Abstract
Awareness is an essential feature of the human mind that can be directed internally, that is, toward our self, or externally, that is, toward the environment. The combination of internal and external information is crucial to constitute our sense of self. Although the underlying neuronal networks, the so‐called intrinsic and extrinsic systems, have been well‐defined, the associated biochemical mechanisms still remain unclear. We used a well‐established functional magnetic resonance imaging (fMRI) paradigm for internal (heartbeat counting) and external (tone counting) awareness and combined this technique with [18F]FMZ‐PET imaging in the same healthy subjects. Focusing on cortical midline regions, the results showed that both stimuli types induce negative BOLD responses in the mPFC and the precuneus. Carefully controlling for structured noise in fMRI data, these results were also confirmed in an independent data sample using the same paradigm. Moreover, the degree of the GABAA receptor binding potential within these regions was correlated with the neuronal activity changes associated with external, rather than internal awareness when compared to fixation. These data support evidence that the inhibitory neurotransmitter GABA is an influencing factor in the differential processing of internally and externally guided awareness. This in turn has implications for our understanding of the biochemical mechanisms underlying awareness in general and its potential impact on psychiatric disorders. Hum Brain Mapp 35:173–184, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: GABA, PET, fMRI, interoception, exteroception, awareness
Abbreviations
- BOLD
blood oxygen level dependent
- BPND
nondisplaceable binding potential (ratio at equilibrium of specifically bound radioligand to nondisplaceable radioligand)
- BPND‐Pons
ratio at equilibrium of specifically bound radioligand to nondisplaceable radioligand using the pons as reference tissue
- BPND‐WM
ratio at equilibrium of specifically bound radioligand to nondisplaceable radioligand using the white matter as reference tissue
- CMS
cortical midline structures
- EA
external awareness
- fMRI
functional magnetic resonance imaging
- FMZ
flumazenil
- GABA
gamma‐aminobutyric acid
- IA
internal awareness
- NBR
negative BOLD response
- PET
positron‐emission‐tomography
- PSC
percent signal change
- ROIfunc/ROIana
region of interest defined by functional MRI contrast or anatomical structure
INTRODUCTION
Awareness is a central, if not defining, feature of the human mind. Through this faculty we can become explicitly aware of both our environment and ourselves, including our body, this having been described in neuroscience as external and internal awareness (IA) [James, 1890; Vanhaudenhuyse et al., 2011]. Whilst external awareness (EA) describes the perception of the environment, IA refers to the perception of self‐related mental processes and awareness related to one's own body, including vegetative measures like one's own heartbeat.
Recent brain imaging studies using positron‐emission‐tomography (PET) or functional magnetic resonance imaging (fMRI) have associated IA and EA with different neuronal networks [Boly et al., 2008, 2009; Fox and Raichle, 2007; Fransson, 2005, 2006; Golland et al., 2007, 2008; Raichle and Snyder, 2007; Vanhaudenhuyse et al., 2011]. EA has been linked to regions in mainly the lateral frontal and parietal cortex, the “extrinsic system,” as they are recruited during external and goal‐oriented behavior [Boly et al., 2007; Fransson, 2005; Golland et al., 2007]. In contrast, IA, as for instance during mind‐wandering or self‐oriented thoughts, recruits a network located along the anterior and posterior cortical midline structures (CMS). This “intrinsic system” includes the anterior and posterior cingulate cortex, the ventro‐ and dorsomedial prefrontal cortex and the retrosplenial cortex [Goldberg et al., 2006; Lou et al., 2004; Northoff et al., 2010, 2011].
The anterior and posterior CMS are also involved in the default‐mode network, a collection of regions that show high metabolic and neuronal activity during the absence of an active task [Buckner et al., 2008; Fox et al., 2005; Raichle, 2010; Raichle and Snyder, 2007; Raichle et al., 2001; Shulman et al., 2009; Vincent et al., 2007]. This network is characterized by task‐induced deactivations (i.e., a decrease of neuronal activity), and accordingly negative BOLD responses (NBRs) in response to external stimuli—that is, awareness of stimuli originating from outside the body [Fransson, 2005; Gusnard and Raichle, 2001; McKiernan et al., 2003; Raichle and Gusnard, 2005]. In addition to stimuli originating from the external environment, the neuronal activity in these midline regions also encounters internal stimuli originating continuously from one's own body, with recent studies suggesting that the awareness of such internal stimuli also induces NBRs in CMS like the mPFC and the precuneus [Schilbach et al., 2012; Wiebking et al., 2011]. With both IA and EA being seen to induce NBRs within midline regions, the question arises as to whether there is a differentiation between these stimulus domains in these regions at the functional level.
Gamma‐aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the brain, is a likely candidate to mediate task‐induced activity along CMS in the brain. This hypothesis is supported by recent work [Alcaro et al., 2010; Northoff et al., 2007]; however, it concerns only comparisons between GABA and NBRs induced by goal‐oriented external stimuli, meaning that details of the role of GABA in processing internal stimuli are lacking. This leaves open the possibility that, should there be a distinction between IA and EA within the CMS as assumed, such a distinction can be related to some degree to differential GABAergic function.
A growing body of literature shows that psychiatric disorders are accompanied by a common pathophysiological pattern of GABAergic deficits [Bajbouj et al., 2006; Kalueff and Nutt, 2007; Luscher et al., 2011; Mohler, 2012; Petty, 1995; Sanacora, 2010; Smith and Rudolph, 2012]. Depression, for example, is characterized by GABAergic deficits, especially in anterior CMS [Bielau et al., 2007; Levinson et al., 2010; Walter et al., 2009] and altered states of awareness (decreased environment‐focus and increased self‐focus [Grimm et al., 2011; Paulus and Stein, 2010; Stewart et al., 2001; Wiersma et al., 2011], which lend support to the hypothesis that GABAergic function might be considered as an influencing factor in the distinction between internally and externally guided awareness within the regions of interest.
