Abstract
Personality traits are multidimensional traits comprising cognitive, emotional, and behavioral characteristics, and a wide array of cerebral structures mediate individual variability. Differences in personality traits covary with brain morphometry in specific brain regions, and neuroimaging studies showed structural or functional abnormalities of cerebellum in subjects with personality disorders, suggesting a cerebellar role in affective processing and an effect on personality characteristics. To test the hypothesis that cerebellar [white matter (WM) and cortex] volumes are correlated with scores obtained in the four temperamental scales of the Temperament and Character Inventory (TCI) by Cloninger, a total of 125 healthy participants aged 18–67 years of both genders (males = 52) completed the TCI and underwent magnetic resonance imaging. The scores obtained in each temperamental scale were associated with the volumes of cerebellar WM and cortex of right and left hemispheres separately by using linear regression analyses. In line with our hypothesis, novelty seeking (NS) scores were positively associated with WM and cortex cerebellar volumes. Harm avoidance (HA) scores were negatively associated with WM and cortex cerebellar volumes. The range of individual differences in NS and HA scores reflects the range of variances of cerebellar volumes. The present data indicating a cerebellar substrate for some personality traits extend the relationship between personality and brain areas to a structure up to now thought to be involved mainly in motor and cognitive functions, much less in emotional processes and even less in personality individual differences. Hum Brain Mapp 35:285–296, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: magnetic resonance imaging, individual differences, morphometry, cerebellum
INTRODUCTION
On the basis of family background, longitudinal developmental, psychometrical, neuropharmacological, and neuroanatomical studies, Cloninger identified primary‐basic personality temperament and character traits [Cloninger, 1987; Cloninger et al., 1993]. Although the character traits are produced by learning, maturation, and sociocultural factors and evolve across lifespan, the temperamental traits are potentially associated with a specific genetic and biological substrate, relatively consistent and stable over time [Cloninger et al., 1993; Comings et al., 2000; Tellegen et al., 1988]. It is postulated that they determine disposition to attachment and early emotions of fear and anger as well as automatic behavioral responses to danger, novelty, and reward. Novelty seeking (NS), harm avoidance (HA), reward dependence (RD), and persistence (P) are the four temperamental traits described in the Temperament and Character Inventory (TCI) [Cloninger et al., 1993]. Because personality traits are multidimensional traits comprising cognitive, emotional, and behavioral characteristics, it may be advanced that a wide array of cerebral circuits mediate the individual variability. Associations have been demonstrated between personality factors and neurobiological measures, as neurotransmitter metabolites [Cloninger, 1987; Cloninger et al., 1993; Kim et al., 2002; Limson et al., 1991] and various markers associated with in vivo neuroimaging [Canli et al., 2001; Kumari et al., 2004; Sugiura et al., 2000; Youn et al., 2002]. Furthermore, neuroanatomical studies indicated that individual differences in personality traits covaried with brain morphometry (e.g., cortical thickness and gray matter volume) in specific brain regions [DeYoung et al., 2010; Gardini et al., 2009; Hu et al., 2011; Yamasue et al., 2008]. Neuroimaging studies emphasized the involvement in the personality traits of the cortico‐limbic pathways involved also in emotional processing and reappraisal [Deckersbach et al., 2006; Gogtay et al., 2004; Kumari et al., 2004], but they have almost ignored cerebellar involvement.
After being connected to motor and cognitive functions, cerebellar structures have been completely reconsidered to address new roles on a variety of domains. Impaired executive and spatial functions, language deficits, and personality changes are described in subjects with lesions of the posterior lobe and vermis (cerebellar cognitive‐affective syndrome) [Schmahmann and Sherman, 1998]. Furthermore, a positive association between pattern of synchronous neuronal activity (indexed by regional homogeneity) and extraversion (temperamental trait similar to NS) was described in the cerebellum that contributes to generally higher hedonic tone of extraverts [Wei et al., 2011]. Magnetic resonance imaging (MRI) studies showed structural or functional abnormalities of the cerebellum in patients with personality disorders or depression [Fitzgerald et al., 2008; Liu et al., 2010; Pillay et al., 1997]. This evidence indicates a cerebellar role in affective processing, which has an effect on one's personality characteristics. Actually, the psychopathological profile of patients affected by cerebellar diseases of different etiology describes them as impulsive, obsessive, hyperactive, disinhibited, and with ruminative and stereotypical behaviors, features affecting their personality style [Schmahmann et al., 2007].
On the basis of this, we tested the hypothesis that cerebellar [white matter (WM) and cortex (Cx)] volumes were correlated with scores obtained in the four TCI temperamental scales even in healthy subjects. To our knowledge, this is the first study addressing the potential relation between cerebellar structures and personality traits in a large cohort of nonpathological differently aged subjects of both sexes. In investigating brain regions more likely associated with a personality scale from a structural point of view, a preliminary question is how brain structure—specifically, the relative volume—relates to brain function. A greater‐than‐average volume may signify greater‐than‐average power to carry out specific functions. Human and experimental evidence favors the larger‐is‐more‐powerful position: training on particular tasks or experiencing complex environment does increase the volume of the functionally related brain structures [Boyke et al., 2008; Di Paola et al., 2012; Pangelinan et al., 2011]. Thus, it seems reasonable to assume that volume tends to positively covary with function. Furthermore, structural neuroimaging studies demonstrated correlations between regional specificity and temperamental traits [DeYoung et al., 2010; Gardini et al., 2009; Yamasue et al., 2008]. In particular, by associating the Big Five personality traits with volume in specific brain regions, it has been reported that the neuroticism (dimension corresponding to HA by TCI) covaries positively with volume of mid‐cingulate gyrus and negatively with volume of dorsomedial prefrontal cortex and posterior hippocampus, regions associated with threat, punishment, and negative affect; extraversion (dimension corresponding to NS by TCI) covaries positively with volume of medial orbitofrontal cortex, region involved in processing reward information [DeYoung et al., 2010]. Furthermore, it has been demonstrated that NS correlates positively with volume of frontal and posterior cingulate cortex; HA negatively correlates with volume of orbitofrontal, occipital, and parietal structures; RD negatively correlates with volume of caudate nucleus and rectal frontal gyrus; finally, P positively correlates with volume of precuneus, paracentral lobule, and parahippocampal gyrus [Gardini et al., 2009]. These results indicate that personality dimensions may reflect structural variance in specific brain areas, supporting systematic studies of individual differences in personality using neuroscience methods.
