Abstract
A set of cortical and sub‐cortical brain structures has been linked with sentence‐level semantic processes. However, it remains unclear how these brain regions are organized to support the semantic integration of a word into sentential context. To look into this issue, we conducted a functional magnetic resonance imaging (fMRI) study that required participants to silently read sentences with semantically congruent or incongruent endings and analyzed the network properties of the brain with two approaches, independent component analysis (ICA) and graph theoretical analysis (GTA). The GTA suggested that the whole‐brain network is topologically stable across conditions. The ICA revealed a network comprising the supplementary motor area (SMA), left inferior frontal gyrus, left middle temporal gyrus, left caudate nucleus, and left angular gyrus, which was modulated by the incongruity of sentence ending. Furthermore, the GTA specified that the connections between the left SMA and left caudate nucleus as well as that between the left caudate nucleus and right thalamus were stronger in response to incongruent vs. congruent endings. Hum Brain Mapp 35:367–376, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: sentence comprehension, semantic integration, independent component analysis, graph theoretical analysis, fMRI
INTRODUCTION
The seminal work of Kutas and Hillyard [2008] has stimulated researchers interested in sentence processing to use violation paradigms which are based on the assumption that neural networks engaged when comprehension fails are identical to the networks underlying the processing of well‐formed sentences. To study semantic processing, a word is presented which cannot be integrated into the preceding context (e.g., I drink my coffee with cream and *mud). This paradigm has been employed in different languages and modalities (visual, auditory, sign language) and has revealed a central‐parietal negativity with a peak latency of about 400 ms (N400) for semantic violations in the event‐related potential and a corresponding N400m effect in magnetoencephalography (MEG) [for reviews, see Kutas and Federmeier, 2000, 1980]. The N400 and N400m effects have been source‐localized to distributed frontal and temporal generators among which the left temporal lobe appeared to be the most prominent source [for a review, see Van Petten and Luka, 2006].
Taking advantage of the better spatial resolution of functional magnetic resonance imaging (fMRI), recent studies have linked a set of brain areas with sentence‐level semantic processes, including the medial prefrontal cortex [Newman et al., 2001; Zhu et al., 2009], inferior frontal gyrus (IFG) [Baumgaertner et al., 2002; Cardillo et al., 2004], anterior temporal lobe [Kiehl et al., 2002], superior and middle temporal gyrus [Friederici et al., 2003; Kuperberg et al., 2000, 2000], temporoparietal junction, and caudate nucleus [Kuperberg et al., 2008; Newman et al., 2001; Ni et al., 2000; Zhu et al., 2009]. The thalamus has also been observed in the processing of semantically anomalous sentence endings in recordings from deep brain stimulation electrodes inserted for the alleviation of movement disorders [Wahl et al., 2008]. Although regions such as the left IFG and left middle temporal gyrus have long been claimed to support lexical access, retrieval, selection, and/or integration [e.g. Friederici, 2002; Lau et al., 2008], it remains unclear how these regions are organized to subserve semantic integration. Previous studies have explored the functional connectivity of up to six regions‐of‐interest during the processing of high‐imagery sentences [Just et al., 2004] or the functional network of spoken sentence comprehension in general [van de Ven et al., 2009]. However, no network analysis has been conducted over the whole brain yet focusing on sentence‐level semantic processes.
In this fMRI study of the semantic violation paradigm introduced by Kutas and Hillyard [2008], we look into this issue by applying two approaches of network analysis, independent component analysis (ICA) and graph theoretical analysis (GTA). These two approaches are complementary in that they look at different aspects of the brain network. The ICA first decomposes the whole brain activity pattern into non‐overlapping groups of brain areas (i.e., components), with each component presented as a spatial map of functionally connected regions along with a time‐course highly correlated with the real fMRI time‐course. In the next step, the component(s) of which the time‐course is dynamically modulated under different conditions is (are) determined. Whereas the ICA focuses on a particular group of brain areas, the GTA measures the functional integration and segregation of all areas as a whole (global level) and the role of a given area within the whole‐brain network (regional level). Functional integration refers to the ability of rapidly combining dedicated information from distributed brain areas, whereas functional segregation reflects the ability of processing this dedicated information within densely interconnected groups of brain areas. In this study, functional integration was quantified by the global efficiency metric and functional segregation by the clustering coefficient and modularity measures. The balance between functional integration and segregation was indicated by the small‐world index (for detailed definitions of these measures, see the Methods). Moreover, the GTA determines the relative importance of a given area by measuring regional properties such as degree, which indicates the number of interregional connections the area has. The GTA has been used to analyze the topological organization of cortical systems in nonhuman primates since the early 90s [Stephan et al., 2000; Young, 1992]. It has recently been applied to human anatomical and functional imaging data as it provides a concise quantification of the extraordinary complexity of interregional connectivity in the human brain [e.g., Bassett et al., 2006, 2008; Liu et al., 2008; for reviews, see Bullmore and Bassett, 2011; Bullmore and Sporns, 2009]. By systematically comparing topological properties between conditions over global and regional levels, we can test whether and how the brain network that supports the processing of semantically congruent stimuli is adjusted for semantically incongruent words in sentences.
