Abstract
Brain effective connectivity can be tracked by cerebral recruitments evoked by transcranial magnetic stimulation (TMS), as measured by simultaneous electroencephalography (TMS‐EEG). When TMS is targeting the primary motor area, motor evoked potentials (MEPs) can be collected from the “target” muscles. The aim of this study was to measure whether or not effective brain connectivity changes with the excitability level of the corticospinal motor pathway (CSMP) as parameterized by MEP amplitude. After averaging two subgroups of EEG‐evoked responses corresponding to high and low MEP amplitudes, we calculated the individual differences between them and submitted the grand average to sLORETA algorithm obtaining localized regions of interest (RoIs). Statistical differences of RoI recruitment strength between low and high CSMP excitation was assessed in single subjects. Preceding the feedback arrival, neural recruitment for stronger CSMP activation were weaker at 6–10 ms of homotopic sensorimotor areas BA3/4/5 of the right nonstimulated hemisphere (trend), weaker at 18–25 ms of left parietal BA2/3/40, and stronger at 26–32 ms of bilateral frontal motor areas BA6/8. The proposed method enables the tracking of brain network connectivity during stimulation of one node by measuring the strength of the connected recruited node activations. Spontaneous increases of the excitation of the node originating the transmission within the hand control network gave rise to dynamic recruitment patterns with opposite behaviors, weaker in homotopic and parietal circuits, stronger in frontal ones. The effective connectivity within bilateral circuits orchestrating hand control appeared dynamically modulated in time even in resting state as probed by TMS. Hum Brain Mapp 35:1740–1749, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: transcranial magnetic stimulation simultaneous to electroencephalographic recordings, cerebral connectivity as localized cortical recruitment, spontaneous fluctuation of excitability
INTRODUCTION
Transcranial magnetic stimulation (TMS) allows noninvasive investigation of the functional state of the human cerebral cortex, especially of the motor cortex. By means of rapidly changing magnetic fields reaching the brain undistorted by extracerebral tissues, electric currents are induced producing trans‐synaptic depolarization of pyramidal neurones located in the superficial cortical layers [Barker et al., 1985; for a review see Rossini and Rossi, 2007]. In recent years, the registration of electroencephalography (EEG) activity during TMS and of the corresponding transcranial evoked potentials elicited by individual stimuli (TMS‐EEG) provided valuable information about the characteristics of cortical reactivity, effective connectivity, and synchronised neuronal firing of different brain areas in response to magnetic stimuli with high spatiotemporal specificity [Bonato et al., 2006; Ilmoniemi et al., 1997; Paus et al., 1997; Siebner et al., 2009; Virtanen et al., 1999]. EEG and Magnetoencephalography (MEG) technologies have demonstrated a good ability to detail connectivity and its time‐varying directionality [for reviews see Siegel et al., 2012, Schnitzler and Gross, 2005]. Therefore, TMS‐EEG is one of the most promising neurophysiological methods because EEG responses evoked by TMS cortical excitation allow the marking of specific patterns of cerebral connectivity. EEG responses at precise and repeatable latencies after stimulation were observed in physiological conditions [Lioumis et al., 2009], proving that specific topographies are relative to the stimulation site [prefrontal cortices: Casarotto et al., 2010; Kähkönen et al., 2004, 2005; Kähkönen and Wilenius, 2007; Rosanova et al., 2009; primary motor area: Bender et al., 2005; Bonato et al., 2006; Ferreri et al., 2010, Huber et al., 2007; Ilmoniemi et al., 1997; Komssi et al., 2004, 2002; Komssi and Kähkönen 2006; Nikulin et al., 2003; Paus et al., 2001].
Coregistration of the EEG activity during the stimulation of the primary motor cortex (M1) allows the study of M1 cortico‐cortical and intrahemispheric and interhemispheric connections in combination with electromyographic (EMG) recordings of the motor evoked potential (MEP) in the target muscle [Bender et al., 2005; Bonato et al., 2006; Ferreri et al., 2010; Huber et al., 2007; Ilmoniemi et al., 1997; Komssi et al., 2002, 2004; Nikulin et al., 2003; Paus et al., 2001]. MEPs induced by TMS are the result of a combination of excitatory/inhibitory phenomena occurring at cortical, spinal, and neuromuscular levels along the motor pathway. Thus, amplitudes and latencies of MEP are parameters that allow the evaluation of the functional state of the corticospinal motor pathway (CSMP) and its relays, also providing valuable information about their function in both physiological and pathological conditions [Barker et al., 1985; Rossini and Rossi, 2007 for a review].
