Abstract
Introduction
Blood oxygenation level‐dependent (BOLD) signal changes at the time of interictal epileptic discharges (IEDs) identify their associated vascular/hemodynamic responses. BOLD activations and deactivations can be found within the epileptogenic zone but also at a distance. Source imaging identifies electric (ESI) and magnetic (MSI) sources of IEDs, with the advantage of a higher temporal resolution. Therefore, the objective of our study was to evaluate the spatial concordance between ESI/MSI and BOLD responses for similar IEDs.
Methods
Twenty‐one patients with similar IEDs in simultaneous electroencephalogram/functional magnetic resonance imaging (EEG/fMRI) and in simultaneous EEG/magnetoencephalogram (MEG) recordings were studied. IEDs in EEG/fMRI acquisition were analyzed in an event‐related paradigm within a general linear model (GLM). ESI/MSI of averaged IEDs was performed using the Maximum Entropy on the Mean. We assessed the spatial concordance between ESI/MSI and clusters of BOLD activations/deactivations with surface‐based metrics.
Results
ESI/MSI were concordant with one BOLD cluster for 20/21 patients (concordance with activation: 14/21 patients, deactivation: 6/21 patients, no concordance: 1/21 patients; concordance with MSI only: 3/21, ESI only: 2/21). These BOLD clusters exhibited in 19/20 cases the most significant voxel. BOLD clusters that were spatially concordant with ESI/MSI were concordant with IEDs from invasive recordings in 8/11 patients (activations: 5/8, deactivations: 3/8).
Conclusion
As the results of BOLD, ESI and MSI are often concordant, they reinforce our confidence in all of them. ESI and MSI confirm the most significant BOLD cluster within BOLD maps, emphasizing the importance of these clusters for the definition of the epileptic focus. Hum Brain Mapp 35:4396–4414, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: electroencephalogram (EEG), EEG‐functional magnetic resonance imaging (EEG/fMRI), electric source imaging, focal epilepsy, intracranial EEG, magnetic source imaging, magnetoencephalogram (MEG)
INTRODUCTION
Combined recording of electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI) noninvasively assesses blood oxygenation level‐dependent (BOLD) signal changes at the time of interictal epileptiform discharges (IEDs) [Gotman and Pittau, 2011]. Positive and negative BOLD responses (activations and deactivations) in EEG/fMRI reflect metabolic changes related to the epileptogenic network [Kobayashi, et al., 2005; Laufs and Duncan, 2007]. BOLD responses usually occur in brain regions associated with the generation of IEDs [Benar et al., 2002; Donaire et al., 2013; Thornton et al., 2010], but they are also often seen outside the epileptogenic region [Fahoum et al., 2012; Kobayashi et al., 2006a, 2009; Wiest et al., 2013]. Deactivation within the epileptic focus is less often observed than activations, and their significance remains not totally understood [Fahoum et al., 2013; Kobayashi et al., 2006b; Pittau et al., 2013; van Houdt et al., 2013].
The clinical value of EEG/fMRI investigations has been demonstrated in the context of presurgical evaluation. EEG/fMRI findings support surgical decision when concordant with other noninvasive examinations like positron emission tomography (PET) and single photon emission tomography (SPECT) [Donaire et al., 2013; Moeller et al., 2009; Zijlmans et al., 2007]. Additional validation comes from EEG/fMRI studies using intracranial EEG electrodes, which revealed BOLD responses close to the most active EEG contacts [Cunningham et al., 2012; Vulliemoz et al., 2011]. BOLD clusters included the seizure onset zone in 83% of the patients in a quantitative comparison with subdural EEG [van Houdt et al., 2012, 2013]. Furthermore, a good surgical outcome has been found when the resection included regions showing activation [Thornton et al., 2010]. A limitation for the interpretation of EEG/fMRI BOLD responses to IEDs is the low temporal resolution of several seconds due to the dynamics of regional vascular changes elicited by increased neuronal activity. As a consequence, BOLD responses might comprise regions involved in the onset but also in propagation of IEDs. The limited temporal resolution might explain the multifocal pattern of BOLD responses in EEG/fMRI including areas within and at a distance from the epileptogenic region.
With a temporal resolution in the order of milliseconds, EEG provides essential information to characterize IEDs that cannot be retrieved from BOLD data. In EEG/fMRI, concordance of BOLD responses with the epileptic focus is usually defined as the region presumed to bear the generator of visually detected scalp EEG IEDs. However, the localization accuracy of scalp EEG is limited (possibly several centimetres) and lobar classification of IED generators from scalp topographies remains difficult.
Simultaneous recording of EEG and magnetoencephalogram (MEG) signals provides complementary and noninvasive depiction of similar IEDs due to their different characteristics. MEG is sensitive to tangentially oriented sources. EEG signals are especially sensitive to radial sources, while the low electric conductivity of the skull will impose more spatial smearing of the scalp signals [Goldenholz, et al., 2009; Hamalainen, 1992; Iwasaki, et al., 2005]. Electric and magnetic source imaging (ESI/MSI) could further help our understanding of the relationship between IED generators and BOLD responses, as it allows us to estimate the cortical sources of EEG/MEG signals in this comparison framework [Baillet et al., 2001]. ESI of scalp EEG IED could provide a better estimate of the generators of IEDs than visual evaluation of EEG traces, allowing comparison of their spatial concordance at a sublobar level [Brodbeck et al., 2011].
MSI can reveal additional sources for the understanding of BOLD responses, as one could expect that parts of the BOLD response, which are not explained by the EEG, could spatially correlate with MEG due to the complementary properties mentioned above. To the best of our knowledge, the correlation of MSI and BOLD responses has so far been reported only for a single epilepsy patient, showing a good correlation between the MEG equivalent current dipole (ECD) source and the BOLD activation [Vulliemoz et al., 2011].
Our objective was to systematically compare the localization of activations and deactivations with ESI and MSI of similar IEDs in a series of drug‐resistant focal epilepsy patients. Our comparison especially focused on the BOLD cluster that was most concordant with ESI and MSI per patient.
MATERIAL AND METHODS
Ethics Approval and Patients' Consent
This study was approved by the Montreal Neurological Institute Research Ethics Board and all patients signed a written informed consent prior to the study.
Patient Selection
We retrospectively selected all patients with drug resistant neocortical focal epilepsy evaluated at the Epilepsy Service from the Montreal Neurological Institute and Hospital who underwent a combined EEG/MEG acquisition and an EEG/fMRI acquisition between 2009 and 2012.
Patients with typical mesial temporal lobe epilepsy were excluded from this study, as the sensitivity for IEDs in the EEG/MEG is different compared to neocortical foci [Agirre‐Arrizubieta et al., 2009].
From 42 patients with a neocortical focus, 21 were excluded because no IEDs were detected during EEG/fMRI (n = 3), there was no significant BOLD response (n = 8), there were no similar IEDs in both EEG/fMRI and EEG/MEG sessions (n = 5), or there were artifacts caused by a vagal nerve stimulator in the MRI signal (n = 1). Four other patients had to be excluded because of other technical problems (artifacts of EEG electrodes in the region of interest for EEG/fMRI, magnetization artifacts in the MEG, unsatisfactory head positioning in the MEG dewar, MEG coregistration problem). For details about patients included in the final comparison, anatomical MRI findings and IEDs used for the comparison please see Tables 1, II and supplementary table 1.
Table 1.
Patients' characteristics
| Age | Mean 30 (range: 19–43) years |
|---|---|
| Epilepsy duration | Mean 15 (range: 1–29) years |
| Number of antiepileptic drugs | Mean 2 (range: 1–4) |
| Interval between EEG/fMRI and EEG/MEG recordings | 1.4 months (range: 0–6 months) |
| 3T structural MRI findings | Non‐lesional: N = 10; Focal cortical dysplasia: N = 4; double cortex: N = 1; gliosis, N = 1; hemimegalencephaly N = 1; polymicrogyria: N = 2; subcortical heterotopia: N = 2 |
| Epileptic focus defined by invasive recordings | 11 patients |
| Epilepsy surgery (follow up >12 months) | 10 patients |
EEG/fMRI Acquisition and Data Analysis
EEG/fMRI acquisition
Simultaneous EEG/fMRI data were acquired according to the institutional standard as described elsewhere [Moeller et al., 2009]. In summary, the EEG was acquired during MRI scans using 25 Ag/AgCl electrodes placed according to the 10/20 international system (19 usual electrodes without Fpz and Oz, reference at FCz) and six additional electrodes (F9, T9, P9, F10, T10, and P10). To improve comfort and reduce artifacts, patients' heads were immobilized with a pillow filled with foam microspheres (Siemens, Germany). EEG data were recorded with an MRI compatible BrainAmp amplifier with a sampling rate of 5 kHz (Brain Products, Munich, Germany).
MRI data were acquired using a 3T Siemens Trio MRI scanner (Siemens, Germany). We acquired a T1‐weighted anatomical MRI sequence (1‐mm slice thickness, 256 × 256 matrix, TE = 4.18 ms, TR = 23 ms, flip angle 9°), followed by runs of 6 min of a T2* weighted echo planar imaging (EPI) ‐sequence (3.7 × 3.7 × 3.7 mm3 voxels, 33 slices, 64 × 64 matrix TE = 25 ms, TR = 1,900 ms, flip angle 90°). Depending on patient's tolerance, nine to 14 EPI runs were acquired.
