Abstract
The cerebral cortex is a distinctive part of the mammalian nervous system, displaying a spatial variety in cyto‐, chemico‐, and myelinoarchitecture. As part of a rich history of histological findings, pioneering anatomists von Economo and Koskinas provided detailed mappings on the cellular structure of the human cortex, reporting on quantitative aspects of cytoarchitecture of cortical areas. Current day investigations into the structure of human cortex have embraced technological advances in Magnetic Resonance Imaging (MRI) to assess macroscale thickness and organization of the cortical mantle in vivo. However, direct comparisons between current day MRI estimates and the quantitative measurements of early anatomists have been limited. Here, we report on a simple, but nevertheless important cross‐analysis between the histological reports of von Economo and Koskinas on variation in thickness of the cortical mantle and MRI derived measurements of cortical thickness. We translated the von Economo cortical atlas to a subdivision of the commonly used Desikan–Killiany atlas (as part of the FreeSurfer Software package and a commonly used parcellation atlas in studies examining MRI cortical thickness). Next, values of “width of the cortical mantle” as provided by the measurements of von Economo and Koskinas were correlated to cortical thickness measurements derived from high‐resolution anatomical MRI T1 data of 200+ subjects of the Human Connectome Project (HCP). Cross‐correlation revealed a significant association between group‐averaged MRI measurements of cortical thickness and histological recordings (r = 0.54, P < 0.001). Further validating such a correlation, we manually segmented the von Economo parcellation atlas on the standardized Colin27 brain dataset and applied the obtained three‐dimensional von Economo segmentation atlas to the T1 data of each of the HCP subjects. Highly consistent with our findings for the mapping to the Desikan–Killiany regions, cross‐correlation between in vivo MRI cortical thickness and von Economo histology‐derived values of cortical mantle width revealed a strong positive association (r = 0.62, P < 0.001). Linking today's state‐of‐the‐art T1‐weighted imaging to early histological examinations our findings indicate that MRI technology is a valid method for in vivo assessment of thickness of human cortex. Hum Brain Mapp 36:3038–3046, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: cortex, cortical thickness, cortical layer, cytoarchitecture, von Economo, Magnetic Resonance Imaging, T1‐weighted imaging, Human Connectome Project
INTRODUCTION
The human cerebral cortex occupies an average 550–600 ml of volume [Miller et al., 1980; Triarhou, 2007b], spans an impressive area of 2,000–2,500 cm2 [Peters and Jones, 1984; Triarhou, 2007b] and includes an estimated, but widely varying, 25,000 neurons per mm3 [Alonso‐Nanclares et al., 2008]. A long history of histological examinations has identified a rich variation in cyto‐, chemico‐, and myelinoarchitecture of the human cortex [Schuz, 2002; Zilles et al., 2002], identifying a large number of distinct cortical regions. Among pioneers of cortical mapping Korbinian Brodmann (1868–1918) [Brodmann, 1909] and Oskar (1870–1959) and Cécile Vogt (1875–1962) (see [Nieuwenhuys et al., 2014] for a recent meta‐analysis), early anatomists Constantin von Economo (1876–1931) and Georg Koskinas (1885–1975, see [Triarhou, 2007a] for a biography of von Economo's life) provided detailed mappings of the complete human cortex by means of histological examination. In 1925 von Economo and Koskinas presented the monumental work “Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen” (translated “Cytoarchitectonics of the Adult Human Cerebral Cortex”, from now on referred to as the von Economo Atlas) [von Economo and Koskinas, 1925] reporting on a complete parcellation of the human cortex in 54 distinct cortical areas (and 107 even more detailed smaller subregions) on the basis of quantified data on multiple aspects of cortical architecture, including neuronal count and width of the cortical mantle.