MEP onset latencies are relatively stable when a cascade of successive stimuli are repeated in time over the primary motor cortex; however, their amplitudes are also highly variable intraindividually in a trial‐to‐trial evaluation even within the same experimental session, without any apparent fluctuation of the stimulus/recording/environment/subject parameters [Ellaway et al., 1998; Starr et al., 1988]. For instance, while minor changes in coil position or modifications of the background tone in the target muscle can significantly modify MEP amplitudes, cardiac or respiratory phases of the TMS instant are noninfluential [Amassian et al., 1989; Filippi et al., 2000; Kiers et al., 1993; Sparing et al., 2008]. Once provided a spatially and physically stable stimulus, the spontaneous fluctuations of neuronal and trans‐synaptic excitation—at cortical [Adrian and Moruzzi, 1939] and spinal levels—remain the main factors underlying MEP amplitude variability. Altogether it is worth understanding the origin and consequences of such involuntary variability of the cortical motor output [Amassian et al., 1989; Magistris et al., 1998; Rossini et al., 1991; Steriade and Llinas, 1988], a goal that can nowadays be pursued by the TMS‐EEG technique.
The aim of this study was to investigate via TMS‐EEG technique whether or not intrasubject and intrasession spontaneous fluctuations of motor pathway excitation can influence the transmission within the cortico‐cortical, cortico‐subcortical, and intraspinal pathways impinged by M1 projections. For this purpose, the MEP amplitude variability was exploited as a marker of different excitation levels of cerebral and spinal neuronal pools, related to the recruitment of corticospinal fibres connected to the target muscle. As different MEP amplitudes can also correspond to different sensory and proprioceptive feedback reaching the relevant brain areas, we investigated the cerebral recruitments separating those occurring before and after the peripheral inflow arrival.
Once the electric potentials directly produced by the TMS impulse are removed, the EEG signals are generated by the excited neuronal pools impinged by the connections originating from the stimulated cortex. Ipsilateral and contralateral cortical areas are recruited by intrahemispheric and interhemispheric cortico‐cortical and subcortico‐cortical connections, with longer latency contributions of sensory feedback from the twitching muscles in the case of stimulation of the motor cortex. The object of this investigation was to quantify whether or not spontaneous modification of the CSMP excitation level—as reflected in MEP amplitude—implies cortical connectivity changes. Previous TMS‐EEG studies approached the same question [Bonato et al., 2006; Mäki and Ilmoniemi, 2010a; Nikulin et al., 2003; Paus et al., 2001] describing the TMS‐induced effects through EEG channel‐derived measures. To enhance the signal to noise ratio and to better understand the behavior of specific cortical areas in response to TMS, we approached the investigation of TMS‐induced time‐varying cortico‐cortical connectivity through the dynamic assessment of serial recruitments of cortical sources as identified by the sLORETA EEG source localization algorithm [Fuchs et al., 2002; Pascual‐Marqui, 2002]. To minimize TMS‐induced artifacts and to enhance sensibility to cortical activations depending on CSMP excitation levels, we searched for localized cortical generators subtending the grand average of the individual differences between high‐ and low‐EEG averages to be our regions of interest (RoIs). Connected cortical area recruitment strength was indexed by the current density peak of the localized source [Casali et al., 2010; Massimini et al., 2005] on an individual basis to assess statistical significance of the recruitment dependence on CSMP excitation level.
MATERIALS AND METHODS
Subjects
Ten healthy volunteers (six males, age range 19–35 years; four females age range 18–30 years) were enrolled after giving written informed consent to the experimental protocol previously approved by the institutional Ethics Committee. Subjects were instructed to abstain from caffeine, alcohol, and medication and to maintain their regular sleep‐wake schedule for 3 days before the experimental session. All subjects were right‐handed as evaluated by the Handedness Questionnaire (0.70 ± 0.08). The exclusion criteria established by international safety standards for TMS were followed [Rossini et al., 1994; Rossi et al., 2009]. No special care was given to fix the phase of menstrual cycle in females as the ovarian cycle effects on the motor cortex excitation [Smith et al., 2002] do not have relevant impact on the present intrasubject study of the cortico‐cortical transmission dependence on CSMP excitation.
TMS‐EEG Experimental Setup and Protocol
TMS‐compatible EEG equipment (BrainAmp 32MRplus, BrainProducts GmbH, Munich, Germany) was used for recording TMS triggered evoked potentials. The EEG activity was continuously acquired from 32 scalp sites (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T7, T8, P7, P8, Fz, Cz, Pz, FC1, FC2, CP1, CP2, FC5, FC6, CP5, CP6, TP9, TP10, FT9, FT10, FCz of the 10‐20 International System) using electrodes mounted on an elastic cap. Additional electrodes were used as ground (Oz to have maximal distance from the stimulating coil) and reference (linked mastoids). To minimize overheating of the electrodes located in the vicinity of the stimulating coil, TMS‐compatible Ag/AgCl‐coated electrodes were used. Skin/electrode impedance was maintained below 5 kΩ. BrainAmp MRplus allowed the fine adaptation to the TMS stimulus magnitude by selecting amplifier sensitivity and operational range to prevent saturation under the specific stimulus conditions: a sensitivity of 100 nV/bit (signal range/resolution) and an analog/digital‐conversion range of 6553.5 mV (± 3276.8 mV) were used.