The aim of this study is to investigate, firstly, the neuronal response to IA and EA in anterior and posterior CMS, and secondly their relationship to the binding potential of GABAA receptors in these regions. We performed an fMRI study in healthy subjects to define CMS showing NBRs in response to internal (counting one's own heartbeat) and external (counting tones) stimuli. Since paradigms dealing with IA are specifically sensitive to artifacts resulting from blood flow, we carefully controlled the fMRI for structured noise to improve the sensitivity and specificity of the results.
Next, we performed high‐resolution [18F]‐labeled flumazenil (FMZ) PET imaging in the same subjects to investigate the binding potential of GABAA receptors. fMRI results were confirmed in an independent data sample using the same paradigm. The combined results of PET and fMRI suggest that neuronal inhibition related to GABAergic function (as reflected indirectly through GABAA receptor binding potential) is more closely associated with processes of EA, rather than IA.
MATERIALS AND METHODS
Subjects
Twenty‐eight healthy subjects (10 female, 18 male, mean age 22.37 years ± 3.77 SD, ranging from 18 to 34 years) underwent fMRI and 24 subjects (9 female, 15 male, mean age 22.6 years ± 3.79 SD, ranging from 18 to 34 years) out of this group also underwent PET scanning. All subjects had a Beck Depression Inventory [Beck et al., 1961] score of ≤ 4 and were questioned about psychiatric, neurological, or medical diseases using a custom‐made semistructured clinical questionnaire. Participants were recruited mainly from McGill University in Montreal and the local community. The study was approved by the local ethics committee. All participants gave their written informed consent before participating in this study and were financially compensated for their participation.
Four subjects were excluded due to structural abnormalities in their anatomical scans or due to motion artifacts (>2 mm), leaving 24 subjects with fMRI scans (9 female, 15 male, mean age 22.71 years ± 3.95 SD, ranging from 18 to 34 years) and 20 subjects with PET scans (8 female, 12 male, mean age 23.05 years ± 4.14 SD, ranging from 18 to 34 years).
Functional MRI data from an independent data sample of 30 healthy subjects (15 female, 15 male, mean age 33.73 years ± 11.62 SD, ranging from 22 to 60 years) were also analyzed.
Paradigm
The fMRI design (Supporting Information Fig. 2) was based on a paradigm introduced by Critchley and Pollatos [Critchley et al., 2004; Pollatos et al., 2007]. Subjects were presented with three separate conditions: an internal task, an external task, and fixation periods, in a pseudo‐randomized order (each jittered between 6 and 10 s).
During the internal task, subjects were asked to silently count their own heartbeat. Similarly, an external stimulus in the form of a tone had to be counted during the external task. In both cases, the subjects reported the number of counted heartbeats or tones after each trial by using a visual analog scale (3.5 s). To make the difficulty of both tasks closely comparable, tones were presented at an individually determined volume (i.e., just noticeable, like the heartbeat). The general presentation frequency of the tones was adapted to correspond to each subject's pulse‐rate. To control for habituation effects, the onset time of the tones was jittered by 200 ms from this general frequency.
During rest conditions—indicated by a dark fixation cross on a light background—subjects were instructed to relax and reduce any cognitive work during these periods. The fMRI paradigm for the independent data sample included the same conditions for fixation, IA, and EA. For a detailed description of the paradigm, please see [Wiebking et al., 2011].
fMRI Data Acquisition and Analysis
Functional scans were acquired on a 3‐Tesla whole body MRI system (Siemens Trio, Erlangen, Germany), using a 32‐channel headcoil. The settings were as follows: 47 T2*‐weighted echo planar images per volume with BOLD contrast; alignment at 30° off the AC‐PC plane in an odd–even interleaved acquisition order; FoV: 205 × 205 mm2; spatial resolution: 3.2 × 3.2 × 3.2 mm3; TE = 25 ms; TR = 2,270 ms; flip angle = 90°. Functional data were recorded in one scanning session containing 580 volumes for each subject. A high resolution T1‐weighted 3D structural image was also acquired.
The fMRI data of 24 subjects were preprocessed and statistically analyzed by the general linear model approach using the SPM8 software package (http://www.fil.ion.ucl.ac.uk) and MATLAB 7.11 (The Mathworks, Natick, MA). All functional images were slice‐time corrected with reference to the first acquired slice, corrected for motion artifacts by realignment to the mean functional image, and spatially normalized to a standard T1‐weighted SPM template [Ashburner and Friston, 1999]. The normalization was generated by warping the coregistered anatomical image to the MNI T1‐template, and applying these parameters to all functional images. The images were resampled to 2 × 2 × 2 mm3 and smoothed with an isotropic 6 mm full‐width half‐maximum (FWHM) Gaussian kernel. The time‐series fMRI data were filtered using a high pass filter (threshold 128 s). A statistical model for each subject was computed by applying a canonical response function [Friston et al., 1998].
Since structured noise still remains in the fMRI data after traditional steps of preprocessing, an independent component analysis (ICA) was applied to denoise the data and hence improve the sensitivity and specificity of the results. Using Probabilistic Independent Component Analysis, which is implemented in the MELODIC toolbox [Beckmann and Smith, 2004] of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl/) [Smith et al., 2004; Woolrich et al., 2009], a group ICA was performed on the preprocessed fMRI data, which were temporally concatenated across subjects. Two independent raters (CW, NWD) visually inspected the resulting components and classified them as noise or signals of interest according to a detailed description of an operationalized denoising procedure [Kelly et al., 2010]. In particular, components were considered as noise when they showed a ring‐like pattern in the periphery of the brain and tightly clustered areas in the frontal regions [McKeown et al., 1998], clusters with a location in the white matter/CSF or an association with blood vessels [Sui et al., 2009; Zou et al., 2009], spotted patterns diffusely spread over the brain, and time courses showing a saw‐tooth pattern or spikes [McKeown et al., 1998]. Thirteen components were independently identified as noise and discarded from the original fMRI data through linear regression.