METHODS
All procedures were cleared by the ethical review board of the University of Magdeburg, the affiliation of the authors at the time of the experiment.
Participants
Twenty native German speakers (10 women, mean age 25 years, age range 21–30 years) participated in this study. They were right‐handed and had normal or corrected‐to‐normal vision. None of them had a history of neurological or psychiatric disorders. All of them gave written informed consent before scanning.
Stimuli and Task
Participants read sentences for comprehension during scanning and completed a recognition test after scanning. They were informed about the recognition test before scanning to make sure they would read the sentences attentively. In the congruent condition, the terminal noun of a sentence matched the semantic specification of the preceding context (e.g., Die Mädchen spielen mit ihren Puppen [The girls played with their dolls]). In the incongruent condition, the terminal noun violated the semantic specification and could not be integrated into the sentential context (e.g., Nachts jagen Katzen nach Brezeln [At night cats chase pretzels]). We created 60 pairs of sentences (the same sentence stem with two different endings) and split them into two lists such that the congruent and the incongruent versions would not appear in the same list. Each participant read only one list comprising 30 sentences per condition. Terminal words of the congruent and incongruent conditions were matched in lexical frequency [Baayen et al., 1995], length (number of letters: congruent 5.78 vs. incongruent 6.07, t < 1), and concreteness [Hager and Hasselhorn, 1994]. Each list was used for ten participants. Sixty filler sentences with congruent terminal words were added to each list. In other words, 25% of sentences had semantically incongruent endings and 75% had semantically congruent endings. We employed a slow event‐related design in which trials had a fixed length of 20 s. In each trial, the stem of a sentence (all words expect the terminal word) was displayed for 2 s. After a variable interval of 1–2 s, the terminal word was displayed for 0.5 s. A fixation cross stayed on the screen during the rest of the trial. There were two sessions, each lasting 20 min.
The recognition task was conducted after the scanning session to assess whether participants had read the sentences attentively. Each participant read 32 old sentences, all from the list he/she had seen during the experiment proper. One half of these sentences had been presented in the first run; the other half had been presented in the second run. One half of them had congruent endings, while the other half had incongruent endings. Participants were asked to judge whether a particular sentence had occurred in the first or the second run.
Data Acquisition
Data were collected in a neuro‐optimized 1.5‐T GE Signa Horizon LX scanner with a standard quadrature head coil in two sessions. Functional images were acquired using a T2*‐weighted echo planar imaging (EPI) sequence, with 2,000 ms time repetition, 35‐ms time echo, and 80° flip angle. Each functional image consisted of 23 transversal slices, with 64 × 64 matrix, 200 mm × 200 mm field of view (FOV), 5‐mm thickness, 1‐mm slice gap, and 3.125 mm × 3.125 mm in‐plane resolution. Structural images were acquired using a T1‐weighted 3D SPGR sequence. Each structural image consisted of 124 contiguous slices, with 256 × 256 matrix, 200 mm × 200 mm FOV, and 1.5‐mm thickness.
Data Analysis
Data were preprocessed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The first four volumes were discarded owing to longitudinal magnetization equilibration effects. Functional images were first time‐shifted with reference to the middle slice in time to correct differences in slice acquisition time. They were then realigned with a least squares approach and a rigid body spatial transformation to remove movement artifacts. Realigned images were normalized to the EPI‐derived MNI template (ICBM 152, Montreal Neurological Institute) and re‐sampled to 3 × 3 × 3 mm3 voxel. Normalized images were smoothed with a Gaussian kernel of 8 mm full‐width half‐maximum and filtered with a high‐pass filter of 128 s.
Univariate analysis
The univariate analysis was performed to examine brain regions differentially activated in the processing of incongruent vs. congruent words. This analysis was implemented with SPM8 on the basis of a general linear model (GLM) by convolving the design matrix with the canonical hemodynamic response function. Four types of events were specified in the design matrix and time‐locked to their onsets: the congruent terminal word, the incongruent terminal word, the congruent terminal words of the filler sentences and the sentence stem. As mentioned in the section of stimuli and task, the filler sentences were used to manipulate the frequency of incongruent sentences. The terminal words of the fillers were separately defined in the design matrix to ensure that the congruent and the incongruent conditions had the same numbers of trials. Estimated movement parameters (six parameters per image: x, y, z, pitch, roll, and yaw) were included as nuisance regressors of no interest to minimize signal‐corrected motion effects. Classical parameter estimation was applied with a one‐lag autoregressive model to whiten temporal noise in the fMRI time‐courses of each participant to reduce the number of false‐positive voxels. Contrast maps were calculated for incongruent > congruent words for each participant and entered into a one‐sample t test at group level (random effect). The resulting map was considered at P < 0.05 (corrected for false discovery rate, FDR‐corrected).