BrainVision Analyser (Brain Products, Munich, Germany) was used for off‐line correction of the gradient artifact and for EEG filtering [Allen et al., 2000]. An additional 50‐Hz low‐pass filter was applied to remove remaining artifacts. The ballistocardiogram artifact was removed using independent component analysis [Benar et al., 2003]. The EEG was reviewed by a neurologist for visual identification and marking of IEDs. A clinical neurophysiologist (M.H.) reviewed the markings to select typical IEDs and to ensure that the comparison with the combined EEG/MEG recordings included similar IED types based on their morphology and topography.
FMRI data analysis
The EPI data underwent first motion correction (realignment with 6‐parameter rigid‐body transformations) and spatial smoothing (6mm full width at half maximum (FWHM)) using the software package of the Brain Imaging Center of the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca/software/). Temporal autocorrelations were accounted for by fitting an autoregressive model of order 1 [Worsley et al., 2002] and low frequency drifts in the signal were modeled with a third‐order polynomial fitted to each run. Regressors for each IED type were built using the timing/duration of each event, convolved with four hemodynamic response functions (HFRs) with peaks at 3, 5, 7, and 9 s [Bagshaw et al., 2004] to increase sensitivity. For each HRF model, the BOLD response to IEDs was analyzed using a General Linear Model (GLM). The six parameters estimated during motion correction were also included in the GLM analysis as confound regressors, in order to account for residual movement artifacts. During standard fMRI analysis, a statistical t‐map was estimated for each regressor using fMRIStat software [Worsley et al., 2002]. At each voxel, a “combined” map was then estimated: for each voxel the maximum t value estimated from the four analyses obtained with the four HRFs was taken [Bagshaw et al., 2004].
EEG/MEG Data Acquisition and Analysis
EEG/MEG data acquisition
Combined EEG/MEG recordings were performed in a 275 channel CTF‐MEG‐ system (MISL, Vancouver, Canada) using a cap with 54 with ceramic EEG electrodes (Easy‐cap, Herrsching, Germany). EEG electrodes were placed according to the 10/20 system with additional inferior temporal electrodes according to the 10/10 system. Anatomical landmarks (nasion, left and right preauricular points), EEG electrodes position and a series of points distributed on the scalp and fronthead (headshape) were digitized prior to the EEG/MEG recordings using a Polhemus 3D localizer (Colchester, NH). EEG/MEG signals were recorded with patients at rest in a supine position. The total duration depended on the patient's comfort and occurrence of clinical events (on average, 8–10 runs of 6 min recording). No filters were applied to the MEG recording and a hardware high pass filter of 0.03 Hz was used for the EEG. The sampling rate was 1,200 Hz. Three coils placed on anatomical landmarks were used for continuous head localization during the recording and runs with head movements exceeding 0.5 mm were discarded.
EEG/MEG data were pre‐processed and evaluated offline. A DC‐offset was removed and data were bandpass filtered between 0.3 and 70 Hz (60‐Hz notch filter was also applied). Bad EEG channels were removed and an average reference montage was created for EEG signal browsing. In one patient, magnetic artifacts created by the presence of a vagal nerve stimulator were removed using principal component analysis prior to the evaluation and source localization of the MEG data (patient 8).
EEG/MEG data were evaluated for presence of IEDs that were similar, in morphology and topography, to those recorded in the EEG/fMRI acquisition. They were visually marked using DataEditor software (MISL, Vancouver, Canada) by two clinical neurophysiologists (M.H., E.K.). EEG/MEG spike marking and source localization procedures were performed blindly to the knowledge of EEG/fMRI results.
Electric and magnetic source imaging (ESI/MSI)
Forward model and EEG/MEG data processing
The anatomical T1‐MRI for source localization consisted of a T1W MPRAGE 1mm isotropic 3D acquisition (192 sagittal slices, 256 × 256 matrix, TE = 2.98 ms, TR = 2.3 s, flip angle adjusted according to AC‐PC), acquired in a Siemens Tim Trio 3T scanner at a separate scanning session without EEG electrodes. For each patient, the surfaces of the skin and of the grey–white matter junction were segmented from the anatomical MRI using BrainVISA‐4.2.1 software [Mangin et al., 1995]. The cortical surface mesh used for the source space (∼8,000 dipolar sources oriented perpendicular to the surface) was tessellated from the grey–white matter interface. The anatomical MRI as well as the skin and cortical surfaces were imported in brainstorm software [Tadel et al., 2011], where individual three‐layers‐BEM‐surfaces were reconstructed (inner‐skull, outer‐skull and skin). A 1‐layer boundary element model (BEM) (conductivity: 0.33 S m−1) was calculated for MEG and a three‐shell BEM (conductivity: brain 0.33 S m−1, skull 0.165 S m−1, and skin 0.33 S m−1; brain‐to‐skull ratio: 1/20) [Chen et al., 2010; Goncalves et al., 2003] was estimated for EEG source localization using OpenMEEG implementation in the Brainstorm software [Gramfort et al., 2010]. The anatomical MRI and the surface segmentations were co‐registered with the EEG/MEG data by surface fitting between the skin surface and a series of points of the headshape digitized on the head of the subject using a Polhemus localization device. Epochs (−100 ms to 300ms) around every IED marked in EEG/MEG and a 2‐s baseline were selected; these were down‐sampled to 600 Hz and imported into brainstorm.
Inverse modeling using the Maximum Entropy on the Mean (MEM) method
Source imaging was performed on averaged IEDs [Bast et al., 2004] using the MEM method [Amblard et al., 2004; Chowdhury et al., 2013; Grova et al., 2006b]. Source imaging findings at the peak of IEDs were used for comparison with BOLD response.
For IEDs occurring across multiple runs, averaged IEDs results were first localized for each run and then source localization results were averaged across runs in the source space. The source volume was restricted to the grey/white matter junction. The MEM method was selected to solve the inverse problem due to its sensitivity for the spatial extent of the underlying sources, as previously evaluated in EEG [Grova et al., 2006b] and in MEG [Chowdhury et al., 2013].
EEG/fMRI and ESI/MSI Comparison
Coregistration of fMRI and ESI/MSI results
To allow quantitative comparison between spatially extended ESI/MSI and fMRI results data needs to be estimated on the same spatial support, i.e., on the cortical surface considered for EEG and MEG source localization as described by our group before [Grova et al., 2008b]. To do so, the anatomical MRI used to extract the cortical surface was first co‐registered with the MRI acquired during the EEG/fMRI session [Collins et al., 1994], by maximizing the correlation coefficient between the two volumes using a rigid transformation (three translations, three rotations, one scaling parameter).
Cluster analysis of the BOLD responses
To specifically determine what BOLD responses and what EEG/MEG sources were spatially concordant, we performed a cluster size test of significant positive and negative BOLD clusters from the “combined” t‐map.
This statistical map was thresholded using the minimum given by a Bonferroni correction and random field theory, taking into account the spatial correlation of the errors [Worsley et al., 2002]. A 6‐mm FWHM Gaussian smoothing was applied during preprocessing. For each of the four HRF analyses, an average brain FWHM was estimated from the residuals of the inter‐run fixed effects analysis [Moeller et al., 2009]. We then considered the averaged value obtained over the 4 HRF analyses, in order to attribute an empirical FWHM value for the combined map (8 mm on average) for the cluster size test. All voxels showing a t value >3.17 or <−3.17 (uncorrected P < 0.001) located within the brain mask were included in this cluster analysis. A cluster was defined as a set of spatially connected voxels passing this criterion. Subsequently a cluster size test was applied, providing for each cluster a corrected p value for its spatial extent [Worsley et al., 2002]. We considered as significant every cluster showing a corrected p < 0.0125 for its spatial extent (i.e., corrected p < 0.05 / 4, Bonferroni correction for the four HRF analysis, as explained above). These selected clusters were then considered for the comparison with ESI/MSI results. Positive clusters obtained from the positive BOLD response were defined as activations and clusters obtained from the negative BOLD responses were defined as deactivations.
An expert reviewer (D.A.) assessed all deactivations (blindly to the results of the ESI/MSI) for their occurrence in the ventricles and within the default mode network topography (defined qualitatively and literature‐based [Meindl et al., 2010; Raichle et al., 2001]). All deactivations completely within posterior cingulate cortex, precuneus, medial prefrontal cortex, and lateral parietal cortex were excluded. They were not considered in the comparison with ESI and MSI, since default mode network deactivations are not considered as a direct consequence of the generation of the IEDs [Fahoum et al., 2012; Gotman et al., 2005; Laufs and Duncan, 2007], whereas ventricles deactivation were considered as artifacts.
Interpolation of significant BOLD clusters on the cortical surface
BOLD results are obtained within a volume space consisting in a 3D grid of voxels. For quantitative comparison with ESI/MSI findings estimated along the cortical surface segmented from the gray–white matter interface, we had to interpolate BOLD results from the volume space to the same cortical surface.
To do so, the binary mask of each significant BOLD cluster as well as the combined t‐map were interpolated on the cortical surface, using the method described in Grova, et al. [2006c] (see also Figs. 2A,3A,4A,5A). The objective of this interpolation is to associate an interpolation kernel (i.e. a list of voxels) to each vertex of the cortical surface over which BOLD results will be averaged. First, only voxels located within a mask obtained by a 5‐mm dilatation of the grey–white‐matter junction were considered for the interpolation. Then, non‐overlapping interpolation kernels (Voronoï cells) were built for each of the 8,000 vertices of the cortical surface. As we demonstrated in Grova et al. [2006b], this method allows generating relatively uniform interpolation kernels around each vertex of the cortical surface, while taking into account the underlying cortical folding of each subject.
Figure 2.