After removing nonbrain voxels using brain extraction tool (BET [Smith, 2002]), all three conditions (Fixation, IA, and EA) were then included in the SPM model as separate events including their feedback phases. Regionally specific condition effects were tested by employing linear contrasts for each subject and different conditions. The resulting contrast images were submitted to a second level random‐effects analysis. Here, one‐sample t‐tests were used on images obtained for each subject's volume set and different conditions. To control for the multiple testing problem, a familywise error rate correction was performed. The anatomical localization of significant neural responses in the main contrast (Fixation > IA/EA; P ≤ 0.05, FWE‐corrected, k ≥ 10) was assessed with reference to the standard stereotactic atlas by superimposition of the SPM maps on a standard brain template provided by SPM8.
Based on the functional results, three clusters of deactivation were found along the cortical midline and defined as regions of interest (ROIfunc). To confirm findings in ROIfunc, additional anatomical regions (ROIana) were identified due to their proximity to ROIfunc. Using the WFU‐pickatlas (AAL atlas of the Wake‐Forrest University, NC), four medial regions were defined: the frontal middle orbital cortex, the frontal superior middle cortex, the anterior cingulate, and the precuneus. For each region, the left and right hemispheres were calculated separately. Percent signal changes (PSC) from these CMS were extracted using the MarsBaR toolbox (http://www.sourceforge.net/projects/marsbar). All data were controlled for possible outliers. Whole brain regressions using BPND of an anatomical region were inclusively masked by ROIana on the whole (P ≤ 0.005, uncorrected, k ≥ 20). Analysis for ROIfunc is set to (P ≤ 0.001, uncorrected, k ≥ 20). Regional specificity was tested by applying ROIfunc and correspondent ROIana that were not located along the midline regions.
Confirming fMRI results, since the physiological basis of deactivation is still a controversial issue in the current literature [Lauritzen et al., 2012], by using an independent set of data, in line with proposed good practice [Kriegeskorte et al., 2009; Poldrack and Mumford, 2009; Vul et al., 2009], these three ROIfunc were also applied to another sample (acquired at the Department of Neurology, Otto‐von‐Guericke University Magdeburg, Germany). Subjects in both studies performed the same tasks (IA, EA, Fix), and have been instructed by the same researcher (CW). Functional measurements of this independent data set were performed on an identical 3‐Tesla whole body MRI system (Siemens Trio, Erlangen, Germany). The headcoils differed across studies (an 8‐channel headcoil compared to a 32‐channel headcoil), but research has been shown that fMRI results across different imaging sites are very well comparable, even when comparing across different headcoils and scanner machines [see for example, Casey et al., 1998; Gountouna et al., 2010; Kaza et al., 2011; Zou et al., 2005]. The settings were as follows: 32 T2*‐weighted echo planar images per volume with BOLD contrast; alignment parallel to the AC‐PC plane in an odd–even interleaved acquisition order; FoV: 224 × 224 mm2; spatial resolution: 3.5 × 3.5 × 4 mm3; TE = 30 ms; TR = 2,000 ms; flip angle = 80°. A total of 1,160 volumes were recorded for each of the 30 healthy subjects. These data were processed in the exact same way (including ICA denoising) as the main data set. This was a reanalysis of a data set presented beforehand [Wiebking et al., 2011].
PET‐Image Acquisition and Reconstruction
Twenty subjects underwent PET imaging with FMZ, a GABA antagonist that binds at the GABAA benzodiazepine receptor. It is a common method to measure GABAA receptor density in vivo in humans [Frey et al., 1991; Salmi et al., 2008]. [18F]FMZ has the advantages of a long half‐life, a good affinity for the benzodiazepine site on the GABAA receptor (Ki = 11.6 nM), and a short positron range, which enables the production of high‐quality images. PET imaging was done randomly either before or after the fMRI (mean duration ± SD between both types of scans: 1.9 ± 3.6 days).
Whole‐brain [18F]FMZ binding potential (BPND) values were obtained using a Siemens ECAT HRRT (High Resolution Research Tomograph) PET system (Siemens Medical Solutions, Knoxville, TN) [de Jong et al., 2007]. [18F]FMZ was synthesized as published previously, with a specific activity between 1,500 and 2,000 Ci/mmol [Massaweh et al., 2009]. Head movement was minimized with a head‐restraining adhesive band. A 6‐min transmission scan (137Cs‐point source) was first acquired for attenuation correction followed by an intravenous tracer injection (over 60 s) of 260.7 MBq (± 21.24 SD) of [18F]FMZ. Subjects were instructed to close their eyes and remain awake.
List‐mode data were acquired for a period of 60 min and then binned into a series of 26 sequential sets of 2,209 span 9 sinograms of increasing temporal duration, ranging from 30 s to 5 min. PET data were reconstructed using a 3D OP‐OSEM algorithm (10 iterations and 16 subsets) [Hong et al., 2007; Hudson and Larkin, 1994] with full accounting for scatter, random coincidences, attenuation, decay, dead‐time, and frame‐based motion correction [Costes et al., 2009]. The images used had a voxel size of 1.22 × 1.22 × 1.22 mm3 (256 × 256 × 207 voxels). GABAA BPND maps were then calculated according to the Logan plot method, using each of the pons (BPND‐Pons) and cerebral white matter as the reference tissue region (BPND‐WM) [Logan et al., 1996]. In a final step, all BPND images were aligned to MNI space.
Two separate reference regions were used as each in itself has limitations in relation to the Logan method. The pons has commonly been used in [18F]FMZ studies [Frankle et al., 2012; Klumpers et al., 2012; Odano et al., 2009; Pearl et al., 2009]; however, recent studies have suggested that it displays a significant degree of specific binding [Frankle et al., 2009, 2012; Klumpers et al., 2008; Millet et al., 2002]. The cerebral white matter has been proposed as an alternative reference region due to its lower degree of specific binding [Klumpers et al., 2008; la Fougere et al., 2011]. This region is not without downsides; however, specifically its location close to gray matter and CSF (leading to potential activity spill‐in from these regions) and the fact that it is not clear that the level of nonspecific binding is the same in gray and white matter, an assumption inherent to the Logan method [Lammertsma and Hume, 1996]. Thus, given these independent limitations of the two reference regions, both were used and the correlation results seen to be consistent across both BPND values taken to be highly reliable.
