1c, FlexiForce®Sensors, Tekscan) were attached to the previously marked spinous processes (Fig. 1b) and the thumb. The sensors were composed of resistive sensors and an amplifier that transformed the resistive changes in an appropriate voltage signal. The signal was digitalized by a micro controller (1 KHz) and sent to a personal computer. The applied PA pressure was 30 N. That force affirmed that the spinal movement was at the beginning of the range, free of resistance and preventing adverse effects [Snodgrass et al., 2006].
The event‐related fMRI experiment consisted of 68 stimuli of 5 s duration each and with a randomized interstimulus interval (ISI) of 6–8 s. The experimenter followed a randomized stimulation protocol with an equal number of stimulations (N = 17) per vertebral level, using the thumb as a control (Fig. 1d). During the ISI, the instructions with the stimulation level (L1, L3, and L5) was projected onto the screen in front of the experimenter in the MR room and followed by the START signal. During the stimulation, the applied force was displayed on screen, thus the experimenter could continuously adjust the pressure on the visual feedback. The end of the stimulation period was indicated by a projected STOP signal.
Data Acquisition and Analysis
A 3‐T (Philips Ingenia, Best, The Netherlands) blood‐oxygen‐level dependent (BOLD) sensitive single‐shot gradient echo planar imaging sequence was used to acquire 32 axial whole brain slices, with a 15‐channel receive‐only head coil. Parameters were as follows: echo time = 30 ms, flip angle = 75°, repetition time = 2,600 ms, slice thickness = 4 mm, inter‐slice gap = 0 mm, field of view = 220 mm, and matrix size in plane = 128 × 128, resulting in a voxel size of 1.72 × 1.72 mm2. Three dummy scans were first acquired to reach steady‐state magnetization and were subsequently discarded. A single run with 341 functional images was performed and the complete scanning phase of lasted about 15 min. SPM8 (http://www.fil.ion.ucl.ac.uk/spm) software package running on MATLAB R2011b was used for functional voxel‐by‐voxel analysis. In a first step, spatial realignment to the first image in the series as reference was performed. Afterward, it was assured that any detected movement did not exceed 2 mm (translational) or 1° (rotational) in relation to the reference. For studying group effects, data were normalized to the MNI (Montreal Neurological Institute) template brain [Evans et al., 1992] followed by smoothing with a Gaussian kernel of 6 mm FWHM.
To reveal significant changes in cortical activity associated with distinct lumbar and thumb stimulations first level analyses were performed on each subject's data versus baseline (no stimulation) by means of the general linear model, using the hemodynamic response function implemented in SPM8. Regressors were separately set to the onset of L1, L3, L5 and thumb stimulation with duration of 5 s. To control for possible head movement effects, individual movement parameters (translations in the x, y, and z direction, as well as rotations around the x, y, and z axis) were implemented in the first level model as regressors of no interest. Statistical parametric maps were then calculated, yielding beta estimates of the model fit for each subject and condition.
Using a random effect model, the second level group activations were computed for the L1, L3, and L5 activation maps. A subsequent repeated measures ANOVA was performed to investigate whether the cortical representation of the various levels could be distinguished. Additionally, a group statistical map was calculated on the pooled FSU stimulations (L1, L3, and L5) in a random effects model using one sample t tests. Resulting voxel T values were color‐coded and superimposed onto the MNI template brain [Evans et al., 1992]. All results were thresholded at P < 0.05 and corrected for multiple testing (family wise error correction, FWE) with a voxel extent of 10 voxels.