TMS was performed over the left M1 during multichannel EEG recording, monitoring the coil position stability along the session by a neuronavigation system (NBS system, SofTaxicOptic, Bologna, Italy) (Fig. 1), which also assured stable coil orientation with respect to the subject's head. EMG activity from the first dorsal interosseous (FDI) muscle of the right hand was recorded via surface electrodes in belly tendon montage. The Magstim SuperRapid magnetic stimulator was used with a figure‐of‐eight coil having an outer wing diameter of 7 cm (Magstim Company, Whitland, UK). After the EEG cap was fitted, the coil was placed tangentially to the scalp on site C3 of the 10‐20 International System with the handle pointing backward and laterally at about a 45° angle from the midline. The coil was moved in steps of approximately 0.5 cm searching for the best coil position to induce maximal MEPs from the right FDI “hot spot.” After having identified the FDI hot spot coil position, the FDI resting motor threshold (RMT) was determined as the lowest stimulus intensity eliciting at least five MEPs of 50 mV out of 10 consecutive stimuli [Rossini et al., 1994, 1999]. EEG and EMG signals were sampled at 5 kHz after band pass filtering at 50–1,000 Hz for EMG and 0.1–500 Hz for EEG recordings.
Figure 1.
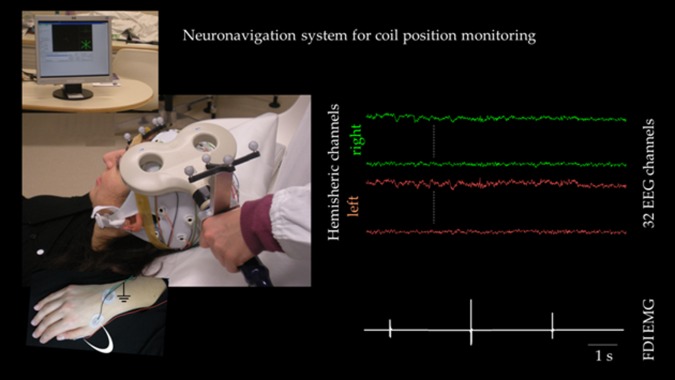
Experimental setup. Left neuronavigated TMS setup: the subject lies relaxed on a semireclined chair and the coil position is monitored throughout the session duration. The hand with surface electrodes for FDI muscle recordings is evidenced in the inset (white arch, ground symbol indicates belly‐tendon montage, while on the wrist dorsum is the ground electrode). Right EEG and EMG signals after artifact removal. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To mask coil‐generated clicks, a white noise was continuously delivered through earphones. We adjusted the masking volume until the subjects reported that the TMS click was not audible (always below 90 dB). To ensure wakefulness throughout the recording sessions, subjects were required to keep their eyes open maintaining the gaze on a fixation point during stimulation subperiods (2–3 min). About 120 magnetic stimuli were delivered at 120% RMT (supra‐threshold stimulation) over the left M1 region with an irregular interstimulus interval of about 5 s.
Data Analysis
Offline analysis of the EEG and EMG data were performed using MATLAB (The Mathworks, Natick, MA). The data were firstly visually inspected to remove excessively noisy channels (no more than two per subject) and to discard trials in which baseline EMG activity revealed muscle contraction in the 300 ms period preceding the TMS. Subsequently, EEG data were zero‐phase filtered using a second‐order Butterworth bandpass (1‐100 Hz) filter and independent component analysis was used to reduce nonbiological (TMS) and biological (eye and muscular) artifacts [Barbati et al., 2004]. The muscular artifacts are also increased by the contribution of cranial muscles, activated because of the stimulation of the lateral sites, and the consequence is an artifact signal lasting tens of milliseconds and few orders of magnitude larger than the recorded cortical signal [Mäki and Ilmoniemi, 2010b].
Identification of high and low CSMP excitation
For each subject, peak‐to‐peak MEP amplitudes were determined over EMG recorded data. TMS‐evoked EEG responses corresponding to high and low CSMP excitation levels were obtained averaging trials corresponding to the smallest (EEG low) and the largest (EEG high) thirds of MEP amplitudes [Mäki and Ilmoniemi, 2010a; Tecchio et al., 2008].
Identification of RoIs (localized cerebral recruitments)
For each subject, the two EEG low and EEG high groups of trials were averaged from 100 ms prestimulus to 500 ms poststimulus with baseline correction in [−100, −10] ms intervals. Thereafter, the individual differences between EEG averages at high and low CSMP excitation levels were estimated and resulted in a signal minimizing common artifacts. The grand average across all subjects of individual differences was then submitted to a source localization algorithm [sLORETA, Pascual‐Marqui, 2002] in the 3–100 ms period after the impulse.
To identify the RoIs, a devoted analysis of the “stability” of cortical recruitment was carried out at each millisecond by selecting the 200 higher intensity voxels among the 6,239 cortical grey matter voxels scanned by sLoreta in the whole brain volume (MNI ‐ Montreal Neurological Institute ‐ coordinates, at 5 mm resolution). A RoI was identified if (1) at least 75% of voxels were common for localizations of two consecutive milliseconds and (2) this happened for at least three consecutive milliseconds. Two parameters were calculated for further analysis: the RoI spatial position (average of contiguous points weighted for the current density, RoI_pos) and the starting and final time points of position stability (RoI_lat). Within this technical framework, the RoI_pos could include more than one cortical region, and the duration of recruitment stability (RoI_lat) could be variable, including all time points of location stability.
