Abstract
Childhood stress and genetic factors like the Val66MET polymorphism of the brain derived neurotrophic factor (BDNF) gene are associated with a higher risk for developing major depressive disorder (MDD) and might also influence hippocampal changes. The aim of this study was to determine which hippocampal dentate gyrus and cornu ammonis subfields are altered in MDD compared to healthy controls and which subfields are affected by the BDNF Val66Met polymorphism and child adversity. Adult patients with MDD and healthy matched controls underwent high‐resolution magnetic resonance imaging. Automatic segmentation using the programme FreeSurfer was used to segment the hippocampal subfields dentate gyrus (DG/CA4), CA1 and CA2/3. The history of possible childhood adversity was assessed using the Childhood Trauma Questionnaire and the Val66Met BDNF SNP (rs6265) genotypes were obtained. Patients with MDD had significantly smaller CA4/DG and CA2/3 volumes compared to healthy controls. Furthermore, there was a significant interactive effect of BDNF allele and childhood adversity on CA2/3 and CA4/DG volumes. Met allele carriers without childhood adversity had larger and with childhood adversity smaller CA4/DG and CA2/3 volumes than Val‐allele homozygotes. Our results highlight stress by gene interactions as relevant for hippocampal volume reductions, in particular for the subfield CA2/3 and dentate gyrus. Hum Brain Mapp 35:5776–5783, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: dentate gyrus, CA2/3, hippocampus, depression, BDNF genotype, childhood maltreatment
INTRODUCTION
Major depressive disorder (MDD) is one of the most prevalent and burdensome of psychiatric illnesses [Goetzel et al., 2003; Murray and Lopez, 1996]. Previous studies have suggested that aberrant neuronal plasticity and neural remodelling play a significant role in the pathophysiology of MDD. Recent studies using behavioural, molecular and electrophysiological techniques have revealed that certain aspects of depression may result from maladaptive, stress‐induced neuroplastic changes in specific neural circuits [Krishnan and Nestler, 2008]. Psychological, behavioural and psychosocial stress is associated with structural changes in the hippocampus [Malberg and Duman, 2003; Pham et al., 2003; Vermetten and Bremner, 2002]. Interestingly, in vivo magnetic resonance imaging studies have also shown a significant reduction in hippocampal volume in patients with MDD [Campbell et al., 2004].
New imaging techniques allow the segmentation of the hippocampus into its main subfields and thus allows investigation of subregions in humans [Van Leemput et al., 2009]. In a recent study which explored hippocampal subfield volumes using high‐resolution MRI, nine unmedicated patients with MDD had lower dentate gyrus volumes in comparison to 27 controls and 11 medicated patients with MDD. Unmedicated patients with MDD had also lower cornu ammonis (CA1‐3) volumes than the control subjects [Huang et al., 2013]. Research into hippocampal subregions like the dentate gyrus and the cornu ammonis is also important as they have different functions and seem to have different stress sensitivities. Smaller volumes in the CA2/3 and dentate gyrus subfields of the hippocampus were linked to depressive symptoms and were associated with hyper‐reactivity of cortisol secretion during the day in patients with multiple sclerosis [Gold et al., 2010], suggesting region specific effects of stress and daily cortisol levels, at least in subjects vulnerable to depression. Interestingly, the reduction of BDNF expression in the dentate gyrus but not in the region CA3 of the hippocampus, reduces neurogenesis and affects behaviours that experimentally have been associated with depression [Taliaz et al., 2010].
Converging evidence suggests that the brain‐derived neurotrophic factor (BDNF) might be involved in synaptic changes in the brain, before, during and after an episode of depression [Gonul et al., 2011]. The gene coding for BDNF is located in chromosome 11 [Liu et al., 2005]. The MET‐allele of the Val66MET polymorphism of BDNF polymorphisms was found to be associated with MDD [Verhagen et al., 2010]. A functional single‐nucleotide polymorphism (SNP) identified in the BDNF gene (rs6265) results in a methionine (Met) to valine (Val) substitution at codon 66 in the pro‐region of the BDNF [Egan et al., 2003]. The Met variant has been associated with impaired intracellular trafficking of pro‐BDNF into dendrites and vesicles as well as a reduction in activity‐dependent secretion, the process that plays a major role in the regulation of extracellular level of BDNF [Egan et al., 2003].