MATERIALS AND METHODS
Participants
A sample of 125 neurologically intact subjects [52 males (42%); mean age ± SD = 35 ± 12 years, range 18–67] was recruited from universities, community recreational centers, and hospital personnel by local advertisement. Educational level ranged from an eighth‐grade education to a postgraduate degree (mean education ± SD = 15.5 ± 2.8 years, range 8–24). All participants were right handed as assessed with the Edinburgh Handedness Inventory [Oldfield, 1971]. The subjects were submitted to MRI. The inclusion criteria were age between 18 and 70 years and suitability for MRI scanning. Exclusion criteria included: (i) suspicion of cognitive impairment or dementia based on Mini Mental State Examination [Folstein et al., 1975] score ≤ 24 [Measso et al., 1993], identifying positive screening for cognitive deterioration in Italian population and confirmed by clinical neuropsychological evaluation by using the Mental Deterioration Battery [Carlesimo et al., 1996] and NINCDS‐ADRDA criteria for dementia [McKhann et al., 1984]; (ii) subjective complaint of memory difficulties or of any other cognitive deficit, interfering or not with the daily living activities; (iii) major medical illnesses, e.g., diabetes (not stabilized), obstructive pulmonary disease, or asthma; hematologic and oncologic disorders; pernicious anemia; clinically significant and unstable active gastrointestinal, renal, hepatic, endocrine, or cardiovascular system disease; and newly treated hypothyroidism; (iv) current or reported psychiatric, assessed by the SCID‐I and the SCID‐II [First et al., 1997a, 1997b] or neurological, assessed by a clinical neurologist disorders (e.g., schizophrenia, mood disorders, anxiety disorders, stroke, Parkinson disease, seizure disorder, head injury with loss of consciousness, and any other significant mental or neurological disorder); (v) known or suspected history of alcoholism or drug dependence and abuse during lifetime; and (vi) MRI evidence of focal parenchymal abnormalities or cerebrovascular diseases: for each subject, a trained neuroradiologist and a neuropsychologist expert in neuroimaging coinspected all the available clinical MRI sequences (i.e., T1 and T2‐weighted and fluid attenuated inversion recovery (FLAIR) images) to ensure that subjects were free from structural brain pathology and vascular lesions (i.e., T2‐weighted hyperintensities or T1‐weighted hypointensities). On the basis of the inclusion criteria, in a quality control previous to sample definition we had excluded 28 subjects (the most elderly) showing WM hyperintensities evident on T2‐weighted MRI sequences.
The study was approved by the local ethics committee of the I.R.C.C.S. Santa Lucia Foundation, and written consent was obtained from all participants after a full explanation of the procedures of the study.
Temperament and Character Inventory
TCI consists of 240 items comprising seven dimensions, including four temperamental scales (NS, HA, RD, and P) and three character scales (self‐directedness, cooperativeness, and self‐transcendence) [Cloninger et al., 1993]. We focused on the scales of the temperaments, known to be stable traits and with high heritability [Cloninger, 1986; Stallings et al., 1996]. NS refers to a tendency to action behaviors and it is expressed as the tendency to exploratory activity in response to novelty, impulsive decision making, extravagant approach to cues of reward, and quick loss of temper. HA refers to a tendency to inhibit behaviors and act with caution, apprehensiveness, and pessimism to respond intensively to aversive stimuli, leading to passive avoidance behavior, fear of uncertainty, shyness of strangers, and rapid fatigability. RD refers to a tendency to maintain ongoing behaviors previously associated with reinforcement and to express persistence, social attachment, and dependence on the others' approval. P refers to the ability to maintain arousal and motivation internally in the absence of immediate external reward.
MRI Acquisition and Processing
All 125 participants underwent the same imaging protocol, which included standard clinical sequences (FLAIR, DP‐T2‐weighted) and a whole‐brain high‐resolution T1‐weighted sequence obtained in the sagittal plane using a modified driven equilibrium Fourier transform sequence (echo time (TE)/repetition time (TR) = 2.4/7.92 ms, flip angle: 15°, and voxel size: 1 × 1 × 1 mm3) using a 3‐T Allegra MR imager (Siemens, Erlangen, Germany) with a standard quadrature head coil. All planar sequence acquisitions were obtained in the plane of the anterior‐posterior commissure line. Particular care was taken to center the subject in the head coil and to restrain the subject's movements with cushions and adhesive medical tape.
MRI‐based quantification of cerebellar WM and Cx was performed using Freesurfer (v. 4.05) software package (http://surfer.nmr.mgh.harvard.edu). The stream consists of five different stages, fully described elsewhere [Dale et al., 1999; Fischl and Dale, 2000]. Initially, the MRI volumes were registered to the Talairach space and the output images were intensity normalized. At the next stage, the skull was automatically stripped off the 3D anatomical data set by using a hybrid method that uses both watershed algorithms and deformable surface models. At this stage, manual intervention is needed to visualize and edit areas of skull and the areas of cortex or cerebellum that should be corrected. After skull stripping, the output brain mask was labeled using a probabilistic atlas [Destrieux et al., 2010] where each voxel in the normalized brain mask volume was assigned one of the following labels: cerebral WM, cerebral cortex, lateral ventricle, inferior lateral ventricle, cerebellum WM, cerebellum Cx, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, accumbens area, third ventricle, fourth ventricle, brainstem, and cerebrospinal fluid. Volumetric data of definite structures (i.e., cerebellar WM and Cx volumes, see Fig. 1) were then extracted (in mm3) using specific algorithms, which count the number of voxels inside the structure and multiple it by the resolution of the MRI image.
Figure 1.