ROI Analysis
In a subsequent ROI analysis, the voxel space was limited to the S1 region, comprising the probability maps of the subareas 3a, 3b, 1, and 2 [Eickhoff et al., 2005]. To identify activation within S2 in the parietal opercular region (OP), a volume of interest, covering the subregions OP1, OP2, OP3, and OP4, was used [Eickhoff et al., 2006, 2007]. Masks were taken from the probabilistic Juelich Histological Atlas (included in FSLview version 3.1: http://www.fmrib.ox.ac.uk/fsl/). Voxels were included in a mask if the probability for belonging to the desired structure was P > 0.25. Within these ROIs, for each subject and FSU stimulation level the center of mass (COM), peak of activation (POA) and activation overlap (AO) were calculated in the MNI space [Plow et al., 2010]. For the COM and POA measures separately, a repeated measures ANOVA was then computed with the factors coordinates (x, y, z) and “vertebral level” (L1, L3, L5), using the software SPSS 20. The 3D Euclidean distance between two individual COM for L1, L3, and L5 were computed using the following distance formula d(x,y,z), (x,y,z) = √(x − x)2 + (y − y)2 + (z − z)2. The significance level was set to p < 0.05.
RESULTS
PA stimulation on the lumbar vertebrae induced a significant increase in the BOLD signal intensity in many cortical areas and the cerebellum. The results of the individual subjects, as well as the group analysis in S1 and S2, are first reported, followed by other brain regions.
Functional Organization of S1 and S2
The application of PA pressure on lumbar vertebrae L1, L3, and L5 revealed bilateral neuronal activity in S1 on the convexity of the postcentral gyrus which is consistent with the expected location in the somatosensory homunculus (Table 1, Fig. 2). The clusters on the right side were larger than in the left hemisphere. On the right hemisphere an 81% activation overlap between L1 and L3 and 76% overlap between L1 and L5 was observed. In the left hemisphere the overlap between L1 and L3 was 63 and 59% between L1 and L5. The mechanical stimulation on the thumb, revealed only activation in the left convexity of the postcentral gyrus, posterior to the motor hand representation known as the hand knob [Yousry et al., 1997].
Table 1.
Coordinates (in MNI standard brain space) of significant cluster maxima, t values, and volumes in the group analysis for pooled PA stimulation of L1, L3, and L5 versus baseline (FWE‐corrected, P < 0.05, voxel extend threshold = 10)
| Region | Left/right | Cluster size | Peak | |||
|---|---|---|---|---|---|---|
| t value | X | Y | Z | |||
| Postcentral gyrus (S1) | R | 834 | 10.64 | 18 | −36 | 66 |
| L | 137 | 6.39 | −16 | −46 | 68 | |
| Operculo‐insular cortex | R | 1008 | 47.6 | 32 | −20 | 10 |
| L | 96 | 20.4 | −44 | −34 | 20 | |
| SMA | R/L | 834 | 7.03 | 0 | −18 | 66 |
| Posterior ACC | R | 105 | 7.91 | 10 | 36 | 20 |
| L | 94 | 6.94 | −12 | 38 | 18 | |
| Anterior MCC | R | 93 | 7.68 | 12 | 26 | 32 |
| L | 90 | 7.12 | −12 | 22 | 32 | |
| Posterior MCC | R | 129 | 8.12 | 8 | −10 | 40 |
| L | 108 | 7.37 | −10 | −10 | 36 | |
| Middle frontal gyrus | L | 120 | 8.28 | −20 | 48 | 28 |
| Cerebellum Larsel II‐III | R | 33 | 7.03 | 8 | −46 | −14 |
| L | 53 | 8.22 | −8 | −44 | −16 | |
Abbreviations: S1, primary somatosensory cortex; SMA, supplementary motor area; ACC, anterior cingulate cortex; MCC, medial cingulate cortex; Larsel = Larsel lobule; L = left; R = right; L/R = bilateral.
Figure 2.