Following in the footsteps of these early pioneers in neuroanatomy, contemporary investigations into the human cortex have utilized technological advances in Magnetic Resonance Imaging (MRI) to investigate the layout of the human cortex. Almost a hundred years after the pioneering work of von Economo and Koskinas MR imaging is arguably one of the most popular techniques for studying the anatomy of the human cerebral cortex, with one of the advantages of this technique including the unique capability of examining cortical structure in vivo. This has led to a wide range of applications, including–among many others–investigations into delineation of distinct cortical regions, examinations of individual differences in cortical layout in relationship to cognitive functioning, mapping of changes in cortical structure in a wide range of psychiatric and neurological brain disorders, and so forth.
The number of studies directly comparing MRI derived estimates of cortical thickness with histological examinations is however surprisingly limited, but a few studies have made some important steps in bringing the two fields together. In their seminal work on the introduction of the FreeSurfer methodology to measure the thickness of the cortical mantle based on T1 MRI images, Fischl and Dale [2000] reported lobe averaged values of imaging derived cortical thickness levels to be within the range of values as reported by the histological measurements of von Economo. Furthermore, comparing postmortem and MRI estimates they reported on a significant correlation between imaging derived estimates of cortical thickness of two postmortem brains and microscopy examinations in 7 brain regions [Rosas et al., 2002]. In addition, a recent study reported overlapping levels of cortical thickness obtained by means of presurgery MRI and subsequent microscopy examination of a specific region removed from the temporal lobe during epilepsy surgery across a group of 26 patients [Cardinale et al., 2014]. However, a systematic, region‐wise whole‐brain comparison between cytoarchitectural observations and MRI‐based estimations of cortical structure remains missing. Connecting data from one of the most detailed –but perhaps by today's neuroscientist sometimes forgotten‐ cytoarchitectonic mappings of the human cortex with the best of what contemporary methods on anatomical MRI measurements have to offer [Van Essen et al., 2013] may provide a better understanding of the biological basis of the in vivo MRI cortical thickness measurements we do today. Here, we report on a cross‐technique validation of cortical thickness estimations derived from MRI T1 imaging data of 215 subjects of the Human Connectome Project with the documented reports of pioneering anatomists von Economo and Koskinas on the cellular structure of the human cerebral cortex.
METHODS
Histological Data
The detailed von Economo atlas, originally published in 1925 by von Economo and Koskinas includes a parcellation of the total human cerebral cortex into 54 “Grundareae” (ground areas) based on a differentiating cytoarchitectonic layout of regions, with a more fine‐grained division into 76 smaller “Varianten” (variants) and finally 107 even finer “Modifikationen” (modifications). As described in von Economo's 1927 writings that accompanied the atlas (titled “Zellaufbau der Grosshirnrinde des Menschen,” based on a series of teaching lectures by von Economo [1927]), von Economo stated that every region in the atlas was consistently observed in all investigated brain samples, with the atlas based on histological examination of the cortex of numerous brains (“zahlreichen Gehirnen”) of healthy (i.e., no reported history of neurological and/or psychiatric disorders) subjects in the age range of 30–40 years. Examples of cause of death as mentioned in the original text are hemoptysis in tuberculosis or a sudden death during surgery; the exact number of investigated samples was, however, not explicitly documented [Triarhou, 2007b; von Economo, 2009]. For the 51 most important regions described in the atlas (being 43 ground areas and 8 variants, of which 48 cortical and 3 hippocampal areas) von Economo and Koskinas included overview tables with detailed quantified measures of cortical thickness and cytoarchitecture. Documentation of quantitative cytoarchitectonic values included reports of left and right hemispheric regions mixed together. A recent book translation by dr. L.C. Triarhou [von Economo, 2009] of the 1927 accompanying textbook to English lists a detailed description of the cellular structure of the brain regions of the 1925 atlas, together with the information of von Economo's 1925 overview tables. In the study here, information on the width of the cortical mantle for the 48 cortical regions (40 ground regions and 8 variants) was included in our MRI versus von Economo cortical thickness comparison, taken as the von Economo and Koskinas reported overall width or the average of the cortical dome and cortical wall when separately provided [von Economo, 2009].
