Abstract
Chronic insomnia is one of the most prevalent central nervous system diseases, however, its neurobiology is poorly understood. Up to now, nothing is known about the integrity of white matter tracts in insomnia patients. In this study, diffusion tensor imaging (DTI) was used in a well‐characterized sample of primary insomnia (PI) patients and good sleeper controls to fill this void. Voxelwise between‐group comparisons of fractional anisotropy (FA) were performed in 24 PI patients (10 males; 14 females; 42.7 ± 14.5 years) and 35 healthy good sleepers (15 males; 20 females; 40.1 ± 9.1 years) with age and sex as covariates. PI patients showed reduced FA values within the right anterior internal capsule and a trend for reduced FA values in the left anterior internal capsule. The results suggest that insomnia is associated with a reduced integrity of white matter tracts in the anterior internal capsule indicating that disturbed fronto‐subcortical connectivity may be a cause or consequence of the disorder. Hum Brain Mapp 35:3431–3438, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: primary insomnia, sleep, diffusion tensor imaging, structural connectivity, MRI
INTRODUCTION
Chronic insomnia is one of the most prevalent central nervous system diseases [Morin and Benca, 2012]. While often viewed as a nuisance symptom in clinical practice, it is associated with a severely reduced quality of life [Kyle et al., 2010b], cognitive impairments [Fortier‐Brochu et al., 2012], physical complaints [Buysse et al., 2007] and poor social functioning [Kyle et al., 2010a]. Furthermore, chronic insomnia confers a substantially increased risk for psychiatric disorders, especially depression [Baglioni et al., 2011; Riemann and Voderholzer, 2003], and cardiovascular morbidity and mortality [Sofi et al., in press]. Accordingly, insomnia is associated with a huge increase in health care consumption, work disability and absenteeism, and, consequently, with very high costs for our societies [Shahly et al., 2012]. For the US, the costs of insomnia due to low work performance and absenteeism have been estimated to exceed 60 billion $ per year [Kessler et al., 2011]. Thus, insomnia significantly contributes to the major diseases of our aging society and to a significant part of the health expenses. Despite the huge socio‐economic impact of chronic insomnia, its neurobiological causes and consequences are poorly understood. Structural neuroimaging studies reported inconsistent results. While a number of cross‐sectional studies suggested that hippocampus volumes may be reduced in insomnia patients [Neylan et al., 2010; Riemann et al., 2007; Taki et al., 2012], other investigations did not find this effect [Altena et al., 2010; Joo et al., 2013; Noh et al., 2012; Spiegelhalder et al., 2013; Winkelman et al., 2010]. Similarly, frontal lobe morphometry may be altered in insomnia according to two investigations [Altena et al., 2010; Joo et al., 2013] but was not found to be different from normal in another one [Spiegelhalder et al., 2013]. In contrast to this body of research, nothing is known about structural connectivity in insomnia although a reduction in white matter integrity is generally assumed to be involved in the pathophyiology of psychiatric disorders [Kanaan et al., 2005; Sexton et al., 2009]. Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique that allows the investigation of white matter integrity. Fractional anisotropy (FA), the most commonly used parameter in DTI studies, indicates the degree of directionality of water diffusion in the tissue and has a range of zero (complete isotropic diffusion, i.e. equal diffusion in all directions) to one (complete anisotropic diffusion, i.e. diffusion in only one direction). Of particular importance for the current study, decreased FA values can be attributable to decreased fiber coherence which may result from a degenerative process. The aim of the current study was to analyze the integrity of white matter tracts in insomnia patients in comparison with good sleeper controls. It was hypothesized that insomnia is associated with abnormal FA levels.
MATERIALS AND METHODS
Participants
Twenty‐eight patients meeting diagnostic criteria for primary insomnia (PI) according to DSM‐IV‐TR (American Psychiatric Association, 2000) and 40 good sleeper controls were included in the present study. Structural brain imaging results of this sample obtained by analyzing T1‐weighted MRI data have been published elsewhere [Spiegelhalder et al., 2013]. Insomnia patients were referred to our sleep disorders clinic by their primary care provider or medical specialist. Healthy controls were recruited through local advertisements. Two control participants were excluded from the analysis because of pathological MRI scans; 4 PI patients as well as 3 further control participants were excluded due to missing data resulting from technical problems during the DTI acquisition. Thus, the final sample consisted of 24 PI patients and 35 good sleeper controls.