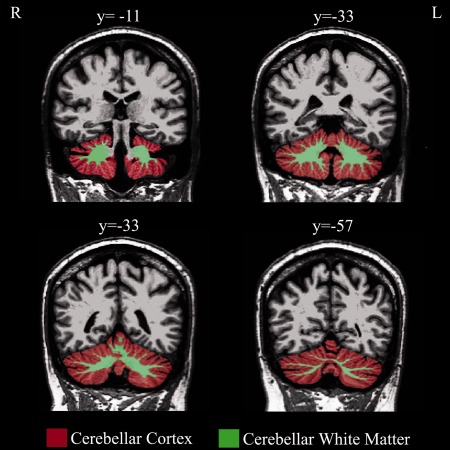
Cerebellar cortex and white matter segmentation. Coronal slices showing the process of segmentation of cerebellar cortex (red) and white matter (green) in a representative subject. Approximate y coordinates are in MNI space. L, left; R, right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical Analysis
Parametric associations between the four TCI temperamental scales or cerebellar volumes and age or years of education were tested by using Pearson product moment correlation (Fisher r to z). Main effects of sex were assessed by using independent‐samples t‐test for each TCI scale or cerebellar volumes. We evaluated the associations between scores in each temperamental scale and volumes of cerebellar WM and Cx of right and left side separately by using linear regression analyses. To rule out that individuals at either extreme end on the scales disproportionately influenced the above described associations, according to Westlye's paper [Westlye et al., 2011], we made an additional outlier rejection analysis on the analyses using a deleted residuals strategy. We omitted cases with ±2 studentized residuals in the main analysis and tested whether the relationships between scale and volume values remained in the reduced sample.
To further analyze whether age‐ or sex‐related processes modulated the results obtained in the main analysis on NS and HA, respectively, and to establish the stability of effects, we reanalyzed the data of male/female subsamples as for NS scale as well as data of five subsamples classified for decades of age as for HA scale.
Additional analyses have been performed to evaluate the associations between scores in TCI temperamental scales and volumes of cerebellar WM and Cx of both hemispheres in the reduced sample as well as cerebral WM and GM of both hemispheres in the entire sample by using linear regression analyses and adding age, sex, and total brain volume as predictive variables. Sex was considered a “dummy variable” given its dichotomic nature. Alpha level for significance testing was Bonferroni corrected at P ≤ 0.01. Power analysis calculated on the linear regressions with 125 participants showed the high statistical power of 0.91.
RESULTS
Relationship Between TCI Scales as Well as Cerebellar Volumes and Sex, Age, and Years of Education
Table 1 gives the sample characteristics for age, sex, education, and TCI scores per age decades and in total. The TCI score distribution is comparable to that reported in a previous study by Cloninger et al. 1993. Female participants showed higher scores than male participants only in HA scale (t = 4.012, P < 0.001), whereas they had similar scores in NS (t = −0.52, P > 0.05), RD (t = 0.56, P > 0.05), and P (t = −0.09, P > 0.05) scales. Furthermore, female participants showed smaller cerebellar volumes than male participants (left WM: t = 75.32, P < 0.0001; right WM: t = 74.55, P < 0.0001; left Cx: t = 90.76, P < 0.0001; and right Cx: t = 93.49, P < 0.0001).
Table 1.
Sample characteristics
| Age group (years) | No. | Age Mean (± SD) | Female (%) | Education Mean (± SD) | NS Mean (± SD) | HA Mean (± SD) | RD Mean (± SD) | P Mean (± SD) |
|---|---|---|---|---|---|---|---|---|
| 18–27 | 43 | 22.7 (2.5) | 63 | 15.4a (1.9) | 22.2 (5.1) | 13.14 (5.7) | 16.0 (3.6) | 4.9 (2.0) |
| 28–37 | 40 | 32.4 (2.8) | 47.5 | 16.8 (3.0) | 22.0 (5.4) | 11.7 (6.9) | 16.13 (3.32) | 5.5 (1.6) |
| 38–47 | 18 | 42.2 (3.1) | 61 | 15.2 (2.3) | 19.6 (5.3) | 13.4 (6.9) | 14.9 (3.12) | 5.3 (1.7) |
| 48–57 | 17 | 53 (2.8) | 70 | 14.1 (2.5) | 19.6 (5.1) | 17.1 (4.2) | 16.24 (3.03) | 4.8 (1.7) |
| 58–67 | 7 | 61.6 (3.4) | 57 | 12.4 (3.7) | 14.3 (5.0) | 15.7 (7.1) | 13.9 (4.2) | 4.9 (1.8) |
| Total | 125 | 34.9 (12.4) | 58.4 | 15.5 (2.8) | 21 (5.5) | 13.4 (6.3) | 15.8 (3.4) | 5.1 (1.8) |
Sample characteristics by age groups, sex, years of education, and TCI temperamental scores (NS, HA, RD, and P).
NS, novelty seeking; HA, harm avoidance; RD, reward dependence; P, persistence.
Many individuals in the group aged 18–27 years were still attending secondary school or university at the time of assessment. Completed years of education at the time of assessment are used in the present study.
No positive correlation between TCI scores and age or education years was found (Table 2), but a significant negative correlation between NS scores and age (r = −0.33, P < 0.001) was observed. NS and HA scores were significantly negatively correlated (r = −0.37, P < 0.0001). Furthermore, although no correlation between cerebellar volumes and education was found, a significant negative correlation between cerebellar volumes and age was observed for left Cx (r = −0.30, P < 0.001) and right Cx (r = −0.32, P < 0.001) and not for the WM of both hemispheres.
Table 2.
Correlations among TCI temperamental scores and age or years of education
| Variable | NS | HA | RD | P |
|---|---|---|---|---|
| Age | −0.33a | 0.15 | −0.09 | −0.002 |
| Education | 0.08 | −0.05 | 0.13 | 0.17 |
Pearson product moment correlations among NS, HA, RD, P and age and years of education.
NS, novelty seeking; HA, harm avoidance; RD, reward dependence; P, persistence.
P < 0.001.
Relationship Between TCI Scores and Cerebellar Volumes
As shown in Figure 2A,B, significant positive associations emerged between NS scores and volumes of cerebellar WM (left side: β = 0.30, F(1,123) = 12.41, P < 0.001 and right side: β = 0.29, F(1,123) = 11.24, P < 0.001) and Cx (left side: β = 0.25, F(1,123) = 8.49, P < 0.01 and right side: β = 0.27, F(1,123) = 9.94, P < 0.01).