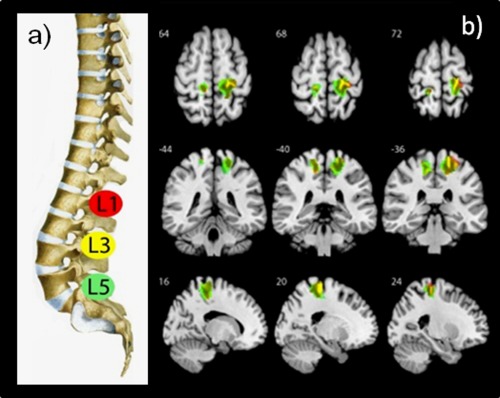
Group activations in S1 regions of interest (ROIs) during PA movement for L1, L3 and L5. (a) Lumbar spine with indication of stimulated lumbar vertebrae in the same color code as illustrated in the activation patterns. (b) Brain activations in healthy subjects during PA movements of lumbar vertebrae L1, L3, and L5 (random‐effects analysis, P < 0.001, uncorrected, voxel extend threshold = 10). All activations are rendered on the surface of the MNI template image displaying axial (above), frontal (middle) and sagittal (lower) sections. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In the ROI analysis, the mean coordinates with standard deviations of individual COM and the POA were computed for the 20 subjects and listed in Table 2. COM measures point towards a low cortical intersubject variability within the different vertebral levels. The repeated measures ANOVA of the COM data did not reveal significant differences between the coordinates of the three lumbar vertebrae representations in both hemispheres. The 3D Euclidian distances based on the COM measures between L1 and L3 was 2.38 mm (SD 1.27), between L3 and L5 was 2.76 mm (SD 1.05) and 2.06 mm (SD 0.73) between L1 and L5, which corresponds to <1.4 voxels on the x, y, and z axis. As displayed in Figure 2, the lumbar vertebrae are not arranged in a medial‐to‐lateral topography as L3 is positioned below L1 and L5. The POA coordinates revealed a larger variability compared to the COM data and similarly no statistical differences were revealed by the repeated measures ANOVA. The representation of the thumb showed the largest variability for the COM coordinates (Table 2).
Table 2.
Mean (± standard deviation) coordinates for the center of mass (COM) and peak of activation (POA) in mm in the MNI space in S1 for both hemispheres for L1, L3, L5 and the thumb
| X | Y | Z | X | Y | Z | X | Y | Z | X | Y | Z | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COM | right hemisphere | left hemisphere | POA | right hemisphere | left hemisphere | ||||||||
| L1 | 19.41 (0.78) | 37.25 (0.51) | 64.99 (0.66) | −15.73 (1.26) | 36.94 (1.30) | 63.30 (0.82) | 21.00 (5.05) | 36.00 (5.54) | 67.50 (5.61) | −18.10 (7.12) | 36.60 (6.16) | 65.40 (6.50) | |
| L3 | 19.26 (0.54) | 37.14 (0.76) | 65.02 (0.79) | −15.28 (1.58) | 36.84 (1.15) | 62.82 (0.62) | 20.70 (5.37) | 36.30 (6.33) | 66.50 (6.01) | −14.70 (6.50) | 36.80 (4.90) | 63.10 (6.63) | |
| L5 | 19.27 (0.53) | 37.03 (0.85) | 65.24 (1.36) | −15.86 (1.50) | 36.88 (1.26) | 63.28 (0.67) | 19.60 (4.70) | 36.03 (5.40) | 67.60 (6.30) | −16.70 (7.40) | 36.70 (5.60) | 64.50 (5.88) | |
| Thumb | −23.34 (5.24) | 37.21 (1.64) | 64.02 (1.76) | −32.30 (5.60) | 36.10 (4.28) | 66.20 (3.94) | |||||||
Abbreviations: COM = center of mass, POA = peak of activation, x,y and z = MNI coordinates, L1 = first lumbar vertebrae, L3 = third lumbar vertebrae, L5 = fifth lumbar vertebrae.
Pressure on the lumbar spine induced bilateral activation in S2, distributed within all four OP subregions. Furthermore, those large clusters extended into the insular cortex. That anatomically contiguous region has previously been named the operculo‐insular cortex [Mazzola et al., 2012]. Similar as in S1 the S2 activation was larger in the right hemisphere (right 1687 voxels versus left 323 voxels, Table 1, Fig. 3).
Figure 3.
