Abstract
Mirror neurons, originally described in the monkey premotor area F5, are embedded in a frontoparietal network for action execution and observation. A similar Mirror Neuron System (MNS) exists in humans, including precentral gyrus, inferior parietal lobule, and superior temporal sulcus. Controversial is the inclusion of Broca's area, as homologous to F5, a relevant issue in light of the mirror hypothesis of language evolution, which postulates a key role of Broca's area in action/speech perception/production. We assess “mirror” properties of this area by combining neuroimaging and intraoperative neurophysiological techniques. Our results show that Broca's area is minimally involved in action observation and has no motor output on hand or phonoarticulatory muscles, challenging its inclusion in the MNS. The presence of these functions in premotor BA6 makes this area the likely homologue of F5 suggesting that the MNS may be involved in the representation of articulatory rather than semantic components of speech. Hum Brain Mapp 36:1010–1027, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: motor control, mirror neurons, Broca's area, language, functional magnetic resonance imaging, intraoperative brain mapping
INTRODUCTION
A large body of research in the past 20 years has identified a frontoparietal network of cortical areas in the human brain, called the Mirror Neuron System (MNS) in homology with a similar circuit in the monkey, which is activated during action execution and observation and includes the inferior section of the precentral gyrus, the inferior parietal lobule, the intraparietal sulcus, and the superior temporal sulcus [Rizzolatti and Craighero, 2004]. At present, the inclusion of a specific portion of the inferior frontal gyrus (BA44/45, Broca's area) within the MNS is still highly controversial [de Zubicaray et al., 2010; Press et al., 2012]. The controversy is in part rooted in earlier debates of anatomical, functional, and theoretical nature, namely about the evolution of Broca's area, its physiological function as a motor/semantic area in speech/language production, and the evolution of human language. The attribution of Broca's area to the human MNS is the cornerstone of the mirror hypothesis of language evolution. This hypothesis, postulating a dual role of Broca's area in action/speech perception and action/speech production [Fazio et al., 2009; Levy, 2012] proposes a close relationship between the evolution of a human manual gesture system and of the open vocalization system, on which human speech is built [Rizzolatti and Arbib, 1998]. In addition, on this conceptual premise other roles have also been proposed for the MNS such as, for example, in the understanding of action‐related language [Hauk et al., 2004; Tettamanti et al., 2005].
From the anatomical point of view, the debate revolves around trying to establish the appropriate homology of human Broca's area with other areas of the non‐human primate brain based on cytoarchitectonic characteristics, particularly with area F5 in the ventral premotor cortex of the macaque brain, where mirror neurons were originally described [Gallese et al., 1996]. There is, however, no definitive consensus and the question remains extremely confused, with multiple and often contradictory interpretations of new and old data [de Zubicaray et al., 2010; Morin and Grezes, 2008; Nelissen et al., 2005; Petrides and Pandya, 2001; Petrides et al., 2005; Press et al., 2012; Toni et al., 2008]. Comparing human and macaque brains based solely on cytoarchitectural properties is extremely difficult for a variety of reasons, such as the independent recent evolution of the two species, the nonlinear effects of body size and the difficulty in accurate three‐dimensional reconstruction of areas and their spatial relations, and great caution must be exercised in interpreting localization of specific Brodmann areas [Mantini et al., 2012; Sereno and Tootell, 2005]. Much evidence suggests that, in humans, the inferior precentral sulcus may represents the homologue of the monkey arcuate sulcus, while the ventral compartment of area 6 (Vogt area 6aα), covering the major part of the precentral gyrus, may correspond to postarcuate macaque ventral premotor cortex (F4–F5). A confounding factor in the monkey/human anatomical matching is that the ventral premotor cortex in humans enlarges dorsolaterally in front of the precentral sulcus including area BA44 [Freund, 1996]. Ultimately, the truly critical property expected in the human homologue of F5 is that it must have a clear motor output as it does in the monkey, where the motor output elicited by electrical stimulation is one of the parameters identifying F5 [Cerri et al., 2003, Kraskov et al., 2009].
From the functional point of view, Broca's area has been famously identified as a key component of the network supporting the exclusively human linguistic ability, but is also recognized as a dishomogeneous area of great functional complexity [Duncan and Owen, 2000; Fedorenko et al., 2012; Koechlin and Joubault, 2006]. The role of Broca's area has been extended to include various abstract motor functions, such as imitation [Buccino et al., 2004; Iacoboni et al., 1999] or imagination [Gerardin et al., 2000; Grafton et al., 1996] of hand actions, and categorization of manipulable objects [Gerlach et al., 2002]. These studies have, thus, suggested that this brain area may include, in addition to the more commonly postulated mouth motor representation, a representation of hand and finger actions, a feature that would reinforce the homology with F5 in the macaque and further support the mirror hypothesis of language evolution. Several studies have also reported the bilateral activation of BA44 during hand and mouth action observation, hence the proposal to include this area in the MNS [Molnar‐Szakacs et al., 2005, Tettamanti et al., 2005]. However, in these studies, the definition of areas activated during action observation was not matched to execution, so that one of the fundamental parameters of the definition of “mirror” function, that is, activation of neural substrates by both observation and execution, was not verified [Dinstein et al., 2007; Turella et al., 2009]. Indeed, a careful scrutiny of the literature reveals that the activation of BA44 during the execution of hand actions is rarely observed and, when it is, always right at the border between BA44 and BA6, based on Talairach coordinate localization [Binkofski et al., 1999; Decety et al., 1997; Gazzola and Keysers, 2009; Molenberghs et al., 2012; Nishitani and Hari, 2000, 2002]. Phonological fluency tasks clearly identify BA44 [Heim et al., 2008] but, given the individual variability in both morphology (Amunts et al., 1999; Amunts and Zilles, 2012) and function [Juch et al., 2005] of this area, the limiting factor of the above methods remains the fact that in group studies, the precise definition of its borders is greatly blurred. For this reason, an additional individual assessment is necessary when trying to localize it more precisely, especially with respect to the neighboring BA6.
This article aims at resolving the question about Broca's area (BA44) alleged role in action execution and observation, and thus, inclusion in the human MNS, with two different and complementary approaches. A functional magnetic resonance imaging (fMRI) study, designed to determine (at the group level) the activation of Broca's area, identified in each subject with a phonological fluency task, during both execution and observation of different hand and mouth actions, and an intraoperative neurophysiological investigation (at the individual level), allowing to disclose the motor properties of Broca's area (also functionally identified) on several different phonoarticulatory and hand muscles. This approach gives a unique opportunity to measure directly, in humans, the motor output of Broca's area, not assessable with fMRI studies, compared to other areas involved in motor control, such as the premotor (BA6) and primary motor (BA4) cortices. In the neurosurgical literature, great attention has been given to the anatomical and functional definition of Broca's area, mostly with experiments involving the interference of electrical cortical stimulation with naming and counting tasks but, to our knowledge, there are no intraoperative studies addressing the specific features of muscle activation elicited from stimulation of premotor and Broca's area in human subjects.
MATERIALS AND METHODS
The Neuroimaging Study
Twenty‐two right‐handed healthy subjects (10 males, mean age 32.4 years, range 20–52), free from any neurologic disorder, underwent fMRI study. Subjects gave their informed consent to the protocol, approved by the ethical committee.
Subjects were asked to execute three tasks in both observation and execution conditions, assessing mirror activity in different motor effectors (hand and mouth), and a fluency task to identify BA44 area. They observed videos (Fig. 1) of (a) a hand grasping objects, (c) a mouth grasping objects, (e) a mouth performing communicative movements, and were asked to execute, (b) hand grasping movements, (d) mouth grasping movements, (f) mouth communicative movements.
Figure 1.
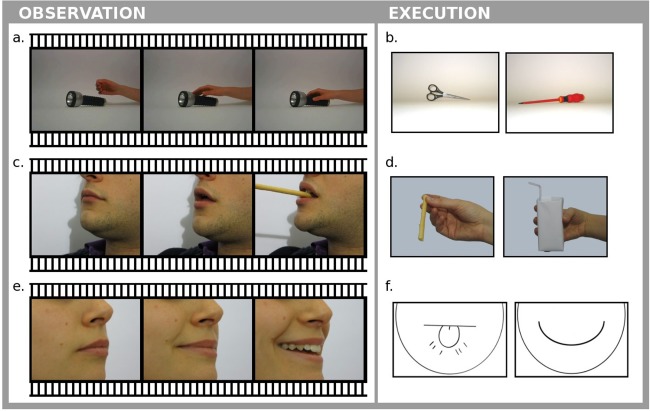
Some examples of stimuli presented to the subjects during the observation (a, c, e) and “execution” (b, d, f) runs for fMRI acquisition.