Figure 2.
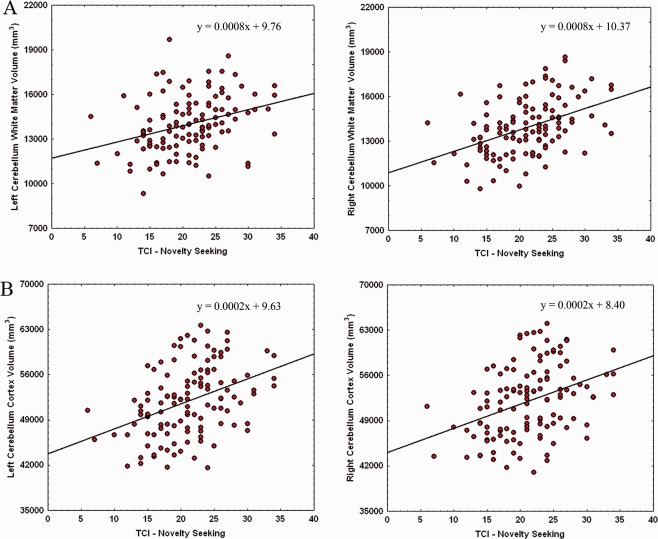
Relationship between cerebellar WM (A) and Cx (B) volumes and NS scores. Scatterplots are separated for left and right volumes. Linear fit (solid black line) and equations are also reported. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
As shown in Figure 3A,B, significant negative associations emerged between HA scores and volumes of cerebellar WM (left side: β = −0.28, F(1,123) = 10.91, P < 0.001 and right side: β = −0.30, F(1,123) = 12.56, P < 0.0005) and Cx (left side: β = −0.31, F(1,123) = 13.48, P < 0.001 and right side: β = −0.30, F(1,123) = 12.58, P < 0.0005).
Figure 3.
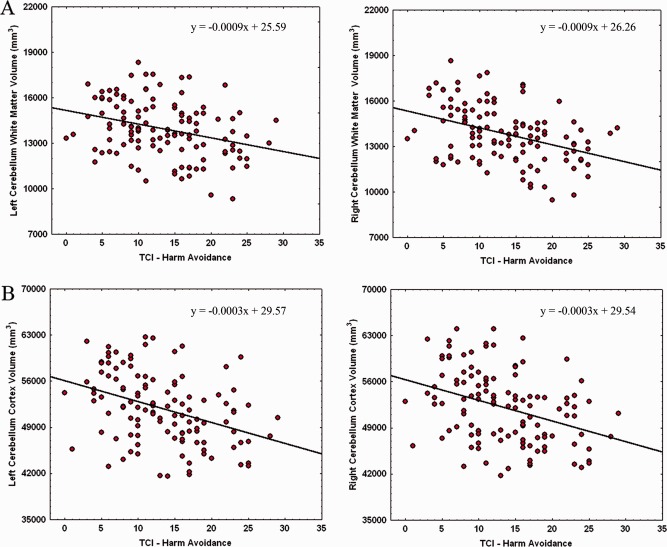
Relationship between cerebellar WM (A) and Cx (B) volumes and HA scores. Scatterplots are separated for left and right volumes. Linear fit (solid black line) and equations are also reported. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
No significant associations emerged between RD scores and volumes of cerebellar WM (left side: β = −0.06, F(1,123) = 0.51, P > 0.05 and right side: β = −0.01, F(1,123) = 0.02, P > 0.05) and Cx (left side: β = 0.07, F(1,123) = 0.64, P > 0.05 and right side: β = 0.05, F(1,123) = 0.35, P > 0.05). No significant associations emerged between P scores and volumes of cerebellar WM (left side: β = −0.11, F(1,123) = 1.59, P > 0.05 and right side: β = −0.08, F(1,123) = 0.87, P > 0.05) and Cx (left side: β = −0.02, F(1,123) = 0.44, P > 0.05 and right side: β = −0.02, F(1,123) = 0.08, P > 0.05).
To rule out that statistically outlying individuals disproportionately influenced the associations, we omitted all cases with ±2 studentized deleted residuals from the main significant analyses and repeated the analyses on the reduced sample. The outlier rejection analyses revealed significant positive associations between NS scores and cerebellar WM (left side: β = 0.32, F(1,118) = 14.0, P < 0.001 and right side: β = 0.43, F(1,116) = 26.4, P < 0.00001) and Cx (left side: β = 0.39, F(1,117) = 21.3, P < 0.00001 and right side: β = 0.37, F(1,118) = 18.7, P < 0.0001).
Similarly, the outlier rejection analyses revealed significant negative associations between HA scores and cerebellar WM (left side: β = −0.31, F(1,118) = 12.7, P < 0.0005 and right side: β = −0.39, F(1,116) = 20.46, P < 0.00001) and Cx (left side: β = −0.38, F(1,116) = 19.9, P < 0.0001 and right side: β = −0.36, F(1,117) =17.77, P < 0.00005).
Because the outlier rejection analyses indicated associations that were robust for statistical outliers, we performed subsequent analyses on the reduced sample.
Relationship Between NS Scores and Cerebellar Volumes in Age Groups
Given the significant correlation between age and NS (Table 2), we recalculated the linear regressions between NS scores and cerebellar volumes according to decades of age (Table 3). In the first decade, the only significant positive association emerged between NS and right WM volume (β = 0.45, F(1,38) = 9.5, P < 0.005). In the second decade, the only significant positive association emerged between NS and left Cx volume (β = 0.43, F(1,36) = 8.3, P < 0.01), whereas no association was found in the third decade of age. Conversely, in the two last decades, pooled together given the scarce number of subjects belonging to fourth and fifth decades, significant positive associations were found between NS and WM volumes of both hemispheres (left side: β = 0.57, F(1,22) = 10.6, P < 0.005 and right side: β = 0.52, F(1,22) = 8.4, P < 0.01).
Table 3.