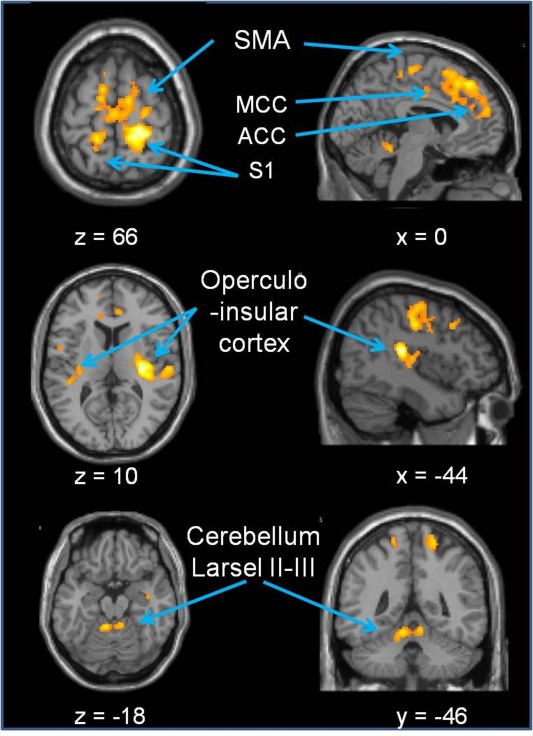
Pooled activation patterns during PA movements on lumbar vertebrae. The significant regions are listed in Table 1. Results are superimposed on the MNI template. Abbreviations: SMA: supplementary motor area; MCC: medial cingulated cortex; ACC: anterior cingulated cortex; S1: primary somatosensory cortex. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Activation in Other Brain Areas
The repeated measures ANOVA did not reveal significant differences for the localization of the vertebral levels in other brain areas. The pooled data of mechanical PA pressure revealed bilateral activation in the supplementary motor area (SMA; Table 1 and Fig. 3). Several bilateral clusters of activation were detected in cingulate regions and middle frontal cortex. As reported for the somatosensory cortices, cluster sizes were always larger in the right hemisphere. Subcortically, significant bilateral activation was revealed in the medial anterior cerebellum (Larsel lobules II–III, Table 1 and Fig. 3).
DISCUSSION
In this study, we investigated the neural correlates of a nonpainful, force controlled mechanical stimulation directly onto lumbar vertebrae in healthy participants that lie in prone position. The results demonstrate that controlled posterior‐to‐anterior pressure induced significant activation patterns. Major new findings include a strong and consistent activation bilaterally in S1 and extensive activation in S2 and the insular cortex. In addition, bilateral activation was located medially in the anterior cerebellum. Furthermore, the activation pattern included other cortical areas, disclosing a large sensorimotor network for the trunk, probably related to postural control.
The applied PA pressure on lumbar vertebrae in healthy subjects revealed strong and robust bilateral activation in S1. The activation occupied bilaterally the appropriate topographic representation of the stimulated body part within the convexity of the postcentral gyrus, in accordance with the homunculus of Penfield [Penfield and Boldrey, 1937; Penfield and Rasmussen, 1950]. Similar coordinates have been reported for unpleasant mechanical stimulation of the lower back in the right hemisphere [Lloyd et al., 2008], and brushing of the lower abdomen and penis revealed activation in an adjacent position [Kell et al., 2005]. Accordingly, the localization of the thumb used as the control stimulation was found more laterally, opposite to the stimulation site and consistent with recent data on tactile thumb stimulation [Martuzzi et al., 2012].