A semi‐standardized psychiatric and sleep‐related interview was conducted by an experienced psychiatrist to rule out any lifetime history of psychiatric disorder, shift work or sleep disorder (including hypersomnia, parasomnia, sleep‐related breathing disorder, sleep‐related movement disorder, and circadian rhythm sleep disorder). Furthermore, all participants underwent a standard physical examination, including electrocardiogram, electroencephalogram (EEG), and routine blood work (blood cell count, liver, renal, and thyroid function) to exclude those with serious medical conditions. All participants were right‐handed, as assessed with the Edinburgh Handedness Inventory [Oldfield, 1971] and free of any psychoactive medication at least 2 weeks prior to and during the study. Participants with a periodic leg movements during sleep arousal index per total sleep time of more than 5.0/h or a sleep apnea index per total sleep time of more than 5.0/h were not included in this study.
All study participants were asked to complete the Insomnia Severity Index [ISI; Bastien et al., 2001], the Pittsburgh Sleep Quality Index [PSQI; Buysse et al., 1989], the brief version of the Dysfunctional Beliefs and Attitudes about Sleep Scale [DBAS‐16; Morin et al., 2007], the Glasgow Sleep Effort Scale [GSES; Broomfield and Espie, 2005], the Pre‐Sleep Arousal Scale [PSAS; Nicassio et al., 1985], the Epworth Sleepiness Scale [ESS; Johns, 1991], the Beck Depression Inventory [BDI; Beck and Steer, 1987], and the State‐Trait Anxiety Inventory [STAI; Spielberger et al., 1983].
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the University Medical Center Freiburg. All participants gave their informed written consent prior to inclusion in the study.
Polysomnography
As outlined in detail in Spiegelhalder et al. [2013], all participants underwent two consecutive nights of PSG sleep monitoring. Sleep was recorded for 8 h from “lights out” (10–11 pm) until “lights on” (6–7 am) and was scored visually by experienced raters according to standard criteria (American Academy of Sleep Medicine, 2007). All participants had to refrain from alcohol, caffeine, and daytime naps during the recording days.
MRI Acquisition and Analysis
T1‐weighted MRI and DTI data were acquired on a 3 Tesla MRI scanner (Magnetom TIM‐Trio, Siemens, Erlangen, Germany). T1 data were acquired using an MPRAGE sequence (TR 2.2 s; TE 2.6 ms; 160 sagittal slices of 256 × 256 voxels, 1.0 × 1.0 × 1.0 mm3; Mugler, 3rd and Brookeman, 1990). All scans were inspected for the absence of pathological findings by a neurologist under the supervision of a board‐certified neuroradiologist.
The diffusion‐weighted data were acquired using a diffusion‐weighted single‐shot spin‐echo echo‐planar sequence along 31 noncollinear directions (TR 9.9 s; TE 101 ms; 16 axial slices of 128 × 128 voxels, 2.0 × 2.0 × 2.0 mm3; effective b‐value of 1,000 mm2/s; motion and distortion correction by scanner software, Zaitsev et al., 2004). An additional measurement without diffusion weighting (b = 0 mm²/s) was performed to allow calculation of the apparent diffusion coefficient values. Head motion was restrained by using cushions and proper instructions.
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) was used for the preprocessing of the data and the generation of FA maps. Preprocessing included segmentation, coregistration with the anatomical image, normalization and smoothing (FWHM, 8 mm). The voxel‐based analyses of the FA maps were conducted using the AFNI software [Cox, 1996]. These analyses were restricted to white matter voxels. The corresponding group‐common white matter mask included 56,181 voxels. It was generated by including all non‐zero voxels after multiplying the normalized individual white matter masks as obtained by the SPM8 segmentation algorithm.