As GABAA receptors have been previously utilized as markers of neuronal density [la Fougere et al., 2011], there is the potential that any relation between BPND and PSC could be the result of the former acting as a proxy ROI neuronal volume measure. To account for this possibility, the segmented MR gray matter image, which contains voxel values between 1 and 0, was convolved with a Gaussian kernel of 2.5 mm FWHM to simulate the spatial resolution of the HRRT PET scanner. The proportion of gray matter in each ROI for each subject was calculated using FSL and included as a control variable in all analysis regarding BPND values. The individual biochemical measures for specific regions were entered into a second‐level correlation analysis in SPM, using the proportion of gray matter as regressor of no interest in all calculations. Controlling for gray matter, BPND values were also correlated (two‐tailed) with PSC derived from each ROI.
RESULTS
Behavioral Data
The mean reaction time for internal stimuli was 0.95 s (±0.18 SD) and for external stimuli 0.98 s (±0.23 SD). Reaction times showed no difference between stimuli types and no significant correlation with PSC or PET‐BPND values in the main regions.
Imaging Results
To investigate BOLD responses in CMS during IA and EA, a well‐established fMRI paradigm was used [Critchley et al., 2004; Pollatos et al., 2007; Wiebking et al., 2010, 2011] (Supporting Information Fig. 2).
As detailed in Figure 1 (see SPM images on the left side), the contrast (Fixation > IA/EA; P ≤ 0.05, FWE‐corrected, k ≥ 10) revealed clusters of deactivation in the left mPFC as well as in the bilateral precuneus. These functional clusters were defined as ROIfunc:
Left mPFC: center of mass (x,y,z in MNI space): −6, 54, 4, volume: 688 mm.
Bilateral precuneus: center of mass (x,y,z in MNI space): −1, −55, 26, volume: 5,624 mm.
Figure 1.
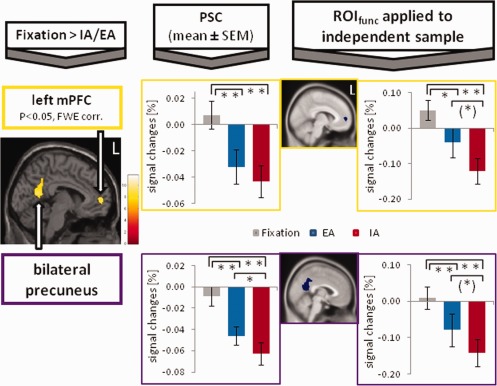
Cortical midline regions of interest (ROIfunc, yellow color) were defined by the contrast [Fixation > Internal (IA)/External Awareness (EA)] (P ≤ 0.05, FWE‐corrected, k ≥ 5, n = 24 subjects; see SPM images on the left side). Bar diagrams, next to the SPM images, show percent signal changes (PSC, mean ± SEM) and accordingly negative BOLD responses (NBRs) during fixation (gray), EA (blue), and IA (red). Paired t‐tests between the PSC were calculated (**P ≤ 0.005, *P ≤ 0.05, (*) P ≤ 0.1). ROIfunc were also applied to an independent data sample (n = 30 subjects), and paired t‐tests between PSC were calculated. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
A third cluster was found in the medial frontal region (see also Supporting Information Fig. 1): the right mPFC, center of mass (x,y,z in MNI space): 9, 54, 10, volume: 96 mm.
Calculating PSC in these functional clusters showed clear task‐induced deactivations during both IA and EA across all regions. During fixation, small NBRs were observed in the bilateral precuneus (see bar diagram on the left side of Fig. 1) and the right mPFC (see bar diagram in Supporting Information Fig. 1); a small positive BOLD response occurred in the left mPFC (Fig. 1). All three regions differed significantly (**P ≤ 0.005) when comparing fixation to IA or EA, respectively (using paired t‐tests, two‐tailed). The distinction between IA and EA was less pronounced across all regions, with significant differences (*P ≤ 0.05) in the bilateral precuneus and the right mPFC and no difference in the left mPFC.
To support the results of task‐induced deactivations in CMS and to test for statistical independence, in line with the recommendations made by Kriegeskorte, Poldrack, and Vul [Kriegeskorte et al., 2009; Poldrack and Mumford, 2009; Vul et al., 2009], these ROIfunc were also applied to an independent data sample that used the same fMRI paradigm and image processing. The PSC of the independent data sample (bar diagrams on the right side of Fig. 1 and Supporting Information Fig. 1) showed a similar pattern between the three conditions. Signal changes for fixation differed significantly from the two conditions (**P ≤ 0.005 or *P ≤ 0.05), except for the right mPFC (Supporting Information Fig. 1), where EA showed a small positive BOLD response and hence no difference between fixation and EA. Compared to fixation, the differentiation between the signal changes for IA and EA were also less pronounced across all regions [*P ≤ 0.05 in the right mPFC and (*) P ≤ 0.1 in the left mPFC and bilateral precuneus].
Having demonstrated in the two samples that both IA and EA induce reliable degrees of NBRs in the regions studied, the potential involvement of GABAA‐related function in these processes was investigated. GABAA BPND‐Pons values for each ROIfunc (see above) were extracted from each subject's [18F]FMZ‐PET scan (Fig. 2a,b, left side) and correlated with the signal differences between fixation and EA. Since the proportion of gray matter in each ROI was included as a control variable (see Methods; Table 1), the correlation graphs use the residuals of BPND‐Pons and PSC. In all ROIfunc, the difference in PSC between fixation and EA was found to be negatively associated with intraregional GABAA BPND‐Pons (Fig. 2a: *P ≤ 0.05; left mPFC, 2b: **P ≤ 0.01, bilateral precuneus). In contrast, no relationship was observed between BPND‐Pons values and the difference between fixation and IA or other signal changes in any of the ROIfunc (Table 1). Calculations using BPND‐WM can confirm this pattern in the anterior midline, but not the posterior region (Table 1). The difference between the two correlations was significant for the bilateral precuneus (P ≤ 0.05, one‐tailed) and marginal significant (P ≤ 0.1, one‐tailed) for the right mPFC. The same calculations were carried out using the brain regions also derived from the main contrast, but which were not located along the cortical midline. None of these areas showed a significant relationship between BPND and PSC (see Supporting Information Table 1).