In nonhuman primates, recordings of single neuronal activity have revealed bilateral receptive fields related to midline structures in S1 [see review by Iwamura et al., 2002]. Together with findings based on retrograde labeling, transcallosal as well as thalamocortical projections seemed to be responsible for the activation in the ipsilateral hemisphere [Conti et al., 1986; Jones and Hendry, 1980]. In humans, fMRI studies investigating unilateral tactile trunk stimulation of either body‐side showed robust bilateral responses within the SI trunk representation [Eickhoff et al., 2008; Fabri et al., 2005] as well as in all S2 subregions [Eickhoff et al., 2008]. Similar activations in callosotomized patients indicate that bilateral cortical trunk representation is at least partially independent of callosal connections [Fabri et al., 2006].The integration of sensorimotor information across the midline at the cortical level is essential for the body parts for which bilateral neuromuscular control is mandatory, such as oral structures [Disbrow et al., 2003] and the trunk's musculoskeletal system [Tsao et al., 2008].
In contrast to the fMRI studies described above, the present investigation directly stimulated midline structures of the lower back. In all cortical regions, larger clusters of activation were found in the right than in the left hemisphere. Interestingly, in the study of Lloyd et al. [2008] bilateral stimulation of the lower back in healthy subjects only revealed activation in the right hemisphere. Right sided lateralization has not been observed in tactile trunk stimulation. Together those data suggest a specific organization of the proprioceptive processing for the lower back.
PA pressure revealed in single subjects COM analysis a remarkable robustness of individual somatotopic maps as confirmed by the quite small standard deviations of the coordinates across the 20 participants. In contrast, COM analysis of the thumb activation revealed more widely distributed activations, a finding concurrent with a study showing tactile finger representation with larger standard deviations [Martuzzi et al., 2012]. The POA coordinates of the lumbar vertebrae revealed larger standard deviations. This deviation can be due to the fact that COM is an absolute average measure of coordinates within a cluster, regardless of the intensity of activation. In contrast, the intensity of activation within the same cluster is the indicator for the POA measures [Plow et al., 2010].
Regarding the COM and POA measures, a somatotopic representation of the three lumbar levels could not be established. This is not surprising regarding the considerable overlap of activation up to 80% between L1, L3, and L5 and their individual representation with L3 positioned below L1 and L5. This lack of a somatotopy as well as the large overlap could be explained by the coarse spatial resolution due to the whole brain analysis. Further investigations focused on a ROI approach in S1 might reveal a somatotopy of lumbar vertebrae in the different Brodmann areas of S1.
Furthermore, the multisegmental input from adjacent FSUs due to the polysegmental innervations and sensorimotor control of spinal structures might have prevented separate representations [Hansen et al., 2006; Holm et al., 2002; Izzo et al., 2013; Sjolander et al., 2002]. Finally, it is also likely that more than just the intended FSUs were moved by the PA pressures. Using dynamic MRI, it has been reported that by applying a much stronger PA force (89 to 110 N), the most prominent motion at the tested segment also included a reduced motion in neighboring segments [Powers et al., 2003].
We assume that the nature of the applied stimulus in the present investigation is responsible for the strong and consistent activation patterns revealed. PA pressure on spinous processes induced a motion within the FSUs that mainly activated proprioceptive sensors, although the positioning and pressure of the thumb grip simultaneously stimulated skin sensors. The anatomical structures of an FSU contain mechanoreceptors that act like transducers by sending a continuous flow of proprioceptive information on loads, motions and posture to the central nervous system [Bogduk, 1983; Izzo et al., 2013; Sjolander et al., 2002]. In addition, the lumbar spine also consists of a wide array of uni‐segmental muscles, probably responsible for the fine tuning of motor responses. Uni‐segmental muscles exert less force but possess a two to six times higher density of muscle spindles compared to multisegmental spinal muscles responsible for movement [Bastide et al., 1989]. Proprioceptive information is mandatory to select neural control strategies for the stabilization of the spine, as accurate muscles have to be facilitated and the magnitude of activation level must be scaled appropriately. On the other hand, the spine needs to allow movements in various directions that is also perceived and performed in the same control areas [Gilchrist et al., 2003; Hansen et al., 2006; Holm et al., 2002].