A Monte‐Carlo simulation using the AFNI program “3dClustSim” was performed to estimate the probability of false positive clusters. According to this, overall statistical significance of P < 0.05 was obtained for whole‐brain analysis by considering cluster sizes of at least 736 mm³ (92 voxels) at a voxel threshold of P < 0.005 (T > 2.93). MRIcron (http://www.mricro.com/mricron/install.html) was used for three‐dimensional visualization of the results on a ch2better.nii.gz atlas.
For each participant, mean FA values were calculated for those areas that were found to have significantly decreased FA values in insomnia patients. Using multiple linear regression analyses with age and sex as covariates, the relationship between these indicators of white matter integrity and the following variables were investigated: (a) total sleep time (TST) of the second sleep laboratory night, (b) ISI scores, (c) BDI scores, and (d) STAI trait scores.
RESULTS
Sample Characteristics
Demographic characteristics and psychometric data of the study sample are presented in Table 1. The groups did not differ significantly in sex distribution, age or body mass index (BMI). The PI patients had significantly higher ISI, PSQI, DBAS‐16, and GSES scores as well as higher scores on the cognitive subscale of the PSAS. Furthermore, BDI scores were increased in PI patients even after excluding the two sleep‐related items [PI patients: 5.2 ± 4.0, good sleeper controls: 3.1 ± 2.9, t(57) = 2.34, P = 0.023] and trait anxiety was increased as indicated by the trait subscale of the STAI. No significant group differences were found for the somatic subscale of the PSAS, the ESS, and the state subscale of the STAI.
Table 1.
Description of the study population (means ± standard deviations)
| PI patients | Healthy controls | t/χ² | P | |
|---|---|---|---|---|
| Sex (M/F) | 10/14 | 15/20 | 0.01 | 0.928 |
| Age (years) | 42.7 ± 14.5 | 40.1 ± 9.1 | 0.84 | 0.402 |
| BMI (kg/m²) | 23.2 ± 2.0 | 23.1 ± 3.4 | 0.17 | 0.868 |
| ISI | 15.9 ± 3.0 | 2.5 ± 2.3 | 19.32 | <0.001 |
| PSQI | 11.2 ± 2.8 | 3.8 ± 1.8 | 12.30 | <0.001 |
| DBAS‐16 | 4.7 ± 1.4 | 2.3 ± 1.0 | 7.57 | <0.001 |
| GSES | 7.0 ± 2.5 | 1.2 ± 1.5 | 11.07 | <0.001 |
| PSAS – cognitive | 17.8 ± 6.1 | 13.6 ± 4.3 | 3.14 | 0.003 |
| PSAS – somatic | 12.0 ± 3.9 | 10.3 ± 3.0 | 1.92 | 0.060 |
| ESS | 8.0 ± 4.5 | 6.9 ± 3.9 | 1.07 | 0.290 |
| BDI | 8.2 ± 4.4 | 3.6 ± 3.3 | 4.62 | <0.001 |
| STAI – state | 34.5 ± 6.7 | 34.6 ± 6.3 | −0.05 | 0.957 |
| STAI – trait | 40.5 ± 7.4 | 33.0 ± 7.3 | 3.85 | <0.001 |
Three insomnia patients suffered from sleep‐onset insomnia, 6 from sleep‐maintenance insomnia, and 14 from mixed insomnia. One patient had a complaint of nonrestorative sleep in the absence of difficulties initiating and maintaining sleep. The average duration of primary insomnia was 12.0 ± 10.9 years.
Polysomnography
The data of the polysomnographic investigation are presented in Table 2. In both nights, PI patients had a significantly lower total sleep time and sleep efficiency compared with good sleeper controls. Additionally, PI patients had a significantly decreased REM % in the first night and a significantly increased wake after sleep onset in the second night.
Table 2.