Independent component analysis
The ICA was performed to examine functional networks dynamically modulated in the processing of incongruent vs. congruent words. This analysis was implemented with the GIFT toolbox (http://mialab.mrn.org/software) using the infomax algorithm [Bell and Sejnowski, 1995]. The fMRI data were split into a set of spatially independent functional networks (i.e., components). Each component was presented as a spatial map along with an associated time‐course. The optimal number of independent components was estimated by using a modified minimum description length algorithm [Li et al., 2007] and found to be 19. To avoid the problem of matching components across participants, the ICA was performed on all participants at once (group ICA). It has been shown that the group ICA does not significantly detract or alter the result in comparison to an ICA performed on each participant separately [Calhoun et al., 2007, 2008, 2009]. On the other hand, the group ICA computes components that are comparable across participants (e.g., Component 1 of Subject 1 is the same as Component 1 of Subject 2). The label of a component has no particular meaning.
Spatial sorting was applied to identify components reflecting head motion, physiological noise, eyeball movement, or other signal artifacts. The spatial sorting was implemented by correlating the spatial map of each component with prior probabilistic maps of gray matter, white matter, and cerebrospinal fluid (MNI templates provided in SPM). If the spatial correlation for white matter was greater than R 2 = 0.02 or greater than R 2 = 0.05 for cerebral spinal fluid, the component was considered to represent artifacts and therefore should be discarded [Kim et al., 2009a, 2007]. The component was also excluded if the spatial correlation for gray matter was smaller than that for white matter or cerebrospinal fluid. Among all 19 components, 11 were identified as artifacts and excluded from further analysis.
Temporal sorting was then applied to the remaining eight components to identify the component of which the modulation was larger for incongruent vs. congruent words. The temporal sorting was implemented by regressing the time‐course of each component with the design matrix, giving rise to one beta weight for each component in each condition. The beta weight indicates the degree to which a particular component was modulated by a particular experimental condition. Higher beta weights suggest larger condition‐specific modulation. The beta weights were tested with permutation (5,000 times, P < 0.05) that is conducted by computing all possible t values (reference distribution) and the possibility of obtaining the real t value from the reference distribution [Blair and Karniski, 1993]. For the selected component, the spatial map was computed at group level (P < 0.05 corrected) and the event‐related average of the associated time‐course was calculated. The spatial map reflects the contribution of a particular region to the associated time‐course. Higher t values suggest greater contributions. The univariate and ICA results were visualized with MRIcron (http://www.cabiatl.com/mricro).
Graph theoretical analysis
The GTA was performed with the Brain Connectivity Toolbox [Rubinov and Sporns, 2010] to examine whether and how the topological organization of the whole‐brain network was changed for incongruent vs. congruent words. The whole‐brain network was constructed to represent functional connectivity between regions with the “beta series correlation” method [Rissman et al., 2004] for two reasons. First, methods in which correlation or coherence of the fMRI time‐courses is computed to measure functional connectivity are based on the assumption that the time‐courses are stationary (i.e., probabilistically unchanging across time). Those methods, when applied to the event‐related design in which the time‐courses are not stationary, will lead to the overestimation of true connectivity. For slow event‐related designs, the “beta series correlation” approach has been proposed as a solution [Bullmore and Bassett, 2011; Zhou et al., 2009]. Second, the “beta series correlation” approach gives rise to stage‐specific functional connectivity (terminal word vs. sentence stem), allowing us to study the brain network underlying a particular process in a complex task of multiple stages. The “beta series correlation” method was implemented on the basis of a GLM by using separate covariates to modal hemodynamic responses of every single event, resulting in a set of stage‐ and condition‐specific beta series.
Each cerebral hemisphere was segmented into 45 regions (number of regions, N = 90) according to the Automated Anatomical Labeling (AAL) tool [Tzourio‐Mazoyer et al., 2002]. Beta series were averaged within each region. Correlation coefficients were computed for every possible pair of regional beta series to generate a N‐by‐N matrix C, where C(i, j) represented the functional connectivity between regions i and j. The connectivity matrix was constructed for congruent and incongruent words separately. A representative adjacency matrix can be derived from the connectivity matrix by preserving 15% of the strongest correlations. This threshold was selected to ensure that (a) the resulting matrix of each participant under each condition passed a FDR‐corrected P < 0.05; (b) the density of the resulting matrix (i.e., the fraction of present connections to possible connections) was comparable among participants. The adjacency matrix can further be represented as an undirected weighted graph G, in which a node indicates a region, an edge drawn between two nodes indicates a connection between two regions, and the edge weight indicates the strength of the interregional connection.