Observation runs
Subjects observed three videos. In run (a), a right hand grasping common objects [Cabinio et al., 2010]; in (c), a mouth (male and female) grasping common objects (e.g., little objects as a straw or a spoon, pieces of fruit, a breadstick); in (e), a mouth (male and female) performing communicative movements (smiling, sending kisses, whistling, snorting, making a “raspberry,” lip licking). During rest blocks, subjects observed still images of the effector just seen in action.
Execution runs
Subjects were asked to perform: (b) hand grasps adequate to the objects presented on the screen [Cabinio et al., 2010] using their right hand, and (d) mouth grasps adequate to the objects (same as in Observation Runs) presented. In both runs, subjects had to execute the movement as though the object were close to the effector. In run (f), subjects were asked to execute, with explicit awareness of their intention, the communicative movements (same as in Observation Runs) indicated by stylized faces presented to them. In all execution runs, subjects repeated iteratively (0.5 Hz) the same movement until the appearance of a different instruction (e.g., a different object). During rest blocks subjects kept the effector relaxed while still images of the effector (or a neutral stylized face) were presented. When testing the grasping conditions (execution with hand or mouth), the presentation of the object by itself (Fig. 1b,d) was used to instruct execution of the appropriate grasp because an actual video presentation of the action would have resulted in an imitation task. Accordingly, the instruction to perform the appropriate communicative movement (Fig. 1f) was neither a verbal instruction (activating language areas), nor a video of an actual face performing the movement (resulting in an imitation task).
Fluency
In one fMRI run, subjects were asked to perform, with eyes closed, a fluency task. At the beginning of each task block, subjects heard a phonological cue and they were asked to covertly think about words beginning with that phoneme. The choice to execute the fluency task only covertly was motivated by the necessity to avoid confounding effects deriving from the activation of the primary motor area. During rest blocks, subjects had to covertly count from 1 to 10 iteratively until the next block.
Subjects were trained for each task about 20 min before the scanning session. They were instructed to keep their gaze on the fixation point, to perform isolated movements of the hand and wrist and to move the mouth minimizing head displacement.
Each run had an A–B structure, with rest blocks alternated with task blocks. Each block started with a written command. The order of blocks (A–B or B–A) and the selection of presented stimuli within each run were randomly chosen. Each fMRI session of observation and execution included seven runs; all but one run were of 84 dynamics. Each observation and execution session had an A–B structure, alternating six rest blocks and six task blocks, each of them having seven dynamics duration. Fluency run were of 100 dynamics, with an A–B structure alternating rest blocks (4 dynamics long) with task blocks (6 dynamics long). The study was fully randomized using Presentation software (http://www.neurobehavioralsystems.com). The stimuli, projected onto a screen at the front side of the magnet bore, were viewed by subjects through a mirror attached to the head coil.
MRI scans were acquired using a 3.0 T scanner (Achieva, Philips Medical System, Best, The Netherlands). A conventional T2‐weighted scan (repetition time (TR) = 3,000; echo time (TE) = 85; field of view (FOV) = 230 mm; slice thickness = 5 mm; number of slices = 22) was performed in each subject to exclude brain abnormalities. A 3D T1‐weighted FFE scan (TR = 8 ms; TE = 3.9; FOV = 230 mm; matrix = 256 × 256; slice thickness = 1 mm; number of slices = 180) was acquired as anatomical reference for fMRI analysis. Functional images were collected by gradient echo‐planar T2 sequence (TR = 3,000 ms; TE = 30 ms; flip angle = 85°; FOV = 240 mm; matrix size = 128 × 128; number of slices = 40; thickness = 3 mm; SENSE factor anterior posterior (AP) = 2) using blood oxygenation level‐dependent contrast. Each fMRI session included seven runs; all but one run comprised 84 dynamics. A single run (Fluency) comprised 100 dynamics.
Data analysis
fMRI data were analyzed in agreement with the General Linear Model using MATLAB 7.1 (MathWork, Natick, MA) and SPM5 (Wellcome Dept. Cogn. Neurol., London; http://www.fil.ion.ucl.ac. uk/spm). Images were first corrected for motion, then they were realigned and movement parameters were estimated. Anatomical and functional images were then spatially normalized to the MNI template using a 2 × 2 × 2 voxel size with a trilinear algorithm. The normalized functional images were spatially smoothed using a 8‐mm full‐width at half‐maximum isotropic Gaussian kernel. We modeled the expected hemodynamic response function of the software package with a block design.
First level analysis
In each subject, seven t‐contrasts were defined: observation of hand movements, execution of hand movements, observation of mouth grasping movements, execution of mouth grasping movements, observation of mouth communicative movements, execution of mouth communicative movements, and fluency.
Second level analysis
All contrasts defined in the First Level were included in the Second Level analysis and a 7 × 1 ANOVA design was run. We then defined seven t‐contrasts, as done in the First Level analysis, and on the basis of these contrasts conjunction analyses were performed (for a schematic representation, see Fig. 2).
Figure 2.
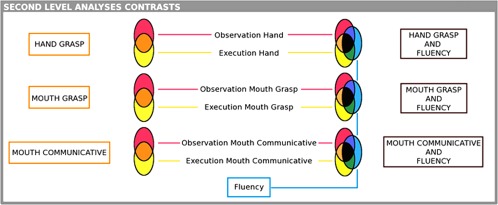
Contrast analysis. Graphical representation of the contrasts defined at the group level. Conjunction analyses between two conditions (action vs. observation) are represented for the three different stimuli (hand grasp, mouth grasp, and mouth communicative) in the orange areas. Conjunction analyses between three conditions (action vs. observation vs. fluency) are represented in the black areas. The only contrast considered per se is fluency, represented in the blue area.
Using different conjunction analyses, we were able to highlight cortical activations common to more than one (two or three) contrasts.
Fluency. Significantly activated were only those voxels surviving at P < 0.05 (family wise error (FWE) corrected) statistical threshold;
-
Conjunction analyses—two contrasts. To identify cortical areas involved in the MNS, that is, active during both observation and execution of the same movements, we performed conjunction analyses between:
Observation and execution of hand movements (Conjunction hand grasp);
Observation and execution of mouth grasping movements (Conjunction mouth grasp);
Observation and execution of mouth communicative movements (Conjunction mouth communicative);
-
Conjunction analyses—three contrasts. To identify which of the MNS areas are involved in phonological fluency, we performed conjunction analyses between:
Observation, execution of hand movements and fluency (Conjunction hand grasp AND Fluency);
Observation, execution of mouth grasping movements and fluency (Conjunction mouth grasp AND Fluency);
Observation, execution of mouth communicative movements and fluency (Conjunction mouth communicative AND Fluency).
Given the definition of the MNS (areas commonly activated in both observation and execution tasks), we limited the conjunction analysis with an inclusive masking (P < 0.05) only to those voxels that were active in the observation condition contrast, to avoid including of voxels not recruited in the observation condition. The results of the conjunction were considered as significant with P < 0.05FWE‐corrected threshold.
The Intraoperative Study
In this study were included 10 patients affected by gliomas, candidate for awake surgery after exposure of the frontal cortex. An extensive and multidisciplinary preoperative study involving neurological, neuropsychological (reported in Table 1) and neuroimaging tests was performed in order for patients to satisfy all inclusion requirements. Eligible for the study were only subjects affected by tumors not infiltrating the areas of interest. The preoperative neuroimaging study included basic morphological and fMRI plus diffusion tensor imaging. Functional tractography was performed on all patients before the surgery to detect the degree of infiltration of corticospinal and language pathways, at both cortical and subcortical levels, and fMRI analysis was conducted to identify possible shifts or disruptions of language and motor functions. None of these problems were reported in the patients included in the study. Although all tumors were (necessarily) located in the frontal lobe, it can be safely concluded that they did not affect the normal organization of Broca–premotor–primary motor cortex circuits, as assessed by all the above techniques. In addition, on admission none of the patients reported neurological impairment either in motor or language abilities and all were extensively investigated with preoperative neuropsychological tests.
Table 1.