MRI Data
High‐resolution T1‐weighted imaging data of the Human Connectome Project was used for reconstruction of the human cerebral cortical mantle [Van Essen et al., 2013] (Q3 release, 215 subjects, voxel‐size 0.7 mm isotropic, age‐range 20–35 years). For each individual dataset, the FreeSurfer software package [Fischl et al., 2004] was used for automatic gray/white/cortical spinal fluid classification and subsequent reconstruction of the cortical mantle. A commonly used 114 region‐subdivision of the Desikan–Killiany atlas [Desikan et al., 2006; Fischl et al., 2004] was used to parcellate the cortex into distinct brain regions, describing 57 unique cortical regions per hemisphere (a cortical parcellation as provided by [Cammoun et al., 2011; Hagmann et al., 2008], being a subdivision based on the original Desikan–Killiany atlas describing 68 (34 single hemisphere) larger cortical regions [Desikan et al., 2006; Fischl et al., 2004]. This subdivision of 57 single hemisphere regions was used as it closely matched the number of cortical regions for which von Economo‐Koskinas cortical thickness was reported (48 regions). [Note that using other finer‐grained subdivisions of the Desikan–Killiany atlas (dividing the original 34 cortical regions of the Desikan–Killiany atlas into respectively 111, 224, or 499 single hemisphere subregions [Hagmann et al., 2008] revealed highly comparable results, as shown in Supporting Information Fig. S1)]. Figure 1 illustrates the 57 single hemisphere subdivision of the Desikan–Killiany parcellation [Desikan et al., 2006].
Figure 1.
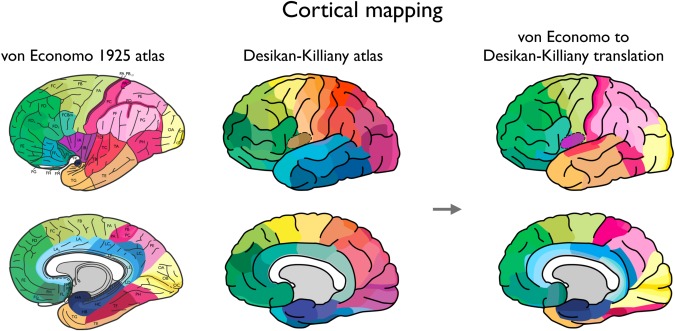
Cortical mapping. Left panel shows a remake of the original von Economo atlas as published in [Triarhou, 2007c] (reprinted with permission), depicting the cortical areas as described and illustrated in [von Economo, 2009; von Economo, 1927]. The middle panel shows the Desikan–Killiany atlas used for the computation of the MRI‐based thickness of 114 cortical regions (57 per hemisphere, left hemisphere is shown). Right panel shows the regions of the Desikan–Killiany atlas (left hemisphere) as colored by the mapped von Economo regions to our atlas. Supporting Information Table 1 gives a textual description of our mapping of the von Economo regions to the Desikan–Killiany atlas. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Next, per individual dataset, FreeSurfer was used to compute the level of vertex‐wise cortical thickness, and for each of the 114 cortical regions the average cortical thickness in that region was taken as the mean value over all cortical thickness measurements in that specific patch of the cortex. For the 114 regions group‐averaged cortical thickness levels were computed as the average values over the total group of 215 subjects. To match the von Economo atlas (which does not distinguish between left and right hemisphere, see description above), values of homologous regions of the left and right hemisphere were averaged to obtain MRI measurements of 57 single hemisphere cortical areas in total.