BDNF is expressed throughout the brain in cortical and subcortical areas, particularly in the hippocampus [Dennis and Levitt, 2005; Lu and Gottschalk, 2000]. In a large population‐based study of 1,435 participants, it was shown that BDNF Met‐allele carriers had reduced serum BDNF levels if they had experienced childhood abuse. Interestingly, Met‐allele carriers had higher BDNF levels than the Val/Val individuals, who did not show decreases in BDNF levels associated with childhood adversity [Elzinga et al., 2011]. Past neuroimaging studies focused on associations between BDNF genotype and the hippocampus. It has been hypothesized that Met‐allele carriers may be at risk of developing smaller hippocampal volumes and thus may be more susceptible to developing depression [Frodl et al., 2007]. Moreover, the Met‐allele seems to affect other brain regions such as the amygdala and the parahippocampal gyrus [Montag et al., 2009]. A recent meta‐analysis not only confirmed that carriers of the Met‐allele have smaller hippocampal volumes but also pointed out that effect size decreased substantially with age, suggesting that the effect might be smaller than indicated by earlier published studies [Molendijk et al., 2012].
In a two‐centre study, we found an interactive effect between BDNF Val66Met and childhood adversity and showed that Met‐allele carriers had significantly smaller hippocampal volumes in both patients with depression and controls with a history of childhood adversity [Carballedo et al., 2013]. A recent study with healthy volunteers explored the association between BDNF genotype and stress, and reported that subjects carrying the Met‐allele had smaller hippocampal volumes when they had more sub‐threshold symptoms of depression and a higher extent of neuroticism [Joffe et al., 2009]. Another relevant study used structural equation modelling to study the interaction between BDNF and childhood maltreatment on brain structure. The authors suggested that the combination between Met carrier status of the BDNF genotype and a history of self‐reported childhood abuse predicted reduced hippocampal volumes, associated reduced lateral prefrontal cortex volumes and, in turn, higher depression rating scores [Gatt et al., 2009]. Latest research by Gerritsen et al. has also shown that BDNF Met‐allele carriers with a history of childhood maltreatment had significantly less grey matter in sub‐genual ACC compared with Met‐allele carriers without such a history of maltreatment and Val/Val homozygotes with childhood maltreatment. Interestingly, they did not find significant differences in the hippocampus, amygdala and prefrontal cortex [Gerritsen et al., 2012], which might be due to methodological differences between voxel‐based morphometry (VBM) used in the study by Gerritsen et al. [2011] compared to segmentation methods used by Gatt et al. [2009] or differences in the kind of child stressors between samples.
Thus, the goal of the present study was to assess changes in the hippocampal subfields of the dentate gyrus and cornu ammonis in patients with MDD compared to healthy controls. A novel aim was to investigate whether there are also effects of BDNF genotype and childhood abuse and/or significant interactions between BDNF genotype and childhood adversity within these hippocampal subfields.
MATERIALS AND METHODS
A total of 82 adult participants (38 patients with MDD and 44 matched healthy controls, HC) were recruited from the mental health services of the Adelaide and Meath Hospital, incorporating the National Children's Hospital and St. James's Hospital, Dublin, Ireland. The research diagnosis of these patients with MDD was based on a clinical diagnosis according to DSM‐IV criteria confirmed by an independent experienced psychiatrist using the SCID interview.
Healthy participants from the local community were recruited via advertisements and the groups were matched for age and sex (Table 1). Exclusion criteria were age <18 or >65, history of neurological, severe medical illness, head injury with loss of consciousness or substance abuse. For patients, exclusion criteria were comorbid psychiatric disorders (Axis I or Axis II) and for healthy participants any psychiatric disorders. Also contraindications for MRI were exclusion criteria. Demographic variables, inclusion and exclusion criteria were documented using a standardised questionnaire and through a structured interview by a psychiatrist.
Table 1.