Identification of high and low current density levels in each RoI
RoI_pos was then used to estimate time courses relative to individual current densities in those RoIs. This RoI single subject evolution was then averaged among high and low trials, and the current density peak within the RoI_lat time window was identified. The mean value of the current density at the peak ± 2 ms was used to estimate the strength of cortical recruitment at high and low CSMP excitability for subsequent statistical analysis ( ).
Statistical Analysis
A possible MEP amplitude modulation related to the repetition of consecutive stimuli was evaluated analyzing MEP's amplitude variability along the session, considering the correlation between MEP amplitudes and the order of stimulation (performed by Sperman's Rho correlation).
Gaussianity of RoIs' current density distributions was checked using the Kolmogorov‐Smirnov test and appropriate transformations were applied if needed. Cerebral recruitment corresponding to high and low CSMP excitation levels in single subjects were submitted to a repeated measure ANOVA model with Connected Node (RoI1, RoI2, RoI3, etc.) and CSMPexc (RoIlow, RoIhigh) as within‐subjects factors to evaluate significance of the dependence of the cortical recruitment strength on the TMS efficacy. Only effects at P <0.05 were reported as results. Trends were indicated when P <0.10.
RESULTS
Identification of High and Low CSMP Excitation
Mean RMT was 45.7 ± 8% of maximal stimulator output.
An Average of 110 ± 6 EEG/MEP Trials Were Obtained Per Subject After Data Cleaning
MEP values were not normally distributed and were therefore log‐transformed y = log(MEP+1) to achieve a good approximation to Gaussianity and to limit the potentially detrimental effect of right‐skewed outliers. Analysis of the evoked motor potentials confirmed, in spite of a high intrasubject stability of the MEP latency (peak MEP latency 24.0 ± 1.6 across subjects), a relatively high variability of MEP amplitude (Fig. 2), with 5.65 ± 0.24 of variation coefficient, that is, the ratio between the standard deviation and the mean, across subjects. We also investigated the correlation between amplitude of MEPs (considered the independent variable) and chronological order of MEPs (considered the dependent variable), and we found no relationship between these two conditions (P = 0.39).
Figure 2.
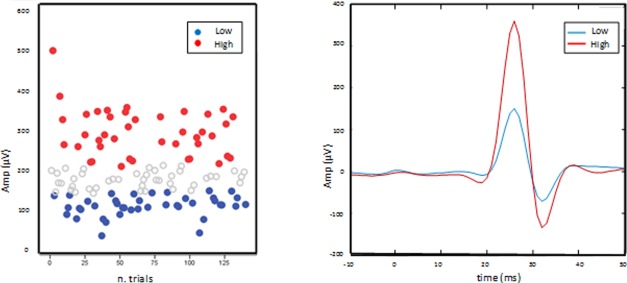
Procedure to identify high and low motor pathway excitation levels. Left MEP peak‐to‐peak amplitude for each trial of a representative subject, with largest/smallest third (red/blue) of trials used to select the EEG epochs to obtain individual EEGhigh and EEGlow averages. Right average of largest/smallest third (red/blue) MEPs in the [−10, 50] ms time window.
Brain Areas Recruited by Left M1‐TMS for Low and High CSMP Excitation Levels
The grand average of individual differences between EEGhigh and EEGlow submitted to sLORETA source localization algorithm evidenced eight RoIs at latencies and sites consistent with previous reports [Bonato et al., 2006; Daskalakis et al., 2008; Ferreri et al., 2010; Lee et al., 2007; Rizzo et al., 2011]: 6–10 ms, 13–16 ms, 18–25 ms, 26–32 ms, 35–39 ms, 44–47 ms, 68–75 ms, and 79–85 ms (Table 1, Fig. 3). It was noteworthy that no RoI was identified in the prestimulus interval ([−10, 0] ms period).
Table 1.
