Abstract
Memory impairments are common in major depression. Neural processing during non‐emotional episodic memory in depressed patients has only sparsely been investigated, since the majority of studies have focused on emotional stimuli. The aim of this study was to explore neural correlates of episodic memory in depressive patients and to assess brain regions related to subsequent memory performance. Forty‐six participants (23 depressed patients) performed a non‐emotional episodic memory encoding and retrieval task while brain activation was measured with functional magnetic resonance imaging. Patients with depression showed decreased activation in the right prefrontal cortex and right cingulate cortex during memory encoding, but increased activation in the right inferior frontal gyrus (IFG) during recognition memory. While a strong association between hippocampal and parahippocampal activation during memory encoding with subsequent memory performance became evident in healthy controls, this relationship was absent in patients with depression. Taken together, these findings demonstrate that memory related brain regions are affected in their appropriate functioning during memory encoding in depressed patients. Therefore, patients with depression may rely to a greater degree on other brain regions such as the IFG during episodic memory retrieval. Hum Brain Mapp 35:4293–4302, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: major depression, episodic memory, fMRI, hippocampal formation, prefrontal cortex
INTRODUCTION
Major depression is a common mental disorder, which is accompanied by cognitive deficits. Meta‐analytical findings indicate that episodic memory problems are among the most impaired cognitive domains in patients with depression [Burt et al., 1995; Lee et al., 2012; McDermott and Ebmeier, 2009; Zakzanis et al., 1998]. Despite a strong neuropsychological tradition of non‐emotional episodic memory research in major depression, only relatively few functional neuroimaging studies have previously been carried out using non‐emotional stimuli [e.g., Fairhall et al., 2010; Kelley et al., 2013; Milne et al., 2012; van Eijndhoven et al., 2011].
Generally, episodic memory has been associated with a widespread network of brain regions including the medial temporal lobe (MTL) and the prefrontal cortex (PFC) [Cabeza and Nyberg, 2000; Dickerson and Eichenbaum, 2010; Eichenbaum et al., 2007; Fernández and Tendolkar, 2001; Nyberg et al., 2003]. Among MTL structures the hippocampus has been shown to be critically involved in episodic memory processing [Kircher et al., 2008; Kirwan and Stark, 2004; Wang and Morris, 2010]. Furthermore, meta‐analytical findings indicate hippocampal activation during memory encoding to be highly predictive for successful memory formation [Kim, 2011; Spaniol et al., 2009]. The PFC, which is strongly connected with MTL structures, has been shown to be crucial for episodic memory processing. The various PFC subregions subserve selection and maintaining of incoming information, forming and organizing associations between items, as well as monitoring and evaluation of items [Blumenfeld and Ranganath, 2007; Cabeza and Nyberg, 2003; Spaniol et al., 2009].
Compared with healthy subjects, there is a growing consensus that patients with depression show neural alterations mainly in the MTL, PFC, and cingulate cortex during non‐emotional episodic memory processing [Bremner et al., 2004; Fairhall et al., 2010; Kelley et al., 2013; Milne et al., 2012; Werner et al., 2009; van Eijndhoven et al., 2011]. In particular, functional neuroimaging studies have provided evidence that depression is associated with hippocampal and cingulate hypoactivity during memory processing [Bremner et al., 2004; Diener et al., 2012; Fairhall et al., 2010; Kelley et al., 2013; Milne et al., 2012; Young et al., 2012]. However, regarding the PFC, significant differences in the functional role of various subregions have been found in depressed patients, which demonstrated hypoactivity as well as hyperactivity during memory encoding and recognition [Aihara et al., 2007; Bremner et al., 2004; Kelley et al., 2013; van Eijndhoven et al., 2011; Werner et al., 2009].
While most studies have explored the neural correlates of non‐emotional memory using verbal memory tasks, there are only two studies applying a face association learning paradigm [Fairhall et al., 2010; Werner et al., 2009]. Fairhall et al. [2010] investigated exclusively encoding processes and identified hippocampal dysregulations during a face‐name association memory task in patients with major depression. However, only eight young depressed outpatients were included in this study, which limits the generalizability of the results. Besides, the authors focused on a priori selected regions‐of‐interest. In contrast, Werner et al. [2009] examined whole brain activation patterns between eleven outpatients with depression and eleven healthy controls during memory encoding and recognition in a face‐profession association task. They found differences in memory‐related brain regions between both groups, but did not show an involvement of the hippocampus. Again, only a small number of depressed outpatients were included. Moreover, the authors used emotional biased stimuli that might confound the outcome.