Figure 2.
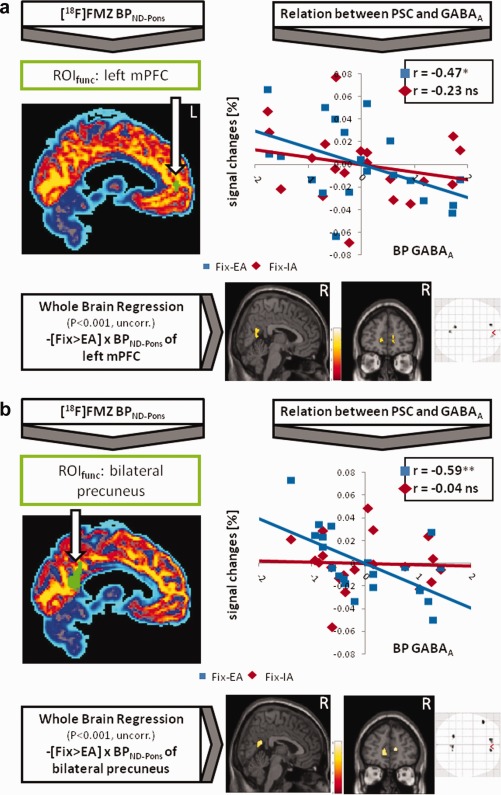
[18F]FMZ‐PET imaging was used to calculate binding potentials (BPND‐Pons) for GABAA receptors, applying functional regions of deactivation (ROIfunc, green color) derived from the contrast [Fixation > Internal/External Awareness] (P ≤ 0.05, FWE‐corrected, k ≥ 5; see also Fig. 1). These values were correlated (controlled for gray matter) with percent signal changes (PSC) of the ROIs (see partial correlation graph showing the residuals of BPND‐Pons and PSC, *P ≤ 0.05, **P ≤ 0.01). Moreover, BPND‐Pons values were entered into a whole brain regression analysis in SPM (controlled for the proportion of gray matter). The lower part shows a negative correlation for the contrast [Fixation > External Awareness] with BPND‐Pons (P ≤ 0.001, uncorrected, k ≥ 20). (a) Shows results for the left mPFC and (b) for the bilateral precuneus. Results for the right mPFC can be found in Supporting Information Figure 1. Calculations applying BPND‐WM can be found in Supporting Information Figure 3.
Table 1.
Three cortical midline regions were defined using the contrast [Fixation > IA/EA] (P ≤ 0.05, FWE‐corrected, k ≥ 10, n = 24 subjects, coordinates in MNI space)
| ROIfunc | BPND‐Pons | PSC | Partial correlation (controlled for gray matter) between PSC and BPND‐Pons | BPND‐WM | Partial correlation (controlled for gray matter) between PSC and BPND‐WM |
|---|---|---|---|---|---|
| (x,y,z)/ k/ T‐value | Fixation | ||||
| IA | |||||
| EA | |||||
| Fix‐IA | |||||
| Fix‐EA | |||||
| Left mPFC | |||||
| (−4,56,6)/ 86/ 9.18 | 6.7 ± 1.1 | 0.01 ± 0.05 | r = −0.31 | 9.0 ± 1.2 | r = −0.05 |
| −0.04 ± 0.05 | r = −0.14 | r = 0.01 | |||
| −0.03 ± 0.05 | r = 0.02 | r = 0.27 | |||
| 0.05 ± 0.03 | r = −0.23 | r = −0.10 | |||
| 0.04 ± 0.03 | r = −0.47* | r = −0.49* | |||
| Right mPFC | |||||
| (8,56,10)/ 12/ 7.08 | 6.4 ± 1.8 | 0.00 ± 0.04 | r = 0.05 | 8.1 ± 1.9 | r = −0.03 |
| −0.04 ± 0.05 | r = 0.10 | r = −0.04 | |||
| −0.02 ± 0.04 | r = 0.29 | r = 0.18 | |||
| 0.04 ± 0.03 | r = −0.11 | r = 0.02 | |||
| 0.02 ± 0.03 | a r = −0.46(*) | r = −0.39(*) | |||
| Bilateral Precuneus | |||||
| (0,−48,38)/ 703/ 8.44 | 6.8 ± 1.0 | 0.00 ± 0.04 | r = −0.11 | 9.1 ± 1.2 | r = 0.04 |
| −0.06 ± 0.05 | r = −0.07 | r = −0.05 | |||
| −0.05 ± 0.04 | r = 0.33 | r = 0.29 | |||
| 0.06 ± 0.02 | r = −0.04 | r = 0.17 | |||
| 0.04 ± 0.03 | r = −0.59** | r = −0.34 | |||
For each region, binding potential values (BPND‐Pons using the pons as reference tissue and BPND‐WM using the white matter as a reference tissue) for GABAA receptors were calculated (n = 20 subjects) and correlated (two‐tailed, controlled for gray matter) with percent signal changes (PSC).
Result for the right VMPFC/DMPFC is due to an outlier regards extracted PSC.
P ≤ 0.05.
P ≤ 0.01.
The individual biochemical measures for each ROIfunc were entered into a second‐level correlation analysis in SPM. In particular, the anterior midline regions showed a negative correlation between BPND and the contrast containing EA [Fixation > EA]. Whole brain regressions using BPND‐Pons are illustrated in the lower parts of Figure 2a,b (P ≤ 0.001, uncorrected, k ≥ 20). Regressions using BPND‐Pons are shown in the right lower corner of Supporting Information Figure 3 (green‐bordered, P ≤ 0.005, uncorrected, k ≥ 40). No results were observed in the contrast containing IA [Fixation > IA]. Detailed results about the conducted calculations are provided in Supporting Information Table 3. Again, as already shown by the previous results, it is specifically EA compared to fixation that showed a negative connection with BPND rather than IA.