Low versus high CSMP excitation RoIs
| RoI_lat (ms) | RoI_pos (mm) | Cytoarchitectonic structure | Recruitment change |
|---|---|---|---|
| 6–10 | 30, −40, 67 | Right BA5 parietal lobe, postcentral gyrus | ∼− |
| 35, −26, 66 | Right BA4 frontal lobe, precentral gyrus | ||
| 35, −21, 66 | Right BA3 parietal lobe, postcentral gyrus | ||
| 13–16 | −5, 13, 64 | Left and right BA6 frontal lobe, superior frontal gyrus | = |
| −10, 13, 64 | |||
| 5, 22, 59 | |||
| 18–25 | −64, −23, 20 | Left BA40 parietal lobe, postcentral gyrus | − |
| −54, −23, 33 | Left BA2 parietal lobe, postcentral gyrus | ||
| −64, −13, 24 | Left BA3 parietal lobe, postcentral gyrus | ||
| 26–32 | −5, 13, 64 | Left and right BA6 frontal lobe, superior frontal gyrus | + |
| −10, 13, 64 | |||
| 5, 22, 59 | |||
| 35–39 | 54, 2, 46 | Right BA6 middle frontal gyrus, frontal lobe | = |
| 50, 2 46 | Right BA6 precentral gyrus, frontal lobe | ||
| 50, −7, 51 | Right BA4 precentral gyrus, frontal lobe | ||
| 44–47 | −25, 32, 49 | Left BA8 superior frontal gyrus, frontal lobe | + |
| −45, 12, 50 | Left BA6 middle frontal gyrus, frontal lobe | ||
| 68–75 | −15, −6, 65 | Left and right BA6 superior, middle and medial frontal gyrus, frontal lobe | ∼+ |
| −10, −7, 60 | |||
| 10, −6, 65 | |||
| 79–85 | −5, 13, 64 | Left and right BA6 frontal lobe, superior frontal gyrus | = |
| −10, 13, 64 | |||
| 5, 22, 59 |
RoI starting and final time points (RoI_lat, t = 0 ms being the TMS stimulation of the left M1 controlling FDI), positions (RoI_pos) in Talairach coordinates and corresponding cytoarchitectonic structure (Brodmann area, BA). RoI's multiple cortical areas are presented in order of their current density. Latencies after 45 ms (thick horizontal line) are successive to somatosensory feedback arrival. Areas activated more (less) for high CSMP excitation are indicated by +(−), ∼ added for P < 0.10, and = is reported when no statistical difference was found.
Figure 3.
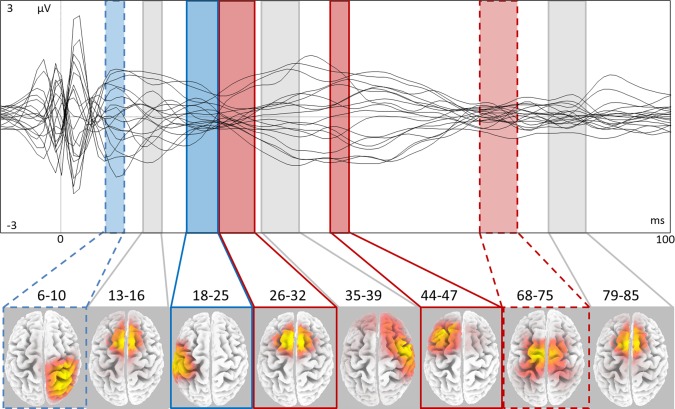
Cerebral areas recruited differently at low and high CSMP excitation levels by left M1‐TMS. Top superimposition in a butterfly plot of the TMS evoked potentials recorded at electrodes common to all subjects in the [−10, 100] ms time window, obtained from grand average of the differences between high and low CSMP. Bottom region of activation at corresponding latencies (ms), with color code referring to lower/higher (blue/red) activation corresponding to higher/lower CSMP excitation level, trends indicated by dashed lines and significant differences by solid lines.
ANOVA model with Component (RoI1, RoI2, RoI3, etc.) and CSMPexc (RoIlow, RoIhigh) as within‐subjects factors indicated different modulations of the different cerebral recruitments [Component*CSMPexc interaction factor (F = 6.648, P < 0.001)].
Recruitments preceding sensory feedback arrival
Cerebral recruitments at 6–10 ms were confined to the right nonstimulated hemisphere. In fact, in this interval cerebral recruitment appeared clearly evident in central parietal and frontal areas of the right nonstimulated hemisphere (BA5, 4, and 3; Fig. 3, Table 1). Post hoc comparisons between individual current densities within the RoI for EEGlow and EEGhigh conditions showed a trend for lower activation associated with higher CSMP excitation [P = 0.08].
At 13–16 ms, the RoI was localized in the frontal lobe bilaterally (BA6 bilaterally; Fig. 3, Table 1). Post hoc comparison showed no significant differences in this RoI [P = 0.24].
At 18–25 ms, interval recruitment appeared clearly evident in the parietal lobe of the left stimulated hemisphere (BA3, 2 and 40; Fig. 3, Table 1). Post hoc comparison showed a stronger RoI recruitment for lower CSMP excitation [P = 0.02].
At 26–32 ms, we found a RoI localized in the frontal BA6 bilaterally (Fig. 3, Table 1). Post hoc comparison showed a stronger SM1 recruitment for higher CSMP excitation [P = 0.03].
At 35–39 ms, cerebral recruitment was again evident in the centro‐frontal areas of the right nonstimulated hemisphere (BA6 and 4; Fig. 3, Table 1). Post hoc comparison showed no dependence on CSMP excitation [P = 0.23].
Recruitments after sensory feedback arrival
The interval at around 50 ms (44–47 ms), which is compatible with sensory feedback from the hand muscles, showed a RoI localized in the left sensory‐motor area (BA6, 4 and 8; Fig. 3, Table 1). Post hoc comparison showed a stronger recruitment for higher CSMP excitation [P = 0.01]. No correlation was observed between the latencies of MEP and this RoI activation (r = 0.120, P = 0.734).