Our study focused on two objectives. First, we explored the neural correlates of memory encoding and recognition of neutral faces in patients with major depression, in order to minimize an emotional related memory bias. Regarding previous findings, we expected neural alterations in prefrontal regions, hippocampal formation, and cingulate cortex during memory encoding and recognition in patients with depression compared with healthy controls [e.g., Bremner et al., 2004; Kelley et al., 2013; Werner et al., 2009]. Second, we assessed brain regions related to the individual memory performance. We expected a strong relationship between MTL structures and memory performance in healthy controls, but not in depressive patients due to disorder‐related aberrant functioning of these structures.
MATERIALS AND METHODS
Participants
A total of 23 inpatients from the Department of Psychiatry and Psychotherapy at the Philipps‐University Marburg, Germany, diagnosed with a current depressive disorder according to the International Classification of Diseases (ICD‐10) and 23 healthy controls participated in this study (see Table 1). Additional affective ratings were assessed with the German versions of the Beck Depression Inventory (BDI) [Hautzinger et al., 1994] and the Hamilton Depression Rating Scale (HAM‐D) [CIPS, 2005]. Patients were included if they suffered primarily from either a first (n = 5) or a recurrent episode (n = 18) of unipolar depression. Ten patients did not have any comorbidities and seven patients additionally fulfilled the criteria for a dysthymic disorder (“double depression”). Patients with another comorbiditiy were only included if the depression was the reason for current hospitalization. Therefore, patients with the following comorbidities were included: social phobia (n = 2), somatoform disorder (n = 2), and dependent or narcissistic personality disorder (n = 2). Three patients did not receive any medication and 20 patients were treated according to the current treatment guidelines at time of inclusion. Patients received an antidepressant monotherapy (n = 13), combined antidepressant therapy (n = 3), or combined antidepressant and antipsychotic pharmacotherapy (n = 4). Patients with a history of manic or psychotic episodes as well as patients with comorbid alcohol or substance dependence (life time diagnosis) were excluded from this study.
Table 1.
Sociodemographic, clinical, and performance variables of the two groups
| Patients | Controls | t ‐value | P | |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| Age | 36.69 (10.35) | 37.13 (10.42) | 0.14 | 0.88 |
| Gender (f/m) | 14/9 | 14/9 | ||
| Years of education | 10.95 (1.71) | 11.43 (1.53) | 0.99 | 0.32 |
| BDIa | 29.39 (5.86) | 2.65 (2.20) | 20.48 | <0.001 |
| HAMDb, c | 17.75 (3.64) | |||
| VLMT d | ||||
| Immediate recall | 7.34 (2.34) | 7.60 (2.12) | 0.39 | 0.65 |
| Delayed recall | 11.61 (2.61) | 12.64 (2.37) | 1.42 | 0.16 |
| Correctly recognized items | 14.21 (1.24) | 14.17 (1.61) | 0.10 | 0.91 |
| Performance during fMRI tasks | ||||
| Encoding task | ||||
| Reaction time (ms) | 852 (150) | 852 (150) | 0.01 | 0.99 |
| Correct gender identification (%) | 98.40 (2.23) | 99.43 (1.63) | 1.77 | 0.08 |
| Recognition task | ||||
| Reaction time (ms) | 1976 (263) | 1876 (237) | 1.36 | 0.18 |
| Correctly recognized items | 23.17 (2.27) | 25.09 (2.56) | 2.68 | 0.01 |
In general, participants were excluded if they had major medical or neurological disorders, mental retardation, medication likely to influence cognitive function, current alcohol or drug abuse, history of lifetime alcohol or drug dependence, were younger than 18 years or older than 60 years, or fulfilled general MRI exclusion criteria. Additional exclusion reasons for healthy controls were a current or a lifetime history of a psychiatric disorder assessed with the German version of the Structured Clinical Interview for DSM‐IV (SCID‐I) [Wittchen et al., 1997] or first‐degree relatives with a psychiatric disorder. None of the healthy controls had scores on the BDI that indicated pathological symptoms. After a complete description of the procedure, all participants gave written informed consent. The protocol was approved by the ethics committee of the Faculty of Medicine, Philipps‐University Marburg, according to the declaration of Helsinki.