Polysomnographic data (means ± standard deviations)
| First Night: | PI patients | Healthy controls | t | P |
|---|---|---|---|---|
| First Night | ||||
| Total sleep time (min) | 351.9 ± 60.0 | 380.9 ± 48.8 | −2.03 | 0.047 |
| Sleep efficiency (%) | 73.3 ± 12.3 | 79.4 ± 10.1 | −2.05 | 0.045 |
| Sleep onset latency (min) | 28.7 ± 18.3 | 21.7 ± 19.9 | 1.36 | 0.180 |
| Wake after sleep onset (min) | 87.8 ± 47.6 | 69.5 ± 39.8 | 1.59 | 0.118 |
| Number of awakenings | 32.2 ± 16.4 | 40.3 ± 15.0 | −1.95 | 0.057 |
| Arousal index/TST (h‐1) | 19.7 ± 7.1 | 20.8 ± 7.8 | −0.54 | 0.591 |
| Sleep apnea index/TST (h‐1) | 0.3 ± 0.8 | 0.4 ± 0.5 | −0.26 | 0.793 |
| PLMS arousal index/TST (h‐1) | 0.3 ± 0.6 | 0.4 ± 0.8 | −0.58 | 0.566 |
| Stage 1 (% SPT) | 10.4 ± 5.2 | 11.4 ± 4.9 | −0.75 | 0.454 |
| Stage 2 (% SPT) | 47.6 ± 9.7 | 49.1 ± 8.6 | −0.63 | 0.534 |
| SWS (% SPT) | 7.7 ± 7.8 | 7.0 ± 6.8 | 0.37 | 0.716 |
| REM (% SPT) | 14.3 ± 4.4 | 17.0 ± 5.2 | −2.03 | 0.047 |
| Second Night | ||||
| Total sleep time (min) | 387.4 ± 53.2 | 415.2 ± 24.6 | −2.71 | 0.009 |
| Sleep efficiency (%) | 80.7 ± 11.1 | 86.5 ± 5.1 | −2.70 | 0.009 |
| Sleep onset latency (min) | 16.6 ± 10.8 | 18.1 ± 17.1 | −0.39 | 0.695 |
| Wake after sleep onset (min) | 65.3 ± 45.3 | 42.1 ± 17.2 | 2.76 | 0.008 |
| Number of awakenings | 31.1 ± 11.9 | 36.5 ± 13.8 | −1.55 | 0.126 |
| Arousal index / TST (h‐1) | 16.9 ± 6.1 | 16.1 ± 6.5 | 0.49 | 0.629 |
| Stage 1 (% SPT) | 8.3 ± 4.0 | 9.1 ± 4.5 | −0.69 | 0.491 |
| Stage 2 (% SPT) | 50.9 ± 9.6 | 53.6 ± 5.8 | −1.37 | 0.177 |
| SWS (% SPT) | 9.5 ± 8.8 | 8.7 ± 7.4 | 0.38 | 0.704 |
| REM (% SPT) | 16.8 ± 6.4 | 19.4 ± 3.8 | −2.00 | 0.050 |
Diffusion Tensor Imaging
Corrected for multiple comparisons, a significant cluster of 101 voxels with reduced FA values in PI patients was found within the right anterior internal capsule (see Fig. 1). Of note, Figure 1 additionally shows a cluster in the left anterior internal capsule which was also found to show reduced FA values in insomnia. However, the size of this cluster (80 voxels) narrowly failed to reach the required cluster size for statistical significance. The mean FA values of all participants in these two areas are presented in Figure 2.
Figure 1.