Associations between NS scores and cerebellar volumes
| Age group (years) | L WM F d.f. β | R WM F d.f. β | L Cx F d.f. β | R Cx F d.f. β |
|---|---|---|---|---|
| 18–27 | F 1,39 = 2.8 | F 1,38 = 9.5b | F 1,40 = 3.6 | F 1,40 = 3.8 |
| 0.26 | 0.45 | 0.29 | 0.29 | |
| 28–37 | F 1,37 = 0.5 | F 1,36 = 2.8 | F 1,36 = 8.3a | F 1,36 = 5.9 |
| 0.11 | 0.27 | 0.43 | 0.37 | |
| 38–47 | F 1,14 = 0.25 | F 1,14 = 0.77 | F 1,15 = 0.01 | F 1,15 = 0.01 |
| 0.13 | 0.23 | 0.02 | 0.02 | |
| 48–67 | F 1,22 = 10.6b | F 1,22 = 8.4a | F 1,20 = 2.26 | F 1,21 = 1.7 |
| 0.57 | 0.52 | 0.27 | 0.32 |
Linear regressions between NS scores and cerebellar volumes in age groups.
NS, novelty seeking; L WM, left white matter; R WM, right white matter; L Cx, left cortex; R Cx, right cortex.
Statistical significance at level of P < 0.01.
Statistical significance at level of P < 0.005.
Relationship Between HA Scores and Cerebellar Volumes in Female and Male Participants
Given the significant correlation between sex and HA, we recalculated the linear regressions between HA scores and cerebellar volumes in females and male, separately (Table 4). Although no association was found in female participants, in males significant negative associations were found between HA and volumes of left cerebellar Cx (β = −0.38, F(1,45) = 7.6, P < 0.01) and right WM (β = −0.39, F(1,45) = 8.4, P < 0.01).
Table 4.
Associations between HA scores and cerebellar volumes
| Sex | L WM F d.f. β | R WM F d.f. β | L Cx F d.f. β | R Cx F d.f. β |
|---|---|---|---|---|
| Females | F 1,68 = 3.6 | F 1,69 = 4.4 | F 1,69 = 2.8 | F 1,68 = 2.9 |
| −0.22 | −0.25 | −0.20 | −0.20 | |
| Males | F 1,48 = 2.1 | F 1,45 = 8.3a | F 1,45 = 7.6a | F 1,47 = 4.2 |
| −0.20 | −0.39 | −0.38 | −0.28 |
Linear regressions between HA scores and cerebellar volumes in female and male participants.
HA, harm avoidance; L WM, left white matter; R WM, right white matter; L Cx, left cortex; R Cx, right cortex.
Statistical significance at level of P < 0.01.
Additional Analyses on TCI Scales and Cerebellar (or Cerebral) Volumes
The additional analyses (Supporting Information) confirmed the significant associations between the NS or HA scores and the cerebellar WM and Cx of both hemispheres, independently from the total brain volume. Furthermore, they failed to reveal any significant association between the four TCI scales and cerebral WM and GM of both hemispheres.
DISCUSSION
Biopsychosocial theories propose that personality traits are related to genetic and neurobiological markers [Cloninger, 1986, 1987; Cloninger et al., 1993]. The main result of the present research is that the range of individual differences in NS and HA scores observed in healthy subjects of both sexes and different ages reflects the range of variances of cerebellar volumes. No relationship between cerebellar volumes and the other two TCI scales (RD and P) was found. To our knowledge, this is the first study that in a large cohort of healthy subjects advances the involvement of cerebellar networks in specific TCI temperamental traits by combining a structural imaging technique and a personality measure. In particular, in line with our hypothesis, we found a powerful positive association between cerebellar volumes and NS scores as well as a powerful negative association between cerebellar volumes and HA scores. Noteworthy, these associations were evidenced either in cerebellar WM and Cx of both hemispheres. Such innovative data indicating a cerebellar substrate for some personality traits extend the relationship between personality and brain areas to a structure up to now thought to be involved mainly in motor and cognitive functions [De Bartolo et al., 2009; Foti et al., 2010; Oliveri et al., 2007, 2009; Torriero et al., 2007, 2011], much less in emotional processes [Schmahmann and Sherman, 1998; Schmahmann et al., 2007; Turner et al., 2007] and even less in personality individual differences [O'Gorman et al., 2006].
NS behavior defined as a proclivity to approach unfamiliar situations encompasses an array of traits, such as explorativity, impulsivity, risk‐taking behaviors linked to approach tendencies, and sensitivity to novelty and reward. HA behavior defined as a proclivity to inhibit behaviors encompasses an array of traits, such as excessive worrying, apprehensiveness, and pessimism. Individuals with high NS scores are exploratory, impulsive, fickle, excitable, quick tempered, and extravagant, whereas those with high HA scores are worried in anticipation of future problems, passive, fearful of uncertainty, shy, and easily fatigued. Although individuals with neuropsychiatric symptoms such as depression [Ono et al., 2002], suicidal behavior [Pompili et al., 2008], bipolar mania [Loftus et al., 2008], schizophrenia [Fresán et al., 2007], substance use disorders [Conway et al., 2003], pathological gambling [Martinotti et al., 2006], and anxiety disorders [Kashdan and Hofmann, 2008] have personality scores that fall at the extreme ends of the normal distribution for each personality trait, NS and HA are also part of not‐dysfunctional behaviors and contribute to adaptive functioning. In fact, NS on one hand and risk avoiding on the other hand provide mechanisms to expand the range of stimuli and possibilities or to protect from potentially aversive contexts supplying thus the appropriate feedback for sculpting the brain and developing interest in specific domains [Koziol et al., 2010; Luna et al., 2001]. As temperament traits are associated with emotional processing, attentional focus, and inhibitory control [Goldsmith et al., 2000, 2008; Vandervert, 2009] that are governed by circuits between the cortex and basal ganglia and the cortex and cerebellum, the involvement of the cerebellar regions in the neuroanatomical geography supporting NS and HA traits appears reasonable. Furthermore, the recently discovered direct reciprocal connections between cerebellum and basal ganglia [Bostan et al., 2010; Hoshi et al., 2005] could provide the neuroanatomical basis for the cerebellum to influence even reward‐driven behavior and processing of information related to motivational valence, functions notoriously attributed to basal ganglia [Delgado, 2007; Palmiter, 2008; Robbins and Everitt, 1996; Wise, 2004]. It is likely that the cerebellum would accelerate the “force” with which the reward was experienced [Schmahmann et al., 2007].