Neuropsychological Memory Assessment
In addition to the scanning procedure, all participants completed the Verbal Learning and Memory Test (VLMT) [Helmstaedter et al., 2001], which was applied to explore the immediate and delayed verbal memory performance as well as verbal recognition memory.
Functional Magnetic Resonance Imaging (fMRI) Task and Stimuli
The paradigm consisted of an encoding and a recognition task in different sessions. Both sessions were divided by a short break of approximately 3 min. During this time, participants stayed in the scanner.
Encoding task
During the encoding task, either single pictures of neutral faces (encoding condition) or scrambled pictures (high‐level baseline condition) were presented on a black background for 4000 ms in a pseudo‐randomized order using Presentation software package (Neurobehavioral Systems, San Francisco, CA). Following this, stimuli were replaced by a blank screen for another 1000 ms, which completed the trial. Participants were instructed to actively memorize each face for later recognition. To ensure their attention, participants were instructed to indicate via button press (LUMItouch™ Lightwave Technologies, Richmond, B.C., Canada) if the presented face was female (right index finger) or male (right middle finger). During the high‐level baseline condition, participants were required to press a button with the right index finger every time a scrambled picture appeared. There were five blocks of each condition, with six responses in each block, resulting in 30 faces (half male and half female) to be encoded. Similarly, during the high‐level baseline condition, 30 button presses had to be accomplished. Each block lasted 30 s.
Recognition task
After the encoding task, a recognition task of equal length and structure was administered. During the recognition condition, two pictures of faces were presented simultaneously side by side, each trial comprising a previously presented face and a new face, randomly positioned at the left or right side. Participants were requested to select the previously presented face by pressing the corresponding button with the right index finger (for the face on the left side) or the right middle finger (for the face on the right side). During the high‐level baseline condition, two scrambled pictures were presented side by side. Participants were instructed to press a button with the right index finger every time the two scrambled pictures appeared. Both tasks (encoding and recognition) have already been applied successfully in different samples [e.g., Jansen et al., 2010; Krach et al., 2010; Krug et al., 2010, 2013; Thimm et al., 2010].
fMRI Data Acquisition
Imaging was performed on a 3‐T Tim Trio MR scanner (Siemens Medical Systems AG, Erlangen, Germany) at the Department of Psychiatry and Psychotherapy, Philipps‐University Marburg. Functional images were collected with echo planar imaging sensitive to blood oxygen level dependent (BOLD) contrast (T2*, 64 × 64 matrix, FoV 224 × 224 mm2, 40 slices, 3.5 mm thickness, TR = 2.5 s, TE = 30 ms, flipangle = 90°). Slices covered the whole brain and were positioned transaxially parallel to the anterior–posterior commissural line (AC–PC). For each task, 132 functional images were collected, and the initial three images were excluded from further analysis to remove the influence of T1 stabilization effects.
Data Analysis
Analysis of fMRI data was performed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) running on MATLAB 7.7.0 (The MathWorks, Natick, MA). Functional images were realigned, normalized (resulting voxel size 2 × 2 × 2 mm3), smoothed (8 mm isotropic Gaussian filter), and high‐pass filtered (cut off period 128 s). Statistical analysis was performed in a 2‐level mixed‐effects procedure. Head movement was visually checked for each participant. All participants had tolerable head movement smaller than one voxel. At the first level, the BOLD responses for the task (encoding and recognition) and the high‐level baseline condition were modeled by a boxcar function convolved with the canonical hemodynamic response function employed by SPM8. Parameter estimate (ß‐) and t‐statistic images were calculated for each participant. On the second level, we conducted a 2 × 2 × 2 analysis of variance (ANOVA), using a full factorial design with group (patients vs. controls), condition (baseline vs. task), and task (encoding vs. recognition) as factors. Additionally, age, gender, and fMRI task performances (hits and reaction time) were entered as variables of no interest into the model to exclude possible differences in brain activation that might interfere with or alter task‐related activity. For whole brain comparisons, group (patients vs. controls) by condition (baseline vs. task) interactions were analyzed. An advantage of this approach is that it takes into account potential differences in baseline conditions between patients and controls [Hanslmayr et al., 2013]. Several studies have shown that differences in baseline conditions are common in patients with depression compared to healthy controls [for meta‐analysis see Fitzgerald et al., 2008a]. Thus, this approach allows for a more delicate analysis of activation patterns compared to subtractive designs.