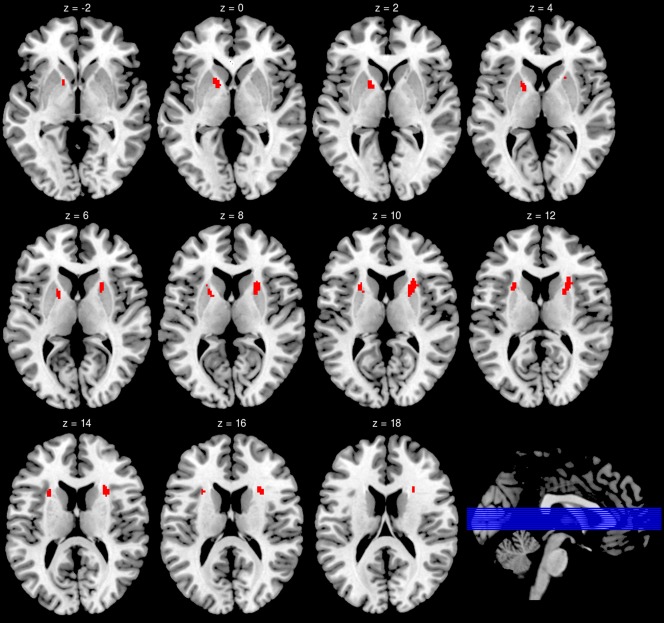
Brain areas that showed reduced (right hemisphere) or a trend for reduced (left hemisphere) FA values for insomnia patients. For the illustration of the cluster in the left hemisphere, the minimal cluster size for this figure was set at 320 mm³ (40 voxels) at a voxel threshold of P < 0.005 (T > 2.93).
Figure 2.
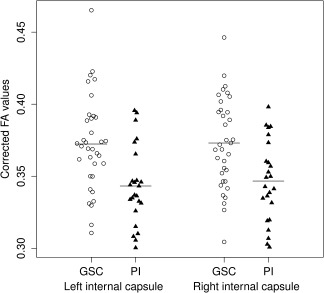
Mean FA values for the those areas within the left and right anterior internal capsule that showed reduced (right hemisphere) or a trend for reduced (left hemisphere) FA values for insomnia patients. Presented FA values are age‐, and sex‐adjusted by using the residual method. Horizontal bars represent group means. GSC, good sleeper controls; PI, primary insomnia.
Mean FA values in the area within the right internal capsule were significantly associated with ISI scores [t = −2.55, P = 0.014; see Fig. 3], but not with TST of the second sleep laboratory night [t(55) = 0.79, P = 0.44], BDI scores [t(55) = −0.13, P = 0.90] or STAI scores [t(55) = −1.23, P = 0.23]. Similarly, mean FA values in the left internal capsule were found to be significantly correlated with ISI scores [t(55) = −3.08, P = 0.003; see Fig. 3], but not with TST of the second sleep laboratory night [t(55) = 0.39, P = 0.70], BDI scores [t(55) = −0.51, P = 0.61], or STAI scores [t(55) = −1.32, P = 0.19].
Figure 3.
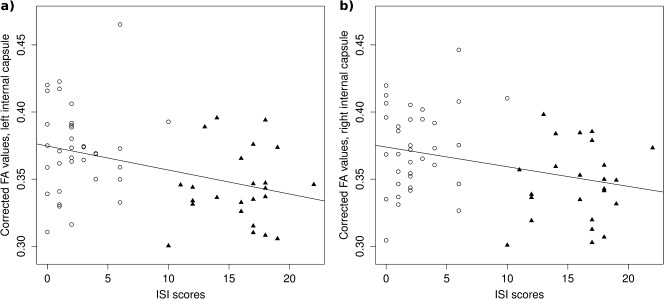
Association between ISI scores and mean FA values for those areas within the left (a) and right (b) anterior internal capsule that showed reduced (right hemisphere) or a trend for reduced (left hemisphere) FA values for insomnia patients. Presented FA values are age‐, and sex‐adjusted by using the residual method. Insomnia patients are presented as closed triangles, good sleeper controls as open circles. Solid lines represent regression lines.
DISCUSSION
This is the first study to report disruptions of white matter integrity in PI, i.e. insomnia in the absence of a somatic or mental illness. The results suggest that insomnia is associated with a reduced integrity of white matter tracts in the anterior internal capsule.
The anterior internal capsule contains fibers connecting subcortical nuclei and the prefrontal cortex including frontothalamic and frontopontine fibers [Alexander et al., 1986, 1990; Axer and Keyserlingk, 2000; Haber and Calzavara, 2009]. The pons, the thalamus and the prefrontal cortex are key structures for the regulation of sleep and wakefulness [Saper et al., 2005], and synchronized activity in a cortico‐thalamic feedback‐loop is thought to underlie the emergence of consolidated NREM sleep [Steriade, 2006]. Furthermore, altered function in fronto‐subcortical circuits has been suggested to be involved in the pathophysiology of insomnia [Cano et al., 2008; Nofzinger et al., 2004]. While the exact neurobiological mechanisms underlying insomnia remain unclear, it seems reasonable to assume that reduced white matter integrity in the anterior internal capsule may contribute to disturbed sleep‐specific fronto‐subcortical neuronal synchronization and, thus, to the experience of insomnia.