The subsequent site of excitation was at about 68–75 ms and 79–85 ms. At both these intervals, we found an excitation in the left and right frontal lobes (BA6 bilaterally Fig. 3, Table 1). Post hoc comparisons showed a trend of stronger activation in correspondence with higher CSMP excitation for the earliest [P = 0.08] and no effect for the latest activation [P = 0.23].
DISCUSSION
To identify whether or not the time‐varying excitability of CSMP—as indexed by the MEP amplitude—corresponds to different effective connectivity patterns within the network to which M1 belongs, four main effects were found corresponding to stronger CSMP excitation (MEPs of larger amplitude): (1) the right nonstimulated primary sensorimotor region homotopic to the stimulated region showed a trend of weaker activation at 6–10 ms; (2) the left parietal area was less activated at 18–25 ms; (3) bilateral motor areas showed a stronger activation at 26–32 ms; (4) the left sensory‐motor area was more activated at 44–47 ms and a trend of stronger activation of bilateral fronto‐medial lobes was found at 68–75 ms.
Among the inhibitory effects in contralateral—through crossed projections—and ipsilateral cortices, which can occur in correspondence of an increased activation at the stimulation site, we documented that a stronger projection to the periphery corresponded to an inhibited central‐parietal projection efficacy at early latencies (<25 ms) followed by stronger centro‐frontal recruitments (26–47 ms).
RoIs Recruitments Preceding Sensory Feedback Arrival
Right nonstimulated homotopic SM1 recruitment
At 6–10 ms after the left M1 stimulation, for both high and low CSMP excitation levels and in all subjects, a clear neuronal excitation was produced in the nonstimulated right region homotopic to the stimulated region. The most conceivable hypothesis is that these observed recruitments were mediated by transcallosal fibres [Daskalakis et al., 2008; Lee et al., 2007; Rizzo et al., 2011, Rizzolatti and Luppino, 2001]. The recruitment of homotopic areas of the nonstimulated hemisphere, as in a previous TMS‐fMRI study [Bestmann et al., 2004], showed a trend for being weaker with higher CSMP excitation, suggesting minor inhibitory effects induced in the contralateral homologous region when stronger cortico‐spinal outputs are generated. We can infer that the crucial role in achieving hemispheric hierarchy while executing unimanual motor tasks [Bender et al., 2005; Hoppenbrouwers et al., in press; Kicić et al., 2008; Nikulin et al., 2003; Voineskos et al., 2010], orchestrated by interhemispheric connectivity regulating inhibition levels acting on the contralateral circuit, is functionally modulated even in resting state and during motor relaxation.
At 13–16 ms, we did not observe dependence of neural recruitment strength on CSMP excitation, in agreement with the results of Bonato and colleagues [2006], who did not find correlation between the amplitude of MEP and the amplitude of EEG deflections peaking at 15 ms from TMS.
Left parietal recruitment
Parietal recruitment in the left hemisphere ipsilateral to TMS at 18–25 ms was weaker with stronger CSMP excitation. The existence of direct connections between the posterior parietal cortex and the ipsilateral M1 [Jones, 1978; Rizzolatti and Luppino, 2001] was recently confirmed by functional studies [Ferreri et al., 2010; Koch et al., 2007; Rizzo et al., 2011] but the latency of the observed activation speaks in favor of reverberant thalamo‐cortical circuits implicated in this effect. Lower recruitment for stronger CSMP excitation at this latency seems to indicate that a prevalent projection to the periphery corresponds to weaker cortico‐subcortical projection efficacy.
The reconstruction of neural recruitments at latencies up to 20–25 ms can be distorted by electrical activity related to head muscle contractions induced by TMS [Kujirai et al., 1993; Mäki and Ilmoniemi, 2010b). If muscular activity had significantly influenced the identification of RoIs, stronger RoI activities would have been paired with stronger CSMP excitations. The contrary occurred, with the RoIs less activated with stronger CSMP excitation at 6–10 ms (a trend) and 18–25 ms. Muscular activities do not conceivably contribute to RoI identification.
Bilateral centro‐frontal recruitment
Stronger recruitment of bilateral motor areas documented for stronger CSMP excitation at 26–32 ms agrees with previous studies [Mäki and Ilmoniemi, 2010a), elucidating the specific structures involved at this poststimulus epoch. The centro‐frontal cortical activation could reveal inhibitory‐excitatory integrative phenomena from right M1 and within left premotor and supplementary motor areas ipsilateral to the left M1 impacted by TMS stimulus, resulting in stronger circuit reverberant involvements for stronger TMS‐induced activation.