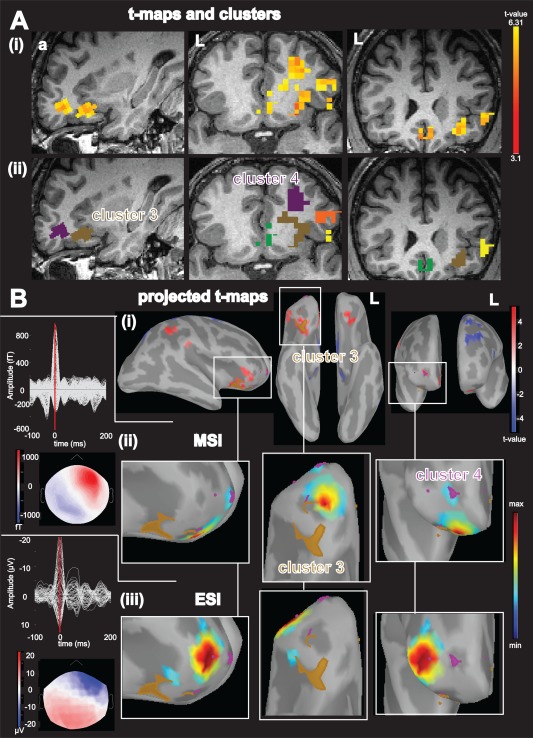
Concordance of different regional BOLD activations with ESI and MSI at the peak of the averaged IED for patient 5, A: (i): Activations (yellow‐red color code) superimposed over the patient's T1 MRI (from left to right: sagittal view, axial view, coronal view); (ii): result of the BOLD cluster size test presented in the volume space super‐imposed over the individual T1 MRI of the patient. Each color labels a specific cluster considered for the comparison with ESI/MSI: cluster 3: brown, cluster 4: purple; B: (i): Combined BOLD t‐map projected over the cortical surface and represented over the inflated brain using Brainstorm software. Activations results are presented using a red‐white color code and deactivations are presented using blue‐white color code. The projection of activation cluster 3 is highlighted in brown, and the activation cluster 4 is highlighted in purple, red arrow points toward the vertex with highest t value, from left to right: right lateral view, lower view, frontal view; (ii): MSI, left: superimposed MEG signals for the averaged IED and bottom corresponding MEG spatial topography at the peak of the IED (blue: negative, red: positive), right: magnified comparison between BOLD activation cluster 3 and 4 and MSI jet‐color scale (maximum red, thresholded at 70% of the maximum (max. 70%), max. 30% marked on colorbar with white rectangle), same orientation as row above. (iii): superimposed EEG signals for the averaged IED and bottom the corresponding EEG spatial topography at the peak of the EEG IED (blue negative, red positive), right: magnified ESI results, same color code, threshold and orientation as for comparison with MSI. The magnified figures are chosen to better illustrate the regional comparison of ESI and MSI with the BOLD responses, all significant source imaging results are shown. Abbreviations: fT: femto‐tesla, µV: micro volt, L: left, a: anterior MSI finding thresholded at maximum 30% (max. 30%) was concordant (Dmin<5mm, AUC >70%) with cluster number 3 and 4, while the ESI finding was concordant with cluster number 4 only. Please note also the BOLD responses involving areas of both hemispheres. For Figures 2, 3, 4, 5, 6 the MSI and ESI findings are thresholded at max. 70% (not max 30% which was used for the concordance comparison with the BOLD) to further illustrate the extent of the source. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com]
Figure 3.
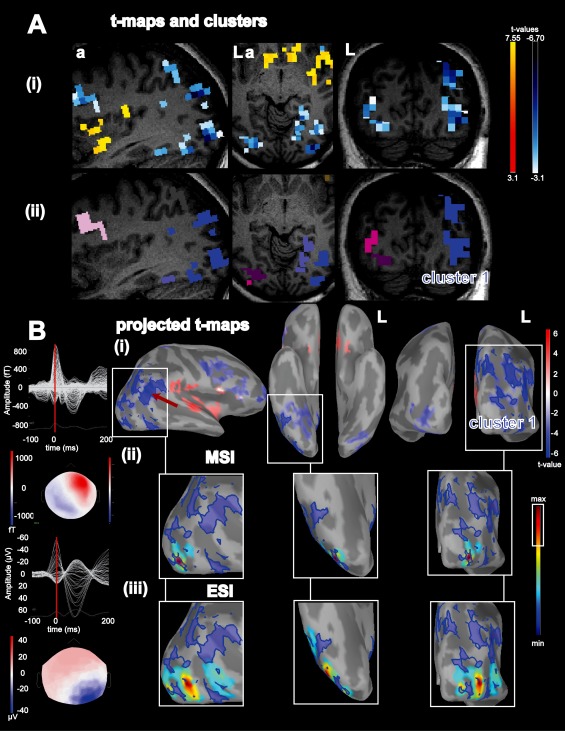
Concordance of BOLD deactivation with ESI/MSI for patient 4: A: (i), Activations (yellow‐red color code) and deactivations (blue‐white colorcode) superimposed over the patient's T1 MRI (from left to right: sagittal view, axial view, coronal view); (ii): results from the BOLD cluster size test presented in the volume space superimposed over the patient's T1 MRI. Each color labels a specific cluster considered for the comparison with ESI/MSI, Cluster 1: blue. B: (i): Combined BOLD t‐map projected over the cortical surface and represented over the inflated brain using Brainstorm software. Activations are presented using a red‐white color code and deactivations are presented using blue‐white color code. The projection of cluster 1 is highlighted in blue, red arrow points towards the vertex with lowest t value, from left to right: right lateral view, lower view, posterior view; (ii) MSI left: superimposed MEG signals of the averaged interictal epileptic discharge (IED) and bottom coresponding spatial topography at the peak of the IED (blue: negative, red: positive), right: magnified comparison between BOLD deactivation cluster 1 and MSI (jet‐colorscale, max. red, thresholded at 70% of the max), same orientation as row above. (iii) ESI left: superimposed signals of the averaged EEG IED and bottom the corresponding EEG spatial topography at the peak of the EEG IED (blue negative, red positive), right: magnified ESI results, same color code, threshold and orientation as for comparison with MSI. abbreviations: fT: femto‐tesla, µV: micro volt, L: left, a: anterior. ESI and MSI thresholded at max. 30% were concordant with deactivation cluster number 1 extending over the right temporo‐parieto‐occipital area. ESI and MSI were concordant with each other as well. For this patient the invasive recordings confirmed right temporo‐parieto‐occipital IED and the seizure onset zone in the same region. Please note also the high amplitude slow wave following the IED, which is more pronounced in EEG than in MEG. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
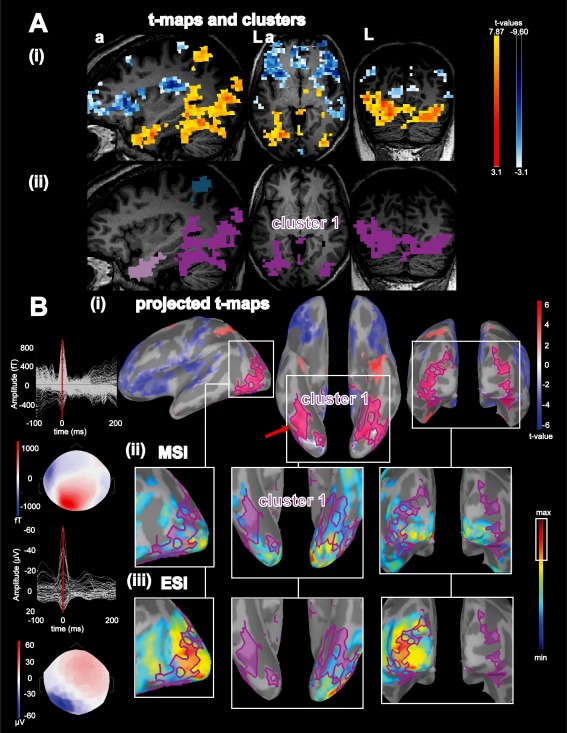
Concordance of BOLD activation with ESI/MSI for patient 15: A: (i): Activations (yellow‐red colorcode) and deactivations (blue‐white colorcode) superimposed over the patient's T1 MRI, from left to right: sagittal view, axial view, coronal view; (ii): results from the BOLD clusters size test presented in the volume space superimposed over the patient's T1 MRI. Each color labels a specific cluster considered for the comparison with ESI/MSI, activation cluster 1: purple. B: (i): Combined BOLD t‐map projected onto the cortical surface and represented over the inflated brain using Brainstorm software. Activations are presented using a red‐white color code and deactivations are presented using blue‐white color code. The projection of activation cluster 1 was highlighted in purple, red arrow points towards the vertex with highest t value, from left to right: left lateral view, lower view, posterior view; (ii) MSI: left: superimposed signals of MEG interictal discharges (IED) and bottom coresponding spatial topography at the peak of the IED (blue: negative, red: positive), right: magnified comparison between activation cluster 1 and magnetic source imaging (MSI) jet‐colorscale (max red, thresholded at 70% of the max, max. 30% marked on colorbar with white rectangle), same orientation as row above. (iii) ESI left: superimposed averaged signals of EEG IED and bottom the corresponding EEG spatial topography at the peak of the EEG IED (blue negative, red positive), right: magnified ESI results same colorscale, threshold and orientation as for comparison with MSI. abbreviations: fT: femto‐tesla, µV: micro Volt, L: left, a: anterior. ESI and MSI thresholded at max. 30% were concordant with the left hemispheric part of cluster 1 over the left lateral occipital lobe. In addition ESI and MSI were concordant with each other although MSI findings extended to the contralateral hemisphere. For this patient the BOLD clusters extended to deep basal occipital and basal temporal structures. Because these parts were not covered by ESI/MSI this suggests the added value of the BOLD response for deep brain regions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 5.