Cortical Mapping of Atlases
The 48 cortical areas of von Economo's atlas were manually mapped to the regions of the commonly used higher resolution 57 single hemisphere subdivision of the Desikan–Killiany atlas [Hagmann et al., 2008] (note that the three hippocampal regions as described by von Economo and Koskinas were not included in our analysis as the hippocampus is not included as a cortical (but instead as a subcortical) structure in FreeSurfer). The von Economo regions were manually mapped to the regions of the Desikan–Killiany atlas (LS, MvdH) using the information on the locations of the cortical regions as provided by visual illustrations of the 1925 atlas and the textual descriptions of the areas [von Economo and Koskinas, 1925]. For each region of the Desikan–Killiany atlas the best matching region(s) of the von Economo atlas were selected, resulting in a mapping of the von Economo regions to the 57 single‐hemispheric regions of the subdivided Desikan–Killiany atlas. Figure 1 displays a one‐to‐one visual comparison between the digitized version of the von Economo atlas and a schematic illustration of the Desikan–Killiany subdivided atlas (left hemisphere, 57 regions). Next, using the von Ecomomo to Desikan–Killiany atlas mapping, quantitative histological values were obtained for the 57 cortical regions: in case multiple von Economo areas mapped to one single Desikan–Killiany region (e.g., regions FH, FL, FM, FN to medial orbitofrontal gyrus) the von Economo and Kosikinas reported levels were averaged; if one von Economo area overlapped with two or more Desikan–Killiany regions (e.g., region FA to three Desikan–Killiany subareas of the precentral gyrus) each of the Desikan–Killiany regions obtained the histological levels as reported by von Economo and Koskinas. Supporting Information Table 1 summarizes the von Economo to Desikan–Killiany mapping in text; Figure 1 illustrates the mapping visually. [A post hoc analysis of regions of which a 1‐to‐1 mapping between the von Economo atlas and the Desikan–Killiany atlas (Supporting Information Table 1) was present (i.e., not involving averaging of cortical values) revealed highly consistent findings (see Supporting Information Results)].
Statistical Analysis
Cross‐technique examination was performed by means of correlation analysis between mapped histological values of the von Economo atlas (i.e., reported width of the cortical mantle) and cortical thickness estimates as derived by MRI. Effects reaching a P < 0.0045 (reflecting a Bonferroni corrected alpha level of < 0.05, correcting for a total of 11 statistical tests performed in this study) were taken as significant.
Von Economo FreeSurfer Atlas
In addition to a cross‐technique analysis using MRI cortical thickness estimates based on the commonly used Desikan–Killiany atlas (see above), a second analysis was performed in which a direct mapping of 48 cortical von Economo regions into FreeSurfer was established. For this, first, we manually segmented the 48 cortical regions of the von Economo atlas on the Colin27 brain (a standard reference brain in the field, consisting of an averaging of an individual brain that was scanned 27 times) [Holmes et al., 1998] using the label segmentation tools included in the FreeSurfer software suite. In this segmentation, region PB1 and PB2 were merged into one region PB (von Economo describes PB2 as being situated in an island‐like fashion within area PB1 which we could not reliably draw onto the cortical surface) resulting in a total of 47 region labels. We note that during segmentation region FM and FN were found to be difficult to reliably draw onto the cortical surface due to their very small size and narrow shape (being the smallest segmented regions in the atlas, including only 2.6 and 3.1% of the average size of all von Economo areas) (see also results). Second, the resulting manual segmentation was used to create a von Economo Freesurfer atlas using the atlas training tools as provided in FreeSurfer. Third, similar to our analysis for the Desikan–Killiany atlas, the von Economo parcellation atlas was run on each of the individual HCP subjects (see Supporting Information Fig. S2 for five exemplary HCP subjects), individual MRI‐based cortical thickness values for the von Economo regions were extracted, and for each region a group average cortical thickness value was computed. Finally, region‐wise group averaged cortical thickness estimates were cross‐correlated with the von Economo and Koskinas reported levels of width of the cortical mantle.
RESULTS
Mapping of Von Economo—Koskinas to Desikan—Killiany
Region‐wise MR T1‐weighted imaging derived cortical thickness significantly correlated to von Economo and Koskinas measurements of cortical width (P = 2.00 × 10−06, r = 0.58, surviving Bonferroni correction, Fig. 2A,B).
Figure 2.