Demographic and clinical data
| Patients (N = 38) | Controls (N = 44) | df | Diagnosis effect | Childhood adversity effect | BDNF genotype effect | |
|---|---|---|---|---|---|---|
| Age | 40.9 (10.8) | 36.2 (13.3) | 80 | T = 1.7, P = 0.08 | T = 0.6, P = 0.56 | T = 0.77, P = 0.45 |
| Sex (Female/Male) | (25/13) | (27/17) | 80 | χ 2 = 0.17, P = 0.68 | χ 2 = 2.6, P = 0.11 | χ 2 = 0.18, P = 0.67 |
| Height | 171.5 (8.2) | (172.1 (10.2) | 80 | T = −0.3, P = 0.76 | T = 1.2, P = 0.23 | T = 0.73, P = 0.47 |
| Weight | 75.4 (15.3) | 70.7 (15.6) | 80 | T = 1.4, P = 0.17 | T = 1.5, P = 0.13 | T = 0,3, P = 0.76 |
| Hamilton depression score | 28.3 (6.7) | 2.8 (2.9) | 80 | T = 21.7, P < 0.001 | T = 4.3, P < 0.001 | T = 1.5, P = 0.15 |
| Beck depression | 32.7 (11.6) | 2.7 (3.5) | 80 | T = 16.2, P < 0.001 | T = 4.6, P < 0.001 | T = 2.2, P = 0.028 |
| Emotional abuse | 10.4 (5.8) | 6.3 (1.8) | 80 | T = 4.5, P < 0.001 | T = 6.3, P < 0.001 | T = 0.74, P = 0.46 |
| Physical abuse | 8.7 (5.8) | 5.8 (1.6) | 80 | T = 3.2, P = 0.004 | T = 4.9, P < 0.001 | T = 1.4, P = 0.17 |
| Sexual abuse | 8.4 (6.0) | 5.6 (1.4) | 80 | T = 3.1, P = 0.006 | T = 3.9, P < 0.001 | T = 0.84, P = 0.40 |
| Emotional neglect | 11.7 (5.8) | 7.2 (2.5) | 80 | T = 4.7, P < 0.001 | T = 5.4, P < 0.001 | T = 1.0, P = 0.33 |
| Physical neglect | 8.5 (3.7) | 6.1 (1.6) | 80 | T = 3.9, P = 0.001 | T = 3.5, P = 0.001 | T = 0.15, P = 0.89 |
| Medication (none/SSRI/dual acting) | 13/13/12 | 36 | χ 2 = 0.68, P = 0.71 | χ 2 = 0.035, P = 0.98 | ||
| Age of Onset | 24.2 (12.9) | 36 | T = 1.5, P = 0.13 | T = 0.7, P = 0.49 | ||
| Cumulative illness duration | 9.4 (9.8) | 36 | T = 1.2, P = 0.24 | T = 0.84, P = 0.41 | ||
| Days treated | 2,385.8 (3,295.7) | 36 | T = 0.76, P = 0.45 | T = 0.14, P = 0.89 | ||
| Days depressed and not treated | 1,564.9 (2,513.3) | 36 | T = 0.55, P = 0.58 | T = 1.7, P = 0.09 |
Shown are mean values and standard deviations for patients and healthy controls. The diagnosis effect scores belong to the differences between patients and healthy controls. Additionally, the statistics for abuse and BDNF genotype effects are shown, whereby mean values for groups are not shown in this table.
Written informed consent was obtained from all participants after being given detailed explanation of the study which was designed and performed in accordance to the ethical standards laid out by the Declaration of Helsinki and also approved by the ethics committee of St. James and the Adelaide and Meath Hospitals, Dublin.
Rating Instruments
Self‐ and observer‐rated scales were filled out for all participants included in the study. The rating scales that were used comprised: the Hamilton Rating Scale for Depression [Hamilton, 1969], Beck's Depression Inventory (BDI‐II) [Beck et al., 1996], Childhood Trauma Questionnaire (CTQ) [Bernstein et al., 1994] and the Structured Clinical Interview for DSM‐IV (SCID‐II) personality questionnaire [Spitzer et al., 1992]. CTQ is a standardised self‐report instrument that assesses five types of childhood maltreatment: emotional, physical and sexual abuse, and emotional and physical neglect. Reliability and validity of the CTQ have been established, including measures of convergent and discriminative validity from structured interviews, stability over time and corroboration [Bernstein et al., 2003].
Childhood adversity was calculated as the sum score of all five categories (physical abuse, emotional abuse, sexual abuse, physical neglect and emotional neglect). Moreover, a threshold was used in order to categories adversity for further analysis when a participant had scores greater than the cut‐off score in at least one of the subscales of physical abuse (≥10) and/or, emotional abuse (≥12) and/or sexual abuse (≥8), and/or emotional neglect (≥15) and/or physical neglect (≥10).