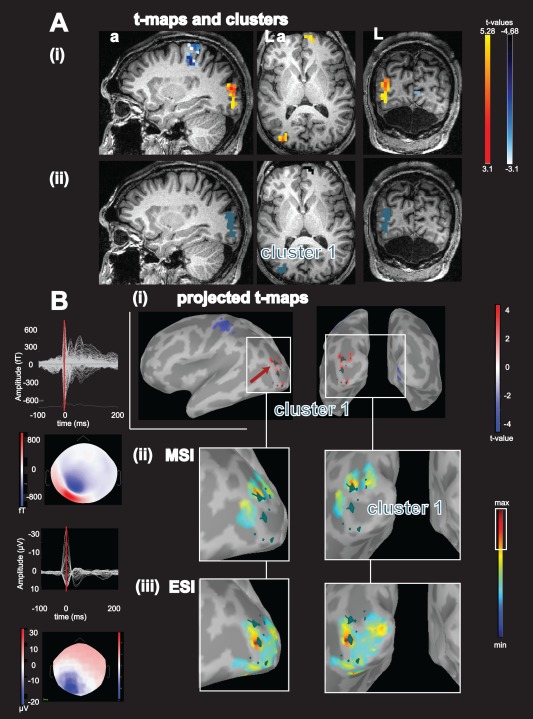
Concordance of BOLD activation with ESI/MSI for patient 17: A: (i):, Activations (yellow‐red color code) and deactivations (blue‐white colorcode) superimposed over the patient's T1 MRI, from left to right: sagittal view, axial view, coronal view; (ii): results from the BOLD cluster size test presented in the volume space superimposed on the patient's T1 MRI. Each color labels a specific cluster considered for the comparison with ESI/MSI, activation cluster 1: blue‐green. B: (i): Combined BOLD t‐map projected over the cortical surface and represented over the inflated brain using Brainstorm software. Activations are presented using a red‐white color code and deactivations are presented using blue‐white color code. The projection of activation cluster 1 is highlighted in blue‐green, red arrow points toward the vertex with highest t value, from left to right: left lateral view, posterior view; (ii) MSI: left: superimposed signals of MEG interictal discharges (IED) and bottom coresponding spatial topography at the peak of the IED (blue negative, red positive), right: magnified comparison between activation cluster 1 and magnetic source imaging (MSI) jet‐colorscale (max red, thresholded at 70% of the max, max. 30% marked on colorbar with white rectangle), same orientation as row above. (iii) ESI left: superimposed averaged signals of EEG IED and bottom the corresponding EEG spatial topography at the peak of the EEG IED, right: magnified ESI results. Abbreviations: fT: femto‐tesla, µV: micro volt, L: left, a: anterior. ESI and MSI thresholded at max 30% were concordant with the left occipital activation cluster 1. This figure nicely illustrates the different characteristics of ESI and MSI: The ESI peak is found on top of the gyration, whereas the peaks of the MSI findings are localized over the borders of the sulci, which are most often the generators of tangential sources. Please note that ESI and MSI are concordant with different parts of the BOLD cluster. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For each vertex of the cortical surface, the t values of the voxels belonging to the corresponding Voronoï cell were then averaged. The corresponding averaged t value was associated to this vertex of the cortical surface, only when at least 30% of the voxels of the interpolation kernel showed a significant response. This allowed us to estimate fMRI results on the same spatial support as the one used for ESI/MSI. The threshold of 30% was chosen to prevent overestimation of the final cluster size after interpolation on the surface.
Subcortical BOLD clusters were not interpolated on the lateral cortical surface, since either they did not lie within the 5‐mm dilated mask or they were interpolated along the midline between the two hemispheres (for thalamic activations notably). In these brain regions it was clearly unlikely to find ESI/MSI results.
Comparison of BOLD Clusters and ESI/MSI Results
To assess the spatial overlap between BOLD clusters and ESI/MSI along the cortical surface, we used as a comparison metric the area under the curve value (AUC) of a receiver operating characteristic (ROC) curve estimated by applying various thresholds on ESI/MSI maps [Grova et al., 2008a], thus avoiding the definition of a statistical threshold on source maps. For this “ROC” analysis, the set of vertices belonging to the selected BOLD cluster were considered as the “Ground Truth.” This method allowed approximating the spatial overlap of the different methods in a single value, while integrating all possible thresholds of ESI/MSI maps, obtained at the peak of the averaged IEDs. A perfect spatial match between a BOLD cluster and ESI or MSI reconstruction should correspond to an AUC value close to 100%. AUC was considered as an interesting metric to assess spatial overlap, because a perfect one‐to‐one correspondence is unlikely to occur since fMRI and ESI/MSI are measuring processes of different natures (hemodynamic response versus electrophysiology). Therefore an AUC value >70% was considered as a good spatial overlap. As this analysis was done for each BOLD cluster, vertices belonging to the cluster of interest were considered as the “truth,” whereas vertices belonging to other clusters were not considered for the AUC estimation (please see the Appendix for details on the AUC comparison).
In addition, we estimated the minimum geodesic distance D min (i.e., distance following sulco‐gyral morphology) between ESI/MSI sources maps thresholded at 70% of their maximum value (max 30%) and the closest vertex belonging to the corresponding BOLD cluster. The arbitrary max 30% was chosen to have a restrictive value to estimate the spatial extent of the electromagnetic source, as there is currently no statistical value to estimate it. The concordance of ESI/MSI and EEG/fMRI activations and deactivations was evaluated using the following criteria:
▪ Concordant: overlap (D min = 0) between the max 30% of the ESI/MSI and a significant BOLD cluster or geodesic distance D min < 5mm together with an AUC value >70%
▪ Regionally concordant: geodesic distance D min < 20 mm, for any AUC value.
▪ Not concordant: comparisons not meeting the above mentioned criteria. If the maximum of the ESI/MSI was found on the other side of the Sylvian fissure or of the interhemispheric fissure when compared to the BOLD cluster, they were also assessed as not concordant.
Within this group of regionally concordant or concordant clusters, we identified in each patient the BOLD cluster, which was most concordant with ESI, MSI and ESI/MSI. In patients with multiple clusters that were concordant with ESI and MSI, the cluster that was concordant with both (i.e., ESI and MSI) was chosen. This cluster was defined as “the most concordant BOLD cluster”.
We also performed a comparison between ESI and MSI results. This evaluation was qualitative and performed on visual inspection.
Comparison of iEEG in Patients With Circumscribed Lesions With ESI/MSI and BOLD Responses
The comparison of ESI/MSI and BOLD responses with iEEG findings and lesions was based on iEEG reports, post‐implantation CT/MRI scans and clinical MRI. This evaluation was qualitative and performed on visual inspection, since the positions of iEEG electrodes were not co‐registered with our functional findings. The additional methodology required to propose a quantitative comparison with iEEG was out of the scope of this study. Concordance was defined as spatial overlap between the modalities. Regional concordance was defined as maximum 2‐cm sublobar distance between the different modalities. As we could not compare all BOLD clusters with invasive EEG, we analyzed only clusters identified as the most concordant with ESI/MSI results. When the BOLD cluster was spatially too extended, we considered the part of the clusters identified by ESI/MSI results.
The epileptic focus was called “defined” by iEEG when electrodes recorded IEDs and the seizure onset zone (SOZ).
RESULTS
Concordance of BOLD Clusters with ESI and MSI
A total of 97 activations/69 deactivations were compared with ESI, whereas 99 activations/71 deactivations were compared with MSI. The high number of BOLD clusters included in this comparison emphasizes the fact that EEG/fMRI data usually provides extended and distributed BOLD responses over many brain regions. Whereas most of these clusters were spatially not closely related to our ESI and MSI results, we identified a subset of 44 comparisons between BOLD activations, deactivations, ESI and MSI satisfying our selection criteria for concordance and regional concordance (Fig. 1, Table 2). There was no difference in cluster volume for activations and deactivations.
Figure 1.