An important region for the processing of proprioceptive information and the fine scaling of movements is the cerebellum. To our knowledge, no previous neuroimaging data are available describing lumbar spine representation within the human cerebellum. The activation in the anterior cerebellum (Larsell lobules II‐III) revealed by PA pressure onto lumbar vertebrae, might represent input from the dorsal spinocerebellar pathways forwarding proprioceptive information from the lower part of the trunk [Proske and Gandevia, 2009], for a review see [Bosco and Poppele, 2001]. The activation further extends the established somatotopy for sensorimotor perception from the lower extremities to a more anterior trunk representation [Grodd et al., 2001; Manni and Petrosini, 2004].
The activation pattern further included extensive bilateral S2 activation in the four cytoarchitecturally parcellated regions (i.e., OP1–OP4) of the parietal operculum. This activation was widely spread into the operculo‐insular cortex. Actually, [Garcia‐Larrea, 2012] suggested that the posterior insular cortex could be considered a third somatosensory area (S3), given the bidirectional relations with multisensory regions involved in integration and higher‐order postprocessing of mechanosensory information [Mazzola et al., 2012; Young et al., 2004]. The activation in the operculo‐insular cortex, together with cingulate and prefrontal cortices form a neuronal circuit that perceives, processes and holds sensory signals [Burton and Sinclair, 2000; Galazky et al., 2009; Goswami et al., 2011; Kostopoulos et al., 2007]. That working memory of sensory information is important for preparatory motor processing [de Graaf et al., 2009; Galazky et al., 2009]. Motor responses are required to counterbalance small or large postural perturbations to maintain postural control of the trunk before an actual movement is performed [Gilchrist et al., 2003; Masse‐Alarie et al., 2012].
PA pressure on the lumbar vertebrae also activated the SMA and related cingulate regions. Neuroimaging studies have described a rostral to caudal topography in SMA for performed foot, hand and face representation [Chainay et al., 2004; Mayer et al., 2001].The location of the sensory activation in the present study is anterior to the foot motor representation of [Chainay et al., 2004]. This finding is important as there is considerable evidence of involvement of SMA in postural control. Repetitive transcranial magnetic stimulation (rTMs) during step initiation revealed SMA involvement in the coordination of timing of anticipatory postural adjustments [Jacobs et al., 2009]. Similarly, functional near‐infrared spectroscopy (fNIRS) showed enhanced activation in SMA during preparation of impending perturbation of postural control in standing position [Mihara et al., 2008]. In the present study, the activation in SMA might indicate subliminary motor preparation, in the absence of an intended or actual performance of trunk stabilization. Together with SMA, the bilateral activations of the anterior cerebellum might be involved in the facilitation of predictive information for anticipatory postural control [Jacobs and Horak, 2007; Wolpert et al., 1998]. Previous investigations in LBP patients reported a lack of postural control of the trunk [Masse‐Alarie et al., 2012; Tsao et al., 2010]. Therefore, this approach represents a promising area for the investigation of the effects of LBP onto the sensorimotor system and subsequent postural disorders.
CONCLUSION
In healthy subjects, direct nonpainful stimulation of lumbar vertebrae induced a strong and extensive activation of sensorimotor networks of the lumbar spine by using fMRI. The applied PA pressure induced activation in cortical and subcortical regions that perceive, process, and retain mechanosensory information that might be used for anticipatory postural adjustments and stabilization of the lumbar spine. These acquired stable somatotopic maps of the lumbar vertebrae in healthy subjects can subsequently be used as a baseline to detect possible cortical plasticity in low back pain patients.
ACKNOWLEDGMENTS
The authors thank Marie‐Claude Hepp‐Reymond for helpful comments and discussion on the manuscript and Eduard Tolsma for the development of the pressure sensor system. They are grateful to Janine Rothmund for editing the English.