RoIs Recruitments After Sensory Feedback Arrival
Primary sensorimotor areas of the left stimulated hemisphere showed higher recruitment at 44–47 ms for higher CSMP excitations. These latencies are consistent with arrival to cortical relays of sensory feedback from the mechano/proprioceptors of the muscles whose contraction was induced by TMS [Desmedt, 1987; Kawamura et al., 1996; Mauguiere et al., 1997; Rossini et al., 1987; Siniatchkin et al., 2007]. It is conceivable that stronger muscle contraction, generating stronger feedback, contributes to this effect. Meanwhile, absence of correlation between MEP and the activation latency of this left sensorimotor region, indicates that a relevant contribution of this RoI activation is due to central projections, in addition to the activation induced by feedback. No effects were observed for neural recruitments later that 80 ms, consistent with previous studies, reporting inhomogeneous observations with channel deflections around 100 ms, associated [Paus et al., 2001] or not [Nikulin et al., 2003] to MEP amplitude.
CONCLUSIONS
The results brought to light how the spontaneous and involuntary fluctuations of the cortico‐spinal neural excitation corresponded to changes of the circuit transmission in the 6–75 ms period within the bihemispheric primary network devoted to hand control. More specifically, at early latencies (<25 ms) a prevalent projection to the periphery corresponded to weaker central‐parietal projection efficacy. Starting from 26 ms, even before feedback arrival, centro‐frontal recruitments revealed stronger circuit reverberant involvements for stronger cortico‐spinal pathway excitation.
Altogether, the proposed method, sensitive to changes of central internodes' effective connectivities associated with different levels of stimulated node excitation, might be useful in studies of neurological diseases in which circuits relevant to motor control are impaired.
ACKNOWLEDGMENTS
The authors thank MD. F. Ferreri and TNFP Rita Fini and Emma Fabrizio for their assistance in data collection and interpretation and for scientific collaboration.
REFERENCES
- Adrian ED, Moruzzi G (1939): Impulses in the pyramidal tract. J Physiol 97:153–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Maccabee PJ, Cracco RQ (1989): Focal stimulation of human peripheral nerve with the magnetic coil: A comparison with electrical stimulation. Exp Neurol 103:282–289. [DOI] [PubMed] [Google Scholar]
- Barbati G, Porcaro C, Zappasodi F, Rossini PM, Tecchio F (2004): Optimization of an independent component analysis approach for artifact identification and removal in magnetoencephalographic signals. Clin Neurophysiol 115:1220–1232. [DOI] [PubMed] [Google Scholar]
- Barker AT, Freeston IL, Jalinous R, Jarratt JA (1985): Clinical evaluation of conduction time measurements in central motor pathways using magnetic stimulation of human brain. Lancet 1:1325–1326. [DOI] [PubMed] [Google Scholar]
- Bender S, Basseler K, Sebastian I, Resch F, Kammer T, Oelkers‐Ax R, Weisbrod M (2005): Electroencephalographic response to transcranial magnetic stimulation in children: Evidence for giant inhibitory potentials. Ann Neurol 58:58–67. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J (2004): Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19:1950–1962. [DOI] [PubMed] [Google Scholar]
- Bonato C, Miniussi C, Rossini PM (2006): Transcranial magnetic stimulation and cortical evoked potentials: A TMS‐EEG co‐registration study. Clin Neurophysiol 117:1699–1707. [DOI] [PubMed] [Google Scholar]
- Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M (2010): General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage 49:1459–1468. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, Defendi S, Mariotti M, Massimini M (2010): EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One 22:e10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R (2008): The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543 (Pt 1):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE (1987): Physiology and physiopathology of somatic sensations studied in man by the method of evoked potentials. J Physiol (Paris) 82:64–136; Review. [PubMed] [Google Scholar]
- Ellaway PH, Davey NJ, Maskill DW, Rawlinson SR, Lewis HS, Anissimova NP (1998): Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol 109:104–113. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Määttä S, Ponzo D, Ferrarelli F, Tononi G, Mervaala E, Miniussi C, Rossini PM (2010): Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage 54:90–102. [DOI] [PubMed] [Google Scholar]
- Filippi MM, Oliveri M, Vernieri F, Pasqualetti P, Rossini PM (2000): Are autonomic signals influencing cortico‐spinal motor excitability? A study with transcranial magnetic stimulation. Brain Res 881:159–164. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS (2002): A standardised boundary element method volume conductor model. Clin Neurophysiol 113:702–712. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, De Jesus DR, Stirpe T, Fitzgerald PB, Voineskos AN, Schutter DJ, Daskalakis ZJ (in press): Inhibitory deficits in the dorsolateral prefrontal cortex in psychopathic offenders. Cortex. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G (2007): TMS‐induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One 2:e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T (1997): Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. NeuroReport 8:3537–3540. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH (1978): Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181:291–347. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Wilenius J (2007): Effects of alcohol on TMS‐evoked N100 responses. J Neurosci Methods 166:104–108. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Wilenius J, Komssi S, Ilmoniemi RJ (2004): Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol 115:583–588. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Komssi S, Wilenius J, Ilmoniemi, RJ (2005): Prefrontal transcranial magnetic stimulation produces intensity‐dependent EEG responses in humans. Neuroimage 24:955–960. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Nakasato N, Seki K, Kanno A, Fujita S, Fujiwara S, Yoshimoto T (1996): Neuromagnetic evidence of pre‐ and post‐central cortical sources of somatosensory evoked responses. Electroencephalogr Clin Neurophysiol 100:44–50. [DOI] [PubMed] [Google Scholar]