MRI Data Acquisition
Magnetic resonance images were obtained with a Philips Achieva MRI scanner (Philips Medical System, Netherland B.V., Veenphuis 4–6, 5684 PC Best, The Netherlands) operating at 3 T. A sagittal T1 three‐dimensional TFE (turbo field echo) was used to scan all participants (TR user defined of 8.5 ms; TE user defined of 3.9 ms; total acquisition time of 7 min; field of view (FOV) of FH (foot to head): 256 mm, AP (anterior to posterior): 256 mm, RL (right to left): 160 mm; and a matrix of 256 × 256). Slice thickness was 1 mm and voxel size was 1 × 1 × 1 mm3.
Definition of Hippocampus
Hippocampal subfields volume was assessed fully automatic with FreeSurfer. FreeSurfer uses a Bayesian modelling approach, which first builds an explicit computational model of how an MRI image around the hippocampal area is generated, and subsequently uses this model to obtain fully automated segmentations for the hippocampal subfields. Here we used the main hippocampal subfields belonging to the cornu ammonis and dentate gyrus: CA1, CA2/3 and CA4/DG (Fig. 1). It was shown that automatically calculated volumes of CA2/3 and CA4/DG are strongly correlated with those volumes derived from manual delineation, with a correlation coefficient of 0.91 (P ≤ 0.0002) and 0.83 (P ≤ 0.0028), respectively [Van Leemput et al., 2009].
Figure 1.
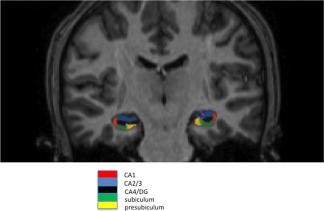
Example for hippocampal subfield delineation. Shown are subfields CA1, CA2/3, CA4/DG, subiculum and presubiculum. The programme FreeSurfer automatically assessed volumes of subfields which are then manually viewed and checked for quality.
Genetic Methods
The Val66Met BDNF SNP (rs6265) was genotyped in this sample using a Taqman® SNP Genotyping Assay on a 7900HT Sequence Detection System (Applied Biosystems). The call rate for the Taqman genotyping was >95% and all samples were in Hardy‐Weinberg equilibrium (P > 0.05). Along with the test samples, a number of HapMap CEU DNA sample positive controls (http://www.hapmap.org) and non‐template negative controls were genotyped for each SNP for quality control purposes. For positive controls, all genotypes were found to be concordant with available online HapMap data. All non‐template samples returned a negative result.
Statistical Analysis
Differences in demographic variables were tested using Student's t‐test and χ 2 test for gender and medication distribution. Morphometric measurements in both groups were normally distributed (using Kolmogorov Smirnov test) and their variances were homogenous (using Levene's test).
First, hippocampal volumes were subjected to a repeated measurement analysis of covariance (ANCOVA) to assess the main and interaction effects of the within‐subjects factor hemisphere (left, right) and region (CA4/DG, CA1, CA2/3) and the between‐subjects factor group (MDD, HC) using total brain volume, age and gender as covariates. Since a previous study found a pronounced difference between unmedicated patients in comparison to controls for hippocampal subfields we also tested additionally for these medication group differences. Differences between hippocampal subfield volumes of unmedicated patients versus medicated patients and healthy controls were tested with analysis of variance followed by Fisher's least significant difference (LSD) post hoc comparison for multiple testing.
A general linear model was then used to assess the main and interactive effects of BDNF genotype and childhood adversity. In this model the hippocampal subfield volumes with differences between patients and controls (CA2/3, CA4/DG) were used as dependent variables and the following variables were predictors: diagnosis (MDD, HC) and BDNF Val66Met polymorphism (Met‐allele carrier, homozygous for Val/Val), total brain volume, age, gender and childhood adversity. In this model, the interactive term BDNF genotype and diagnosis was also evaluated. The mean of left and right hemispheres was taken since there was no significant difference between hemispheres. Since two regions were tested a P < 0.025 was considered as surviving bonferroni correction for multiple testing.
RESULTS
There were no statistically significant demographic differences between diagnostic groups, history of abuse and BDNF genotype. Current medication did not differ between patients carrying the Met‐allele in comparison to those patients homozygous for the Val‐allele. Subjects carrying the Met‐allele of the BDNF Val66Met polymorphism had significantly higher BDI depression scores compared to subjects homozygous for the Val allele (Table 1).
In the overall, repeated measurement ANCOVA a significant three‐way interaction was observed for the factors region, hemisphere and diagnosis (F(1/77) = 5.6, P = 0.021). Significant smaller left CA2/3 volumes (F(1/77) = 8.3, P = 0.005, power: 0.81) and left CA4/DG volumes (F(1/77) = 5.8, P = 0.018, power: 0.66) were found for patients with MDD compared to healthy controls (Fig. 2).