Cerebellar activity signals when sensory input differs from memory‐driven expectations, providing thus a sensory prediction error, guides exploratory drive in novel environments, allows a flexible switching among multiple tasks or alternatives, and makes functions faster and more adaptive. Cerebellum performs these roles by refining the rate, rhythm, and force of behavior and adjusting it for given situations. In essence, cerebellar function is to receive information from cortex and basal ganglia and to send a “corrected” signal back to them [Bostan et al., 2010; Hoshi et al., 2005]. These widespread two‐way communications sustain the cerebellar involvement not only in motor functions but also in cognitive and behavioral processing [Andrease and Pierson, 2008; Ben‐Yehudah et al., 2007; Daum et al., 1993; Foti et al., 2010; Oliveri et al., 2007, 2009; Torriero et al., 2007, 2011]. We are proposing that the cortico‐basal‐cerebellar communications influence and sustain even the processes linked to temperamental individual differences. It has been already established that when the cerebellum works in motor and cognitive domains, it develops both forward and inverse models. In the forward model, the cerebellum is informed by cortex (namely, prefrontal and posterior cortex) and basal ganglia on information load, plans, and intentions about the behavior to do as well as about the characteristics of the environment in which the behavior is done, developing progressive, short‐cut, anticipatory model [Seidler, 2010; van Schouwenburg et al., 2010; Wymbs and Grafton, 2009]. As the behavior and cognition are repeated and the anticipatory predicted feedback is received, the cerebellum becomes increasingly accurate in its predictive capacities. This allows behavioral execution to become more precise and fast and allows behavior to become independent of cortical control. With successful repetitions, behavior governed consciously by cerebellar forward model becomes more and more automated, developing the cerebellar “inverse” model that permits rapid and skilled behavior to occur at an unconscious level. The cerebellum is constantly constructing multipairs of models that constitute a complex modular architecture for adaptively regulating motor, cognitive, and emotional material. Intriguingly, the cerebellum could play a comparable role in facilitating motivation that sustains and reinforces the temperamental features. The present data evidencing a positive correlation between cerebellar volumes and NS scores and a negative one between cerebellar volumes and HA scores appear fully consistent with the different engagement the subjects with different styles of personality require to cerebellar circuitries. In fact, a subject who searches for unfamiliar situations, makes the unknown known, is motivated to explore new environments, displays increased tendency toward risk‐taking, sensation‐seeking, and immediate reward‐seeking, lacks inhibition, as novelty seekers do, needs very rapid detection of unfamiliar events, flexible switching among tasks, alternatives, and contexts, and fast adaptation to change. All these functions seem to require heavy engagement of the cerebellum. Whether as hypothesized “larger is more powerful,” the higher requests a seeker subject makes to his cerebellum, could enlarge it, and vice versa the lower requests an avoiding subject makes to his cerebellum, could reduce it. In fact, the cerebellum is able to provide the fast computational system for timing, sequencing, and modeling and thus could process the novelty‐related information and render the processes increasingly more efficient and adaptive [O'Reilly et al., 2008; Restuccia et al., 2007].
Previous structural neuroimaging studies pertaining the regional specificity of brain‐temperament relationships demonstrated positive correlation between NS and gray matter volume in frontal and posterior cingulate regions [Gardini et al., 2009] as well as between HA or anxiety‐related traits and reduced volumes of whole brain [Knutson et al., 2001], orbitofrontal [DeYoung et al., 2010], occipital, parietal [Gardini et al., 2009], and left anterior prefrontal [Gardini et al., 2009; Yamasue et al., 2008] cortex. Furthermore, increased HA is demonstrated to be associated with decreased microstructure in widely distributed WM tracts, including cortico‐limbic pathways [Westlye et al., 2011]. It is far to be clarified whether temperamental traits determine the size of brain regions or conversely, the differently sized brain regions determine temperamental traits, and limited data are available to clarify which comes first. The development of the neural circuitry organization is influenced by genetic predispositions and environmental events as well as by neuroplastic responses to experiences determining how neurons are connected and how they communicate within individual brain regions and circuits and across neural pathways. Future studies are needed to delineate the contribution of genetic, epigenetic, and environmental variables that may together develop and modulate individual differences.
Among healthy individuals, one of the major factors contributing to individual differences in HA and anxiety‐related traits is gender difference. In accord with previous studies [Cloninger et al., 1993; Fresán et al., 2011; Westlye et al., 2011], female participants of the present research had HA scores higher than male participants. More importantly, although no association was found in female participants, in males there was a negative association between HA scores and cerebellar volumes, indicating that to reach higher HA scores the males had to have lower cerebellar volumes. As HA scores were related to gender differences, NS scores were related to age differences. In fact, in accord with previous studies [Cloninger et al., 1993; Fresán et al., 2011], in the present research, young participants had higher NS scores than old participants. Interestingly, we found that only the cerebellar cortical volumes were negatively correlated with age, whereas the cerebellar WM volumes did not exhibit age‐related differences. This datum perfectly fits with very recently reported findings [Sullivan et al., 2000; Walhovd et al., 2011]. Once again more importantly, the positive association between NS scores and cerebellar volumes found in the first two decades of age was encountered even in the last two decades, indicating that in the oldest participants lower NS scores were associated with reduced cerebellar volumes. The above findings suggest that it is necessary to consider also gender and age effects in attempting to uncover the neuroanatomical underpinnings of personality traits.
CONCLUSIONS
Experimental and brain imaging studies have analyzed the brain‐behavior relationships and have indicated that cortical and subcortical structures play integrated roles in building personality [Ebstein, 2006; Koziol et al., 2010; Westlye et al., 2011]. The models that have emerged go over the traditional cortico‐centric view of personality. By including cerebellar areas, it is possible to characterize facets of both normal and abnormal development of temperamental traits and related individual differences.
The current findings advance the specific function of the cerebellar system in sustaining aspects of motivational network that characterizes the different temperamental traits. In this regard, the individual differences might be interpreted as a product of the cerebellar ability to enhance motivation and model information processing in relation to domains that the cortex and basal ganglia keep in sharp focus. In other words, the personality seems to be a manifestation of the dynamic interplay between cortical and subcortical networks.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Prof. Fabio Ferlazzo for his kind and expert support in statistical analyses. All authors declare no potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations relevant to the subject of their manuscript.