Clinical classification of the 10 patients undergoing the analysis
| Name | Age | Handdominance | Preop neurologicexam | Lesion site | Lesion side | Who grade | Anaesthesia | n‐Psychoassessment | Intraoperatory test |
|---|---|---|---|---|---|---|---|---|---|
| Pz 1 | 71 | R | Normal | F | L | 2 | Asleep–awake | Normal | LFcn‐HF |
| Pz 2 | 35 | Corrected L | Normal | F | R | 3 | Asleep–awake | Normal | LFcn‐LF‐HF |
| Pz 3 | 23 | R | Normal | T | L | 2 | Asleep–awake–asleep | Normal | LFcn‐LF‐HF |
| Pz 4 | 41 | R | Normal | TI | L | 2 | Asleep–awake–asleep | Normal | LFcn‐LF‐HF |
| Pz 5 | 61 | R | Normal | F | L | 3 | Asleep–awake | Normal | LFcn‐LF‐HF |
| Pz 6 | 31 | R | Normal | F | L | 2 | Asleep–awake | Normal | LFcn‐HF |
| Pz 7 | 42 | R | Normal | F | L | 4 | Asleep–awake | Normal | LFcn‐HF |
| Pz 8 | 57 | R | Normal | F | L | 3 | Asleep–awake | Normal | LFcn‐HF |
| Pz 9 | 34 | R | Normal | F | L | 2 | Asleep–awake | Normal | LFcn‐HF |
| Pz 10 | 43 | R | Normal | T | L | 3 | Asleep–awake | Normal | LFcn‐LF‐HF |
For each patient, the handedness, the clinical parameters related to the tumor, the anesthetic protocol, the clinical scores obtained in the preoperatory neurological and neurophsycological assessments are reported together with the intraoperative neurophysiological test allowed by the procedure.
LFcn: Low Frequency counting‐naming.
LF: Low Frequency rest‐precontracted.
HF: High Frequency rest‐precontracted.
The study was performed following the routine procedure normally used for surgical tumor removal, without introducing any even minimal variation. Accordingly, all data were recorded using electrophysiological monitoring and stimulating protocols adopted for clinical mapping. Subjects gave their informed consent to the procedure.
Presurgical routine
Before surgery, all patients were submitted to handedness assessment, neurological examination and a neuropsychological evaluation of nonverbal intelligence, memory, apraxia, and language abilities. Patients' scores were all in the normal range.
The neuroradiological examination included basic morphological T1, T2, FLAIR, DWI, and post contrast T1 images. To determine the functional and anatomical relationship between the tumor and the cortical–subcortical areas important for hand movement and language, patients underwent fMRI and diffusion tensor imaging—Fiber tracking (DTI‐FT). During fMRI, subjects performed a finger tapping task to localize primary motor cortex and supplementary motor area and two language tasks (covert visual naming and fluency task or covert auditory verb generation) to localize premotor and prefrontal cortices, including Broca's area. The language hemispheric dominance was calculated by the laterality index on the basis of the fMRI results in both language tasks.
The DTI‐FT techniques allow reconstructing and visualizing the fiber tracts running around or inside the tumor, identifying the anatomical and functional boundaries of the lesion needed for the surgery. The MRI and the DTI‐FT images were loaded into the neuronavigation system for surgical purpose. DTI‐FT and fractional anisotropy measures excluded the infiltration of the corticospinal tract, inferior fronto‐occipital and superior longitudinal fasciculi (Table 1).
Routine intraoperative protocol
The intraoperative protocol includes asleep–awake (–asleep) anesthesia and functional brain mapping by means of electrophysiological and neuropsychological investigation. Direct Electrical Stimulation (DES) for cortical and subcortical mapping was performed, using both a bipolar and monopolar hand‐held probe connected to an Osiris stimulator [INOMED; Bello et al., 2014].
Functional identification of Broca's area
fMRI preoperative images loaded on the navigation system oriented the surgeon over the frontal “language areas.” The localization of Broca's area was confirmed with a neurophysiological intraoperative test, during which the patient performed a counting test and a naming test based on images presented on a computer screen. During the test, the putative Broca's area indicated by fMRI images was stimulated with the bipolar probe delivering DES with trains of stimuli at 60 Hz (average train duration ± standard deviation [SD]: 2.3 ± 0.9 s; average stimulation intensity ± SD: 3.9 ± 0.6 mA). When the stimulation stopped the patient's counting/naming at least three consecutive times, the localization of Broca's area was considered reliable. The initial current intensity applied to test Broca's area corresponded to the intensity needed to evoke motor responses from the primary motor cortex and progressively increased up to the value successful in aborting the counting task and then used for language testing at cortical and subcortical level.
Neurophysiological study
During surgery, cortical activity was monitored by Electroencephalography and Electrocorticography (EEG, ECoG, Comet, Grass); ECoG from a cortical region adjacent to the area to be stimulated was recorded by subdural strip electrodes (4–8 contacts, monopolar array referred to a midfrontal electrode) through the whole procedure to monitor the basal electrical activity of the brain and to detect afterdischarges or any epileptic activity during the resection. EEG was recorded with electrodes placed over the scalp in standard array. In these procedures, the electromyographic (EMG) responses to stimulation of the motor areas as well as the voluntary motor activity of the patient were recorded, during the procedure, by pairs of subdermal hook needle electrodes inserted into 16 muscles contralateral to the hemisphere to be stimulated, from face, upper, and lower limb, plus four ipsilateral muscles connected to a multichannel EMG recording (ISIS, INOMED).
Motor output assessment
Once the premotor (vPM/BA6) and primary motor cortices (M1) were exposed and Broca's area (BA44/45) functionally identified, the following protocol was applied.
Functional testing of M1, vPM/BA6, and Broca's area by means of DES (bipolar probe) with trains of biphasic shocks at 60 Hz, “low‐frequency paradigm, LF.”
Functional testing of M1, vPM/BA6, and the Broca's area with DES (monopolar probe, reference electrode inserted in the scalp as close as possible to the motor cortex) with trains of monopolar anodal shocks at 250 Hz, “high‐frequency paradigm, HF.”
LF paradigm
In each subject, LF stimulation was applied to M1, vPM/BA6 and Broca's area during picture naming and counting tasks (average train duration ± SD: 2.0 ± 0.8 s; average stimulation intensity ± SD: 3.5 ± 0.8 mA) monitoring both errors and EMG activity during stimulation.
In five subjects, LF was also applied to M1 (average train duration ± SD: 1.7 ± 1.1 s; average stimulation intensity ± SD: 3.2 ± 1.1 mA), over the upper limb and face motor areas in resting and tonic precontracted state. In the last condition, the level of EMG activation was monitored in real time and a degree of variability was unavoidable. The same protocol was applied to the vPM/BA6 (average train duration ± SD: 2.1 ± 0.5 s; average stimulation intensity ± SD: 3.6 ± 0.6 mA) and to Broca's area over the sites effective in blocking counting/naming tasks (average train duration ± SD: 2.3 ± 0.9 s; average stimulation intensity ± SD: 3.9 ± 0.6 mA). For each area and condition, all the muscles were simultaneously recorded during the stimulation.
HF paradigm
HF trains were applied to M1 (average stimulation intensity ± SD: 19.1 ± 8.5 mA) over the upper limb and face motor areas in resting state and precontracted state. During the stimulation, all set of muscles was simultaneously recorded. The number of the stimuli was adjusted to the minimum needed to elicit a motor evoked potential (MEP) in target muscles (train of 1–3 shocks). The current intensity was set at the minimum inducing a response. The same protocol was applied to vPM/BA6 (average stimulation intensity ± SD: 20.7 ± 8.7 mA, train of 1–5 shocks) and to Broca's area (average stimulation intensity ± SD: 16.3 ± 5.3 mA, train 1–7 shocks). The complex condition and the primary aim to avoid any impact on the procedure time did not allow performing all the tests in each subject (Table 1).
Data analysis
Muscular activity (EMG) was acquired by means of a specific software (ISIS, INOMED, sampling rate 20 KHz, notch filter at 50 Hz). In each subject, the occurrence of a MEP following DES stimulation applied on the three cortical areas (M1, vPM, and Broca) was assessed offline with a dedicated software. Due to the clinical condition, the number of trials (stimulations over the same area) acquired for each area varied from 10 to 25 in the different subjects (mean ± SD: 16.2 ± 6.3). Among the whole population of 10 patients and considering all the muscles recorded (orbicularis oris [OO], the mylohyoid [MYLO], the extensor digitorum communis [EDC], the abductor digiti minimi [ADM], the abductor pollicis brevis [APB], and the first dorsal interosseus [FDI]), a total of 346 EMG recordings were acquired from M1 stimulation, 270 from vPM and 569 trials from Broca. In the M1 area, 90 recordings were taken from ADM_APB, 59 from EDC, 21 from FDI, 112 from OO, and 64 from MYLO. In vPM, 43 recordings were taken from ADM_APB, 35 from EDC, 21 from FDI, 109 from OO, and 62 from MYLO.