Figure 2.
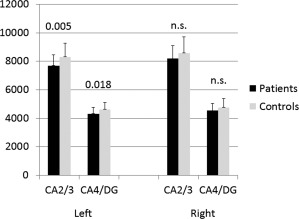
Smaller left CA4/DG and left CA2/3 volumes in patients with MDD compared to healthy controls. Shown are mean values (bars) and standard deviations (on top of the bars).
For medication status, there was a significant main effect of group (unmedicated patients, medicated patients, controls) for left CA2/3 volumes (F(2/76) = 5.0, P = 0.009) and left CA4/DG volumes (F(2/76) = 3.5, P = 0.035). Hippocampal subfield volumes did not differ between patients scanned drug‐free compared to those currently on medication. Compared to healthy controls both groups of patients had smaller hippocampal CA2/3 (unmedicated patients versus HC: CA2/3: P = 0.032 medicated patients versus HC: P = 0.005) and CA4/DG volumes (unmedicatd patients versus HC: CA2/3: P = 0.062 medicated patients versus HC: P = 0.014) using post hoc LSD test.
The general linear model demonstrated a significant effect for BDNF genotype on CA2/3 (F(1/75) = 6.6, P = 0.01) and CA4/DG (F(1/75) = 5.0, P = 0.026) volumes. Moreover, a significant interaction was found between BDNF genotype and childhood adversity on CA2/3 (F(1/75)=5.2, P = 0.023) and CA4/DG (F(1/75)=4.3, P = 0.038) volumes (Fig. 3a,b). The results on CA2/3 volumes survived bonferroni correction for multiple comparison based on the 2 regions (Ca2/3, CA4/DG). Moreover, a trend towards a significant independent effect was found for childhood adversity on CA2/3 (F(1/75) = 3.4, P = 0.064) and CA4/DG (F(1/75) = 3.6, P = 0.056) volumes.
Figure 3.
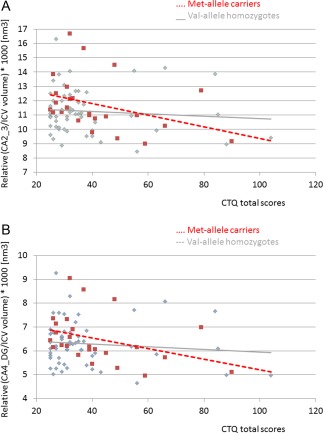
Scatterplott for the association between childhood adversity and hippocampal subfield volumes moderated by BDNF Val66 Met genotype. Shown are the interactive effects of BDNF Val 66 Met genotype and childhood adversity on hippocampal subfield CA2/3 (a) and CA4/DG (b). Met allele carriers with self‐reported childhood adversity show smallest CA2/3 and CA4/DG volumes, while they have larger volumes compared to Val homozygotes with and without childhood abuse. Depicted are the relative CA2/3 to total intracranial volume and relative CA4/DG to total intracranial volume multiplied by 1,000. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The present study shows a significant interaction between childhood adversity and BDNF Val66Met polymorphism on cornu ammonis and dentate gyrus volumes. It, furthermore, confirms that cornu ammonis and dentate gyrus volumes are significantly smaller in patients with MDD when compared to healthy controls. Therefore, our results are in agreement with the study by Huang et al. [2013], who reported smaller dentate gyrus and cornu ammonis (CA1‐3) volumes in patients with MDD. These effects were found in particular in unmedicated patients compared to control subjects [Huang et al., 2013]. In our study, we did not see any differences between patients unmedicated compared to those currently on antidepressants. In addition, unmedicated as well as currently medicated patients had smaller CA4/DG and CA2/3 volumes compared to healthy controls. This effect is seen in particular in the left hemisphere, where stronger differences are usually detected in patients with MDD [Campbell et al., 2004; Frodl et al., 2002; MacQueen and Frodl, 2011]. In line with our finding, there is also consistent evidence in the current literature of patients with MDD having smaller hippocampal volumes compared to healthy individuals.