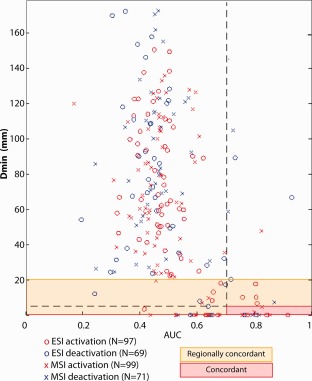
The geodesic distance (D min) is illustrated as a function of the spatial overlap measurement AUC for all comparisons between electric and magnetic source imaging (ESI/MSI) and BOLD activation/deactivation in all patients (ESI: X, MSI: O, activation: red, deactivation: blue). Regionally concordant comparison are highlighted by an orange rectangle and concordant comparisons are highlighted by the red rectangle and the red horizontal line starting at D min and AUC = 0. Please note that only a minority of comparisons was found to be at least regionally concordant. Many comparisons between ESI/MSI and BOLD clusters show a AUC value of about 0.5, meaning that they were not obviously related. However the most concordant clusters were usually the ones associated with the most significant BOLD response. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Summary of the multimodal comparison per patient
| ID | Spike types selected | No. of IED EEG/MEG | No. IED EEG/fMRI | Anatomical MRI | Activation‐ESI | Activation‐MSI | Deactivation‐MSI | Deactivation‐ESI | MSI‐ESI | MSI‐iEEG/ circumscribed lesion | ESI‐iEEG/ circumscribed lesion | Activation iEEG/circumscribed lesion | Deactivation ‐ iEEG/ circumscribed lesion | Type of surgery | Engel Outcome | Follow up (months) | Histology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LF | 37/30 | 165 | Normal | rc (cl 1) | n | % | % | rc | Focus nd | Focus nd | Focus nd | % | % | % | ||
| 2 | RFT | 24/48 | 299 | Normal | n | n | n | n | n | c IED/ SOZ | ESI focus nc | nc by iEEG | nc by iEEG | % | % | ||
| 3 | LFT | 424/240 | 387 | Normal | rc (cl 1) | n | c (cl 1) | rc (cl 1) | n | c IED/ SOZ | ESI focus nc | nc by iEEG | c | % | % | ||
| 4 | RTPO | 327/58 | 112 | FCD RTP | n | n | c (cl 1) | c (cl 1) | c | c IED/ SOZ, rc lesion | c IED/ SOZ, rc lesion | nc by iEEG | c IED/SOZ, lesion | resection of lesion RTPO | 1a | 12 | FCD 2a |
| 5 | RF | 37/21 | 10 | FCD RF | c (cl 4), (rc: cl 5, cl 9) | c (cl 3 & cl 4) | n | n | c | rc lesion | rc lesion | no iEEG, rc lesion | % | RF resection lesion | 1a | 12 | FCD 2a |
| 6 | RF | 102/159 | 68 | FCD RF | rc (cl 1) | rc (cl 1) | n | n | rc | c IED/ SOZ, lesion | c IED/ SOZ, lesion | c IED/SOZ, lesion | % | RF resection lesion | 1a | 12 | FCD 2b |
| 7 | LT | 106/35 | 85 | Normal | c (cl 1) | rc (cl 1) | n | n | rc | % | % | no iEEG | % | % | |||
| 8 | LF | ‐/19 | 91 | Normal | % | c (cl 1) | n | % | % | focus nd | focus nd | focus nd | focus nd | % | |||
| 9 | RF | 163/16 | 87 | Double cortex | n | c (cl 1) | c (cl 1) | c (cl 1) | rc | % | % | % | % | % | |||
| 10 | RF | 200/60 | 161 | Normal | c (cl 1) | c (cl 1) | n | rc (cl 1) | c | % | % | % | % | % | |||
| 11 | LT | 17/17 | 15 | L hem. PMG | n | rc (cl 2) | n | n | n | c IED/ SOZ | c IED, but not SOZ | rc IED/SOZ | % | LF resection | 4 | 12 | PMG |
| 12 | RT | 28/64 | 89 | Extended subcortical heterotopia right posterior lateral ventricle | n | n | c (cl 2) | c (cl 2) | c | % | % | % | % | % | |||
| 13 | LTP | 14/26 | 275 | Extended subcortical heterotopia left posterior horn lateral ventricle | rc (cl 2) | c (cl 2) | n | n | n | c IED, but not SOZ | c IED/ SOZ | rc IED/SOZ | no covered by iEEG | % | |||
| 14 | RFT | 9/5 | 25 | R hemispheric PMG | n | c (cl 1) | % | % | n | MSI focus nc | ESI focus nc | nc by iEEG | % | 1.) RTP 2.) RT anterior resection | 3 | 18 (after second surgery) | FCD 2a (PMG) |
| 15 | LPO | 30/23 | 39 | Normal | c (cl 1) | c (cl 1) | c (cl 3) | rc (cl 4) | c | % | % | no iEEG | no iEEG | % | |||
| 16 | LP | 12/6 | 27 | Normal | rc (cl 2) | n | n | n | c | c IED/ SOZ | c IED/ SOZ | rc IED/SOZ | nc by iEEG | 2 surgeries LC | 4 | 12 (after second surgery) | migrational type disorder |
| 17 | LPO | 432/95 | 328 | Normal | c (cl 1) | c (cl 1) | n | n | c | % | % | no iEEG | no iEEG | % | |||
| 18 | RFT | 119/478 | 192 | RF FCD | c (cl 2) | c (cl 2) | n | n | c | c IED, SOZ, lesion | c IED, SOZ, lesion | c IED, SOZ, lesion | c IED, SOZ, lesion | LF resection lesion | 1a | 24 | FCD 2a |
| 19 | RF | 32/18 | 53 | R HME | n | n | rc (cl 3) | rc (cl 3) | c | MSI focus nc | ESI focus nc | nc by iEEG | nc by iEEG | RF resection | 3 | 12 | MCD consistent with HME |
| 20 | RPO | 120/9 | 897 | normal | n | n | c (cl 1) | c (cl 1) | rc | c IED/ SOZ | c IED/ SOZ | nc by iEEG | c IED/SOZ | 2 surgeries (RO & RTO) | 4 | 12 (after second surgery) | Gliosis |
| 21 | RT | 9/6 | 74 | R parahippocampal dysplasia | c (cl 1) | c (cl 1) | n | rc (cl 4) | rc | % | % | no iEEG | no iEEG | RT anterior neocortical resection | 4 | 15 (after second surgery) | migrational type disorder |
The most concordant clusters per patient is highlighted in italics. For all patients but patient 19 the cluster marked in italics is also the most significant cluster (patient 19: deactivation cluster 1 most significant). Abbreviations: c: concordant, F: frontal lobe, FCD: focal cortical dysplasia, HME: hemimegalencephaly, iEEG: invasive EEG, IED: interictal epileptic discharges, n: not concordant, nc: not covered, nd: not defined, MCD: malformation of cortical development, PNH: periventricular nodular heterotopia, PMG: polymicrogyria, L: left, O: occipital lobe, OF: orbito‐frontal, P: parietal lobe, R: right, rc: regionally concordant, SOZ: seizure onset zone, T: temporal lobe, %: not available.
In 19/21 patients the BOLD cluster showing the best spatial concordance with ESI/MSI (N = 14 patients), ESI only (N = 2 patients), and MSI only (N = 3 patients) was also the one corresponding to the most significant t value, including 14 activations and five deactivations.
In addition to the comparison of ESI/MSI with the BOLD cluster that was found to be the most concordant per patient (N = 20/21 patients, each with one most concordant BOLD cluster, i.e. 35/44 comparisons between ESI, MSI and BOLD), 9/44 comparisons between ESI/MSI and BOLD clusters fulfilled our criteria of concordance or regional concordance (Table 2, Fig. 1). The importance of these findings is not known and it was not the primary aim of this study to further evaluate this matter.
Concordance of ESI with activations and deactivations
In 17/20 patients (all except patient 2, 11, 14), ESI results were concordant or regionally concordant with one activation (N = 11 patients; Figs. 2, 4, and 5) or with one deactivation (N = 6 patients, Figs. 3 and 6). Note that for patient 3, ESI results showed a source that was concordant with both an activation and a deactivation. Because AUC was larger for the deactivation than for the activation in this patient, better spatial overlap was obtained with the deactivation.
Figure 6.
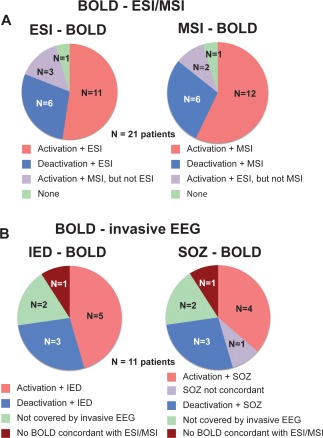
A: Comparison of ESI and MSI with most concordant BOLD cluster per patient (N = 21), left: ESI‐BOLD comparison: The most concordant BOLD cluster (activations: 11/21 patients, deactivations: 6/21 patients) is usually concordant with ESI (N = 16/21 patients). Right: comparison between MSI and BOLD clusters. The comparison between MSI and BOLD confirmed the comparison of ESI and BOLD clusters (15/21 patients concordance of MSI with same cluster as ESI). The BOLD cluster, which is most concordant with ESI, MSI or both contains the most significant voxel, with only one exception (patient 19). B: Comparison of the BOLD cluster, which is most concordant with ESI/MSI, with invasive EEG (iEEG) in patients with defined epileptic focus (N = 11), left: Comparison of BOLD clusters with IED. In eight patients the most concordant BOLD cluster was confirmed by IED from iEEG. Right: Comparison of the same BOLD clusters with the seizure onset zone (SOZ) recorded by iEEG. The SOZ recorded by iEEG was concordant with the same BOLD clusters as the IED recorded by iEEG with one exception. Only for one patient the SOZ differed (patient 13) from the comparison with the IED. For this patient the SOZ recorded by iEEG was within the subcortical heterotopia. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Concordance of MSI with activations and deactivations
MSI was most concordant with either an activation (N = 12 patients; Figs. 2, 4, and 5) or a deactivation (N = 6 patients; Figs. 3 and 6) in 18/21 patients. For two of these patients (patients 15 and 9), MSI results were concordant with both an activation and a deactivation. For patient 15, the concordant activation (cluster 1) was actually the one showing the highest t value, which was also concordant with ESI results (Fig. 4). For patient 9, there was a slight spatial overlap between the concordant activation and deactivation due to the method used to project the clusters on the cortical surface. In this patient the deactivation exhibited the most significant response (highest absolute t value) and ESI was also concordant with the same deactivation (Fig. 6a).
Complementarity between ESI and MSI results in the evaluation of activations and deactivations
MSI confirmed the concordances observed between ESI and BOLD clusters in 15/20 patients with ESI and MSI findings (activation: N = 9 patients, deactivations: N = 6 patients). For 2/20 patients only MSI was concordant or regionally concordant with an activation and for two patients only ESI was concordant or regionally concordant with an activation.