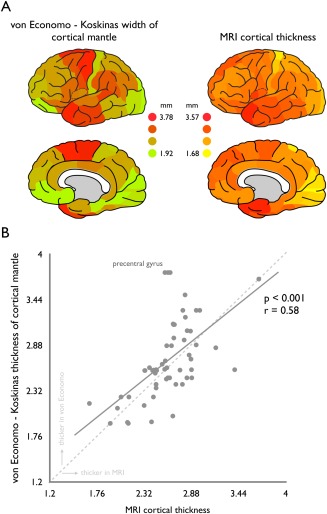
Association between von Economo—Koskinas width of cortical mantle and MRI derived cortical thickness. Left panel of A shows the levels of total width of the cortical mantle as provided by von Economo in millimeters (described as the average of the dome and wall width levels provided); right panel shows the cortical thickness as computed based on T1‐weighted MRI data of 200+ subjects of the Human Connectome Project (see Methods) also in millimeters. B Correlation between region‐wise von Economo width of the cortical mantle (y‐axis) and MRI derived cortical thickness (x‐axis). Cross‐technique comparison shows a strong relationship between von Economo cortical width and MRI cortical thickness (P < 0.001, surviving Bonferroni correction for multiple testing). Points above the dashed middle line reflect regions of which the von Economo histological measurements exceed the MRI derived estimates, points below this dashed line reflect regions of which MRI derived estimates of cortical thickness exceed those of the reports of von Economo and Koskinas (see also Fig. 3). Regions of the precentral gyrus (marked) show a clear underestimation of cortical thickness as observed by means of MRI as compared to von Economo reported cortical width (see results for a further investigation and possible explanation of this effect). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The von Economo data does not distinguish between left and right hemisphere and in the mean analysis MRI data of the left and right hemisphere was thus averaged. Post hoc analysis, correlating von Economo–Koskinas cortical width to MRI derived thickness of the left and right hemisphere separately, revealed similar strong correlations (right: P = 2.69 × 10−06, r = 0.57 | left: P = 3.80 × 10−06, r = 0.57).
Supplementary analyses describing (1) a subset of only the oldest HCP subjects (age range 31–35 years, 96 subjects) and (2) data of a set of n = 40 subjects drawn from a population of healthy participants of recent publications of our group [van den Heuvel and Sporns, 2011, 2013; van den Heuvel et al., 2012, 2013; Walhout et al., 2015] in the same age range as the von Economo‐Koskinas dataset (30–40 years) revealed highly consistent results to the main analysis (HCP 30–35 years: r = 0.64, P = 1.15 × 10−07; Utrecht set 30–40 years: r = 0.56, P = 7.97 × 10−06, data shown in Supporting Information Fig. S3).
Primary motor regions (region FA in the von Economo 1925 atlas and subareas of the precentral gyrus in the Desikan–Killiany atlas, Supporting Information Table 1) showed deviating behavior from the general relationship, showing a clear underestimation of the thickness of the cortex as measured by MRI (over 31%, MRI: 2.60 mm, von Economo total: 3.77 mm) as compared to the histological findings of von Economo and Koskinas (points marked in Fig. 2B). Studies have noted a general difficulty of providing accurate MR measurements of cortical thickness of primary motor regions based on T1‐weighted imaging [Han et al., 2006]. This effect is possibly related to a suggested gradual transition of layer 6 to the white matter in the precentral gyrus [Brodmann, 1909] and thus relatively high T1 signal [Steen et al., 2000], making an accurate segmentation of the cortical and white matter boundary and subsequent estimates of the thickness of the cortical mantle particularly difficult in these regions [Han et al., 2006]. Indeed, taking the von Economo reports of the width of layer 1–layer 5 (i.e., thus excluding the heavily myelinated layer 6 of the precentral gyrus regions) revealed a much better overlap with the MR estimates of precentral gyrus thickness, reducing the effect to a small overestimation of 3.7% (MRI: 2.60 mm, von Economo layer 1–5: 2.46 mm).