Finally, to support findings in ROIfunc, the biochemical measures (BPND‐Pons and BPND‐WM) for anatomical‐defined midline regions (ROIana) were calculated and partial correlated with signal differences (see Supporting Information Table 2) as well as entered into a second‐level correlation analysis in SPM (Supporting Information Fig. 3). Results in Supporting Information Table 2 focus on reporting the central values, namely, the signal differences [Fix‐IA] and [Fix‐EA], since signal changes for fixation, IA, or EA showed no correlation with BPND. Specifically the anterior midline regions, the frontal middle orbital cortex and the frontal superior middle cortex, showed consistent negative correlations between BPND and [Fix‐EA] (see Supporting Information Table 2). Correlation analysis in SPM confirmed this pattern along CMS between BPND and [Fix‐EA] (Supporting Information Fig. 3). Also in accordance to ROIfunc (Table 1), the posterior region of the precuneus revealed not a clear pattern. BPND values of the anterior cingulate cortex did not lead to significant results in neither [Fix‐EA] nor [Fix‐IA] as well as nonmedial ROIana, like the amygdala and hippocampus (see Supporting Information Table 3).
DISCUSSION
The fMRI data from this study demonstrated the induction of NBRs during both IA and EA in anterior and posterior CMS (mPFC and precuneus) when compared to fixation periods (i.e., the absence of specific external stimuli). Whilst the induction of NBRs during external stimuli is in accordance with previous studies focusing on different kinds of external stimuli or goal‐oriented tasks [Gusnard et al., 2001; Raichle and Snyder, 2007; Simpson et al., 2001], the present study extends these findings by showing the induction of NBRs also during IA. This was suggested by a previous exploratory fMRI study [Wiebking et al., 2011], and is in accordance with a specific reanalysis of this data set, as well as with a recent meta‐analyses [Schilbach et al., 2012].
Combining fMRI with biochemical measures derived from PET imaging, this study also investigated a possible differential association between the availability of GABAA receptors (as reflected by the binding potential) and NBRs in CMS. BPND values in the anterior cortical regions, such as the mPFC (ROIfunc), frontal superior medial cortex, and frontal middle orbital cortex (both ROIana) showed a negative relationship to NBRs induced by EA rather than IA when compared to fixation.
Since correlation results across both BPND values are closely comparable, especially in the anterior cortical regions, our results can be considered as valid and not due to advantages or disadvantages of one or the other reference tissue regions for the BPND calculations. However, results concerning posterior regions were less stringent and need to be interpreted with caution.
Of note was the absence of correlating brain regions when BPND values of the anterior cingulate were applied. This can potentially be explained by the size of the anatomical region, which includes both the task‐negative pgACC (perigenual anterior cingulate cortex) and the task‐positive sgACC (supragenual anterior cingulate cortex) [see for example, Duncan et al., 2011]. The opposite neuronal behavior of these regions might lead to a nonresult in the SPM analysis.
These findings indicate that GABAA receptor availability within the regions studied is more closely associated with the processing of external, rather than internal, stimuli when compared to fixation in the context of the task used. Since there is evidence that NBRs can be mediated, at least in part, by GABA [Northoff et al., 2007], the processing of internal stimuli might have a more complex relationship with GABA that is not captured by the methods employed here.
Our findings in healthy subjects might help to form concepts about pathophysiological mechanisms of altered states of awareness as observed in psychiatric disorders. Although speculative at this point, the GABAergic deficit hypotheses of anxiety and depression [Kalueff and Nutt, 2007; Levinson et al., 2010; Mohler, 2012; Pilc and Nowak, 2005; Sanacora, 2010] might in future research be linked to their altered awareness states [Grimm et al., 2011; Paulus and Stein, 2010; Wiersma et al., 2011].
Several limitations of the study should be noted. Firstly, a linear relationship between the BPND of GABAA receptors and the degree of neuronal inhibition obtained in fMRI remains speculative at this point [for more details, see also Donahue et al., 2010; Shmuel et al., 2002]. This assumption needs to be confirmed in future studies in animals and humans, as does how GABAA receptor availability is exactly related to intracellular and extracellular concentrations of GABA. Secondly, apart from reaction time during fMRI, no other measures concerning the degree of awareness were recorded. Whilst reaction time was not related to either the degree of induced NBR or the GABAA receptor BPND, further studies need to provide a more extensive and detailed monitoring during fMRI (e.g., heart rate or galvanic skin response).
One may argue that our measurement of IA still requires a lot of EA. To underline our result that GABA BPND is more closely associated with mediating EA rather than IA, the inclusion of different types of IA, for example, mind wandering, should be considered in future experiments. Moreover, we defined a default‐mode of the brain as the neuronal activity during the absence of an active task [Shulman et al., 2009], which was operationalized using the fixation cross period. Even viewing this cue may, however, induce some relevant activity, thus precluding what has been described as a “pure” default‐mode [Logothetis et al., 2009]. Since we were interested in the induction of positive and negative BOLD responses in CMS during the conditions of interest, the fixation periods used were rather short. We are aware that our results must be replicated for longer rest periods in order to be sure that they are not due to overlapping BOLD responses from preceding activation periods. Finally, the fact that IA‐induced NBRs did not correlate with the BPND for GABAA leaves open the exact underlying differential mechanism, which ties into the point that a linear relationship between BPND and neuronal responses in fMRI is tentative. Also, the exact physiological mechanism underlying the BOLD signal, and especially the NBRs, remains unclear [Lauritzen et al., 2012; Logothetis, 2008]. As such, future investigations may wish to test for the distinction between IA and EA by measuring single unit activity and local field potentials during, for instance, GABAergic modulation. In addition, future ideas to investigate the relationship between changes in BOLD signal in CMS in response to IA and EA and changes in GABAA BPND may wish to include a challenge study [e.g., with tiagabine, Frankle et al., 2012]. Conceivably, even a behavioral paradigm during [18F]flumazenil PET scans can be applied, as changes in BPND would represent a more dynamic component of the GABAergic system.