Furthermore, we conducted a regression analysis in SPM8 to identify brain regions that were functionally correlated with subsequent memory performance. Therefore, we entered the number of correctly recognized items into the model to regress against the parameter estimates (beta values) during memory encoding. To investigate the direct relationship between the identified brain regions and subsequent memory performance, beta values were extracted from the resulting brain regions using the volume of interest function implemented in SPM8. In a second step, these beta values as well as age, gender, and years of education of each participant were used as predictors in a multiple linear regression model using the Statistical Package for the Social Sciences (SPSS 20, IBM, Armonk, N.Y.) to predict later memory performance.
To correct for multiple comparisons within a search volume, we applied a cluster extend threshold determined by Monte Carlo [Slotnick et al., 2003]. For a threshold at the voxel level P = 0.001 and spatial properties as present in this study, 10.000 simulations resulted in an extent threshold of 43 resampled voxels. This procedure prevented a false positive rate above 5% due to multiple testing. The anatomical localization of activated brain regions was assessed by the SPM anatomy toolbox [Eickhoff et al., 2005]. Brain activations were plotted on the anatomical MRIcroN (http://www.mccauslandcenter.sc.edu/mricro/mricron) template.
RESULTS
Behavioral Performance
With respect to the VLMT [Helmstaedter et al., 2001], patients with depression did not differ from healthy controls considering immediate and delayed recall as well as recognition memory (t 44 = 0.39, P = 0.65; t 44 = 1.42, P = 0.16; t 44 = 0.10, P = 0.91, respectively). During both fMRI tasks patients with depression did not differ from healthy controls with respect to reaction time (t 44 = 0.01, P = 0.99; t 44 = 1.36, P = 0.18, respectively) or correct gender judgment as part of the encoding task (t 44 = 1.77, P = 0.08). While patients with depression remembered significantly fewer faces during the recognition task (t 44 = 2.68, P = 0.01), both groups performed significantly above chance (see Table 2).
Table 2.
Brain areas activated during episodic memory encoding and recognition as well as brain regions resulting from the regression analysis
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical region | Hemisphere | x | y | z | Z‐value | No. voxels | |
| Encoding | |||||||
| Middle Frontal Gyrus | Right | 44 | 22 | 50 | 4.11 | 119 | |
| Medial Frontal Gyrus | Right | 12 | 42 | 32 | 3.68 | 102 | |
| Cingulate Cortex | Right | 8 | 34 | 34 | 3.66 | ||
| Recognition | |||||||
| Inferior Frontal Gyrus | Right | 44 | 28 | 20 | 4.18 | 95 | |
| Regression | |||||||
| Hippocampus | Right | 28 | −14 | −18 | 3.99 | 124 | |
| Parahippocampal Gyrus | Right | 30 | −8 | −30 | 3.40 | ||
The x, y, and z coordinates of the local maxima are listed according to the Montreal Neurological Institute (MNI) coordinate system (P < 0.001, corrected by Monte Carlo cluster simulations; cluster extend threshold = 43 voxels).
fMRI Data
Encoding task
The main effect of task revealed an activation pattern of bilateral prefrontal, medial temporal, and occipital regions in accordance with previously reported results [e.g., Jansen et al., 2010; Krach et al., 2010; Krug et al. 2013]. With respect to the experimental objective, we conducted a group (patients vs. controls) by condition (encoding vs. baseline) interaction. It revealed a significant differential activation between patients with depression and healthy controls in a set of regions comprising the right middle frontal gyrus (MiFG), right medial frontal gyrus (MeFG), and right anterior cingulate cortex (ACC; see Fig. 1 and Table 3). Within the patient group, memory performance was significantly correlated with the activation in the MiFG (r 23 = 0.40, P = 0.029) and right MeFG (r 23 = 0.36, P = 0.046). Additionally, there was a trend for the correlation between memory performance and activation in the right ACC (r 23 = 0.31, P = 0.076).
Figure 1.