- Kicić D, Lioumis P, Ilmoniemi RJ, Nikulin VV (2008): Bilateral changes in excitability of sensorimotor cortices during unilateral movement: Combined electroencephalographic and transcranial magnetic stimulation study. Neuroscience 152:1119–1129. [DOI] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J (1993): Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89:415–423. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC (2007): Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci 27:6815–6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S, Aronen HJ, Huttunen J, Kesäniemi M, Soinne L, Nikouline VV, Ollikainen M, Roine RR, Karhu J, Savolainen S, Ilmoniemi RJ (2002): Ipsi and contralateral EEG reactions to transcranial magnetic stimulation. Clin Neurophysiol 113:175–184. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kähkonen S, Ilmoniemi RJ (2004): The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp 21:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S, Kähkönen S (2006): The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev 52:183–192. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Sato M, Rothwell JC, Cohen LG (1993): The effect of transcranial magnetic stimulation on median nerve somatosensory evoked potentials. Electroenceph clin Neurophysiol 89:227–234. [DOI] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R (2007): The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol 580:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioumis P, Kičić D, Savolainen P, Mäkelä JP, Kähkönen S (2009): Reproducibility of TMS‐evoked EEG responses. Hum Brain Mapp 30:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Myers JP (1998): Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 121:437–450. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ (2010a): The relationship between peripheral and early cortical activation induced by transcranial magnetic stimulation. Neurosci Lett 478:24–28. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ (2010b): Projecting out muscle artifacts from TMS‐evoked EEG. Neuroimage 54:2706–2710. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G (2005): Breakdown of cortical effective connectivity during sleep. Science 309:2228–2232. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Kicic D, Kahkonen S, Ilmoniemi RJ (2003): Modulation of electroencephalographic responses to transcranial magnetic stimulation: Evidence for changes in cortical excitability related to movement. Eur J Neurosci 18:1206–1212. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD (2002): Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin Pharmacol 24:5–12. [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC (1997): Transcranial magnetic stimulation during positron emission tomography: A new method for studying connectivity of the human cerebral cortex. J Neurosci 17:3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP (2001): Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J Neurophysiol 86:1983–1990. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Bove M, Naro A, Tacchino A, Mastroeni C, Avanzino L, Crupi D, Morgante F, Siebner HR, Quartarone A (2011): Associative cortico‐cortical plasticity may affect ipsilateral finger opposition movements. Behav Brain Res 216:433–439. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001): The cortical motor system. Neuron 31:889–901. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M (2009): Natural frequencies of human corticothalamic circuits. J Neurosci 29:7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A, Safety of TMS Consensus Group (2009): Safety, ethical consideration, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S (2007): Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology 68:484–488; Review. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caramia M, Zarola F (1987): Central motor tract propagation in man: Studies with non‐invasive, unifocal, scalp stimulation. Brain Res 415:211–225. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Lavaroni F, Caramia MD (1991): Brain excitability and electroencephalographic activation: Non‐invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Res 567:111–119. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RG, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C (1994): Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Tecchio F, Sabato A, Finazzi‐Agrò A, Pasqualetti P, Rossi S (1996): The role of cutaneous inputs during magnetic transcranial stimulation. Muscle Nerve 19:1302–1309. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout AM, Paulus W, Pauri F (1999): Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol 52(Suppl.):171–185. [PubMed] [Google Scholar]
- Schnitzler A, Gross J (2005): Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6:285–296. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC (2009): How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex 45:1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012): Spectral fingerprints of large‐scale neuronal interactions. Nat Rev Neurosci 13:121–134. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Groppa S, Jerosch B, Muhle H, Kurth C, Shepherd AJ, Siebner H, Stephani U (2007): Spreading photoparoxysmal EEG response is associated with an abnormalcortical excitability pattern. Brain 130 (Pt 1):78–87. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM (2002): Effects of ovarian hormones on human cortical excitability. Ann Neurol 51:599–603. [DOI] [PubMed] [Google Scholar]
- Sparing R, Buelte D, Meister IG, Paus T, Fink GR (2008): Transcranial magnetic stimulation and the challenge of coil placement: A comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp 29:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Caramia M, Zarola F, Rossini PM (1988): Enhancement of motor cortical excitability in humans by non‐invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol 70:26–32. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas R (1988): The functional state of the thalamus and the associated neuronal interplay. Physiol Rev 68:649–742 [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Pasqualetti P, De Gennaro L, Pellicciari MC, Ercolani M, Squitti R, Rossini PM (2008): Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol 119:675–682. [DOI] [PubMed] [Google Scholar]
- Virtanen J, Ruohonen J, Naatanen R, Ilmoniemi RJ (1999): Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med Biol Eng Comput 37:322–326. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Farzan F, Barr MS, Lobaugh NJ, Mulsant BH, Chen R, Fitzgerald PB, Daskalakis ZJ (2010): The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry 68:825–831. [DOI] [PubMed] [Google Scholar]