At the global level, we measured the functional integration (global efficiency) and functional segregation (clustering coefficient and modularity) of the whole‐brain network [for mathematical definitions, see Rubinov and Sporns, 2010]. The functional integration refers to the ability of rapidly combining the specialized information from distributed brain areas. The global efficiency is defined as the average inverse number of edges in the shortest path between two nodes. The functional segregation refers to the ability of processing the specialized information within densely interconnected groups of brain areas. The clustering coefficient is defined as the proportion of node i's neighbors (i.e., immediately connected nodes) that are also neighbors of one another. The modularity measure quantifies the degree to which the network can be subdivided into non‐overlapping groups of nodes (i.e., modules) in a way that maximizes the number of within‐group edges and minimizes the number of between‐group edges.
As defined by Watts and Strogatz [2009a], small‐world networks have a large clustering coefficient C(G) but a small average shortest path length L(G), as compared to random networks. The small‐world index σ(G), by its definition, reflects the balance between functional integration and segregation.
where C(R) is the clustering coefficient of a comparable random graph and L(R) is the average shortest path length of the random graph. If the small‐world index is larger than 1, the corresponding network is generally accepted as a small‐world network [Humphries et al., 2006]. To estimate the small‐world index, 1,000 random graphs with the same number of nodes, same number of edges, and same degree of distribution were sampled.
At the regional level, we focused on the left supplementary motor area (SMA) and left IFG (BA 45), which were consistently observed in the univariate analysis and ICA (see Results). Two regional parameters were measured: strength, which is defined as the sum of edge weights of a given node, and degree, which is defined as the number of edges of that node. Permutation tests (1,000 times, P < 0.05) were used due to the lack of knowledge concerning the distribution of network parameters [Bullmore and Sporns, 2009]. To avoid multiple testing problems, between‐condition differences were considered at P < 0.025 for regional measures (i.e., adjusted for two regions).
Finally, to visualize the whole‐brain network underlying the semantic integration, we plotted out the interregional connections that were observed in the adjacency matrix of at least 75% participants [van den Heuvel and Sporns, 2011]. For a better presentation of the topological organization, hubs (i.e., relative important regions) were additionally determined as regions that showed degrees one standard deviation larger than the mean of all regions [Sporns et al., 2007]. The GTA result was visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
RESULTS
Recognition Test
Recognition accuracy was 71% (SE = 3%) for sentences with semantically congruent endings and 72% (2%) for sentences with incongruent endings (paired‐sample t test, two‐tailed: t < 1) indicating that participants did indeed pay attention to the sentences, and did so for both sentence types.
Univariate and ICA Results
The univariate analysis and the ICA revealed the activity pattern and the connectivity pattern, respectively. A particular region may exhibit changes in activity, connectivity or both. Figure 1A and Table 1 show brain regions more activated for incongruent vs. congruent words, including the SMA and bilateral IFG. Figure 1B and Table 2 show the spatial map and the event‐related average of Component 12 (determined by the ICA), which was the only component sensitive to the incongruity of sentence endings. This component revealed a functional network comprising the left caudate nucleus, medial superior frontal gyrus/SMA, bilateral IFG, left middle temporal gyrus, and left angular gyrus, displaying a stronger positive modulation for incongruent vs. congruent words. In other words, regions such as the SMA and bilateral IFG show increased activations as well as enhanced connections with other relevant regions, of which only the connectivity was significantly changed in response to incongruent sentence endings (e.g., left caudate nucleus). The results for the filler sentences are presented as Supporting Information. Visual inspection suggested that the remaining non‐artifact components represented the typical resting‐state networks such as the default‐mode network, sensorimotor network, medial visual cortical areas, lateral visual cortical areas, dorsal visual stream, and auditory system [Beckmann et al., 2005], as well as networks featuring the medial temporal lobe and cerebellum. As they were not modulated by the experimental manipulation, these components will not be considered further.
Figure 1.
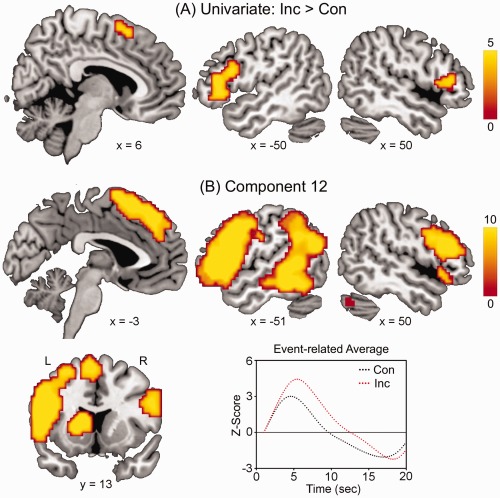
A: The SMA and bilateral IFG showed greater activations for incongruent vs. congruent words (Inc > Con, P < 0.05 corrected). Color scale indicates t values. Coordinates are in MNI. B: The functional network of the left basal ganglia, medial prefrontal cortex, IFG, middle temporal gyrus, and angular gyrus (Component 12) was more activated for incongruent vs. congruent words (P < 0.05). The corresponding event‐related average was more positive for incongruent vs. congruent words. L, left; R, right.