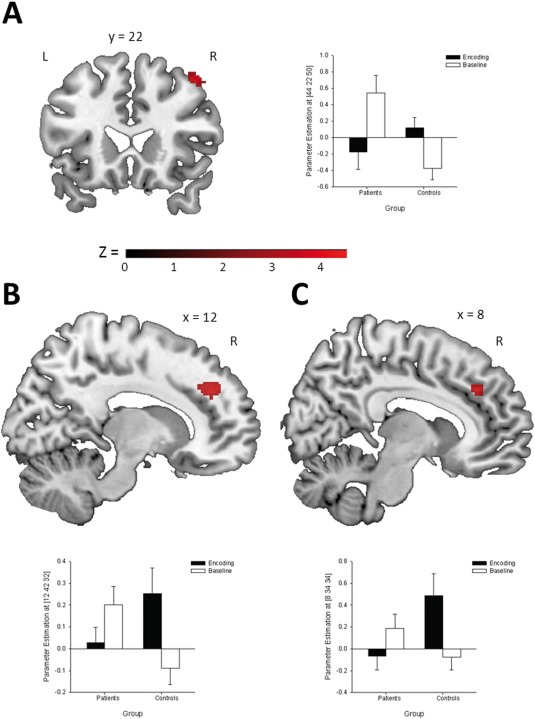
Brain areas activated during memory encoding and magnitudes of the mean BOLD activity at the peak voxel of the associated cluster: (A) middle frontal gyrus, (B) medial frontal gryrus, and (C) anterior cingulate cortex (P < 0.001, corrected by Monte Carlo cluster simulations; cluster extend threshold = 43 voxels). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Recognition task
Again, the main effect of task depicted a commonly observed activation pattern of bilateral prefrontal, occipital, and right hippocampal regions [e.g., Jansen et al., 2010; Krach et al., 2010; Krug et al., 2013]. A group (patients vs. controls) by condition (encoding vs. baseline) interaction revealed that patients with depression and healthy controls differed in the right inferior frontal gyrus (IFG; see Fig. 2 and Table 2).
Figure 2.

(A) Right IFG activation during memory retrieval and (B) magnitude of the mean BOLD activity at the peak voxel of the associated cluster (P < 0.001, corrected by Monte Carlo cluster simulations; cluster extend threshold = 43 voxels). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Regression analysis
To examine the specific functional relationship between the whole brain activation pattern and individual memory performance, we conducted a regression analysis with SPM8. The right hippocampus and the right parahippocampal gyrus were the only regions correlated with subsequent memory performance (see Fig. 3 and Table 2). Following multiple linear regression analysis demonstrated that hippocampal (β = 0.65, t 22 = 3.78, P = 0.001) and parahippocampal gyrus (β = 0.59, t 22 = 3.00, P = 0.008) engagement during encoding were significantly predicting the subsequent memory outcome for healthy controls. In contrast, there was no significant functional relationship between neither hippocampal (β = 0.31, t 22 = 1.55, P = 0.139) nor parahippocampal gyrus (β = 0.19, t 22 = 0.85, P = 0.405) engagement with subsequent memory outcome in the patient group. Group comparisons showed statistical trends for stronger associations between hippocampal (Z = 1.44, P = 0.075) and parahippocampal gyrus (Z = 1.51, P = 0.066) activation during memory encoding with later recognition performance in healthy controls compared with patients with depression.
Figure 3.

(A) Brain areas related to subsequent recognition memory performance. Magnitudes of the mean BOLD activity at the peak voxel of the associated cluster: (B) hippocampus and (C) parahippocampal gyrus (P < 0.001, corrected by Monte Carlo cluster simulations; cluster extend threshold = 43 voxels). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
In the current study, we examined the neural correlates of episodic memory encoding and retrieval of neutral faces in patients with a current depressive disorder. On the behavioral level, both groups did not differ with respect to memory performance in the VLMT [Helmstaedter et al., 2001] as well as according to reaction time and gender judgment during the fMRI tasks. Although all participants performed above chance, patients with depression recognized less items in the fMRI task. These findings represent evidence that memory impairments in depressive patients are associated with more demanding tasks, but not necessarily in automatic aspects of these tasks [Hartlage et al., 1993].