As illustrated in Figure 2, ESI and MSI results for patient 5 were concordant with multiple regional BOLD clusters. Whereas EEG sources were concordant with a single activation, MEG sources were concordant with the same activation (cluster 3) and with an additional one (cluster 4). These findings suggest again that ESI and MSI are complementary in the explanation of BOLD responses. In this case, cluster that was concordant with both ESI and MSI was also the one exhibiting the most significant t value.
Validation with iEEG
Thirteen patients underwent iEEG recordings. For 11/13 patients the epileptic focus was defined. The epileptic focus could not be defined by iEEG for patients 1 and 8 (Table 2). Invasive EEG investigations consisted in the implantation of 4 to 11 intracerebral electrodes (12 patients) and a subdural grid with 64 electrodes was used for one patient.
Concordance between iEEG and activations and deactivations
As described in the methods section, this comparison was performed only for the BOLD clusters which were most concordant with ESI/MSI results. For 8/11 patients with defined epileptic focus by iEEG, at least one BOLD cluster was concordant or regionally concordant with ESI and/or MSI results. IEDs from iEEG for all eight patients were concordant or regionally concordant with that BOLD cluster. BOLD results corresponded to an activation in 5/8 patients and to a deactivation in 3/8 patients. Such a “validated” cluster was found by both ESI and MSI for six patients, and only by MSI for patient 11 and only by ESI for patient 16. In the remaining 3/11 patients, the BOLD cluster highlighted by ESI/MSI was either not covered by iEEG (patients 14 and 19) or there was no concordance between ESI/MSI and BOLD (patient 2) (see Table 2 and Fig. 6b).
In 8/11 patients with the SOZ defined by iEEG, the BOLD cluster identified as the most concordant with ESI/MSI results was covered by iEEG implantation. Among seven of these eight patients, the SOZ was concordant with the “most concordant BOLD cluster”. Only for patient 8, the SOZ was localized within the subcortical heterotopia and not in the more superficial brain area where an activation was concordant with ESI/MSI results.
Concordance between iEEG and ESI/MSI results
ESI results were validated by the localization of IEDs recorded in iEEG for 7/11 patients with defined epileptic focus. Note that for one of the patients (patient 13), the SOZ and the brain area where IEDs are generated were located in two different regions (IEDs: left lateral posterior temporal lobe/SOZ: subcortical heterotopia). For 4/11 patients, the iEEG electrodes did not cover the ESI findings.
The localization of IEDs recorded in iEEG confirmed the MSI results in 9/11 patients with defined epileptic focus. For 8/9 patients the SOZ was recorded with the same iEEG contacts as the IED. For patient 13, the SOZ differed from the region, where IEDs confirmed the MSI. The MSI focus was not covered by iEEG in 2/11 patients (patient 14 and 19).
Lesional occurrence of the most concordant BOLD cluster
In 4/21 patients, who had a focal cortical dysplasias (FCDs), the most concordant BOLD cluster, ESI and MSI results were concordant or regionally concordant with the lesion. In 3/4 patients this was an activation and in 1/4 a deactivation. ESI and MSI were also concordant or regionally concordant with the lesion in the same patients.
Postsurgical outcome
For 10/21 patients the postsurgical outcome with a follow‐up of at least 12 months was known.
For 3/10 patients the most concordant BOLD cluster was validated by iEEG (IED and SOZ) and resected. It was associated with an excellent postsurgical outcome (Engel 1a for patients 4, 6, 18). An additional patient (patient 5) with concordance of the most concordant BOLD cluster (activation), ESI and MSI (no iEEG) also had a postsurgical outcome of Engel 1a. However, patient 20 had a poor postsurgical outcome (Engel 4), despite the fact that BOLD, ESI and MSI were in agreement with iEEG (IED and SOZ).
The remaining 5/10 patients consisted of very complex epilepsy patients with extended lesions or multifocal epilepsy. Three of them underwent two epilepsy surgeries (patient 14, 16, 20). The resections were not always in the same brain area as the BOLD clusters concordant with ESI/MSI (patient 14, 20), which makes it more difficult to correlate with the post‐surgical outcome. Two of them had multiple SOZs (patient 14, 19). In addition, either the iEEG recordings did not cover the BOLD cluster (patient 19), or the BOLD responses were only regionally concordant with the iEEG findings (patients 11, 13, 16) or the patient did not undergo iEEG (patient 21).
For the whole group, the concordance of ESI and MSI with iEEG recordings was less obviously correlated with the postsurgical outcome. Although ESI and MSI were concordant or regionally concordant with iEEG in all four patients with Engel 1a postsurgical outcome, they were as well concordant with iEEG in patient 11 and patient 16 (Engel 4). For a more detailed comparison with postsurgical outcome the functional imaging results should be coregistered with the postsurgical MRI. This was out of the scope of this project.
DISCUSSION
In this study, we showed that there is a close spatial relation between ESI/MSI and the most significant BOLD activation or deactivation in 20/21 patients with focal epilepsy. It is important because the two approaches measure different aspects of IEDs, and the degree to which these two aspects correspond is not yet clear. The good concordance between ESI/MSI and the most significant BOLD cluster is very useful in the further interpretation of BOLD responses, even when no source localization method is available. It confirms that the BOLD cluster with the most significant t‐value is the one which is of highest importance among the different very distributed clusters found for each patient.
ESI and MSI confirm each other and ESI achieves similar results in many patients with a much lower number of EEG channels. In addition either ESI or MSI are independently concordant with the most significant BOLD clusters in some patients. For one patient (patient 11), the BOLD cluster only concordant with MSI was confirmed by iEEG. The concordance between BOLD, MSI and iEEG suggests that MSI adds valuable information to the non‐invasive definition of the epileptic focus due to different sensitivities of MSI compared to ESI. An explanation may be that the IED‐related BOLD responses cover brain areas involved in the generation of IED, for which ESI was not as sensitive as MSI (notably neocortical tangential sources, see e.g. Fig. 5). For some of the patients not MSI but ESI was concordant with the most significant BOLD cluster. This again emphasizes the complementarity of both methods.
Whenever available iEEG validated the findings of noninvasive methods used. It was not the aim of this study to determine which of EEG/fMRI, ESI or MSI is better to detect the epileptic focus as defined by iEEG. This has been done for EEG/fMRI and ESI [Benar et al., 2006; Grova et al., 2008a; Seeck et al., 1998]. Our study aimed at evaluating whether there is a close relation of the most significant BOLD clusters with ESI/MSI. In addition, it was our objective to assess the added value of MSI in this comparison, as a continuation from our previous study focusing on fMRI and ESI [Grova et al., 2008a].
Widespread BOLD Clusters
In addition to the most concordant BOLD clusters, we found widespread BOLD clusters and BOLD clusters that met our criteria of concordance and regional concordance with ESI and also MSI. They may reflect propagation phenomena or changes at a distance from the epileptic focus. Indeed, changes in the epileptic network [Stefan and Lopes da Silva, 2013] are often found at a distance from epileptic focus. It has already been suggested that changes in the theta and delta frequency bands occur in the contralateral homologous regions in patients with focal IEDs [Yu et al., 2009] and that epileptic activity influences the default mode network [Fahoum et al., 2013]. As our findings show that the comparison of BOLD with subsequent simultaneous ESI/MSI is feasible, the spatial relationship between BOLD and ESI/MSI at a distance from the epileptic focus should be evaluated in further details. Such a study should also comprise investigations on the propagation of IED by simultaneous ESI/MSI and BOLD clusters as done for ESI and BOLD clusters alone [Vulliemoz et al., 2009]. Because MSI confirmed ESI findings and even added valuable information, MSI might further help to understand the epileptic network.
Colocalization of ESI/MSI with Deactivations
It remains unclear why ESI and MSI sometimes co‐localize with activations and other times with deactivations. It is easier to understand the occurrence of activation in regions that generate IEDs. The close spatial relation of the epileptic focus with a deactivation can be explained by increased local inhibition [Kobayashi et al., 2006b; Pittau et al., 2013]. An association of the epileptic focus with a deactivation could be explained with highly effective inhibition, which does not increase the metabolic demand by itself [Chatton et al., 2003]. A different explanation might be that constant IEDs lead to a sustained decrease in oxygenation, which is not sufficiently compensated by the physiological increase in regional blood. The lack of oxygenation is measured as deactivation in EEG/fMRI. It has been observed during oxymetric recordings of epilepsy patients [Suh et al., 2006]. In our series the BOLD cluster most concordant with ESI/MSI was a deactivation for 6/20 patients. In these patients, the highlighted deactivations were found over the posterior quadrant (N = 3 patients) and over frontal/temporal lobes (N = 3 patients). Interestingly the surface EEG and also the MEG IEDs in all these patients had a strong slow wave component (see Fig. 3B). In 5/6 cases the amplitude of the slow wave even exceeded the amplitude of the preceding EEG IED. These findings were more obvious in the EEG than in the MEG data. They support the hypothesis of increased inhibition in the area of the epileptic focus in some patients [Pittau et al., 2013]. For one patient the good correlation between deactivation, ESI/MSI results and iEEG findings (patient 4, Fig. 3) was associated with an excellent postsurgical outcome. This further validates that some of these deactivations colocalize with the epileptic focus.
Interestingly the rate of concordance between ESI/MSI and negative BOLD response was 29% (6/21 patients). This was considerably higher than the reported 8% in the recent study focusing on the evaluation of negative BOLD response [Pittau et al., 2013]. Although there could be a selection bias, the source localization using the MEM may have increased the rate of concordances, because it determines more accurately the origin of the activity than the visual evaluation of EEG and also MEG topographies in sensor space.