Table 1.
Brain regions more activated for incongruent vs. congruent words
| Region | BA | H | x | y | z | t | Size |
|---|---|---|---|---|---|---|---|
| Supplementary motor area | 6 | L/R | 6 | 16 | 58 | 5.01 | 41 |
| Inferior frontal gyrus | 45/47 | L | −44 | 26 | −2 | 5.93 | 290 |
| R | 56 | 19 | 1 | 4.70 | 57 |
BA, Brodmann area; H, hemisphere; coordinates in MNI; t, statistic values; L, left; R, right; Size, number of voxels; P < 0.05 corrected.
Table 2.
Regions of component 12
| Region | BA | H | x | y | z | t | Size |
|---|---|---|---|---|---|---|---|
| Medial superior frontal gyrus/ Supplementary motor area | 6/8/9 | L/R | −6 | 44 | 46 | 14.04 | 430 |
| Inferior frontal gyrus | 44/45 | L | −47 | 13 | 31 | 18.50 | 973 |
| R | 47 | 26 | 19 | 14.35 | 518 | ||
| Middle temporal gyrus | 21 | L | −53 | −37 | −2 | 18.50 | 630 |
| R | 56 | −31 | −8 | 8.25 | 27 | ||
| Angular gyrus | 39 | L | −47 | −68 | 25 | 12.17 | 243 |
| Caudate nucleus/ putamen | L | −12 | 7 | 16 | 15.57 | 161 | |
| Cerebellum | R | 35 | −68 | −38 | 13.61 | 567 |
BA, Brodmann area; H, hemisphere; coordinates in MNI; t, statistic values; L, left; R, right; Size, number of voxels; P < 0.05 corrected.
Graph Theoretical Results
Figure 2 shows the whole‐brain network underlying the processing of congruent or incongruent words. Each node represents an AAL region, with the light blue node indicating the relative important regions (i.e., hubs) and the dark blue node indicating non‐hubs. Each edge represents an interregional connection obtained in the thresholded connectivity matrix (i.e. the adjacency matrix) of at least 75% participants. Visual inspection suggested that there were more anterior hubs in the incongruent vs. congruent condition (11% vs. 0% hubs located at y > 0).
Figure 2.
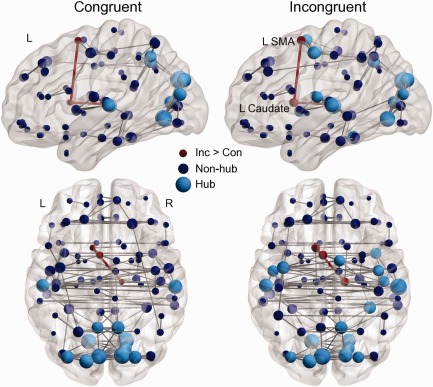
The whole‐brain networks supporting the processing of congruent or incongruent words. Each node represents an AAL region. Light blue nodes indicate hub regions. Dark blue nodes indicate non‐hub regions. Each gray edge represents an interregional connection observed in 75% participants. Red links indicate interregional connections stronger for incongruent vs. congruent words (Inc > Con, P < 0.05 corrected). L, left; R, right; SMA, supplementary motor area.
Table 3 lists the mean and standard deviation of the global and regional parameters in each condition. The incongruent condition was topologically similar to the congruent condition at the global level. No difference was observed between conditions regarding functional integration (global efficiency: t < 1, permutation 5,000 times), functional segregation (clustering coefficient: t < 1; modularity: t < 1), or integration–segregation balance (small‐world index: t < 1). In both conditions, the whole‐brain networks could be accepted as small‐world networks as their small‐world indexes were larger than one.
Table 3.
Means (and standard deviations) of global and regional topological parameters
| Parameter | Congruent | Incongruent |
|---|---|---|
| Global | ||
| Global efficiency | 0.35 (0.03) | 0.35 (0.03) |
| Clustering coefficient | 0.42 (0.05) | 0.42 (0.05) |
| Modularity | 0.35 (0.05) | 0.35 (0.05) |
| Small‐world index | 1.58 (0.28) | 1.59 (0.25) |
| L Supplementary motor area | ||
| Degreea | 15.40 (8.49) | 21.60 (8.18) |
| Strengtha | 12.06 (7.25) | 16.60 (6.99) |
P < 0.05 corrected.