It has also been suggested that disturbed fronto‐subcortical connectivity is a key factor of psychopathology [Mega and Cummings, 1994]. In line with this, decreased FA values in the internal capsule were found in affective disorders [Haznedar et al., 2005; Sussmann et al., 2009; Zhang et al., 2013; Zou et al., 2008], schizophrenia [Guo et al., 2012; Levitt et al., 2012; Lee et al., 2013; Sussmann et al., 2009], and ADHD [van Ewijk et al., 2012]. Furthermore, deep brain stimulation of the anterior internal capsule has been reported to ameliorate symptoms in obsessive‐compulsive disorder [Abelson et al., 2005] and depression [Malone et al., 2009]. Most psychiatric disorders are characterized by sleep disturbances [Benca et al., 1992] with a bidirectional relationship between insomnia and depression [Baglioni et al., 2011]. The current study suggests that PI, a sleep disorder without any underlying psychiatric comorbidity, may be associated with disturbed white matter integrity in the anterior internal capsule. Therefore it may be important to measure and control for insomnia when investigating white matter integrity in other psychiatric disorders.
Mean FA values in the left and right anterior internal capsule were significantly related to ISI scores, but not to the TST of the second sleep laboratory night. With respect to these analyses, it has to be noted that the same dataset was used for selection and selective analysis, thus resulting in circularity [Kriegeskorte et al., 2009]. Consequently, the more interesting finding is the absence of a significant relationship between polysomnographically determined TST and white matter integrity in the internal capsule suggesting a specific role of this area for subjective aspects of insomnia.
Two limitations of the current investigation have to be acknowledged. First, this was a cross‐sectional study. Therefore, conclusions about causality can not be drawn and the direction of the association between insomnia and disturbed white matter integrity in the anterior internal capsule remains unclear. Second, primary insomnia patients had higher depression and trait anxiety scores than good sleeper controls. Although none of our participants had an affective or anxiety disorder, a subtle impact of subclinical depression or anxiety on white matter integrity can not be excluded. However, in the current study, BDI and STAI values were not correlated with FA values in the internal capsule.
In summary, our study suggests that insomnia may either result from, or contribute to, decreased white matter integrity in the anterior internal capsule. While the significance of this finding for pathophysiological models of insomnia [Espie et al., 2006; Harvey, 2002; Morin, 1993; Perlis et al., 1997; Riemann et al., 2010, 2011, 2012] remains to be elucidated, the current result may implicate that insomnia is characterized by disturbed fronto‐subcortical connectivity.
ACKNOWLEDGMENTS
Dr Spiegelhalder and Dr Riemann have received funding by the Else Kröner‐Fresenius‐Stiftung (2011_A208). Additionally, Dr Spiegelhalder has been supported by a habilitation grant of the “Walter und Sibylle Kalkhof‐Rose‐Stiftung der Akademie der Wissenschaften und der Literatur Mainz”. Dr Baglioni and Dr Riemann have received funding from the European Community's Seventh Framework Programme (People, Marie Curie Actions, Intra‐European Fellowship, FP7‐PEOPLE‐IEF‐2008) under grant agreement n. 235321.