Examining this effect across all regions, an underrepresentation of the thickness of the cortical mantle by MRI as compared to von Economo–Koskinas reported values was mostly found in motor frontal, lateral parietal and primary visual cortical regions (e.g., pink to magenta regions, supramarginal cortex showing a von Economo—MRI ratio: 1.17, pericalcarine gyrus: 1.30, Fig. 3), while an overestimation of MRI cortical thickness (i.e., von Economo < MRI) was observed in lateral frontal and medial regions of the insular, frontal, and temporal cortex (yellow regions, ratio: 0.77, lateral orbitofrontal cortex, Fig. 3).
Figure 3.
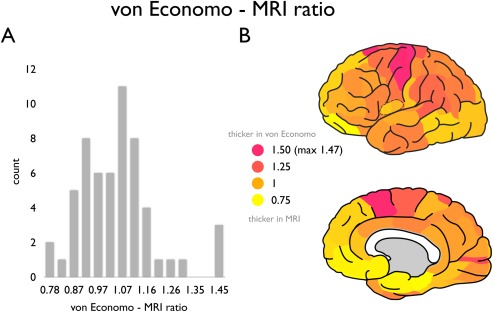
von Economo—MRI ratio across regions. Panel A shows the distribution of the ratio between the von Economo and Koskinas estimate of cortical width and MRI derived cortical thickness across all cortical regions of the 57 region Desikan–Killiany atlas; panel B shows the von Economo—MRI ratio plotted on the cortical surface (left hemisphere). A ratio of >1 reflects a level of cortical thickness higher in the von Economo mapping as compared to the MRI derived estimate (pink to magenta regions). A ratio of < 1 reflects a level of cortical thickness higher as estimated by MRI as compared to the reports of von Economo and Koskinas (yellow regions). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Von Economo FreeSurfer Atlas
Consistent with the presented findings above, correlating MRI cortical thickness values extracted from the manually segmented vonEconomo FreeSurfer atlas with the von Economo‐Koskinas histological reports revealed a positive correlation (P = 0.0016, r = 0.45). Note that as illustrated in Figure 4, two points (indicated in pink in the figure) formed clear outliers to the overall correlation. Indeed, as noted, regions FM and FN were identified during manual segmentation process as difficult to segment subregions due to their very small size and narrow shape as compared to other cortical areas (see methods). Labeling FM and FN as outliers in the correlation analysis resulted in a correlation of r = 0.62 (P = 5.76 × 10−06, Fig. 4). In addition, similar as for the mapping to the 57 single hemisphere Desikan–Killiany regions, the precentral gyrus (region FA in the von Economo atlas) showed a clear underestimation by MRI (von Economo/MRI ratio: 1.35). Further consistent with the results for the mapping of the von Economo atlas to the Desikan–Killiany atlas (Fig. 3), MRI analysis resulted in an underestimation of the thickness of the cortical mantle for frontal motor, lateral parietal, and primary visual regions (e.g., frontal region FB showing a von Economo—MRI ratio of 1.04, region PF 1.12, and region OC 1.09) and an overestimation of MRI derived thickness of medial temporal, frontal, and insular cortex (e.g., region FL showing a ratio of 0.75, region IB 0.90, region HC 0.76).
Figure 4.