Interestingly, our study was novel and showed a significant interaction between BDNF Val66Met and history of childhood adversity in a sample of patients with MDD and healthy controls. Met‐allele carriers showed larger CA4/DG and CA2/3 volumes when they had no history of childhood adversity and smaller CA4/DG and CA2/3 volumes when they had a history of childhood adversity compared to participants homozygous for the Val‐allele. Thus, both the dentate gyrus as well as the cornu ammonis subfield CA3 seems to be affected by childhood maltreatment and the risk‐allele of BDNF. Very few studies have looked at the interaction between BDNF and childhood abuse so far. We have replicated, through a different method, the findings of a recent study of 89 participants, where significant interactions between BDNF genotype and abuse were apparent in hippocampus and amygdala volumes, heart rate and working memory [Gatt et al., 2009]. Here we have developed this knowledge further, and have reported that interactions between childhood adversity and BDNF genotype are particularly seen in the subfields CA2/3 and CA4/DG. However, a recent VBM study in a large sample of healthy subjects did not find differences in the hippocampus with regards to the BDNF Val66Met polymorphism and childhood abuse, but demonstrated that Met‐allele carriers with a history of childhood maltreatment had smaller sub‐genual ACC volumes [Gerritsen et al., 2011].
Interestingly, in the general linear model the effects from BDNF Val66met polymorphism and childhood adversity seem to be most relevant for the hippocampal subfield differences found between patients and healthy controls. As expected, childhood adversity was significantly higher in patients with MDD compared to controls and in patients there was—although not significantly—a larger number of Met‐allele carriers compared to controls. This could suggest that the cause of the structural brain changes seen between patients with MDD and healthy controls might be at least partially due to the effects of childhood stress and genetic factors. Stress is believed to play an important role in the pathogenesis of MDD. Currently, there is much debate about the effects of childhood maltreatment and the potential association between childhood maltreatment and the specific course of illness, long‐lasting emotional problems [Carboni et al., 2010; Heim C, 2001; Mann and Currier, 2010], and hippocampal volumetric changes [Chen et al., 2010]. There is growing evidence that childhood adversity, defined as maltreatment or trauma in the form of emotional, physical or sexual abuse, or emotional or physical neglect, could have detrimental effects on brain structure. In a recent review, it was demonstrated that eight studies in adult samples using manual hippocampal tracing pointed towards an effect of childhood adversity on hippocampal volumes, whereas two studies with traumatization at later ages and pre‐pubertal age did not find such an association [Frodl and O'Keane, 2013]. Vythilingam et al. compared 32 women with recurrent unipolar depression and pre‐pubertal physical or sexual abuse, to 11 depressed women but without pre‐pubertal abuse and to 14 healthy controls. They found that the left hippocampus was 18% smaller in women with depression and pre‐pubertal abuse than those without abuse and 15% smaller than healthy controls [Vythilingam et al., 2002]. Emotional neglect has also been found to be associated with smaller hippocampal volumes. Smaller left hippocampal white matter volumes were reported in MDD patients who had experienced childhood emotional neglect compared to those without a history of emotional neglect. Both emotional neglect and brain structural abnormalities predicted cumulative illness duration [Frodl et al., 2010]. Eighty‐four healthy controls and patients with MDD who reported a history of emotional maltreatment during childhood had smaller left dorsomedial prefrontal cortex (DMPFC) volumes compared to 96 comparison subjects without maltreatment [van Harmelen et al., 2010]. At present, there is not enough data to conclude on which kind of maltreatment, e.g., neglect, abuse, emotional, physical or sexual might contribute to which regional structural changes. One VBM study in 42 healthy participants found that self‐reported childhood adversity was associated with reductions in grey matter volume in prefrontal cortical areas, striatum, amygdala and hippocampus, as well as parietal, temporal and occipital association cortices and cerebellum. In an exploratory analysis, these associations were detected for abuse as well as for neglect [Edmiston et al., 2011]. Moreover, in 145 healthy participants childhood adversity was significantly negatively associated with VBM grey matter volume in the hippocampus, insula, orbitofrontal cortex, anterior cingulate gyrus and caudate. Physical neglect, emotional abuse, sexual abuse, physical abuse and emotional neglect showed similar associations with hippocampal volume, whereby the strongest predictor was physical neglect followed by emotional abuse [Dannlowski et al., 2012]. Also the intensity and duration of maltreatment might be important to consider in future studies.