REFERENCES
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, Martis B, Giordani B (2005): Deep brain stimulation for refractory obsessive‐compulsive disorder. Biol Psychiatry 57:510–516. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR (1990): Basal ganglia‐thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJW (2010): Reduced orbitofrontal and parietal gray matter in chronic insomnia: A voxel‐based morphometric study. Biol Psychiatry 67:182–185. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine (2007): The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester: AASM. [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders DSM‐IV‐TR Fourth Edition (Text Revision), 4th ed. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Axer H, Keyerlingk DG (2000): Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods 94:165–175. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D (2011): Insomnia as a predictor of depression: A meta‐analytic evaluation of longitudinal epidemiological studies. J Affect Disord 135:10–19. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, Morin CM (2001): Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2:297–307. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA (1987): Beck Depression Inventory. San Antonio: The Psychological Corporation. [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC (1992): Sleep and psychiatric disorders. A meta‐analysis. Arch Gen Psychiatry 49:651–668; discussion 669–670. [DOI] [PubMed] [Google Scholar]
- Broomfield NM, Espie CA (2005): Towards a valid, reliable measure of sleep effort. J Sleep Res 14:401–407. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds, III CF , Monk TH, Berman SR, Kupfer DJ (1989): The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res 28:193–213. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall M, Moul DE, Nofzinger EA, Kupfer DJ (2007): Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Med 8:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Mochizuki T, Saper CB (2008). Neural circuitry of stress‐induced insomnia in rats. J Neurosci 28, 10167–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Espie CA, Broomfield NM, MacMahon KMA, Macphee LM, Taylor LM (2006): The attention‐intention‐effort pathway in the development of psychophysiologic insomnia: A theoretical review. Sleep Med Rev 10:215–245. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J (2012): Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta‐analysis. Neurosci Biobehav Rev 36:1093–1106. [DOI] [PubMed] [Google Scholar]
- Fortier‐Brochu E, Beaulieu‐Bonneau S, Ivers H, Morin CM (2012): Insomnia and daytime cognitive performance: A meta‐analysis. Sleep Med Rev 16:83–94. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Liu Z, Gao K, Xiao C, Chen H, Zhao J (2012): Right lateralized white matter abnormalities in first‐episode, drug‐naive paranoid schizophrenia. Neurosci Lett 531:5–9. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R (2009): The cortico‐basal ganglia integrative network: The role of the thalamus. Brain Res Bull 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2002): A cognitive model of insomnia. Behav Res Ther 40:869–893. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Roversi F, Pallanti S, Baldini‐Rossi N, Schnur DB, Licalzi EM, Tang C, Hof PR, Hollander E, Buchsbaum MS (2005): Fronto‐thalamo‐striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry 57:733–742. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991): A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14:540–545. [DOI] [PubMed] [Google Scholar]
- Joo EY, Noh HJ, Kim J‐S, Koo DL, Kim D, Hwang KJ, Kim ST, Kim MR, Hong SB (2013): Brain Gray Matter Deficits in Patients with Chronic Primary Insomnia. Sleep 36:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RAA, Kim J‐S, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK (2005): Diffusion tensor imaging in schizophrenia. Biol Psychiatry 58:921–929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Coulouvrat C, Hajak G, Roth T, Shahly V, Shillington AC, Stephenson JJ, Walsh JK (2011): Insomnia and the performance of US workers: Results from the America insomnia survey. Sleep 34:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI (2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle SD, Espie CA, Morgan K (2010a): “…Not just a minor thing, it is something major, which stops you from functioning daily”: Quality of life and daytime functioning in insomnia. Behav Sleep Med 8:123–140. [DOI] [PubMed] [Google Scholar]
- Kyle SD, Morgan K, Espie CA (2010b): Insomnia and health‐related quality of life. Sleep Med Rev 14:69–82. [DOI] [PubMed] [Google Scholar]
- Lee S‐H, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam‐Gately RI, McCarley RW, Shenton ME (2013): Extensive white matter abnormalities in patients with first‐episode schizophrenia: A Diffusion Tensor Imaging (DTI) study. Schizophr Res 143:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Alvarado JL, Nestor PG, Rosow L, Pelavin PE, McCarley RW, Kubicki M, Shenton ME (2012): Fractional anisotropy and radial diffusivity: Diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr Res 136:55–62. [DOI] [PubMed] [Google Scholar]