In contrast to other studies [e.g., Fairhall et al., 2010; Werner et al., 2009], we explored differential neural activation patterns using group by condition interactions. This takes into account that differences in baseline conditions are common in patients with depression compared with healthy controls (for meta‐analysis see [Fitzgerald et al., 2008a]). Depressive patients, compared with healthy controls, revealed a decreased encoding related BOLD response in the right middle frontal gyrus, right medial frontal gyrus, and right dorsal anterior cingulate cortex. Neuroimaging studies in healthy subjects have highlighted the importance of the PFC and the cingulate cortex in selection and maintaining of incoming information as well as forming and organizing associations between items during encoding [Blumenfeld and Ranganath, 2007; Cabeza and Nyberg, 2000; Dickerson and Eichenbaum, 2010; Eichenbaum et al., 2007; Hofer et al., 2007; Torta and Cauda, 2011]. Thus, the decreased BOLD response during encoding in these regions observed in depressed patients may provide evidence for inefficient cognitive strategies, which may lead to impoverished recognition memory. This interpretation is supported by the finding that the PFC activation was significantly correlated with memory performance in the patient group, that is higher activation was associated with better performance. Interestingly, the depressed patients showed significantly enhanced neural activation in the PFC and cingulate cortex during baseline condition compared to task related activation, while healthy controls exhibited the opposite activation pattern. Again, it is plausible that these differential activation patterns between groups may relate to different cognitive strategies. Moreover, such activation during the baseline condition might represent continuing cognitive processing that may reduce the activation during a cognitive task in terms of an overreaching compensatory response [Diener et al., 2012; Stark and Squire, 2001].
During recognition memory, the BOLD response was significantly increased in the right IFG in depressed patients compared with healthy controls. Previous studies in healthy subjects have suggested that the right IFG belongs to a neural network underlying memory retrieval and is associated with cognitive control demands [Aron et al., 2004; Chikazoe et al., 2007; Greenberg et al., 2005; Svoboda et al., 2006]. Additionally, in healthy individuals the right IFG has been hypothesized to be involved in retrieval effort, independently whether an item is recognized successfully or not [Wagner et al., 1998]. This particular function has been supported by a recent meta‐analysis examining neural correlates of cognition in major depression [Diener et al., 2012]. Diener and colleagues [2012] reported an increased activity in depressive patients, compared with healthy controls, predominantly in the right IFG during working memory. Thus, the larger engagement of the right IFG in depressed patients in our study may indicate that more neural resources are used to perform the recognition memory task. It is plausible that the difference between groups relates to the degree of effort required for this task. Taking into account that patients showed inefficient encoding strategies; it is likely that they relied more on the right IFG during the recognition task, but failed to achieve the same performance as healthy controls. Thus, the enhanced engagement of the IFG in depressive patients could not compensate for encoding deficits. Taken together, these findings can be interpreted in terms of inefficient cognitive processing. This interpretation is in line with other imaging studies suggesting cognitive performance in patients with depression during several demanding tasks to be associated with enhanced neural activation [e.g., Fitzgerald et al., 2008b; Harvey et al., 2005; Matsuo et al., 2007].
In addition to differential cortical activation patterns, we explored the relationship between whole brain activation and memory performance. As expected, depressed patients showed lower levels of activation in the hippocampus and the parahippocampal gyrus during memory encoding when compared with healthy controls. These findings are in line with previous studies demonstrating decreased hippocampal activity in depressive patients [Bremner et al., 2004; Fairhall et al., 2010; Kelley et al., 2013; Milne et al., 2012; Young et al., 2012]. However, the linear relationship between the activation of the hippocampal formation during encoding and subsequent memory performance has not yet been explored in depressed patients. Therefore, we investigated the specific functional relationship of the identified hippocampus and parahippocampal gyrus activation with the individual memory performance of each participant using regression analyses. As hypothesized, hippocampal and parahippocampal gyrus engagement were significantly predicting the recognition outcome for healthy controls. These findings are in line with previous studies suggesting that the strength of hippocampal activity during encoding is the most robust predictor for memory performance in healthy participants [for meta‐analyses see Kim, 2011; Spaniol et al., 2009]. In contrast to healthy subjects, this linear relationship was not significant in patients with depression. Thus, our results propose an aberrant functioning of the hippocampal formation during memory encoding in depressive patients, which may also be responsible for recognition memory deficits. While a lot of research has been done with emphasis on hippocampal volume, our findings extend the understanding of altered hippocampal functioning in major depression [MacQueen and Frodl, 2011].