The raw data relative to each stimulation trial were extracted from the acquisition system and analyzed with a dedicated software (MathWork, Natick, MA), after resampling at 4 KHz. For each trial, a window of interest of 100 ms from the stimulus onset was defined. The average background EMG activity and its standard deviation (BG ± 1SD) were then calculated on the last 25 ms of EMG acquired in the window of interest (from 75 to 100 ms). The emergence of the EMG signal from the BG ± 1SD was set as the beginning of the response (MEP's detection). The absolute latency of each MEP was calculated from the effective stimulus and its amplitude was calculated as the peak‐to‐peak (µV) amplitude. The intensity required to evoke single MEP (mA) was then reported. When, following the stimulation, the EMG signal did not exceed the background activity (i.e., did not even reach the BG ± 1SD), the trial was considered negative for a motor response.
Statistical analysis
Statistical analysis was performed to compare the main MEPs parameters (latency, amplitude) among the three areas. Notably, the “MEP detection” analysis demonstrated that when (HF) DES was applied over Broca's area, no responses could be detected (i.e., the EMG signal did not exceed the BG ± 1SD in any of the trials or subjects, see Results section). The absence of a motor response excluded the possibility of including Broca's area in the Statistical Analysis, given that neither latency nor amplitude could be computed in absence of a response. Normal distribution of data was assessed using the Kolmogorov–Smirnov test (KS test), and latency and amplitude data were transformed using different methods to achieve a normal distribution. Given the biological factors (i.e., the different physical distance from the central nervous system of the cranial muscles with respect to the forearm/hand muscles) underlying the difference in MEP latencies obtained in the muscles belonging to the two effectors (face/mouth vs. forearm/hand), the normal distribution was not achieved even after trying several data transformations, therefore, we split the data into two subsamples including two muscles (Subsample A, including the OO and the MYLO) and four muscles (Subsample B, including the EDC; the ADM; the APB; and the FDI) and perform a natural logarithm (ln) transformation of latency and amplitude data. For univariate analysis, a Student's t‐test was used when comparing quantitative data of a dichotomous variable, and the ANOVA test in case of a nominal variable with >2 categories. For multivariate analysis, a generalized linear model analysis of variance was used using latency and amplitude converted into ln score in two different models and in two subsamples testing the impact of brain area (vPM and M1), muscles (the OO; the MYLO; the EDC; the ADM; the APB; and the FDI), and subject variability. We included in the model interaction terms between independent variables. We performed post hoc tests using Bonferroni correction. SPSS statistical software (IBM) version 20 was used for statistical analyses.
RESULTS
The Neuroimaging Study
The two conditions essential for inclusion in the MNS were enforced by the fMRI experimental protocol and conjunction analysis, which identifies areas active during both observation and execution of hand and mouth grasping actions and communicative mouth actions (conjunction analysis, two contrasts).
Conjunction hand grasps
The analysis of areas active in observation and execution of right hand grasping actions (Fig. 3a) showed the activation of a bilateral, though strongly left‐lateralized, frontoparietal network, including the anterior bank of the precentral gyrus (vPM/BA6), the middle frontal gyrus (BA9), the left postcentral gyrus (BA2), and the inferior parietal lobule (BA40). The left middle occipital gyrus (BA19) was also recruited in this analysis.
Figure 3.
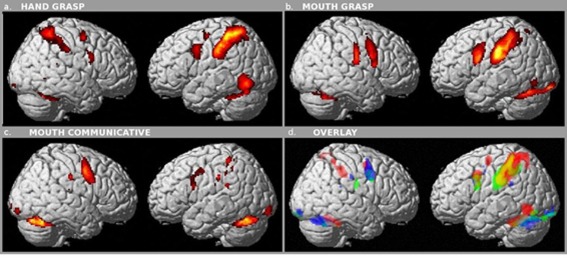
Conjunction, two conditions. Brain areas commonly activated during observation and execution of (a) hand grasp movements (P < 0.05 FWE correction), (b) mouth grasp movements (P < 0.05 FWE correction), (c) mouth communicative movements (P < 0.05 FWE correction). (d) Overlay of the three set of activation: red conjunctions hand grasp (panel a), yellow conjunctions mouth grasp (panel b), and blue conjunctions communicative (panel c).
Conjunction mouth grasps
Processing of mouth grasping actions (Fig. 3b) revealed a bilateral, but still left‐lateralized, frontal network including, in the left hemisphere, vPM/BA6 here recruited more ventrally than for hand grasp and just up to the border with BA44 (Fig. 3a), and BA9. The bilateral activation of the parietal cortex involved the postcentral gyri (BA2–3) and BA40. Left occipital areas (BA17 and19), as well as bilateral cerebellum, were also involved. Most interesting is the clear‐cut somatotopic activation of the vPM/BA6, mapping hand and mouth in dorsoventral fashion, paralleled by a similar somatotopic activation in the primary somatosensory cortex (Fig. 3d).
Conjunction mouth communicative actions
Processing of mouth communicative actions (Fig. 3c) resulted in the activation of a network including bilaterally vPM/BA6, here recruited ventrally just to the border with BA44, BA2–3, BA40, and BA9. Bilateral involvement was also found for occipital (BA18) and cerebellar cortices.
Except for the ambiguous posterior border with vPM/BA6, area BA44 did not emerge as one of the areas activated in either hand or mouth conjunction analyses. However, to further investigate possible mirror properties of the frontal network underlying language functions, the “mirror areas,” activated with the above protocols, were matched with the areas activated during a language production functional test (conjunction analysis, three contrasts).
Fluency and conjunction analyses
The phonological fluency task strongly activated a left lateralized cortical network, which includes the inferior frontal gyrus (BA44, 45, 46, and 47), vPM/BA6, BA9, and the right cerebellum (Fig. 4). However, no common activation emerged in the inferior frontal gyrus when matching the areas activated during the fluency task with those activated during hand or mouth action execution/observation. In all experimental conditions, (hand grasping, mouth grasping, and mouth communicative, Fig. 5a–c) the only area selected by the 3‐contrast conjunction analysis, that is, active in all three conditions (observation, execution, and fluency), was vPM/BA6. The conjunction analysis of the mouth‐grasp/fluency and mouth communicative/fluency tasks produced only a slight, small activation at the BA6‐BA44 border, compared to the massive activation of the inferior operculum (BA44–45) during the fluency task. The positive match observed for BA6, activated in all the three conditions tasks, suggests that the area actually displaying mirror properties within the language network is BA6, rather than BA44.
Figure 4.

Fluency. Brain areas activated (P < 0.05 FWE correction) by the Fluency task in the left hemisphere were: the inferior frontal gyrus (Broca's area, BA44), the precentral gyrus (ventral premotor cortex, BA6) and the supplementary motor area (SMA, not visible on render).
Figure 5.
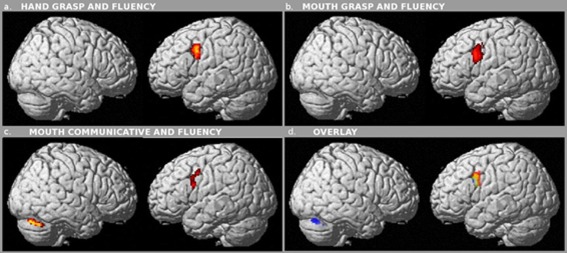
Conjunction, three conditions. Brain areas commonly activated during (a) observation, execution of hand grasp movements and fluency (P < 0.05 FWE correction), (b) observation, execution of mouth grasping movements and fluency P < 0.05 FWE), (c) observation, execution of communicative mouth movements and fluency P < 0.05 FWE). (d) Overlay of the three set of activation: red conjunctions hand grasp (panel a), yellow conjunctions mouth grasp (panel b) and blue conjunctions communicative (panel c).
The Intraoperative Study
The fMRI protocol allowed for the functional separation of ventral premotor vPM/BA6, with mirror properties, from BA44/Broca, without mirror properties. To confirm this result with a more direct approach, we investigated the motor output of these same areas on the premise that, in the monkey, electrical stimulation of the ventral premotor area F5, containing mirror and canonical neurons, results in recordable muscle activity [Cerri et al., 2003; Kraskov et al., 2009].