However, the whole‐brain network was dynamically modulated by the incongruity of the terminal words at the regional level. The left SMA showed an increased degree (t = 3.10, P < 0.01, permutation 5,000 times) and an increased strength (t = 2.66, P < 0.02) in the incongruent vs. congruent conditions, although the left IFG (BA45) did not. In other words, the left SMA was more strongly connected with other brain areas for incongruent vs. congruent words. The strengthened SMA connectivity appeared to be largely due to a condition‐related change of left SMA‐left caudate connectivity. This hypothesis was supported by the finding that the connection between the left SMA and left caudate nucleus was increased for incongruent vs. congruent words (t = 2.08, P < 0.05, congruent = 0.15, incongruent = 0.34). We further tested the caudate‐thalamus connectivity and observed an increased connection between the left caudate nucleus and the right thalamus (t = 3.22, P < 0.01, congruent = 0.32, incongruent = 0.64). The left thalamus showed a similar pattern (t = 2.27, P < 0.05, congruent = 0.40, incongruent = 0.62) although it failed to pass the adjusted threshold of P < 0.025.
DISCUSSION
In this study, we investigated the brain network of semantic integration in sentence reading with two distinct approaches over three different levels. First, the GTA was applied to measure the functional integration (global efficiency), functional segregation (clustering coefficient and modularity), and integration–segregation balance (small‐world index) of the whole‐brain network that represented the functional connections of all possible pairs of brain areas. The whole‐brain network was topologically stable across conditions, although more anterior hubs (i.e., relative important regions) were observed in the incongruent condition. Second, the ICA detected the same group of functionally connected brain areas across conditions, including the SMA, the left IFG, the left middle temporal gyrus, the left angular gyrus, and the left caudate nucleus (represented by Component 12 of the ICA). Those regions simultaneously contributed to the fMRI time‐courses, showing larger activity for incongruent than congruent terminal words. The network stability observed at the global and component/module levels supports the fundamental assumption underlying the use of the violation paradigm, i.e., that the same cognitive operations and networks (not language‐specific) are engaged by incongruent and congruent stimuli. Moreover, the GTA specified the connectivity changes at the regional level, revealing an increased SMA connectivity in general and a strengthened SMA‐caudate‐thalamus pathway.
The GTA and ICA consistently emphasized the importance of the frontal‐striatal circuits in sentence‐level semantic integration. This observation is in line with animal work showing that the caudate nucleus and putamen receive cortical projections and project back to cortical regions via the thalamus, forming parallel [Middleton and Strick, 2002] and integrative circuits in support of motor, cognitive, and emotional processes [Bar‐Gad and Bergman, 2001]. The medial prefrontal cortex has been extensively explored in cognitive control and behavioral adaptation and is assumed to monitor and adjust cognitive processes in sensory, memory, and motor systems [Botvinick et al., 2001; Dosenbach et al., 2007]. The lateral prefrontal cortex, on the other hand, has been associated with a diversity of functions. For example, Hagoort [2006] in his memory‐unification‐control (MUC) model proposed the left IFG to be an active workspace unifying lexical information retrieved from memory in parallel at the semantic, syntactic, and phonological levels [Baggio and Hagoort, 2011]. In their model of semantic processing, Lau et al. [2007] proposed that the anterior IFG is responsible for controlled retrieval of lexical information and the posterior IFG for selection of lexical representations. In a more general sense, those proposals converge in that the prefrontal cortex is associated with executive aspects of semantic processing. As Friederici [2002] and Martin [2002] pointed out, the frontal areas are recruited when strategic and executive manipulations come into play in semantic processes. Such a hypothesis is in line with studies on the involvement of executive control functions in sentence reading [Kuperberg, 2007; Novick et al., 2005; Thompson‐Schill et al., 2005; Ye and Zhou, 2009a], word production [Badre et al., 2005; Badre and Wagner, 2007; Thompson‐Schill et al., 1997], and bilingual language processing [Abutalebi and Green, 2007; Rodriguez‐Fornells et al., 2006; for a review, see Ye and Zhou, 2009b].
In the ICA, the left middle temporal gyrus and left angular gyrus were also revealed as parts of the critical network. However, neither of them showed increased activation in the univariate analysis or changed interregional connections in response to incongruent vs. congruent endings in the GTA (even with a lowered threshold of P < 0.05 uncorrected). Although different in details, recent models of language processing commonly attribute pure semantic processes to the superior/middle temporal cortex. For example, Friederici [2002] considered the middle temporal gyrus as the primary neural basis of the integration of semantic information. In their recent review, Kutas and Federmeier [1980] suggested that the superior/middle temporal gyrus, together with the medial temporal lobe and temporal‐parietal junction subserve semantic memory processes. Lau et al. [2007] distinguished between the posterior portion, which they linked to lexical storage and access, and the anterior portion, which they associated with the integration of lexical information into larger units. The role of the angular gyrus (or temporal‐parietal junction) in semantic processing is less well characterized as that of the frontal and temporal areas. Lau et al. [2007] suggested that this region accompanies the anterior temporal lobe in playing a part in integrating semantic information into context. This integration function may not be constrained to linguistic inputs, as the angular gyrus has also been observed to respond to the mismatch between pictures (e.g., picture of cat) and environmental sounds (e.g., sound “humming of a bee” instead of “meow”) [Noppeney et al., 2008]. The absence of the left middle temporal gyrus and left angular gyrus in the univariate analysis and the GTA suggested that these regions might be recruited to retrieve the meaning of the incoming word and to integrate the meaning into sentential context but may not be sensitive to the overall wellformedness of sentence, which is likely monitored by the prefrontal cortex.