REFERENCES
- Andreasen NC, Pierson R (2008): The role of the cerebellum in schizophrenia. Biol Psychiatry 64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Yehudah G, Guediche S, Fiez JA (2007): Cerebellar contributions to verbal working memory: Beyond cognitive theory. Cerebellum 6:193–201. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2010): The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A (2008): Training‐induced brain structure changes in the elderly. J Neurosci 28:7031–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD (2001): An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci 115:33–42. [DOI] [PubMed] [Google Scholar]
- Carlesimo G, Caltagirone C, Gainotti G (1996): The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384. [DOI] [PubMed] [Google Scholar]
- Cloninger CR (1986): A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev 4:167–226. [PubMed] [Google Scholar]
- Cloninger CR (1987): A systematic method for clinical description and classification of personality variants: A proposal. Arch Gen Psychiatry 44:573–588. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR (1993): A psychobiological model of temperament and character. Arch Gen Psychiatry 50:975–990. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade‐Andavolu R, Gonzalez N, Wu S, Muhleman D, Blake H, Mann MB, Dietz G, Saucier G, MacMurray JP (2000): A multivariate analysis of 59 candidate genes in personality traits: The temperament and character inventory. Clin Genet 58:375–385. [DOI] [PubMed] [Google Scholar]
- Conway KP, Kane RJ, Ball SA, Poling JC, Rounsaville BJ (2003): Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug Alcohol Depend 71:65–75. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Daum I, Ackermann H, Schugens MM, Reimold C, Dichgans J, Birbaumer N (1993): The cerebellum and cognitive functions in humans. Behav Neurosci 107:411–419. [DOI] [PubMed] [Google Scholar]
- De Bartolo P, Mandolesi L, Federico F, Foti F, Cutuli D, Gelfo F, Petrosini L (2009): Cerebellar involvement in cognitive flexibility. Neurobiol Learn Mem 92:310–317. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Dougherty DD, Rauch SL (2006): Functional imaging of mood and anxiety disorders. J Neuroimaging 16:1–10. [DOI] [PubMed] [Google Scholar]
- Delgado MR (2007): Reward‐related responses in the human striatum. Ann N Y Acad Sci 1104:70–88. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR (2010): Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci 21:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Caltagirone C, Petrosini L (2012): Prolonged rock climbing activity induces structural changes in cerebellum and parietal lobe. Hum Brain Mapp doi: 10.1002/hbm.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP (2006): The molecular genetic architecture of human personality: Beyond self‐report questionnaires. Mol Psychiatry 11:427–445. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS (1997a): Structured Clinical Interview for DSM‐IV Axis II Personality Disorders (SCID‐II).Washington, DC:American Psychiatric Press. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (1997b): Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I), Clinician Version.Washington, DC:American Psychiatric Press. [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P (1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Foti F, Mandolesi L, Cutuli D, Laricchiuta D, De Bartolo P, Gelfo F, Petrosini L (2010): Cerebellar damage loosens the strategic use of the spatial structure of the search space. Cerebellum 9:29–41. [DOI] [PubMed] [Google Scholar]
- Fresán A, Apiquian R, Nicolini H, Cervantes JJ (2007): Temperament and character in violent schizophrenic patients. Schizophr Res 94:74–80. [DOI] [PubMed] [Google Scholar]
- Fresán A, Robles‐García R, López‐Avila A, Cloninger CR (2011): Personality differences according to age and sex in a Mexican sample using the Temperament and Character Inventory‐Revised. Compr Psychiatry 52:774–779. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A (2009): Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull 79:265–270. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Nugent TF III, Greenstein D, Nicolson R, Giedd JN, Lenane M, Gochman P, Evans A, Rapoport JL (2004): Comparison of progressive cortical gray matter loss in childhood‐onset schizophrenia with that in childhood‐onset atypical psychoses. Arch Gen Psychiatry 61:17–22. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS, Aksan N, Buss KA (2000): Temperamental substrates of personality development In: Molfese V, Molfese D, editors. Temperamental Substrates of Personality Development.Mahwah, NJ:Lawrence Erlbaum; pp1–32. [Google Scholar]
- Goldsmith HH, Pollak SD, Davidson RJ (2008): Developmental neuroscience perspectives on emotion regulation. Child Dev Perspect 2:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL (2005): The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- Hu X, Erb M, Ackermann H, Martin JA, Grodd W, Reiterer SM (2011): Voxel‐based morphometry studies of personality: Issue of statistical model specification‐effect of nuisance covariates. Neuroimage 54:1994–2005. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Hofmann SG (2008): The high‐novelty‐seeking, impulsive subtype of generalized social anxiety disorder. Depress Anxiety 25:535–541. [DOI] [PubMed] [Google Scholar]
- Kim MS, Cho SS, Kang KW, Hwang JL, Kwon JS (2002): Electrophysiological correlates of personality dimensions measured by Temperament and Character Inventory. Psychiatry Clin Neurosci 56:631–635. [DOI] [PubMed] [Google Scholar]
- Knutson B, Momenan R, Rawlings RR, Fong GW, Hommer D (2001): Negative association of neuroticism with brain volume ratio in healthy humans. Biol Psychiatry 50:685–690. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding DE, Chidekel D (2010): Adaptation, expertise, and giftedness: Towards an understanding of cortical, subcortical, and cerebellar network contributions. Cerebellum 9:499–529. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Williams SC, Gray JA (2004): Personality predicts brain responses to cognitive demands. J Neurosci 24:10636–10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limson R, Goldman D, Roy A, Lamparski D, Ravitz B, Adinoff B, Linnoila M (1991): Personality and cerebrospinal fluid monoamine metabolites in alcoholics and controls. Arch Gen Psychiatry 48:437–341. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y, Cao X, Zhang K, Du C (2010): Decreased regional homogeneity in insula and cerebellum: A resting‐state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res 182:211–215. [DOI] [PubMed] [Google Scholar]