To this aim, we were able to test the motor output elicited by electrical stimulation of vPM/BA6 as well as of Broca's area, on several different phonoarticulatory and hand muscles, using an intraoperative mapping procedure performed in a group of awake patients. Stimulation of the primary motor cortex (M1) was performed to obtain, in most subjects, a reference muscular output from stimulation of the most effective motor area. In this setting, it was crucial to identify Broca's area with respect to the vPM. In each subject, the identification of Broca's area was first based on neuroimaging data and then confirmed by intraoperative functional testing. Two different stimulating protocols, LF (60 Hz bipolar probe) and HF (250 Hz monopolar probe) DES trains, were adopted in the surgical mapping procedure.
Functional identification of Broca's area
During the procedure, the patient performed a naming and a counting task, while Broca's area, as indicated by fMRI images loaded on the navigation system, was stimulated with LF DES trains. Broca's area, and its functional boundaries, was identified as the site where DES induced speech arrest at least three consecutive times.
Motor output of M1, BA6, and Broca's area with LF stimulation during naming/counting task
When LF stimulation was applied to M1 during the naming or counting task, speech arrest was induced due to the interference of the electrically recruited muscular activity (OO, and suprahyoid muscles, Fig. 6A) onto the phonoarticulatory output. When LF stimulation was applied to vPM/BA6, speech arrest or dysarthria (groping articulatory movements, distortions of speech sounds, voice emission, utterances) occurred. Stimulation here induced either block or tonic recruitment of EMG activity in different phonoarticulatory muscles active during the task (contralateral OO, Fig. 6B), in some cases also recruiting muscles (as the suprahyoid) not significantly active during the task. While stimulation over vPM/BA6 interferes with phonoarticulatory movements inducing an aberrant EMG activity, a different effect was observed when LF stimulation was applied to Broca's area, which resulted in speech arrest without affecting the ongoing EMG activity, but seemingly aborting speech at a much earlier stage than muscle activation (Fig. 6C).
Figure 6.
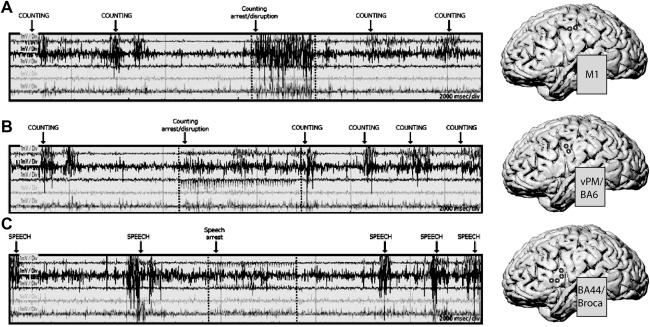
Low Frequency cortical stimulation during speech. LF stimulation applied to primary motor cortex (M1, panel A), to premotor cortex (vPM/BA6, panel B) and to Broca'a area (panel C) in one exemplificative subject while performing counting (A and B) and picture naming (C) tasks. 3D brain reconstruction of the subject's brain, with the superimposed site of stimulation (circles), are shown on the right of each EMG recording. EMG traces refer to (top to bottom): orbicularis oris (ipsi and contralateral), masseter, infrahyoid, and suprahyoid muscles. Stimulus onset and duration are indicated with dotted lines. The effect of LF stimulation on M1 and vPM/BA6 caused speech arrest by disrupting the orderly activity of muscles involved in the phonoarticulatory process, while stimulation on Broca's area induced speech arrest without affecting muscle activation.
Motor output of M1, BA6, and Broca's area with LF stimulation in resting and precontracted conditions
In all subjects tested, LF stimulation of M1 elicited EMG responses in contralateral resting as well as precontracted hand and face muscles consistently with the somatotopic site of cortical stimulation. The EMG onset of the muscles represented in the cortical stimulation site (hot spot) displayed a fast recruitment (Fig. 7, left panel). When stimulating in precontracted conditions, the amount of EMG activation in contralateral muscles progressively increased with respect to the background activity (not shown), as expected by the summation of the voluntary with the DES‐induced corticospinal drive.
Figure 7.

Low frequency cortical stimulation in resting and pre‐activated conditions. LF stimulation applied to M1, vPM/BA6 and BA44/Broca in one exemplificative subject. Stimulus onset and duration are indicated with dotted lines. The subject was stimulated in resting state. Stimulation of M1 (left panel) induced contraction in several upper limb muscles (arrows); stimulation of vPM/BA6 (middle panel) induced a weak activation of forearm muscles (arrows), while stimulation of Broca/BA44 (right panel) failed to elicit responses in any of the recorded muscles. 3D brain reconstruction of the subject's brain, with the superimposed site of stimulation (circles) of M1, vPM/BA6, and BA44/Broca, are shown in the lower panel.
LF stimulation of vPM/BA6 could elicit motor responses in resting forearm muscles only in one subject (Fig. 7, middle panel), with a very slow EMG onset, suggesting that vPM is a less excitable area with respect to M1, needing multisynaptic summation to trigger a response. As described above, a positive motor response was more frequently observed when stimulating vPM during the counting task (Fig. 6B), when the interference with the prime movers of the mouth corresponded to the simultaneous recruitment of muscular activity from other resting muscles (e.g., suprahyoid), again with a very slow onset. The stimulation of the vPM/BA6 in tonically preactivated muscles resulted in a disruption or inhibition of the ongoing EMG activity, usually with a concomitant recruitment of other muscles not involved in the tonic contraction, rather than in a powerful muscle recruitment. This effect was observed only when the stimulation involved face–mouth muscles.
When applied over Broca's area, the LF stimulation failed to induce any motor effects both in resting (Fig. 7, right panel) and in preactivated state in all subjects (not shown).
Motor output of M1, BA6, and Broca's area with HF stimulation in resting and precontracted conditions
Trains of HF DES were applied at rest and in precontracted state. Stimulation over M1 elicited MEPs both in resting and in active target muscles, according to somatotopic cortical representation (see the exemplificative subject in Fig. 8a, upper traces). The number of shocks needed to elicit MEPs varied from 1 in preactivated muscles, to 1–2 (rarely 3) in resting muscles. Stimulation of vPM/BA6 required a higher number of shocks (3–5, rarely 1–2), to elicit responses in hand, forearm, and head muscles (Fig. 8b, middle traces). Stimulation of Broca's area (Fig. 8c, lower traces) always failed to elicit any response in any muscle, both in resting and in active state, even when increasing the number of shocks (up to 7).
Figure 8.

High frequency cortical stimulation in resting condition. HF stimulation applied to M1, vPM/BA6, and BA44/Broca in one exemplificative subject. On the right, MEPs recorded in ADM, EDC, and Mylohyiod (MYLO) following the stimulation of M1 (1 shock; average of 10 trials), vPM/BA6 (5 shocks, interstimulus interval 4 ms in ADM and EDC; 3 shocks, interstimulus interval 4 ms in MYLO; average of 15 trials) and BA44/Broca (7 shocks, interstimulus interval 4 ms;, average of 20 trials) are shown. On the left, the sites of stimulation eliciting MEPs in the hand region of M1, hand/mouth region of vPM/BA6 and unaffected Broca's region are shown. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Considering the results obtained with LF and HF stimulation, it can be summarized that the LF stimulation (although with few subjects; n = 5) is always effective in eliciting motor output from M1 while, when applied to vPM/BA6, it rarely induces motor responses in resting or slightly tonically contracted hand (at best delayed and weak responses), exerting its motor effect mainly over the oro‐facial muscles during phonoarticulatory tasks (naming/counting), normally altered by the vPM stimulation. Differently, the HF stimulation seems to be the most adequate stimulus to evoke motor responses from motor and premotor cortices.
Given that the stimulations were performed in a clinical setting, that limitations were imposed by the dimension of the surgical flap, and that the surgeon was unrelenting in denying the application of the stimulation in sites not needed for the procedure, it was not possible to systematically test all the recorded muscles in all patients, but certainly in all patients representative muscles of face and hand were collected. Despite these constraints, responses to stimulation of M1 could be collected in all patients from mouth and hand muscles. When stimulating vPM/BA6 with HF (depending on the sites available for stimulation), it was possible to collect responses with a clear onset in face and upper limb muscles in four patients, only in face muscles in five patients, and only in upper limb muscles in one patient. The muscles showing the most reliable responses were the OO, the MYLO, the EDC, the ADM, the APB, and the FDI. No responses were detected and therefore recorded in the lower limb following vPM stimulation. As a first observation, the responses obtained from stimulation of vPM were delayed with respect to those obtained from stimulation of M1 (average ± SD: 2.99 ± 1.23 ms).