Prior Studies Comparing ESI with BOLD Responses
Our findings were consistent with results from prior studies comparing IED‐related BOLD clusters with distributed source models for ESI [Grova et al., 2008a; Vulliemoz et al., 2009]. The use of distributed source models and notably the MEM‐method might have improved the comparison with BOLD clusters compared to equivalent current dipoles (ECD). For ECD, it was more difficult to show a clear spatial correlation with BOLD responses [Bagshaw et al., 2006; Benar et al., 2006; Lemieux et al., 2001]. The mean distance between activation and ECD in the largest of these studies was 60 mm [Bagshaw et al., 2006]. Our results support the idea that assessing accurately with MEM the spatial extent of the IED generator when using ESI or MSI is of great importance in the context of this multimodal comparison. It is known from combined surface EEG/iEEG recordings [Tao et al., 2005] and combined MEG/iEEG recordings [Mikuni et al., 1997; Oishi et al., 2002] that the generators of IEDs recorded by surface EEG and MEG are spatially extended. The use of the MEM approach, as a source localization method that was carefully evaluated for its ability to detect the spatial extent of the IED generators in EEG and MEG [Chowdhury et al., 2013; Grova et al., 2006b], allowed us to characterize accurately this concordance between ESI/MSI and BOLD responses. This is the main reason why in addition to a comparison metric based on distance (D min), we proposed the AUC metric to take into account the spatial overlap along the cortical surface between the different modalities.
Concordance of ESI/MSI with EEG/fMRI and Postsurgical Outcome
An excellent postsurgical outcome was achieved in three patients with resection of activations or deactivations identified by ESI and MSI that were concordant with iEEG. This is however largely influenced by the fact that these patients had a histologically proven FCD type II. Its complete resection is in general a good predictor of postsurgical outcome [Wagner et al., [Link]]. In addition a fourth patient with FCD type IIa, who did not undergo iEEG, became seizure free after complete resection of the lesion.
Not all patients with concordance between ESI/MSI and BOLD had good postsurgical outcome, e.g. patients 11, 14, and 19 with extended anatomical lesions (polymicrogyria and hemimegalencephaly). These patients remain difficult to treat. Therefore, as usual, non‐invasive methods should primarily guide invasive recordings [Ramantani et al., 2013] for surgical planning.
Future Perspectives
Our results also suggest that combined source localization of EEG and MEG within a fusion framework might further improve localization accuracy, while taking full benefit of the complementarity between EEG and MEG. Preliminary results of such a fusion proposed within the MEM framework provided promising results [Chowdhury et al., 2012]. Compared to ESI/MSI of averaged IED used in our study, single IED source imaging would allow estimating the variance between single IEDs, thus allowing taking into account the variability between different IEDs. This would probably allow a further analysis of concordances between spatio‐temporal source localization in the IEDs and BOLD clusters distant from the most concordant cluster.
CONCLUSION
The comparison between visually selected IEDs in simultaneous ESI/MSI and IED‐associated BOLD activations and deactivations of similar IEDs in EEG/fMRI recordings is feasible. The most significant BOLD activations or deactivations are usually spatially concordant with ESI and MSI findings. Concordant findings between BOLD and ESI/MSI were often confirmed by iEEG findings. The postsurgical outcome was excellent for patients with FCD and concordance of the functional methods. Thus IED‐related BOLD responses and ESI/MSI of IEDs provide confirmatory information about their origin and add valuable information to delineate the epileptic focus according to their respective characteristics. Despite a much lower density of recording sites in EEG (54 electrodes versus 275 MEG sensors), ESI performed nearly as well as compared to MEG and MSI in this study. This is important to emphasize, considering the less frequent availability of MEG.
As EEG/fMRI becomes more widely used, the concordance of the most significant among many BOLD clusters and ESI/MSI is important for the definition of the focus, and it facilitates its future clinical application in the investigation of epilepsy patients.
Supporting information
Supplementary Information Table 1.
ACKNOWLEDGMENTS
The authors thank François Tadel from the Brainstorm group for his help in the projection of the BOLD clusters on the cortical surface, Manon Robert for her assistance in simultaneous EEG/MEG data acquisition and Natalja Zazubovits for helping to collect and analyze EEG/fMRI data.
AUC Comparison
A receiver operating characteristic (ROC) curve strategy was used to compare the spatial extent between cMEM ESI/MSI findings at the peak of the IED with BOLD clusters in this study. Each BOLD cluster was considered as reference for the comparison. To assess the detection accuracy of a method when a reference is available, ROC curves are generated by plotting the sensitivity against the false positive detection rate for different detection thresholds. The Area Under the ROC Curve (AUC) is a well‐known criterion to assess detection accuracy [Metz, 1986]. Usually, the reference reflects some ground truth regarding the object to be detected, and detection errors are assessed by comparing this reference to the results of the method for different thresholds. Whereas no ground truth was available for our comparison, we used ROC methodology to assess the concordance between each BOLD cluster and the spatial extent of cMEM sources estimated at the peak of the IED.
The AUC comparison has been applied on the comparison of ESI with simulated sources and BOLD clusters [Grova et al., 2006a, 2008b] as well as on the comparison of MSI and simulated sources [Chowdhury et al., 2013]. Each BOLD cluster was then considered as the reference for the estimation of sensitivity and false positive rate at different thresholds of the cMEM source, ranging between 0 and the maximum amplitude of the source. To interpret AUC as the probability that ESI/MSI findings inside the fMRI cluster have higher amplitude than sources located outside the fMRI cluster, one should theoretically provide the same number of sources inside and outside the BOLD cluster to estimate ROC parameters. In Grova et al. [2006b], we proposed a method specifically dedicated to this issue. A nonbiased estimation of AUC is obtained by randomly drawing outside the BOLD cluster as many fictitious sources as sources located inside the BOLD cluster. The AUC is then measured using this set of pairs of sources. This random drawing is repeated 100 times and the proposed metric of ESI/MSI BOLD concordance is the average AUC over these 100 trials. AUC should be interpreted carefully, because we are not measuring detection errors, as is usually the case when using ROC curves. On the other hand, in the present context, AUC allows assessing the overall spatial overlap along the cortical surface between significant BOLD clusters and ESI/MSI results at the peak of the IED, as a method to quantify the contrast of cMEM reconstructed sources.
REFERENCES
- Agirre‐Arrizubieta Z, Huiskamp GJM, Ferrier CH, van Huffelen AC, Leijten FSS (2009): Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain 132:3060–3071. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R (2000): A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage 12:230–239. [DOI] [PubMed] [Google Scholar]
- Amblard C, Lapalme E, Lina JM (2004): Biomagnetic source detection by maximum entropy and graphical models. IEEE Trans Biomed Eng 51:427–442. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Aghakhani Y, B nar C‐G, Kobayashi E, Hawco C, Dubeau F, Pike GB, Gotman J (2004): EEG‐fMRI of focal epileptic spikes: Analysis with multiple haemodynamic functions and comparison with gadolinium‐enhanced MR angiograms. Hum Brain Mapp 22:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw AP, Kobayashi E, Dubeau F, Pike GB, Gotman J (2006): Correspondence between EEG‐fMRI and EEG dipole localisation of interictal discharges in focal epilepsy. NeuroImage 30:417–425. [DOI] [PubMed] [Google Scholar]
- Baillet S, Mosher JC, Leahy RM (2001): Electromagnetic brain mapping. IEEE Signal Process Mag 18:14–30. [Google Scholar]
- Bast T, Oezkan O, Rona S, Stippich C, Seitz A, Rupp A, Fauser S, Zentner J, Scherg M (2004): EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia 45:621–631. [DOI] [PubMed] [Google Scholar]
- Benar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J (2002): The BOLD response to interictal epileptiform discharges. NeuroImage 17:1182–1192. [DOI] [PubMed] [Google Scholar]
- Benar CG, Aghakhani Y, Wang Y, Izenberg A, Al‐Asmi A, Dubeau F, Gotman J (2003): Quality of EEG in simultaneous EEG‐fMRI for epilepsy. Clin Neurophysiol 114:569–580. [DOI] [PubMed] [Google Scholar]
- Benar CG, Grova C, Kobayashi E, Bagshaw AP, Aghakhani Y, Dubeau F, Gotman J (2006): EEG–fMRI of epileptic spikes: Concordance with EEG source localization and intracranial EEG. NeuroImage 30:1161–1170. [DOI] [PubMed] [Google Scholar]
- Brodbeck V, Spinelli L, Lascano AM, Wissmeier M, Vargas MI, Vulliemoz S, Pollo C, Schaller K, Michel CM, Seeck M (2011): Electroencephalographic source imaging: A prospective study of 152 operated epileptic patients. Brain 134:2887–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ (2003): GABA uptake into astrocytes is not associated with significant metabolic cost: Implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA 100:12456–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hallez H, Staelens S (2010): Influence of skull conductivity perturbations on EEG dipole source analysis. Med Phys 37:4475. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Zerouali Y, Lina JM, Kobayashi E, Grova C (2012): Multimodal EEG/MEG Fusion Within the Maximum Entropy on the Mean (MEM) Framework. Paris. [Google Scholar]