In conclusion, this study demonstrates that both IA and EA induce NBRs in anterior and posterior midline regions. Moreover, IA and EA, when taken in comparison to fixation, each show a different association pattern to the BPND of GABAA receptors. Hence, GABAergic receptor availability, and thus potentially GABAergic activity, may be considered as an influencing factor in the mediation of a distinction between external, as compared to internal, processing in CMS. This might have implications for psychiatric disorders like MDD, which show a combination of altered awareness and GABAergic function.
Supporting information
Supplement Legends
Supplement Figure 1
Supplement Figure 2
Supplement Figure 3
Supplement Table 1
Supplement Table 2
Supplement Table 3
ACKNOWLEDGMENTS
The authors thank the staff from the MNI for their excellent technical support as well as K. Dedovic and A. Perna for helping with the subjects' recruitment and screening procedures. We would like to thank the reviewers for their constructive comments and suggestions, and Wm. Duncan for appraisal of the manuscript.
REFERENCES
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G (2010): Is subcortical‐cortical midline activity in depression mediated by glutamate and GABA? A cross‐species translational approach. Neurosci Biobehav Rev 34:592–605. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker‐Hopfe H, Heuser I, Neu P (2006): Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59:395–400. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961): An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM (2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23:137–152. [DOI] [PubMed] [Google Scholar]
- Bielau H, Steiner J, Mawrin C, Trubner K, Brisch R, Meyer‐Lotz G, Brodhun M, Dobrowolny H, Baumann B, Gos T, et al. (2007): Dysregulation of GABAergic neurotransmission in mood disorders: A postmortem study. Ann N Y Acad Sci 1096:157–169. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S (2007): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104:12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang‐Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S (2008): Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Ann N Y Acad Sci 1129:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Tshibanda L, Vanhaudenhuyse A, Noirhomme Q, Schnakers C, Ledoux D, Boveroux P, Garweg C, Lambermont B, Phillips C, et al. (2009): Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp 30:2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, O'Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, et al. (1998): Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage 8:249–261. [DOI] [PubMed] [Google Scholar]
- Costes N, Dagher A, Larcher K, Evans AC, Collins DL, Reilhac A (2009): Motion correction of multi‐frame PET data in neuroreceptor mapping: Simulation based validation. Neuroimage 47:1496–1505. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA (2007): Performance evaluation of the ECAT HRRT: An LSO‐LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol 52:1505–1526. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Near J, Blicher JU, Jezzard P (2010): Baseline GABA concentration and fMRI response. Neuroimage 53:392–398. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Enzi B, Wiebking C, Northoff G (2011): Involvement of glutamate in rest‐stimulus interaction between perigenual and supragenual anterior cingulate cortex: A combined fMRI‐MRS study. Hum Brain Mapp 32:2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Narendran R, Mason NS, Vora S, Litschge M, Price JC, Lewis DA, Mathis CA (2009): Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology 34:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Mason NS, Chen CM, Himes M, Walker C, Lewis DA, Mathis CA, Narendran R (2012): [11C]flumazenil binding is increased in a dose‐dependent manner with tiagabine‐induced elevations in GABA levels. PLoS One 7:e32443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44:2836–2845. [DOI] [PubMed] [Google Scholar]
- Frey KA, Holthoff VA, Koeppe RA, Jewett DM, Kilbourn MR, Kuhl DE (1991): Parametric in vivo imaging of benzodiazepine receptor distribution in human brain. Ann Neurol 30:663–672. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998): Event‐related fMRI: Characterizing differential responses. Neuroimage 7:30–40. [DOI] [PubMed] [Google Scholar]
- Goldberg, II , Harel M, Malach R (2006): When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron 50:329–339. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R (2007): Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex 17:766–777. [DOI] [PubMed] [Google Scholar]
- Golland Y, Golland P, Bentin S, Malach R (2008): Data‐driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia 46:540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gountouna VE, Job DE, McIntosh AM, Moorhead TW, Lymer GK, Whalley HC, Hall J, Waiter GD, Brennan D, McGonigle DJ, et al. (2010): Functional magnetic resonance imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage 49:552–560. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G (2011): Reduced negative BOLD responses in the default‐mode network and increased self‐focus in depression. World J Biol Psychiatry 12:627–637. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong IK, Chung ST, Kim HK, Kim YB, Son YD, Cho ZH (2007): Ultra fast symmetry and SIMD‐based projection‐backprojection (SSP) algorithm for 3‐D PET image reconstruction. IEEE Trans Med Imaging 26:789–803. [DOI] [PubMed] [Google Scholar]
- Hudson HM, Larkin RS (1994): Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 13:601–609. [DOI] [PubMed] [Google Scholar]
- James W (1890): The Principles of Psychology.New York:Dover Publications; pp402–458. [Google Scholar]
- Kalueff AV, Nutt DJ (2007): Role of GABA in anxiety and depression. Depress Anxiety 24:495–517. [DOI] [PubMed] [Google Scholar]
- Kaza E, Klose U, Lotze M (2011): Comparison of a 32‐channel with a 12‐channel head coil: Are there relevant improvements for functional imaging? J Magn Reson Imaging 34:173–183. [DOI] [PubMed] [Google Scholar]
- Kelly RE Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ (2010): Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods 189:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers UM, Veltman DJ, Boellaard R, Comans EF, Zuketto C, Yaqub M, Mourik JE, Lubberink M, Hoogendijk WJ, Lammertsma AA (2008): Comparison of plasma input and reference tissue models for analysing [(11)C]flumazenil studies. J Cereb Blood Flow Metab 28:579–587. [DOI] [PubMed] [Google Scholar]