Statistical Analysis
A total number of 616 trials have been examined in two brain area (346 for M1, 270 for vPM) from five muscles (mean number of trials ± SD: 123.2 ± 65.4) of 10 subjects (mean number of trials ± SD: 61.6 ± 47.9).
As stated in the Materials and Methods section, the “MEP detection” analysis demonstrated that when HF‐DES was applied over Broca's area, no MEPs were elicited (i.e., the EMG signal never exceeded the BG ± 1SD noise, see Materials and Methods section) in any of the trials analyzed and subjects. The absence of a motor response excluded the possibility to include Broca's area in the Statistical analysis, which was instead performed to compare responses obtained from M1 to those obtained from the vPM.
As described in Materials and Methods section, latency and amplitude were not normally distributed (P < 0.0001, KS test) even after applying several data conversion (logarithm conversion, ln conversion, inverse, and square root transformation; P < 0.0001, KS test). Therefore, we splitted our sample into two subsamples and use ln converted latency and amplitude data (Subsample A: P = 0.07 and P = 0.1; Subsample B: P = 0.20 and P = 0.05; KS test).
Univariate analysis shows that, in Subsample A (cranial muscles), the latency was different according to brain area (M1 = 2.34 vs. vPM = 2.52; t(330.8) = −7.6, P < 0.001) and subjects [F(8,338) = 30.6, P < 0.001], but not to muscles (OO = 2.44 vs. MYLO = 2.41; t(345) = 1.3, P = NS). In Subsample B (hand/forearm muscles), latency was different according to brain area (M1 = 3.07 vs. vPM = 3.21; t(267) = −10.3, P < 0.001), muscles (EDC = 3.06, ADM_ APB =3.15 and FDI = 3.16; [F(2,266) = 14.5, P < 0.001]) and subjects [F(4,264) = 17.3, P < 0.001]. In the multivariate model, after adjustment for muscle type and type of subject, brain area was confirmed to be a significant predictor of the model in Subsample A [F(1,347) = 220.9, P < 0.001] and Subsample B [F(1,269) = 90.5, P < 0.001] with a good overall explanation of the two models (global R2 = 0.77 and 0.68). Significant terms were also found for muscles in Subsample B only (P < 0.001) and in subjects (P < 0.001), as well as for the interaction terms brain area × subject (P < 0.001) and subject × muscle (P < 0.001), but not for brain area × muscle.
As regards amplitude, in Subsample A (cranial muscles), it was different according to muscles (OO = 5.7, MYLO = 4.8; t(303.5) = 10.3, P = 0.004) and subjects [F(8,338) = 32.2, P < 0.001] but not to brain area (M1 = 5.48 vs. vPM = 5.31; t(345) = 1.68, P = NS). In Subsample B (hand/forearm muscles), amplitude was different according to brain area (M1 = 5.73 vs. vPM = 4.67; t(242.9) = 13.7, P < 0.001), muscles (EDC = 5.51, ADM_APB = 5.34, and FDI = 5.01; [F(2,266) = 5.2, P = 0.006]) and subjects [F(4,264)=7.7, P < 0.001].
In the multivariate model, after adjustment for muscle type and type of subject, brain area was found to be a significant predictor of the model in Subsample A [F(1,347) = 9.3, P = 0.003] and Subsample B [F(1,269) = 127.5, P < 0.001] with a good overall explanation of the two models (global R2 = 0.77 and 0.60). Significant terms were also found for muscles (P < 0.001) and subjects (P < 0.001), as well as for the interaction terms brain area × subject (P < 0.001) and subject × muscle (P < 0.001), and for the interaction term brain area × muscle only in Subsample A (P = 0.003).
The significantly longer latency observed when MEPs were evoked by stimulating vPM versus M1, supports the view that indeed motor output originated in vPM (when the stimulating electrode was placed there) rather than from current spreading and reaching M1.
Interestingly based on the analysis of the amplitude of responses, it could be suggested that the sector of the vPM controlling phonoarticulatory muscles shows similar excitability to its analogue in M1, while the sector controlling hand muscles seems to be, on average, less excitable than M1. However, the experimental design was not defined to test this hypothesis and a dedicated study is needed.
Overall the data suggest that Broca's area does not provide any motor output while vPM/BA6 shows a motor effect with different features, depending on the activation state of muscles (preactivated or resting) and task performed (language production) during stimulation.
DISCUSSION
The goal of this article is to assess mirror properties, that is, activation during action execution and observation, of the human Broca's area (BA44), in homology with the monkey ventral premotor area F5, where mirror neurons were originally described. To this aim, an fMRI study was designed to assess the activation of brain areas during a fluency task and during the execution and observation of hand and mouth grasping actions as well as nonverbal mouth communicative actions. Subsequently, an intraoperative neurophysiological study was designed to investigate whether the electrical stimulation of Broca's area elicited a motor output in hand or mouth muscles. The results challenge the inclusion of Broca's area in the human MNS.
In the neuroimaging experiments, cortical areas belonging to both the MNS and to the language production system were identified based on activation during three conditions: action execution, action observation, and phonological fluency. This approach was needed to restrictively select areas satisfying both functional requirements: the “mirror” requirement, that is, the activation during both action observation and execution, and the “language” requirement, that is, the activation during phonological fluency. It emerged that vPM/BA6 fully and consistently satisfies the above requirements, while BA44/Broca does not. Even defining BA44 based on more general Talairach coordinates resulted in a borderline activation, in both hemispheres, for both mouth tasks, and only in the right hemisphere for the hand grasping task. The conjunction analysis of the mouth‐grasp/fluency and mouth communicative/fluency tasks produced only small activation at the BA6‐BA44 border, compared to the massive activation of the inferior operculum (BA44–45) during the fluency task. While it is perhaps reasonable to expect that BA44 would be activated less during action observation than during phonological fluency, this marginal activation seems to exclude any significant overlap between mirror and language cortical areas. What is activated at the BA6–BA44 border may be the nonlanguage part of Broca, a “domain‐general sub‐region” which, adjoining posteriorly the language‐selective subregion, extends toward the inferior precentral sulcus, and is activated by a variety of broad cognitive tasks [Fedorenko et al., 2012]. Alternatively, the activation at the BA6–BA44 border could simply result from the somatotopic ventral spreading of the BA6 activity toward the inferior frontal gyrus, rather than from a true BA44 activation. In fact, one of the most interesting results of this study is the effector‐dependent somatotopic activation within the ventral premotor cortex, BA6, mapping hand and mouth in a dorsoventral fashion, paralleled by similar somatotopic activation in the primary somatosensory cortex (Fig. 3).
Several studies have shown the activation of primary somatosensory areas during action observation [for a discussion, see Gazzola et al., 2007]. The activation of the sensory cortex is particularly interesting, given that it occurs without contact with a real object. Using the same paradigm adopted in a previous study, where it is extensively discussed, in this experiment, subjects were instructed to make the grasping movement adequate for the object presented on the screen, without visual access to their moving limb, matching the proprioceptive information coming from the moving limb and possibly creating a sensory–motor neural representation of their hand grasping that specific object [see Cabinio et al., 2010]. The somatosensory activation emerging in the conjunctions mouth and hand grasps was significantly reduced in the conjunction mouth‐communicative, suggesting perhaps a lower expectation for sensory tactile feedback compared to a grasping action, where the contact with the object is the main goal.