This study has several limitations. Almost all patients received antidepressant medication at time of inclusion, which may confound the group differences. Furthermore, we included patients with either a first or a recurrent depressive episode. However, a recent meta‐analysis indicates that patients with a first depressive episode show already memory impairments, especially with respect to visual memory [Lee et al., 2012]. In addition, we also included patients with comorbid disorders, but none of these comorbidities were the reason of current hospitalization. Furthermore, our fMRI task did not enable us to investigate brain activation related to episodic memory encoding and retrieval vs. non‐explicit forms of memory (e.g., recognition based on feelings of familiarity). Finally, we used a block design to increase the power of both tasks. Hence, we were not able to directly analyze successful encoding in terms of contrasting activation during remembered vs. forgotten items. However, our multiple regression approach revealed neural activity in brain regions that have consistently been implicated in successful memory encoding [for meta‐analysis see Kim, 2011].
CONCLUSION
In summary, we investigated neural correlates of non‐emotional episodic memory encoding and retrieval in an adequate sample size of currently depressed patients. While depressed patients, compared with healthy controls, showed decreased activation in the right PFC and cingulate cortex during encoding, they demonstrated an increased activity in the right IFG during recognition memory. Based on these findings, it could be hypothesized that the observed altered activation pattern during encoding leads to impoverished memory, which cannot be compensated by increased prefrontal activity in depressed patients. Furthermore, this study provides evidence for an aberrant linear relationship between hippocampal formation activity during encoding and memory performance in patients with depression. Thus, our findings suggest that the appropriate functioning of the hippocampal formation is impaired in depressed patients.
Conflict of Interest: The authors report no conflict of interest.
REFERENCES
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M (2007): HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res 155:245–256. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C (2007): Prefrontal cortex and long‐term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13:280–291. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS (2004): Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry 161:637–645. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G (1995): Depression and memory impairment: A meta‐analysis of the association, its pattern, and specificity. Psychol Bull 117:285–305. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2003): Functional neuroimaging of memory. Neuropsychologia, 41:241–244. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y (2007): Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci 19:69–80. [DOI] [PubMed] [Google Scholar]
- CIPS (2005): Internationale Skalen für Psychiatrie. Göttingen: Hogrefe. [Google Scholar]
- Dickerson BC, Eichenbaum H (2010): The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology 35:86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H (2012): A meta‐analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage 61:677–685. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C (2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Sharma S, Magnusson J, Murphy B (2010): Memory related dysregulation of hippocampal function in major depressive disorder. Biol Psychol 85:499–503. [DOI] [PubMed] [Google Scholar]
- Fernández G, Tendolkar I (2001): Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: Human declarative memory formation at the system level. Brain Res Bull 55:1–9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008a): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, Egan GF (2008b): An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp 29:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS (2005): Co‐activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 43:659–674. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Backes H, Straub S, Popov T, Langguth B, Hajak G, Bäuml KH, Landgrebe M (2013). Enhanced resting‐state oscillations in schizophrenia are associated with decreased synchronization during inattentional blindness. Hum Brain Mapp 34:2266–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlage S, Alloy LB, Vázquez C, Dykman B (1993): Automatic and effortful processing in depression. Psychol Bull, 113:247–278. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehéricy S, Allilaire JF, Dubois B (2005): Cognitive control and brain resources in major depression: An fMRI study using the n‐back task. Neuroimage 26:860–869. [DOI] [PubMed] [Google Scholar]
- M Hautzinger, M Bailer, H Worall, F Keller (1994): Beck‐Depressions‐Inventar (BDI). Testbuch. Bern: Hans Huber. [Google Scholar]
- Helmstaedter C, Lendt M, Lux S (2001): Verbaler Lern‐ und Merkfähigkeitstest (VLMT). Göttingen: Hogrefe. [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Golaszewski SM, Felber S, Fleischhacker WW (2007): Neural substrates for episodic encoding and recognition of unfamiliar faces. Brain Cogn 63:174–181. [DOI] [PubMed] [Google Scholar]