- Loftus ST, Garno JL, Jaeger J, Malhotra AK (2008): Temperament and character dimensions in bipolar I disorder: A comparison to healthy controls. J Psychiatr Res 42:1131–1136. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (2001): Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13:786–793. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Andreoli S, Giametta E, Poli V, Bria P, Janiri L (2006): The dimensional assessment of personality in pathologic and social gamblers: The role of novelty seeking and self‐transcendence. Compr Psychiatry 47:350–356. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E (1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Measso G, Cavarzeran F, Zappalà G, Lebowitz B, Crook T, Pirozzolo F, Amaducci L, Massari D, Grigoletto F (1993): The Mini‐Mental State Examination: Normative study of an Italian random sample. Dev Neuropsychol 9:77–85. [Google Scholar]
- O'Gorman RL, Kumari V, Williams SC, Zelaya FO, Connor SE, Alsop DC, Gray JA (2006)Personality factors correlate with regional cerebral perfusion. Neuroimage 31:489–495. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Torriero S, Koch G, Salerno S, Petrosini L, Caltagirone C (2007): The role of transcranial magnetic stimulation in the study of cerebellar cognitive function. Cerebellum 6:95–101. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Bonnì S, Turriziani P, Koch G, Lo Gerfo E, Torriero S, Vicario CM, Petrosini L, Caltagirone C (2009): Motor and linguistic linking of space and time in the cerebellum. PLoS One 4:e7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Ando J, Onoda N, Yoshimura K, Momose T, Hirano M, Kanba S (2002): Dimensions of temperament as vulnerability factors in depression. Mol Psychiatry 7:948–953. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Mesulam MM, Nobre AC (2008): The cerebellum predicts the timing of perceptual events. J Neurosci 28:2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD (2008): Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine‐deficient mice. Ann N Y Acad Sci 1129:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangelinan MM, Zhang G, VanMeter JW, Clark JE, Hatfield BD, Haufler AJ (2011): Beyond age and gender: Relationships between cortical and subcortical brain volume and cognitive‐motor abilities in school‐age children. Neuroimage 54:3093–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay SS, Yurgelun‐Todd DA, Bonello CM, Lafer B, Fava M, Renshaw PF (1997): A quantitative magnetic resonance imaging study of cerebral and cerebellar gray matter volume in primary unipolar major depression: Relationship to treatment response and clinical severity. Biol Psychiatry 42:79–84. [DOI] [PubMed] [Google Scholar]
- Pompili M, Rihmer Z, Akiskal HS, Innamorati M, Iliceto P, Akiskal KK, Lester D, Narciso V, Ferracuti S, Tatarelli R, De Pisa E, Girardi P (2008): Temperament and personality dimensions in suicidal and nonsuicidal psychiatric inpatients. Psychopathology 41:313–321. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Della Marca G, Valeriani M, Leggio MG, Molinari M (2007): Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch‐negativity study. Brain 130:276–287. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ (1996): Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6:228–236. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC (1998): The cerebellar cognitive affective syndrome. Brain 121:561–579. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC (2007): The neuropsychiatry of the cerebellum‐insights from the clinic. Cerebellum 6:254–267. [DOI] [PubMed] [Google Scholar]
- Seidler RD (2010): Neural correlates of motor learning, transfer of learning, and learning to learn. Exerc Sport Sci Rev 38:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings MC, Hewitt JK, Cloninger CR, Heath AC, Eaves LJ (1996): Genetic and environmental structure of the Tridimensional Personality Questionnaire: Three or four temperament dimensions? J Pers Soc Psychol 70:127–140. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakagawa M, Okada K, Sato T, Goto R, Sato K, Ono S, Schormann T, Zilles K, Fukuda H (2000): Correlation between human personality and neural activity in cerebral cortex. Neuroimage 11:541–546. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000): Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology 14:341–352. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Lykken DT, Bouchard TJ Jr, Wilcox KJ, Segal NL, Rich S (1988): Personality similarity in twins reared apart and together. J Pers Soc Psychol 54:1031–1039. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Petrosini L, Caltagirone C (2007): Cortical networks of procedural learning: Evidence from cerebellar damage. Neuropsychologia 45:1208–1214. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Ferlazzo F, Caltagirone C, Petrosini L (2011): Changes in cerebello‐motor connectivity during procedural learning by actual execution and observation. J Cogn Neurosci 23:338–348. [DOI] [PubMed] [Google Scholar]
- Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD, Robinson RG (2007): The cerebellum and emotional experience. Neuropsychologia 45:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg M, Aarts E, Cools R (2010): Dopaminergic modulation of cognitive control: Distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des 16:2026–2032. [DOI] [PubMed] [Google Scholar]
- Vandervert LR (2009): The appearance of the child prodigy 10,000 years ago: An evolutionary and developmental explanation. J Mind Behav 30:15–32. [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM (2011): Consistent neuroanatomical age‐related volume differences across multiple samples. Neurobiol Aging 32:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Duan X, Yang Y, Liao W, Gao Q, Ding JR, Zhang Z, Zeng W, Li Y, Lu G, Chen H (2011): The synchronization of spontaneous BOLD activity predicts extraversion and neuroticism. Brain Res 1419:68–75. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Bj⊘rnebekk A, Grydeland H, Fjell AM, Walhovd KB (2011): Linking an anxiety‐related personality trait to brain white matter microstructure: Diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry 68:369–377. [DOI] [PubMed] [Google Scholar]
- Wise RA (2004): Rewards wanted: Molecular mechanisms of motivation. Discov Med 4:180–186. [PubMed] [Google Scholar]
- Wymbs NF, Grafton ST (2009): Neural substrates of practice structure that support future off‐line learning. J Neurophysiol 102:2462–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato NK (2008): Gender‐common and ‐specific neuroanatomical basis of human anxiety‐related personality traits. Cereb Cortex 18:46–52. [DOI] [PubMed] [Google Scholar]
- Youn T, Lyoo IK, Kim JK, Park HJ, Ha KS, Lee DS, Abrams KY, Lee MC, Kwon JS (2002): Relationship between personality trait and regional cerebral glucose metabolism assessed with positron emission tomography. Biol Psychol 60:109–120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