While important criticisms have been raised regarding the limited power of fMRI to identify mirror activity in the human brain [Dinstein et al., 2008] and several other more sophisticated techniques have been developed in an effort to rigorously define the human MNS [see Oosterhof et al., 2013, for a review], the primary effort of these experiments was to test the hypothesis that Broca's area has both motor and language properties. These are essential requirements for the definition of a “mirror” area (i.e., that it can be identified by activation during action execution/observation, and that it shows a clear motor output when electrically stimulated), and of a “language” area (i.e., it can be identified by activation during phonological tasks). In this context, compelling imaging results were empowered by a neurophysiological investigation of the functional properties of Broca's area and vPM/BA6. The intraoperative mapping, by DES of the cortical surface and recording from a large number of speech and hand muscles, aimed at verifying the motor output of Broca's area, a functional property expected from the homologue of monkey F5. Similarly to healthy subjects involved in the fMRI study, patients involved in the intraoperative investigation performed phonological fluency tasks, providing a common functional identification of Broca's area. The peculiar setting of data collection, that is, patients affected by brain tumors, deserves a brief discussion of the possible alterations in localization and function of brain areas in proximity of a tumor mass. It has been reported in the neurosurgical literature [Skirboll et al., 1996] that even with large infiltrating tumors Broca's area may be intact, and that even when a tumor is within 1–2 cm from the area, the anatomical and functional landmarks considered by the neurosurgeons are still valid for accurate localization of Broca's area. Moreover, the existence of intersubjects variability in Broca's area is also well known in the neurosurgical literature [Skirboll et al., 1996; Quiñones‐Hinojosa et al., 2003]. Functional activation, therefore, is the best tool to identify Broca's area, explaining our choice to identify it first with fMRI images obtained during a phonological fluency task and then with the functional intraoperative stimulation inducing speech arrest (without motor effects).
The data presented suggest that Broca and vPM/BA6 behave very differently when electrically stimulated during speech: while stimulation of BA6 resulted in speech arrest as a consequence of motor impairment, Broca's stimulation did not have any direct effect on phonoarticulatory muscles but induced speech arrest as a consequence of halting the naming process, that is, at a cognitive rather than motor level. These results confirm earlier behavioural observations [Duffau et al., 2003; Tandon et al., 2003], which, however, were not supported by the EMG recordings needed to demonstrate the actual impairment of motor activity following the stimulation of BA6 but not of Broca's area, thus, suggesting a different role of the two areas in speech production. The results are also in line with a previous, repetitive transcranial magnetic stimulation study (rTMS) [Aziz‐Zadeh et al., 2005] showing that both overt and covert speech arrest can be induced by stimulating both an anterior/IFG site and a more posterior/motor site in the left inferior frontal cortex, and suggesting that both sites may be involved in language production even when motor output is not directly necessary but only simulated.
Despite intersubject variability, unavoidable in a complex surgical setting, our data consistently failed to show any motor output from BA44/Broca's area, irrespective of the stimulation paradigm (LF or HF), of the excitability state (resting or preactivated), and of the functional state (picture naming or counting task).
LF stimulation of vPM/BA6 resulted in both excitatory and inhibitory effects on distal muscles. The excitatory effect, that is, the muscular activation, was rarely evoked in resting or slightly tonically precontracted muscles and, when it was the case, it showed a very slow recruitment. The inhibitory effect on tonic muscle preactivation may recall the “stimulus‐induced negative responses” evoked by stimulation of the negative motor areas (NMAs) in human brain [Lüders et al., 1995], that is, areas that, when stimulated, produce an inability to perform voluntary movement or to sustain an ongoing muscle contraction. Stimulation of NMAs, identified in the inferior frontal gyrus just anterior to the primary facial motor area and at a medial position in the superior frontal gyrus, typically induces speech arrest and arrest of movements of the limbs. However, while for the limbs a “negative response” clearly implies the inhibition of an ongoing muscle activity, the speech arrest could derive from either the impairment of the execution of articulatory movements (dysarthria), or the suppression of the naming action prior to its motor articulation (anomia), difficult to discriminate without EMG monitoring. Our data suggest a functional difference between the inhibitory effect obtained from stimulation of Broca's area, which interrupts speech without altering muscle activity, and stimulation of vPM/BA6 affecting muscle activity. These results challenge the view of Broca's area as a proper motor area, hosting muscle representations, or providing a crucial node in neuronal circuits controlling the motor drive to phonoarticulatory muscles [Espadaler et al., 2012].
Based on animal studies, high frequency stimulation (repetitive intracortical microstimulation) is the most effective paradigm to elicit responses from primary and nonprimary motor cortices [Porter and Lemon, 1993]. Coherently, as a main result, in our subjects, HF stimulation of vPM/BA6 evoked positive motor responses in face and hand muscles, but failed to induce any motor response when applied to BA44/Broca's area. In humans, both “canonical” and “mirror” stimuli activated the ventral limb of the precentral sulcus [Grèzes et al., 2003], suggesting that mirror and canonical neuronal populations do not segregate in two separate areas and that they both must be searched in one area, that is, the homologue of F5. The expectation of a motor output from the putative human mirror area is supported by the observation that when stimulating the monkey's F5, containing mirror and canonical neurons, muscular activation occurs [Cerri et al., 2003; Kraskov et al., 2009]. Moreover, the excitatory and inhibitory effects of F5 mirror neurons on M1 descending projections are well demonstrated [Kraskov et al., 2011]. This evidence reinforces the expectation of a motor output from the human homologue of F5.
The main result of this study indicates the vPM/BA6 as the functional homologue of F5, thus, to be included in the human MNS, while there is no substantial involvement of a functionally defined Broca's area in the MNS. This observation leads to reconsider the role of the MNS in human language, suggesting its action as “executive motor” control of speech production, rather than “prelexical semantic” control of language generation. It has to be considered, on one side, that the role of MNS in the evolution of “prelexical semantic” aspect of language is based on the hypothesis that actions are organized according to the same architectural model as the syntax of human language, analogy strongly challenged in recent literature [Moro, 2014]. Moreover, speech production is a multistage process, from the prelexical intention to the final motor output, a highly skilled motor task involving an impressive number of muscles [Kent, 2000]. In a recent model of neural circuitry for speech production [Hickok, 2012], the word representation (lemma) feeds the circuit at the opercolar level (BA44) and the translation from syllable to the articulatory feature clusters occurs through a Broca‐vPM‐M1 interaction, so that Broca's area would exert its control on the vocal tract via an additional station, BA6, where the motor plan is assembled [Bouchard et al., 2013]. Therefore, an arrest in speech production, but not a direct motor output, would be expected following stimulation of Broca's area, as indeed observed in the present recordings. Interestingly, the possible involvement of the MNS in motor control of speech production, has been already suggested in the neurosurgical literature [Duffau et al., 2003], where the vPM, rather than Broca's area, was spontaneously associated with the MNS system.
The role of BA6/vPM in the articulatory process is also strongly suggested when considering the apraxia of speech syndrome (AOS), that is, failure in translating a phonological frame into kinematic parameters. AOS is frequently associated with lesions in the left vPM or posterior portions of the inferior frontal gyrus [Hillis et al., 2004] and of the anterior left insula [Dronkers, 1996]. Speech deficits, resembling AOS, can be induced with rTMS over vPM [Tandon et al., 2003]. Moreover, the clinical observation of a double dissociation between aphasia and apraxia suggests that language and phonoarticulation (praxis) are controlled by two different, partly overlapping networks [Mengotti et al., 2013; Papagno et al., 1993].
Thus, the same system involved in speech production overlaps in BA6 with the neural premotor circuit involved in the control of hand/arm actions and belonging to the MNS, suggesting that the role of the MNS in language may concern more the representation of motor than the semantic components of speech.
CONCLUSIONS
Our results show that Broca's area is minimally involved in action observation/execution and has no motor output on hand or phonoarticulatory muscles, challenging its inclusion in the MNS. The presence of these functions in premotor BA6 makes this area the likely homologue of F5 suggesting that the MNS may be involved in the representation of articulatory rather than semantic components of speech.
FUNDING
The work was supported by grants from the Italian Ministry of Health, Ricerca Finalizzata RF‐2009‐1530888 (A.F. and L.B.), Associazione Italiana Ricerca sul Cancro (AIRC) (L.B.) and Fondazione Berlucchi (L.B.).
ACKNOWLEDGMENTS
The authors are deeply grateful to R. Lemon and S.G. Grafton for critical reading of the manuscript. The authors thank A. Castellano for routine DTI analysis and M. Montagna for technical assistance in data processing.