- Jansen A, Krach S, Krug A, Markov V, Thimm M, Paulus FM, Zerres K, Stöcker T, Shah NJ, Nöthen MM, Treutlein J, Rietschel M, Kircher T (2010): The effect of G72 genotype on neural correlates of memory encoding and retrieval. Neuroimage 53:1001–1006. [DOI] [PubMed] [Google Scholar]
- Kelley R, Garrett A, Cohen J, Gomez R, Lembke A, Keller J, Reiss AL, Schatzberg A (2013): Altered brain function underlying verbal memory encoding and retrieval in psychotic major depression. Psychiatry Res 211:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2011): Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. Neuroimage 54:2446–2461. [DOI] [PubMed] [Google Scholar]
- Kircher T, Weis S, Leube D, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Krach S (2008): Anterior hippocampus orchestrates successful encoding and retrieval of non‐relational memory: An event‐related fMRI study. Eur Arch Psychiatry Clin Neurosci 258:363–372. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE (2004): Medial temporal lobe activation during encoding and retrieval of novel face‐name pairs. Hippocampus 14:919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krach S, Jansen A, Krug A, Markov V, Thimm M, Sheldrick AJ, Eggermann T, Zerres K, Stöcker T, Shah NJ, Kircher T (2010): COMT genotype and its role on hippocampal‐prefrontal regions in declarative memory. Neuroimage 53:978–984. [DOI] [PubMed] [Google Scholar]
- Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stöcker T, Shah NJ, Nöthen MM, Treutlein J, Rietschel M, Kircher T (2010): The effect of Neuregulin 1 on neural correlates of episodic memory encoding and retrieval. Neuroimage 53:985–991. [DOI] [PubMed] [Google Scholar]
- Krug A, Krach S, Jansen A, Nieratschker V, Witt SH, Shah NJ, Nöthen MM, Rietschel M, Kircher T (2013): The effect of neurogranin on neural correlates of episodic memory encoding and retrieval. Schizophr Bull 39:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Porter MA, Redoblado‐Hodge MA (2012): A meta‐analysis of cognitive deficits in first‐episode Major Depressive Disorder. J Affect Disord 140:113–124. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T (2011): The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16:252–264. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao JH, Soares JC (2007): Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 12:158–166. [DOI] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP (2009): A meta‐analysis of depression severity and cognitive function. J Affect Disord 119:1–8. [DOI] [PubMed] [Google Scholar]
- Milne AM, MacQueen GM, Hall GB (2012): Abnormal hippocampal activation in patients with extensive history of major depression: An fMRI study. J Psychiatry Neurosci 37:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M (2003): Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia 41:371–377. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J (2003): Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 17:75–82. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL (2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47:1765–1779. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR (2001): When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci 98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B (2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm M, Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stöcker T, Shah NJ, Nöthen MM, Rietschel M, Kircher T (2010): The impact of dystrobrevin‐binding protein 1 (DTNBP1) on neural correlates of episodic memory encoding and retrieval. Hum Brain Mapp 31:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Cauda F (2011): Different functions in the cingulate cortex, a meta‐analytic connectivity modeling study. Neuroimage 56:2157–2172. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, Fernandez G, Rijpkema M, Verkes RJ, Buitelaar J, Tendolkar I (2011): Amygdala responsivity related to memory of emotionally neutral stimuli constitutes a trait factor for depression. Neuroimage 54:1677–1684. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli JD (1998): Prefrontal cortex and recognition memory. Functional‐MRI evidence for context‐dependent retrieval processes. Brain 121:1985–2002. [DOI] [PubMed] [Google Scholar]
- Wang SH, Morris RG (2010): Hippocampal‐neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61:49–79. [DOI] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Materne J, Engel RR, Huber D, Riedel M, Reiser M, Hennig‐Fast K (2009): Functional MRI study of memory‐related brain regions in patients with depressive disorder. J Affect Disord 119:124–131. [DOI] [PubMed] [Google Scholar]
- Wittchen H‐U, Wunderlich U, Gruschwitz S, Zaudig M (1997): SKID‐I. Strukturiertes Klinisches Interview für DSM‐IV. Achse I: Psychische Störungen. Interviewheft. Göttingen: Hogrefe. [Google Scholar]
- Young KD, Erickson K, Nugent AC, Fromm SJ, Mallinger AG, Furey ML, Drevets WC (2012): Functional anatomy of autobiographical memory recall deficits in depression. Psychol Med 42:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E (1998): On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 11:111–119. [PubMed] [Google Scholar]