Mapping large‐scale networks woven of structural and functional brain connections has become a central task in neuroscience. Different network modeling approaches provide profiles of complex brain networks from different perspectives. The ICA allows us to extract regions whose time‐courses are highly correlated, whereas the GTA enables to infer the function of a particular region on the basis of its role in brain architecture [e.g. Sporns et al., 2007]. Because of the different types of information revealed by the different methods, it seems promising to combine different modeling methods rather than to rely on one single approach. The GTA is still at its initial stage of development with regard to brain imaging and therefore needs to be refined in future neuroimaging studies. One of the essential issues is the parcellation problem. Anatomical atlases such as the AAL are often used to segment the whole brain into regions and regional time‐courses are then averaged across all voxels within these regions to generate a representative time‐courses. These data are subsequently used to construct a matrix of connections between the identified cortical and subcortical structures. Due to the lack of detailed subdivision of large regions such as the left IFG and the left middle temporal gyrus, the AAL might lead to the underestimation of interregional interaction and/or connectivity changes. The parcellation approach of the GTA, at the current stage, is not as sensitive as the region‐of‐interest approach (e.g., defining small spheres of high homogeneity). It might explain why the enhanced connectivity between the posterior portions of the left IFG and left middle temporal gyrus, which has been reported in a previous study [Snijders et al., 2010], was not observed in this study.
In conclusion, we delineated functional networks subserving sentence‐level semantic integration with two distinct approaches over three different levels. The GTA suggested that the whole‐brain network subserving the integration of incongruent sentence endings was topologically similar to that mediating the processing of congruent endings, supporting the fundamental assumption underlying the use of the violation paradigm. The ICA revealed a group of regions, of which the overall activity was dynamically modulated for incongruent vs. congruent endings, including the medial and lateral prefrontal cortex, left middle temporal gyrus, left angular gyrus, and left caudate nucleus. Moreover, the GTA specified the connectivity changes at the regional level, demonstrating a strengthened prefrontal‐striatal‐thalamic loop in response to the incongruity of sentence ending. Further studies should consider using different approaches of network analysis to better characterize the interregional interactions under cognitive tasks.
Supporting information
Supporting Information
REFERENCES
- Abutalebi J, Green D (2007): Bilingual language production: The neurocognition of language representation and control. J Neurolinguist 20:242–275 [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers R (1995):The CELEX Lexical Database [CD‐ROM].Philadelphia:University of Pennsylvania, Linguistic Data Consortium. [Google Scholar]
- Badre D, Wagner AD (2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45:2883–2901 [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré‐Blagoev EJ, Insler RZ, Wagner AD (2005): Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47:907–918. [DOI] [PubMed] [Google Scholar]
- Baggio G, Hagoort P (2011): The balance between memory and unification in semantics: A dynamic account of the N400. Lang Cogn Process 26:1338–1367. [Google Scholar]
- Bar‐Gad I, Bergman H (2001): Stepping out of the box: Information processing in the neural networks of the basal ganglia. Curr Opin Neurobiol 11:689–695. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Meyer‐Lindenberg A, Achard S, Duke T, Bullmore ET (2006): Adaptive reconfiguration of fractal small‐world human brain functional networks. Proc Natl Acad Sci USA 103:19518–19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Verchinski BA, Mattay VS, Weinberger DR, Meyer‐Lindenberg A (2008): Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgaertner A, Weiller C, Büchel C (2002): Event‐related fMRI reveals cortical sites involved in contextual sentence integration. NeuroImage 16:745. [DOI] [PubMed] [Google Scholar]
- Beckmann C, DeLuca M, Devlin JT, Smith SM (2005): Investigations ino resting‐state connectivity using independent component analysis. Phil Trans R Soc B 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comp 7:1159. [DOI] [PubMed] [Google Scholar]
- Blair RC, Karniski W (1993): An alternative method for significance testing of waveform difference potentials. Psychophysiology 30:518–524. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001): Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS (2011): Brain graphs: Graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA (2007): Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp 29:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD (2008): Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 29:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T (2009): A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage 45 (1 Suppl):S163–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo ER, Aydelott J, Matthews PM, Devlin JT (2004): Left inferior prefrontal cortex activity reflects inhibitory rather than facilitatory priming. J Cogn Neurosci 16:1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2002): Towards a neural basis of auditory sentence processing. Trends Cogn Sci 6:78–84. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rüschemeyer S‐A, Hahne A, Fiebach CJ (2003): The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cereb Cortex 13:170–177. [DOI] [PubMed] [Google Scholar]
- Hager W, Hasselhorn M (1994):Handbuch deutschsprachiger Wortnormen.Göttingen:Hogrefe Verlag. [Google Scholar]