Previously, it was shown that healthy Met‐allele carriers had relatively small hippocampal volumes compared to subjects homozygous for the Val‐allele [Bueller et al., 2006; Pezawas et al., 2004]. An effect of the BDNF Met‐allele on hippocampal volume was also apparent in patients with MDD and controls [Frodl et al., 2007]. However, in a recent report a main effect of BDNF Val66Met polymorphism without taking the interactions with childhood abuse into account was not observed [Gonul et al., 2011]. Indicative from a recent meta‐analysis is that the effect of the BDNF Val66Met genotype might be smaller than previously suggested and thus larger sample sizes might be necessary to detect pure effects from one genetic polymorphism [Molendijk et al., 2012]. This is mainly the case because of the publication bias in reporting more significant studies then non‐significant findings. Thus, the results from the present study will require replication in an independent sample.
It is important to note that there were some limitations present in the conduct of this study. Firstly, the CTQ was filled out in retrospect, which could lead to inaccurate recollection of events affecting CTQ scores. For the future investigating, a study population that already had been investigated for maltreatment during childhood might be able to overcome this issue and ideally the history of child abuse should be confirmed by both self‐report and conclusion of forensic psychiatric assessment. Secondly, the depressed group with a self‐reported history of childhood abuse showed higher CTQ scores compared to the non‐depressed people with a history of childhood maltreatment. Thus, the groups are not matched on self‐reported severity of the trauma. The difference between patients with MDD and controls might be due in part to childhood adversity. It is worth noting that although the present sample is far too small for a clinical genetic study, there was a difference in the frequencies of BDNF genotype in patients (15 Met carriers/23 Val/Val) compared to controls (10 Met carriers/34 Val/Val), however, this did not reach significance (χ 2 = 2.7, P = 0.10). Subjects carrying the Met‐allele of BDNF had significantly higher depression scores compared to those homozygous for the Val‐allele demonstrating an impact at the behavioural level. In the statistics, we used overall designs to avoid issues with multiple testing as much as possible. The interactive effect of BDNF Val66Met genotype and childhood adversity on CA2/3 volumes survived bonferroni correction; however, this was marginally not the case for CA4/DG volumes. Although this sample had the same size than comparable imaging candidate genetics studies a larger sample would be needed to explore the genetic effect on the behavioural and imaging level in tandem.
CONCLUSION
Hippocampal volume is decreased in patients with MDD compared to healthy controls, and this seems to affect mainly the dentate gyrus and cornu ammonis subfields with pronounced differences on the left hemisphere. Interestingly, a significant interaction is observed between BDNF Val66Met polymorphism and self‐reported childhood adversity on these hippocampal subfields. Having both genetic and stress risk factors seems to impact on structural changes whereby, participants without childhood abuse did not show any significant alterations, even when they were carrying the risk BDNF Met‐allele.
ACKNOWLEDGMENTS
The neuroimaging subfield analysis was part of the medical doctoral thesis by Eva‐Maria Frey. Authors declare no conflict of interest.
REFERENCES
- Beck AT, Steer RA, Brown GK (1996): BDI‐II Manual. San Antonio, TX: Harcourt Brace and Company. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994): Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W (2003): Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27:169–190. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez‐Hassan D, Burmeister M, Zubieta JK (2006): BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry 59:812–815. [DOI] [PubMed] [Google Scholar]
- Campbell S, Mariott M, Nahmias C, MacQueen GM (2004): Lower hippocampal volume in patients suffering from depression: A meta‐analysis. Am J Psychiatry 161:598–607. [DOI] [PubMed] [Google Scholar]
- Carballedo A, Morris D, Zill P, Fahey C, Reinhold E, Meisenzahl E, Bondy B, Gill M, Moller HJ, Frodl T (2013): Brain‐derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Med Genet B Neuropsychiatr Genet 162B:183–190. [DOI] [PubMed] [Google Scholar]
- Carboni L, Becchi S, Piubelli C, Mallei A, Giambelli R, Razzoli M, Mathe AA, Popoli M, Domenici E (2010): Early‐life stress and antidepressants modulate peripheral biomarkers in a gene‐environment rat model of depression. Prog Neuropsychopharmacol Biol Psychiatry 34:1037–1048. [DOI] [PubMed] [Google Scholar]
- Chen MC, Hamilton JP, Gotlib IH (2010): Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry 67:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012): Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. [DOI] [PubMed] [Google Scholar]
- Dennis KE, Levitt P (2005): Regional expression of brain derived neurotrophic factor (BDNF) is correlated with dynamic patterns of promoter methylation in the developing mouse forebrain. Brain Res Mol Brain Res 140:1–9. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP (2011): Corticostriatal‐limbic gray matter morphology in adolescents with self‐reported exposure to childhood maltreatment Arch Pediatr Adolesc Med 165:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003): The BDNF val66met polymorphism affects activity‐dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Molendijk ML, Oude Voshaar RC, Bus BA, Prickaerts J, Spinhoven P, Penninx BJ (2011): The impact of childhood abuse and recent stress on serum brain‐derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology (Berl) 214:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ (2002): Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159:1112–1118. [DOI] [PubMed] [Google Scholar]