REFERENCES
- Amunts K, Zilles K (2012): Architecture and organizational principles of Broca's region. Trends Cogn Sciences 16:418–426. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HBM, Zilles K (1999): Broca's region revisited: Cytoarchitecture and intersubject variability. J Comput Neurol 412:319–341. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Cattaneo L, Rochat M, Rizzolatti G (2005): Covert speech arrest induced by rTMS over both motor and non motor left hemisphere frontal sites. J Cogn Neurosci 17:928–938. [DOI] [PubMed] [Google Scholar]
- Bello L, Riva M, Fava E, Ferpozzi V, Castellano A, Raneri F, Pessina F, Bizzi A, Falini A, Cerri G (2014): Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro Oncol 16:1110–1128. doi: 10.1093/neuonc/not327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ (1999): A fronto‐parietal circuit for object manipulation in man: Evidence from an fMRI‐study. Eur J Neurosci 11:3276–3286. [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF (2013): Functional organization of human sensory motor cortex for speech articulation. Nature 495:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Rizzolatti G (2004): Neural circuits underlying imitation learning of hand actions: An event‐related fMRI study. Neuron 42:323–334. [DOI] [PubMed] [Google Scholar]
- Cabinio M, Blasi V, Borroni P, Montagna M, Iadanza A, Falini A, Cerri G (2010): The shape of motor resonance: Right or left handed? NeuroImage 51:313–323. [DOI] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN (2003): Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol 90:832–842. [DOI] [PubMed] [Google Scholar]
- de Zubicaray G, Postle N, McMahon K, Meredith M, Ashton R (2010): Mirror neurons, the representation of word meaning and the foot of the third left frontal convolution. Brain Lang 112:77–84. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F (1997): Brain activity during observation of actions: Influence of action content and subject's strategy. Brain 120:1763–1777. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ (2007): Brain areas selective for both observed and executed movements. J Neurophysiol 98:1415–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ (2008): A mirror up to nature. Curr Biol 18:R13–R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers N (1996): A new brain region for coordinating speech articulation. Nature 384:159–116. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, Sichez J‐P, Van Effenterrea R (2003): The role of dominant premotor cortex in language: A study using intraoperative functional mapping in awake patients. NeuroImage 20:1903–1914. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Espadaler J, Rogiæ M, Deletis V, Leon A, Quijada C, Conesa G (2012): Representation of cricothyroid muscles at the primary motor cortex (M1) in healthy subjects mapped by navigated transcranial magnetic stimulation (nTMS). Clin Neurophysiol 123:2205–2211. [DOI] [PubMed] [Google Scholar]
- Fazio P, Cantagallo A, Craighero L, D'Ausilio A, Roy AC, Pozzo T, Calzolari F, Granieri E, Fadiga L (2009): Encoding of human action in Broca's area. Brain 132:1980–1988. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N (2012): Language‐selective and domain‐general regions lie side by side within Broca's area. Curr Biol 22:2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund HJ (1996): Functional organization of the human supplementary motor area and dorsolateral premotor cortex. Adv Neurol 70:263–269. [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996): Action recognition in the premotor cortex. Brain 119:593–609. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C (2009): The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single‐subject analyses of unsmoothed fMRI data. Cereb Cortex 19:1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C (2007): The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. NeuroImage 35:1674–1684. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehericy S, Pauline J‐B, Gaymard B, Marsault C, Agid Y, Le Bihan D (2000): Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 10:1093–1104. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Law I, Paulson OB (2002): When action turns into words. Activation of motor‐based knowledge during categorization of manipulable objects. J Cogn Neurosci 14:1230–1239. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G (1996): Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res 112:103–111. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE (2003): Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. NeuroImage 18:928–937. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F (2004): Somatotopic Representation of action words in human motor and premotor cortex. Neuron 41:301–307. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Amunts K (2008): Specialization in Broca's region for semantic phonological and syntactic fluency? NeuroImage 40:1362–1368. [DOI] [PubMed] [Google Scholar]
- Hickok G (2012): Computational neuroanatomy of speech production. Nat Rev Neurosci 13:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K (2004): Re‐examining the brain regions crucial for orchestrating speech articulation. Brain 127:1479–1487. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999): Cortical mechanisms of human imitation. Science 286:2526–2528. [DOI] [PubMed] [Google Scholar]
- Juch H, Zimine I, Seghier ML, Lazeyras F, Fasel JHD (2005): Anatomical variability of the lateral frontal lobe surface implication for intersubject variability in language neuroimaging. NeuroImage 24:504–514. [DOI] [PubMed] [Google Scholar]
- Kent RD (2000): Research on speech motor control and its disorders: A review and prospective. J Commun Disord 33:391–427. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T (2006): Broca's area and the hierarchical organization of human behavior. Neuron 50:963–974. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN (2009): Corticospinal neurons in macaque ventral premotor cortex with mirror properties: A potential mechanism for action suppression? Neuron 64:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraskov A, Prabhu G, Quallo MM, Lemon RN, Brochier T (2011): Ventral premotor‐motor cortex interactions in the macaque monkey during grasp: Response of single neurons to intracortical microstimulation. J Neurosci 31:8812–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F (2012): Mirror neurons, birdsong and human language: A hypothesis. Front Psychiatry 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders HO, Dinner DS, Morris HH, Wyllie E, Comair YG (1995): Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol 67:115–129. [PubMed] [Google Scholar]
- Mantini D, Hasson U, Betti V, Perrucci MG, Romani GL, Corbetta M, Orban GA, Vanduffel1 W (2012): Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nat Methods 9:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengotti P, Corradi‐Dell'Acqua C, Negri GAL, Ukmar M, Pesavento V, Rumiati RI (2013): Selective imitation impairments differentially interact with language processing. Brain 136:2602–2618. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB (2012): Brain regions with mirror properties: A meta‐analysis of 125 human fMRI studies. Neurosci Biobehav Rev 36:341–349. [DOI] [PubMed] [Google Scholar]
- Molnar‐Szakacs I, Iacoboni M, Koski L, Mazziotta JC (2005): Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action observation. Cereb Cortex 15:986–994. [DOI] [PubMed] [Google Scholar]
- Morin O, Grezes J (2008): What is “mirror” in the premotor cortex? A review. Clin Neurophysiol 38:189–195. [DOI] [PubMed] [Google Scholar]
- Moro A (2014): On the similarity between syntax and actions. Trends Cogn Sci 18:109–110. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban GA (2005): Observing others: Multiple action representation in the frontal lobe. Science 310:332–336. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R (2000): Temporal dynamics of cortical representation for action. Proc Natl Acad Sci USA 97:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Hari R (2002): Viewing lip forms: Cortical dynamics. Neuron 36:1211–1220. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, Tipper SP, Downing PE (2013): Crossmodal and action‐specific: Neuroimaging the human mirror neuron system. Trends Cogn Sci 17:311–318. [DOI] [PubMed] [Google Scholar]
- Papagno C, Della Sala S, Basso A (1993): Ideomotor apraxia without aphasia and aphasia without apraxia: The anatomical support for a double dissociation. J Neurol Neurosurg Psychiatry 56:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya ND (2001): Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16:291–310. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S (2005): Orofacial somatomotor responses in the macaque monkey homologue of Broca's area. Nature 435:1235–1238. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN (1993): Corticospinal Function and Voluntary Movement. Oxford: Oxford University Press. [Google Scholar]
- Press C, Weiskopf N, Kilner JM (2012): Dissociable roles of human inferior frontal gyrus during action execution and observation. Neuroimage 15:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones‐Hinojosa A, Ojemann SG, Sanai N, Dillon WP, Berger MS (2003): Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg 99:311–318. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA (1998): Language within our grasp. Trends Neurosci 21:188–204. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L (2004): The mirror‐neuron system. Ann Rev Neurosci 27:169–192. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Tootell RBH (2005): From monkeys to humans: What do we now know about brain homologies? Curr Opin Neurobiol 15:135–144. [DOI] [PubMed] [Google Scholar]
- Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR (1996): Functional cortex and subcortical white matter located within gliomas. Neurosurgery 38:678–685. [PubMed] [Google Scholar]
- Tandon N, Narayana S, Lancaster JL, Brown S, Dodd S, Vollmer DG, Ingham R, Ingham J, Liotti M, Fox PT (2003): CNS Resident Award: Role of the lateral premotor cortex in articulation. Clin Neurosurg 50:341–349. [PubMed] [Google Scholar]
- Tettamanti M, Buccino M, Saccuman, MC , Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D (2005): Listening to action‐related sentences activates fronto‐parietal motor circuits. J Cogn Neurosci 17:273–281. [DOI] [PubMed] [Google Scholar]
- Toni I, de Lange FP, Noordzij ML, Hagoort P (2008): Language beyond action. J Physiol Paris 102:71–79. [DOI] [PubMed] [Google Scholar]
- Turella L, Pierno A, Tubaldi F, Castiello U (2009): Mirror neurons in humans: Consisting or confounding evidence? Brain Lang 108:10–21. [DOI] [PubMed] [Google Scholar]