REFERENCES
- Bastide G, Zadeh J, Lefebvre D (1989): Are the “little muscles” what we think they are? Surg Radiol Anat 11:255–256. [DOI] [PubMed] [Google Scholar]
- Bogduk N (1983): The innervation of the lumbar spine. Spine 8:286–293. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Wilson AS, Tynan W (1982): The human lumbar dorsal rami. J Anat 134 (Part 2):383–397. [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Poppele RE (2001): Proprioception from a spinocerebellar perspective. Physiol Rev 81:539–568. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ (2000): Attending to and remembering tactile stimuli: A review of brain imaging data and single‐neuron responses. J Clin Neurophysiol 17:575–591. [DOI] [PubMed] [Google Scholar]
- Chainay H, Krainik A, Tanguy ML, Gerardin E, Le Bihan D, Lehericy S (2004): Foot, face and hand representation in the human supplementary motor area. Neuroreport 15:765–769. [DOI] [PubMed] [Google Scholar]
- Conti F, Fabri M, Manzoni T (1986): Bilateral receptive fields and callosal connectivity of the body midline representation in the first somatosensory area of primates. Somatosens Res 3:273–289. [DOI] [PubMed] [Google Scholar]
- de Graaf JB, Frolov A, Fiocchi M, Nazarian B, Anton JL, Pailhous J, Bonnard M (2009): Preparing for a motor perturbation: Early implication of primary motor and somatosensory cortices. Hum Brain Mapp 30:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow EA, Hinkley LB, Roberts TP (2003): Ipsilateral representation of oral structures in human anterior parietal somatosensory cortex and integration of inputs across the midline. J Comp Neurol 467:487–495. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K (2006): Identifying human parieto‐insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp 27:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR (2007): The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17:1800–1811. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Fink GR, Zilles K (2008): Functional lateralization of face, hand, and trunk representation in anatomically defined human somatosensory areas. Cereb Cortex 18:2820–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Salvolini U, Manzoni T (2005): Bilateral cortical representation of the trunk midline in human first somatic sensory area. Hum Brain Mapp 25:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Mascioli G, Paggi A, Salvolini U, Manzoni T (2006): Contribution of the corpus callosum to bilateral representation of the trunk midline in the human brain: An fMRI study of callosotomized patients. Eur J Neurosci 23:3139–3148. [DOI] [PubMed] [Google Scholar]
- Flor H, Braun C, Elbert T, Birbaumer N (1997): Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224:5–8. [DOI] [PubMed] [Google Scholar]
- Galazky I, Schutze H, Noesselt T, Hopf JM, Heinze HJ, Schoenfeld MA (2009): Attention to somatosensory events is directly linked to the preparation for action. J Neurol Sci 279:93–98. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea L (2012): The posterior insular‐opercular region and the search of a primary cortex for pain. Neurophysiol Clin 42:299–313. [DOI] [PubMed] [Google Scholar]
- Gilchrist RV, Frey ME, Nadler SF (2003): Muscular control of the lumbar spine. Pain Phys 6:361–368. [PubMed] [Google Scholar]
- Goswami R, Frances MF, Shoemaker JK (2011): Representation of somatosensory inputs within the cortical autonomic network. Neuroimage 54:1211–1220. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M (2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, de Zee M, Rasmussen J, Andersen TB, Wong C, Simonsen EB (2006): Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine 31:1888–1899. [DOI] [PubMed] [Google Scholar]
- Holm S, Indahl A, Solomonow M (2002): Sensorimotor control of the spine. J Electromyogr Kinesiol 12:219–234. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Iriki A, Taoka M, Toda T (2002): Processing of tactile and kinesthetic signals from bilateral sides of the body in the postcentral gyrus of awake monkeys. Behav Brain Res 135:185–190. [DOI] [PubMed] [Google Scholar]