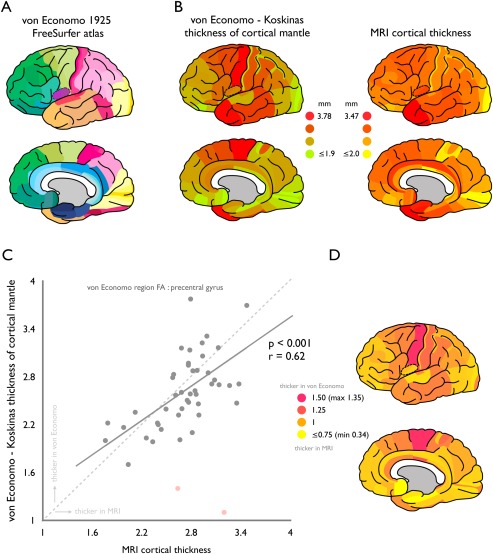
von Economo—FreeSurfer atlas. Panel A illustrates the regions of the manually segmented von Economo 1925 FreeSurfer atlas. Panel B shows for this atlas the von Economo and Koskinas reported cortical width values (left panel) and MRI derived thickness estimates (right panel) of the cortical mantle. Panel C shows the significant region‐wise correlation between von Economo—Koskinas measurements of width of the cortical mantle (y‐axis) and MRI derived measurements of cortical thickness (x‐axis). Panel D shows the ratio between the von Economo and Koskinas reported cortical width values and MRI derived estimates of cortical thickness (with a ratio >1 (pink‐magenta) indicating von Economo > MRI, and a ratio of <1 (yellow) indicating von Economo < MRI). [Note the high overlap with the results shown for the manual mapping to the Desikan–Killiany atlas as presented in Fig. 3B]. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The main finding of our study is a positive correlation between MRI derived measures of cortical thickness and width of the cortical mantle as reported in the histological work of pioneering neuroanatomists von Economo and Koskinas [von Economo, 1925, 1927]. With MRI currently being one of today's most used technologies to measure structure of the human cortex, linking and validating MRI derived metrics to histological reports is of importance to the use of such metrics in clinical, translational and cognitive neuroscience studies. Our technical report shows a simple, but potentially important correlation between MRI derived metrics of cortical thickness and cortical width as measured by histological techniques.
At least three technical remarks have to be kept in mind when interpreting the findings of this study. First, postmortem examinations are known to suffer from volume shrinkage after death related to dehydration of the tissue, thus leading to a general underestimation of measurements of size of postmortem tissue. The data of von Economo and Koskinas have not been corrected for shrinkage effects, which could be argued as having led to a possible global underestimation of measurements of volume and thickness [Amunts et al. 2007]. However, the section procedure as used by von Economo and Koskinas has been noted to lead to an overestimation of tissue volume, factors argued to counter balance shrinkage effects [Amunts et al., 2007]. Amunts et al. [2007], documenting and analyzing the used procedures in detail, thus concluded the von Economo and Koskinas estimates to be accurate and comparable to other techniques. Second, how the brain samples included in the von Economo 1925 atlas exactly correspond to HCP MRI data can unfortunately not be quantified (as trivially no MRI data of the samples as examined by von Economo and Koskinas exists), but it is clear from their detailed writings on differences between individual samples that they investigated a number of brains, presumably of both men and women combined in an age range of 30–40 years. Concerning the latter, supplementary analysis of examining HCP and Utrecht MRI samples in the age range of 30–40 years revealed consistent findings (See Supporting Information Materials and Supporting Information Fig. 3). Third, MRI estimations of the cortical thickness of regions were taken as an average over a large group of subjects thus ignoring individual differences in thickness of the cortical mantle related to for example gender (e.g., [Amunts et al., 2007; Sowell et al., 2007]) and/or variation in cognitive abilities [Karama et al., 2011; Schnack, et al., in press].
Our conclusion is threefold. First, today's MRI technology is a good method for a quick, automated estimation of thickness of the cortical mantle in vivo. Second, translating detailed histology data to an MRI framework provides new opportunities to test algorithms for automated gray/white matter segmentations. And third, findings show that MRI‐based observations can be examined and interpreted in light of cytoarchitectonic observations, suggesting that the “gap” between MRI derived and histology derived measurements of human cortical structure may not be as big as sometimes thought.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Leonard van den Berg, and Ruben Schmidt for kindly sharing T1 MRI data (as part of the analyzed supplementary Utrecht dataset). We thank Claus Hilgetag for fruitful and enjoyable discussions on this topic.