- Frodl T, O'Keane V (2013): How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 52:24–37. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010): Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 44:799–807. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Möller HJ, Meisenzahl EM (2007): Association of the brain‐derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry 64:410–416. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson‐Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM (2009): Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry 14:681–695. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Tendolkar I, Franke B, Vasquez AA, Kooijman S, Buitelaar J, Fernandez G, Rijpkema M (2012): BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol Psychiatry 17:597–603. [DOI] [PubMed] [Google Scholar]
- Goetzel RZ, Hawkins K, Ozminkowski RJ, Wang S (2003): The health and productivity cost burden of the “top 10” physical and mental health conditions affecting six large U.S. employers in 1999. J Occup Environ Med 45:5–14. [DOI] [PubMed] [Google Scholar]
- Gold SM, Kern KC, O'Connor MF, Montag MJ, Kim A, Yoo YS, Giesser BS, Sicotte NL (2010): Smaller cornu ammonis 2–3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms. Biol Psychiatry 68:553–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonul AS, Kitis O, Eker MC, Eker OD, Ozan E, Coburn K (2011): Association of the brain‐derived neurotrophic factor Val66Met polymorphism with hippocampus volumes in drug‐free depressed patients. World J Biol Psychiatry 12:110–118. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1969): Standardised assessment and recording of depressive symptoms. Psychiatr Neurol Neurochir 72:201–205. [PubMed] [Google Scholar]
- Heim C NC (2001): The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry 49:1023–1039. [DOI] [PubMed] [Google Scholar]
- Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, Malykhin NV (2013): Structural changes in hippocampal subfields in major depressive disorder: A high‐field magnetic resonance imaging study. Biol Psychiatry 74:62–68. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Gatt JM, Kemp AH, Grieve S, Dobson‐Stone C, Kuan SA, Schofield PR, Gordon E, Williams LM (2009): Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: Implications for depressive illness. Hum Brain Mapp 30:1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2008): The molecular neurobiology of depression. Nature 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, Strain KJ, de Andrade M, Bower JH, Maraganore DM, Uhl GR (2005): Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson's Disease. Am J Med Genet B Neuropsychiatr Genet 134B:93–103. [DOI] [PubMed] [Google Scholar]
- Lu B, Gottschalk W (2000): Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res 128:231–241. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T (2011): The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16:252–264. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS (2003): Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier DM (2010): Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur Psychiatry 25:268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Kaimatzoglou A, Oude Voshaar RC, Penninx BW, van IMH, Elzinga BM (2012): A systematic review and meta‐analysis on the association between BDNF val(66)met and hippocampal volume—A genuine effect or a winners curse? Am J Med Genet B Neuropsychiatr Genet 159B:731–740. [DOI] [PubMed] [Google Scholar]
- Montag C, Weber B, Fliessbach K, Elger C, Reuter M (2009): The BDNF Val66Met polymorphism impacts parahippocampal and amygdala volume in healthy humans: Incremental support for a genetic risk factor for depression. Psychol Med 39:1831–1839. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD (1996): Evidence‐based health policy—Lessons from the Global Burden of Disease Study. Science 274:740–743. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer‐Lindenberg A, Weinberger DR (2004): The brain‐derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci 24:10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS (2003): Repeated restraint stress suppresses neurogenesis and induces biphasic PSA‐NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17:879–886. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB (1992): The Structured Clinical Interview for DSM‐III‐R (SCID) I: History, rationale, and description. Arch Gen Psychiatry 49:624–629. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A (2010): Knockdown of brain‐derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BW, Elzinga BM (2010): Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry 68:832–838. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B (2009): Automated segmentation of hippocampal subfields from ultra‐high resolution in vivo MRI. Hippocampus 19:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias‐Vasquez A, Buitelaar JK, Franke B (2010): Meta‐analysis of the BDNF Val66Met polymorphism in major depressive disorder: Effects of gender and ethnicity. Mol Psychiatry 15:260–271. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD (2002): Circuits and systems in stress. I. Preclinical studies. Depress Anxiety 15:126–147. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD (2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]