- Malone DA Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD (2009): Deep brain stimulation of the ventral capsule/ventral striatum for treatment‐resistant depression. Biol Psychiatry 65:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL (1994): Frontal‐subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 6:358–370. [DOI] [PubMed] [Google Scholar]
- Morin CM (1993): Insomnia: Psychological Assessment and Management. New York: Guilford Press. [Google Scholar]
- Morin CM, Benca R (2012): Chronic insomnia. Lancet 379:1129–1141. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Ivers H (2007): Dysfunctional beliefs and attitudes about sleep (DBAS): Validation of a brief version (DBAS‐16). Sleep 30:1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler, 3rd J , Brookeman JR (1990): Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE). Magn Reson Med 15:152–157. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N (2010): Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry 68:494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L (1985): The phenomenology of the pre‐sleep state: The development of the pre‐sleep arousal scale. Behav Res Ther 23:263–271. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ (2004): Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 161, 2126–2128. [DOI] [PubMed] [Google Scholar]
- Noh HJ, Joo EY, Kim ST, Yoon SM, Koo DL, Kim D, Lee G‐H, Hong SB (2012): The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol 8:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK (1997): Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. J Sleep Res 6:179–188. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Espie C, Pollmächer T, Léger D, Bassetti C, Someren E van (2011): Chronic insomnia: Clinical and research challenges–an agenda. Pharmacopsychiatry 44:1–14. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C (2010): The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev 14:19–31. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B (2012): REM sleep instability—A new pathway for insomnia? Pharmacopsychiatry 4 5:167–176. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U (2003): Primary insomnia: A risk factor to develop depression? J Affect Disord 76:255–259. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, Hennig J, Perlis ML, Tebartz van Elst L, Feige B (2007): Chronic insomnia and MRI‐measured hippocampal volumes: a pilot study. Sleep 30:955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J (2005): Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257–1263. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP (2009): A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry 66:814–823. [DOI] [PubMed] [Google Scholar]
- Shahly V, Berglund PA, Coulouvrat C, Fitzgerald T, Hajak G, Roth T, Shillington AC, Stephenson JJ, Walsh JK, Kessler RC (2012): The associations of insomnia with costly workplace accidents and errors: Results from the america insomnia survey. Arch Gen Psychiatry 69:1054–1063. [DOI] [PubMed] [Google Scholar]
- Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF: Insomnia and risk of cardiovascular disease: A meta‐analysis. Eur J Prev Cardiol (in press). Available at: http://dx.doi.org/ 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Baglioni C, Klöppel S, Abdulkadir A, Hennig J, Nissen C, Riemann D, Feige B (2013): Insomnia does not appear to be associated with substantial structural brain changes. Sleep 36:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vogg PR, Jacobs GA (1983): Manual for the State‐Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Steriade M (2006): Grouping of brain rhythms in corticothalamic systems. Neuroscience 137:1087–1106. [DOI] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Muñoz Maniega S, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM, McIntosh AM (2009): White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord 11:11–18. [DOI] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, Kotozaki Y, Nouchi R, Asano M, Asano K, Fukuda H, Kawashima R (2012): Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage 60:471–475. [DOI] [PubMed] [Google Scholar]
- Winkelman JW, Benson KL, Buxton OM, Lyoo IK, Yoon S, O'Connor S, Renshaw PF (2010): Lack of hippocampal volume differences in primary insomnia and good sleeper controls: An MRI volumetric study at 3 Tesla. Sleep Med 11:576–582. [DOI] [PubMed] [Google Scholar]
- Zaitsev M, Hennig J, Speck O (2004): Point spread function mapping with parallel imaging techniques and high acceleration factors: Fast, robust, and flexible method for echo‐planar imaging distortion correction. Magn Reson Med 52:1156–1166. [DOI] [PubMed] [Google Scholar]
- Zhang A, Ajilore O, Zhan L, GadElkarim J, Korthauer L, Yang S, Leow A, Kumar A (2013): White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology 38:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Huang X, Li T, Gong Q, Li Z, Ou‐Yang L, Deng W, Chen Q, Li C, Ding Y, Sun X (2008): Alterations of white matter integrity in adults with major depressive disorder: A magnetic resonance imaging study. J Psychiatry Neurosci 33:525–530. [PMC free article] [PubMed] [Google Scholar]