REFERENCES
- Alonso‐Nanclares L, Gonzalez‐Soriano J, Rodriguez JR, DeFelipe J (2008): Gender differences in human cortical synaptic density. Proc Natl Acad Sci USA 105:14615–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Armstrong E, Malikovic A, Homke L, Mohlberg H, Schleicher A, Zilles K (2007): Gender‐specific left‐right asymmetries in human visual cortex. J Neurosci 27:1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K (1909): Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig. Johann Ambrosius Barth.
- Cammoun L, Gigandet X, Meskaldji D, Thiran JP, Sporns O, Do KQ, Maeder P, Meuli R, Hagmann P (2011): Mapping the human connectome at multiple scales with diffusion spectrum MRI. J Neurosci Methods 203:386–397. [DOI] [PubMed] [Google Scholar]
- Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, Lo Russo G, Castana L, Colombo N, Caborni C, De Momi E, Ferrigno G (2014): Validation of freesurfer‐estimated brain cortical thickness: Comparison with histologic measurements. Neuroinformatics 12:535–542. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B (2006): Reliability of MRI‐derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage 32:180–194. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333. [DOI] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC, Brain Development Cooperative Group (2011): Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. NeuroImage 55:1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AK, Alston RL, Corsellis JA (1980): Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: Measurements with an image analyser. Neuropathol Appl Neurobiol 6:119–132. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Broere CA, Cerliani L (2014): A new myeloarchitectonic map of the human neocortex based on data from the Vogt‐Vogt school. Brain Struct Func. [DOI] [PubMed] [Google Scholar]
- Peters A, Jones EG (1984): Comparative Structure and Evolution of Cerebral Cortex, Part I. Springer US. [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002): Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58:695–701. [DOI] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE (2014): Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex (in press). [DOI] [PubMed] [Google Scholar]
- Schuz A (2002): Cortical Areas: Unity and Diversity. London: Taylor & Francis. [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW (2007): Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Reddick WE, Ogg RJ (2000): More than meets the eye: Significant regional heterogeneity in human cortical T1. Magn Reson Imaging 18:361–368. [DOI] [PubMed] [Google Scholar]
- Triarhou LC (2007a): History of Neuroscience: Constantin von Economo (1876‐1931). IBRO History of Neuroscience. [Google Scholar]
- Triarhou LC (2007b): The economo‐Koskinas atlas revisited: cytoarchitectonics and functional context. Stereotact Funct Neurosurg 85:195–203. [DOI] [PubMed] [Google Scholar]
- Triarhou LC (2007c): A proposed number system for the 107 cortical areas of Economo and Koskinas, and Brodmann area correlations. Stereotact Funct Neurosurg 85:204–215. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K (2013): The WU‐Minn Human Connectome Project: An overview. NeuroImage 80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2011): Rich‐club organization of the human connectome. J Neurosci 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013): An anatomical infrastructure for integration between functional networks in human cerebral cortex. J Neurosci 33:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goñi J, Sporns O (2012): High‐cost, high‐capacity backbone for global brain communication. Proc Natl Acad Sci USA 109:11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RCW, Cahn W, Goñi J, Hulshoff Pol HE, Kahn RS (2013): Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry (Chicago, IL) 70:783–792. [DOI] [PubMed] [Google Scholar]
- von Economo C (1927): Zellaufbau der Grosshirnrinde des Menschen. Berlin: Springer. [Google Scholar]
- von Economo C (2009): Cellular structure of the human cerebral cortex In Triarhou LC, editor and Translator. Translation of Zellaufbau der Grosshirnrinde des Menschen (von Economo 1927). Basel: Karger. [Google Scholar]
- von Economo CF, Koskinas GN (1925): Die cytoarchitektonik der hirnrinde des erwachsenen menschen. Berlin: Springer. [Google Scholar]
- Walhout R, Westeneng H‐J, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015): Cortical thickness in ALS: Towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86:288–294. [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero‐Gallagher N, Grefkes C, Scheperjans F, Boy C, Amunts K, Schleicher A (2002): Architectonics of the human cerebral cortex and transmitter receptor fingerprints: Reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol 12:587–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