- Hagoort P (2005): On Broca, brain, and binding: A new framework. Trends Cogn Sci 9:416–423. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Gurney K, Prescott TJ (2006): The brain stem reticular formation is a small‐world, not scale‐free, network. Proc R Soc B 273:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA (2004): Imagery in sentence comprehension: An fMRI study. NeuroImage 21:112–124. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF (2002): Reading anaomalous sentences: An event‐related fMRI study of semantic processing. NeuroImage 17:842–850. [PubMed] [Google Scholar]
- Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, Belger A, Gollub R, Lauriello J, Wible C, O'Leary D, Lim K, Toga A, Potkin SG, Girn F, Calhoun VD (2009a): Auditory oddball deficits in schizophrenia: An independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull 35:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub R, White T, Wible C, Belger A, Bockholt HJ, Clark VP, Lauriello J, O'Leary D, Mueller BA, Lim KO, Andreasen N, Potkin SG, Calhoun VD (2009b): Dysregulation of working memory and default‐mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp 30:3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR (2007): Neural mechanisms of language comprehension: Challenges to syntax. Brain Res 1146:23–49. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe‐Hesketh S, Wright IC, Lythgoe DJ, Williams SCR, David AS (2000): Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: An fMRI study. J Cogn Neurosci 12:321–341. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM (2008): Neuroanatomical distinctions within the semantic system during sentence comprehension: Evidence from functional magnetic resonance imaging. NeuroImage 40:367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD (2000): Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci 4:463–470. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD (2011): Thirty years and counting: Finding meaning in the N400 component of the event‐related brain potential (ERP). Annu Rev Psychol 62:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA (1980): Reading senseless sentences: Brain potentials reflect semantic incongruity. Science 207:203–205. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D (2008): A cortical network for semantics: (de)Constructing the N400. Nat Rev Neurosci 9:920–933. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD (2007): Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T (2008): Disrupted small‐world networks in schizophrenia. Brain 131:945–961. [DOI] [PubMed] [Google Scholar]
- Martin RC (2003): Language processing: Functional organization and neuroanatomical basis. Annu Rev Psychol 54:55–89. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2002): Basal‐ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex 12:926–935. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT (2001): An event‐related fMRI study of syntactic and semantic violations. J Psycholinguist Res 30:339–364. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D (2000): An event‐related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci 12:120–133. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Hocking J, Price CJ, Friston KJ (2008): The effect of prior visual information on recognition of speech and sounds. Cereb Cortex 18:598–609. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson‐Schill SL (2005): Executive control and parsing: Reexaming the role of Broca's area in sentence comprehension. Cogn Affect Behav Neurosci 5:263–281. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M (2004): Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage 23:752–763. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Fornells A, De Diego Balaguer R, Münte TF (2006): Executive control in bilingual language processing. Lang Learn 56 (Suppl 1):133–190. [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Snijders TM, Petersson KM, Hagoort P, (2010): Effective connectivity of cortical and subcortical regions during unification of sentence structure. Neuroimage 52:1633–1644. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kötter R (2007)Identification and classification of hubs in brain networks. PLoS One 2:e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Hilgetag CC, Burns GA, O'Neill MA, Young MP, Kötter R (2000): Computational analysis of functional connectivity between areas of primate cerebral cortex. Philos Trans R Soc Lond B Biol Sci 355:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94:14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Bedny M, Goldberg RF (2005): The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 15:219–224. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- van de Ven V, Esposito F, Christoffels IK (2009): Neural network of speech monitoring overlaps with overt speech production and comprehension networks: A sequential spatial and temporal ICA study. NeuroImage 47:1982–1991. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2011): Rich‐club organization of the human connectome. J Neurosci 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ (2006): Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang 97:279–293. [DOI] [PubMed] [Google Scholar]
- Wahl M, Marzinzik F, Friederici AD, Hahne A, Kupsch A, Schneider G‐H, Saddy D, Curio G, Klostermann F (2008): The human thalamus processes syntactic and semantic language violations. Neuron 59:707. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH (1998): Collective dynamics of ‘small‐world’ networks. Nature 393:440442. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhou X (2009a): Conflict control during sentence comprehension: fMRI evidence. NeuroImage 48:280–290. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhou X (2009b): Executive control in language processing. Neurosci Biobehav Rev 33:1168–1177. [DOI] [PubMed] [Google Scholar]
- Young MP (1992): Objective analysis of the topological organization of the primate cortical visual system. Nature 358:152–155. [DOI] [PubMed] [Google Scholar]
- Zhou D, Thompson WK, Siegle G (2009): MATLAB toolbox for functional connectivity. NeuroImage 47:1590–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhang JX, Wang X, Xiao Z, Huang H, Chen H‐C (2009): Involvement of left IFG in sentence‐level semantic integration. NeuroImage 47:756–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