- Chowdhury RA, Lina J‐M, Kobayashi E, Grova C (2013): MEG source localization of spatially extended generators of epileptic activity: Comparing entropic and hierarchical Bayesian approaches. PLoS One 8:e55969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comp Assisted Tomogr 18:192–205. [PubMed] [Google Scholar]
- Cunningham CB, Goodyear BG, Badawy R, Zaamout F, Pittman DJ, Beers CA, Federico P (2012): Intracranial EEG‐fMRI analysis of focal epileptiform discharges in humans. Epilepsia 53:1636–1648. [DOI] [PubMed] [Google Scholar]
- Donaire A, Capdevila A, Carreño M, Setoain X, Rumià J, Aparicio J, Campistol J, Padilla N, Sanmartí F, Vernet O, Pintor L, Boget T, Ortells J, Bargalló N (2013): Identifying the cortical substrates of interictal epileptiform activity in patients with extratemporal epilepsy: An EEG‐fMRI sequential analysis and FDG‐PET study. Epilepsia 54:678–690. [DOI] [PubMed] [Google Scholar]
- Fahoum F, Lopes R, Pittau F, Dubeau F, Gotman J (2012): Widespread epileptic networks in focal epilepsies‐EEG‐fMRI study. Epilepsia 53:1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahoum F, Zelmann R, Tyvaert L, Dubeau F, Gotman J (2013): Epileptic discharges affect the default mode network—fMRI and intracerebral EEG evidence. PLoS One 8:e68038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenholz DM, Ahlfors SP, Hämäläinen MS, Sharon D, Ishitobi M, Vaina LM, Stufflebeam SM (2009): Mapping the signal‐to‐noise‐ratios of cortical sources in magnetoencephalography and electroencephalography. Hum Brain Mapp 30:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves SI, de Munck JC, Verbunt JPA, Bijma F, Heethaar RM, Lopes da Silva F (2003): In vivo measurement of the brain and skull resistivities using an eit‐based method and realistic models for the head. IEEE Trans Biomed Eng 50:754–767. [DOI] [PubMed] [Google Scholar]
- Gotman J, Pittau F (2011): Combining EEG and fMRI in the study of epileptic discharges. Epilepsia 52 (Suppl 4):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F (2005): Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102:15236–15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Papadopoulo T, Olivi E, Clerc M (2010): OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomed Eng Online 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova C, Daunizeau J, Lina JM, Benar CG, Benali H, Gotman J (2006a): Evaluation of EEG localization methods using realistic simulations of interictal spikes. NeuroImage 29:734–753. [DOI] [PubMed] [Google Scholar]
- Grova C, Makni S, Flandin G, Ciuciu P, Gotman J, Poline JB (2006b): Anatomically informed interpolation of fMRI data on the cortical surface. NeuroImage 31:1475–1486. [DOI] [PubMed] [Google Scholar]
- Grova C, Daunizeau J, Kobayashi E, Bagshaw AP, Lina JM, Dubeau F, Gotman J (2008a): Concordance between distributed EEG source localization and simultaneous EEG‐fMRI studies of epileptic spikes. NeuroImage 39:755–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova C, Daunizeau J, Kobayashi E, Bagshaw AP, Lina JM, Dubeau F, Gotman J (2008b): Concordance between distributed EEG source localization and simultaneous EEG‐fMRI studies of epileptic spikes. Neuroimage 39:755–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen MS (1992): Magnetoencephalography: A tool for functional brain imaging. Brain Topogr 5:95–102. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Pestana E, Burgess RC, Lüders HO, Shamoto H, Nakasato N (2005): Detection of epileptiform activity by human interpreters: Blinded comparison between electroencephalography and magnetoencephalography. Epilepsia 46:59–68. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Jansen A, Andermann F, Andermann E, Gotman J, Dubeau F (2005) Intrinsic epileptogenicity in polymicrogyric cortex suggested by EEG‐fMRI BOLD responses. Neurology 64:1263–1266. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Bénar C‐G, Aghakhani Y, Andermann F, Dubeau F, Gotman J (2006a): Temporal and extratemporal BOLD responses to temporal lobe interictal spikes. Epilepsia 47:343–354. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J (2006b): Negative BOLD responses to epileptic spikes. Hum Brain Mapp 27:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Grova C, Tyvaert L, Dubeau F, Gotman J (2009): Structures involved at the time of temporal lobe spikes revealed by interindividual group analysis of EEG/fMRI data. Epilepsia 50:2549–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Duncan JS (2007): Electroencephalography/functional MRI in human epilepsy: What it currently can and cannot do. Curr Opin Neurol 20:417–423. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Krakow K, Fish DR (2001): Comparison of spike‐triggered functional MRI BOLD activation and EEG dipole model localization. NeuroImage 14:1097–1104. [DOI] [PubMed] [Google Scholar]
- Mangin J‐FO, Frouin V, Bloch I, Rgis J, Lpez‐Krahe J (1995): From 3D magnetic resonance images to structural representations of the cortex topography using topology preserving deformations. J Math Imaging Vis 5:297–318. [Google Scholar]
- Meindl T, Teipel S, Elmouden R, Mueller S, Koch W, Dietrich O, Coates U, Reiser M, Glaser C (2010): Test‐retest reproducibility of the default‐mode network in healthy individuals. Hum Brain Mapp 31:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni N, Nagamine T, Ikeda A, Terada K, Taki W, Kimura J, Kikuchi H, Shibasaki H (1997): Simultaneous recording of epileptiform discharges by MEG and subdural electrodes in temporal lobe epilepsy. NeuroImage 5:298–306. [DOI] [PubMed] [Google Scholar]
- Moeller F, Tyvaert L, Nguyen DK, LeVan P, Bouthillier A, Kobayashi E, Tampieri D, Dubeau F, Gotman J (2009): EEG‐fMRI: Adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. Neurology 73:2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Otsubo H, Kameyama S, Morota N, Masuda H, Kitayama M, Tanaka R (2002): Epileptic spikes: Magnetoencephalography versus simultaneous electrocorticography. Epilepsia 43:1390–1395. [DOI] [PubMed] [Google Scholar]
- Pittau F, Fahoum F, Zelmann R, Dubeau F, Gotman J (2013): Negative BOLD response to interictal epileptic discharges in focal epilepsy. Brain Topogr 26:627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramantani G, Koessler L, Colnat‐Coulbois S, Vignal JP, Isnard J, Catenoix H, Jonas J, Zentner J, Schulze‐Bonhage A, Maillard LG (2013): Intracranial evaluation of the epileptogenic zone in regional infrasylvian polymicrogyria. Epilepsia 54:296–304. [DOI] [PubMed] [Google Scholar]
- Seeck M, Lazeyras F, Michel CM, Blanke O, Gericke CA, Ives J, Delavelle J, Golay X, Haenggeli CA, de Tribolet N, Landis T (1998): Non‐invasive epileptic focus localization using EEG‐triggered functional MRI and electromagnetic tomography. Electroencephalogr Clin Neurophysiol 106:508–512. [DOI] [PubMed] [Google Scholar]
- Stefan H, Lopes da Silva FH (2013): Epileptic neuronal networks: Methods of identification and clinical relevance. Front Neurol 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh M, Ma H, Zhao M, Sharif S, Schwartz TH (2006): Neurovascular coupling and oximetry during epileptic events. Mol Neurobiol 33:181–197. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM (2011): Brainstorm: A user‐friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011:879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes‐Ebersole S, Ebersole JS (2005): Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 46:669–676. [DOI] [PubMed] [Google Scholar]
- Thornton R, Laufs H, Rodionov R, Cannadathu S, Carmichael DW, Vulliemoz S, Salek‐Haddadi A, McEvoy AW, Smith SM, Lhatoo S, Elwes RDC, Guye M, Walker MC, Lemieux L, Duncan JS (2010): EEG correlated functional MRI and postoperative outcome in focal epilepsy. J Neurol Neurosurg Psychiatry 81:922–927. [DOI] [PubMed] [Google Scholar]
- van Houdt PJ, Ossenblok PP, Colon AJ, Boon PA, de Munck JC (2012): A framework to integrate EEG‐correlated fMRI and intracerebral recordings. Neuroimage 60:2042–2053. [DOI] [PubMed] [Google Scholar]
- van Houdt PJ, de Munck JC, Leijten FS, Huiskamp GJ, Colon AJ, Boon PA, Ossenblok PP (2013): EEG‐fMRI correlation patterns in the presurgical evaluation of focal epilepsy: A comparison with electrocorticographic data and surgical outcome measures. Neuroimage 75:238–248. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Thornton R, Rodionov R, Carmichael DW, Guye M, Lhatoo S, McEvoy AW, Spinelli L, Michel CM, Duncan JS, Lemieux L (2009): The spatio‐temporal mapping of epileptic networks: Combination of EEG–fMRI and EEG source imaging. NeuroImage 46:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, McEvoy AW, Lemieux L (2011): Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. NeuroImage 54:182–190. [DOI] [PubMed] [Google Scholar]
- Wagner J, Urbach H, Niehusmann P, von Lehe M, Elger CE, Wellmer J: Focal cortical dysplasia type IIb: Completeness of cortical, not subcortical resection is necessary for seizure freedom. Epilepsia 52:1418–1424. [DOI] [PubMed] [Google Scholar]
- Wiest R, Estermann L, Scheidegger O, Rummel C, Jann K, Seeck M, Schindler K, Hauf M (2013): Widespread grey matter changes and hemodynamic correlates to interictal epileptiform discharges in pharmacoresistant mesial temporal epilepsy. J Neurol 260:1601–1610. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC (2002): A general statistical analysis for fMRI data. NeuroImage 15:1–15. [DOI] [PubMed] [Google Scholar]
- Yu JM, Tyvaert L, LeVan P, Zelmann R, Dubeau F, Gotman J, Kobayashi E (2009): EEG spectral changes underlying BOLD responses contralateral to spikes in patients with focal epilepsy. Epilepsia 50:1804–1809. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FSS (2007): EEG‐fMRI in the preoperative work‐up for epilepsy surgery. Brain 130:2343–2353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Table 1.