- Izzo R, Guarnieri G, Guglielmi G, Muto M (2013): Biomechanics of the spine. Part I: Spinal stability. Eur J Radiol 82:118–126. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB (2007): Cortical control of postural responses. J Neural Transm 114:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Lou JS, Kraakevik JA, Horak FB (2009): The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience 164:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Hendry SH (1980): Distribution of callosal fibers around the hand representations in monkey somatic sensory cortex. Neurosci Lett 19:167–172. [DOI] [PubMed] [Google Scholar]
- Kell CA, von Kriegstein K, Rosler A, Kleinschmidt A, Laufs H (2005): The sensory cortical representation of the human penis: Revisiting somatotopy in the male homunculus. J Neurosci 25:5984–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kurata J, Sekiguchi M, Kokubun M, Akaishizawa T, Chiba Y, Konno S, Kikuchi S (2009): Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: An fMRI study. Spine 34:2431–2436. [DOI] [PubMed] [Google Scholar]
- Kostopoulos P, Albanese MC, Petrides M (2007): Ventrolateral prefrontal cortex and tactile memory disambiguation in the human brain. Proc Natl Acad Sci USA 104:10223–10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D, Findlay G, Roberts N, Nurmikko T (2008): Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine 33:1372–1377. [DOI] [PubMed] [Google Scholar]
- Manni E, Petrosini L (2004): A century of cerebellar somatotopy: A debated representation. Nat Rev Neurosci 5:241–249. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, van der Zwaag W, Farthouat J, Gruetter R, Blanke O (2012): Human finger somatotopy in areas 3b, 1, and 2: A 7T fMRI study using a natural stimulus. Hum Brain Mapp 35:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse‐Alarie H, Flamand VH, Moffet H, Schneider C (2012): Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments. Exp Brain Res 218:99–109. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Zimbelman JL, Watanabe Y, Rao SM (2001): Somatotopic organization of the medial wall of the cerebral hemispheres: A 3 Tesla fMRI study. Neuroreport 12:3811–3814. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R (2012): Spatial segregation of somato‐sensory and pain activations in the human operculo‐insular cortex. Neuroimage 60:409–418. [DOI] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S (2008): Role of the prefrontal cortex in human balance control. Neuroimage 43:329–336. [DOI] [PubMed] [Google Scholar]
- Panjabi MM (2003): Clinical spinal instability and low back pain. J Electromyogr Kinesiol 13:371–379. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E (1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443. [Google Scholar]
- Penfield W, Rasmussen T (1950): The Cerebral Cortex of Man: A Clinical Study of Localization of Function; New York: Macmillan. [Google Scholar]
- Plow EB, Arora P, Pline MA, Binenstock MT, Carey JR (2010): Within‐limb somatotopy in primary motor cortex—Revealed using fMRI. Cortex 46:310–321. [DOI] [PubMed] [Google Scholar]
- Powers CM, Kulig K, Harrison J, Bergman G (2003): Segmental mobility of the lumbar spine during a posterior to anterior mobilization: Assessment using dynamic MRI. Clin Biomech 18:80–83. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC (2009): The kinaesthetic senses. J Physiol 587 (Part 17):4139–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolander P, Johansson H, Djupsjobacka M (2002): Spinal and supraspinal effects of activity in ligament afferents. J Electromyogr Kinesiol 12:167–176. [DOI] [PubMed] [Google Scholar]
- Snodgrass SJ, Rivett DA, Robertson VJ (2006): Manual forces applied during posterior‐to‐anterior spinal mobilization: A review of the evidence. J Manipulative Physiol Ther 29:316–329. [DOI] [PubMed] [Google Scholar]
- Tsao H, Galea MP, Hodges PW (2008): Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 131 (Part 8):2161–2171. [DOI] [PubMed] [Google Scholar]
- Tsao H, Galea MP, Hodges PW (2010): Driving plasticity in the motor cortex in recurrent low back pain. Eur J Pain 14:832–839. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M (1998): Internal models in the cerebellum. Trends Cogn Sci 2:338–347. [DOI] [PubMed] [Google Scholar]
- Young JP, Herath P, Eickhoff S, Choi J, Grefkes C, Zilles K, Roland PE (2004): Somatotopy and attentional modulation of the human parietal and opercular regions. J Neurosci 24:5391–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997): Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120 (Part 1):141–157. [DOI] [PubMed] [Google Scholar]