- Klumpers UM, Boellaard R, Veltman DJ, Kloet RW, Hoogendijk WJ, Lammertsma AA (2012): Parametric [11C]flumazenil images. Nucl Med Commun 33:422–430. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI (2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A (2011): Where in‐vivo imaging meets cytoarchitectonics: The relationship between cortical thickness and neuronal density measured with high‐resolution [18F]flumazenil‐PET. Neuroimage 56:951–960. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies. Neuroimage 4 (3 Part 1):153–158. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ (2012): Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage 62:1040–1050. [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ (2010): Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 67:458–464. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996): Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2008): What we can do and what we cannot do with fMRI. Nature 453:869–878. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A (2009): How not to study spontaneous activity. Neuroimage 45:1080–1089. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH (2004): Parietal cortex and representation of the mental self. Proc Natl Acad Sci USA 101:6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N (2011): The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaweh G, Schirrmacher E, la Fougere C, Kovacevic M, Wangler C, Jolly D, Gravel P, Reader AJ, Thiel A, Schirrmacher R (2009): Improved work‐up procedure for the production of [(18)F]flumazenil and first results of its use with a high‐resolution research tomograph in human stroke. Nucl Med Biol 36:721–727. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ (1998): Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6:160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15:394–408. [DOI] [PubMed] [Google Scholar]
- Millet P, Graf C, Buck A, Walder B, Ibanez V (2002): Evaluation of the reference tissue models for PET and SPECT benzodiazepine binding parameters. Neuroimage 17:928–942. [PubMed] [Google Scholar]
- Möhler H (2012): The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62:42–53. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P (2007): GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci 10:1515–1517. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T (2010): Rest‐stimulus interaction in the brain: A review. Trends Neurosci 33:277–284. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE (2011): Brain imaging of the self—Conceptual, anatomical and methodological issues. Conscious Cogn 20:52–63. [DOI] [PubMed] [Google Scholar]
- Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen AJ, Krasikova RN, Farde L (2009): [18F]flumazenil binding to central benzodiazepine receptor studies by PET—Quantitative analysis and comparisons with [11C]flumazenil. Neuroimage 45:891–902. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2010): Interoception in anxiety and depression. Brain Struct Funct 214:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Gibson KM, Quezado Z, Dustin I, Taylor J, Trzcinski S, Schreiber J, Forester K, Reeves‐Tyer P, Liew C, et al. (2009): Decreased GABA‐A binding on FMZ‐PET in succinic semialdehyde dehydrogenase deficiency. Neurology 73:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F (1995): GABA and mood disorders: A brief review and hypothesis. J Affect Disord 34:275–281. [DOI] [PubMed] [Google Scholar]
- Pilc A, Nowak G (2005): GABAergic hypotheses of anxiety and depression: Focus on GABA‐B receptors. Drugs Today (Barc) 41:755–766. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA (2009): Independence in ROI analysis: Where is the voodoo? Soc Cogn Affect Neurosci 4:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C (2007): Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 1141:178–187. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2010): Two views of brain function. Trends Cogn Sci 14:180–190. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA (2005): Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol 493:167–176. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ (2007): A default mode of brain function: A brief history of an evolving idea. Neuroimage 37:1083–1090. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi E, Aalto S, Hirvonen J, Langsjo JW, Maksimow AT, Oikonen V, Metsahonkala L, Virkkala J, Nagren K, Scheinin H (2008): Measurement of GABAA receptor binding in vivo with [11C]flumazenil: A test‐retest study in healthy subjects. Neuroimage 41:260–269. [DOI] [PubMed] [Google Scholar]
- Sanacora G (2010): Cortical inhibition, γ‐aminobutyric acid, and major depression: There is plenty of smoke but is there fire? Biol Psychiatry 67:397–398. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB (2012): Introspective minds: Using ALE meta‐analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 7:e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K (2002): Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36:1195–1210. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL (2009): Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci USA 106:11096–11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR Jr, Snyder AZ, Gusnard DA, Raichle ME (2001): Emotion‐induced changes in human medial prefrontal cortex. I. During cognitive task performance. Proc Natl Acad Sci USA 98:683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Rudolph U (2012): Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology 62:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Buffett‐Jerrott SE, Kokaram R (2001): Heartbeat awareness and heart rate reactivity in anxiety sensitivity: A further investigation. J Anxiety Disord 15:535–553. [DOI] [PubMed] [Google Scholar]
- Sui J, Adali T, Pearlson GD, Calhoun VD (2009): An ICA‐based method for the identification of optimal FMRI features and components using combined group‐discriminative techniques. Neuroimage 46:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G, et al. (2011): Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci 23:570–578. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME (2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H (2009): Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition (a.k.a. ″Voodoo Correlations in Social Neuroscience). Perspect Psychol Sci 4:274–290. [DOI] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G (2009): The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66:478–486. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G (2010): Abnormal body perception and neural activity in the insula in depression: An fMRI study of the depressed "material me". World J Biol Psychiatry 11:538–549. [DOI] [PubMed] [Google Scholar]
- Wiebking C, de Greck M, Duncan NW, Heinzel A, Tempelmann C, Northoff G (2011): Are emotions associated with activity during rest or interoception? An exploratory fMRI study in healthy subjects. Neurosci Lett 491:87–92. [DOI] [PubMed] [Google Scholar]
- Wiersma JE, van Oppen P, van Schaik DJ, van der Does AJ, Beekman AT, Penninx BW (2011): Psychological characteristics of chronic depression: A longitudinal cohort study. J Clin Psychiatry 72:288–294. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45 (1 Suppl):S173–S186. [DOI] [PubMed] [Google Scholar]
- Zou KH, Greve DN, Wang M, Pieper SD, Warfield SK, White NS, Manandhar S, Brown GG, Vangel MG, Kikinis R, et al. (2005): Reproducibility of functional MR imaging: Preliminary results of prospective multi‐institutional study performed by Biomedical Informatics Research Network. Radiology 237:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Wu CW, Stein EA, Zang Y, Yang Y (2009): Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 48:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Legends
Supplement Figure 1
Supplement Figure 2
Supplement Figure 3
Supplement Table 1
Supplement Table 2
Supplement Table 3


