Abstract
We investigated the lateralization of brain activity pattern during performance of unilateral movement in drug‐naïve Parkinson's disease (PD) patients with only right hemiparkinsonian symptoms. Functional MRI was obtained when the subjects performed strictly unilateral right hand movement. A laterality index was calculated to examine the lateralization. Patients had decreased activity in the left putamen and left supplementary motor area, but had increased activity in the right primary motor cortex, right premotor cortex, left postcentral gyrus, and bilateral cerebellum. The laterality index was significantly decreased in PD patients compared with controls (0.41 ± 0.14 vs. 0.84 ± 0.09). The connectivity from the left putamen to cortical motor regions and cerebellum was decreased, while the interactions between the cortical motor regions, cerebellum, and right putamen were increased. Our study demonstrates that in early PD, the lateralization of brain activity during unilateral movement is significantly reduced. The dysfunction of the striatum–cortical circuit, decreased transcallosal inhibition, and compensatory efforts from cortical motor regions, cerebellum, and the less affected striatum are likely reasons contributing to the reduced motor lateralization. The disruption of the lateralized brain activity pattern might be a reason underlying some motor deficits in PD, like mirror movements or impaired bilateral motor coordination. Hum Brain Mapp 36:1878–1891, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: Parkinson's disease, lateralization of brain activity, basal ganglia circuits, transcallosal inhibition, compensation
INTRODUCTION
In human, the execution of strictly unilateral motor tasks requires inhibition produced by complex interhemispheric interactions between motor areas. These interactions are needed to restrict motor output only from the primary motor cortex (M1) contralateral to the intended hand movement [Carson, 2005]. EEG studies found that during performance of unilateral movements, the motor potential is localized in a restricted area of the contralateral motor cortex [Shibasaki and Hallett, 2006]. Neuroimaging studies have shown that unilateral hand movements usually lead to activation of the contralateral M1, premotor cortex (PMC), supplementary motor area (SMA), basal ganglia, and ipsilateral cerebellum [Catalan et al., 1998; Colebatch et al., 1991; Mattay et al., 1998; Solodkin et al., 2001]. This lateralized brain activity pattern should be the reflection of the so‐called “non‐mirror transformation” networks [Chan and Ross, 1988; Cincotta and Ziemann, 2008], which results in facilitation of intended movements, and inhibition of unnecessary movements of the other hand. It has been suggested that this nonmirror transformation network relies on the SMA, PMC, M1, and basal ganglia [Chan and Ross, 1988; Cincotta et al., 2004, 2006; Hubers et al., 2008].
Loss of this lateralized activity pattern may result in some motor problems, like involuntary movements in the other hand (mirror movements) or difficulty in coordination of bimanual hand movements [Beaulé et al., 2012]. For example, mirror movements can be present in stroke patients [Oliveri et al., 1999] or in normal aging condition [Baliz et al., 2005], and disruption of the non‐mirror transformation network has been suggested as a possible reason [Beaulé et al., 2012]. It has been shown that following unilateral brain lesions or stroke, the ipsilateral motor areas are more activated, or even motor representations of both hands are located in the contralesional hemisphere [Carr et al., 1993, Guzzetta et al., 2007; Rehme et al., 2011; Shimizu et al., 2002]. This pattern of bilateral brain activation during unilateral motor tasks also has been reported in normal aging [Naccarato et al., 2006; Wu and Hallett, 2005b].
In patients with Parkinson's disease (PD), mirror movements are frequent [Borgheresi et al., 2010; Espay et al., 2005; Ottaviani et al., 2008; van den Berg et al., 2000; Vidal et al., 2003]. Mirror movements in PD are typically observed in the less affected hand during voluntary movement of the more affected hand [Espay et al., 2005; Li et al., 2007; Vidal et al., 2003], and majority of PD patients with mirror movements are in the early stage of the disease [Espay et al., 2005]. PD patients also commonly show impaired bimanual hand coordination; this problem is more obvious when they perform bimanual anti‐phase movements than in‐phase movements [Almeida et al., 2002; Johnson et al., 1998; Wu et al., 2010]. Our neuroimaging study found that during performing bimanual movements, PD patients had a different pattern of brain activity compared with healthy controls, including decreased activity in the SMA, but increased activity in the PMC [Wu et al., 2010]. As the SMA and PMC are parts of the nonmirror transformation network [Beaulé et al., 2012], these findings imply that the disrupted lateralized brain activity pattern might be a reason for the problem of motor coordination in PD.
Extensive neuroimaging studies have investigated neural correlates during performance of various movements in PD [Buhmann et al., 2003; Haslinger et al., 2001; Sabatini et al., 2000; Wu and Hallett, 2005a, 2008; Wu et al., 2010]. These reports revealed some typical motor‐related neural features in PD, like hypoactivation in the striatum and SMA, with hyperactivation in the PMC, parietal cortex, and cerebellum compared with normal subjects. These studies have provided important knowledge on our understanding of several aspects of motor control in PD, from simple motor execution to more complex behaviors, like motor coordination or automation. In contrast, the lateralization of brain activity pattern, a basic and important feature of the motor system, has never been systematically analyzed in PD.
A clearer understanding about motor laterality would provide new insight into the neural mechanisms underlying motor deficits in PD. In this study, we used functional MRI (fMRI) to investigate the lateralization of brain activity pattern during performing strictly unilateral movement in PD. Moreover, neural reasons contributing to the PD‐related laterality change were explored.
METHODS
Subjects
Twenty‐six drug‐naïve PD patients were involved in this study, aged 49–67 years (mean 58.96 years) and included 16 males and 10 females. We also investigated 26 age‐ and sex‐matched normal subjects (aged 49–68 years, mean 58.92) as controls. All subjects were right‐handed according to the Edinburgh Inventory to exclude the influence of handedness [Oldfield, 1971]. The diagnosis of PD was based on the UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria [Hughes et al., 1992]. Patients were assessed with the Unified Parkinson's Disease Rating Scale (UPDRS) [Lang and Fahn, 1989], the Hoehn and Yahr disability scale [Hoehn and Yahr, 1967] and Mini‐Mental State Examination (MMSE). Akinesia/rigidity was the predominant symptom, and tremor was no more than mild. To improve the homogeneity of the results, all patients had right hemiparkinson symptoms (Hoehn and Yahr Stage 1–1.5). We chose drug‐naïve patients to exclude the influence of long‐term dopamine exposure on motor networks.
In this study, we focused on the lateralization of neural activity during unilateral hand movement. Because mirror movements in the opposite hand could activate the motor cortex ipsilateral to the movement, only subjects without mirror movements in the left hand confirmed by electromyogram (EMG) were included in this study to avoid the contaminating of imaging results from mirror movements (see the following motor task section). The demographics and clinical details from the patients and controls are shown in Table 1. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board. All subjects gave their written informed consent for the study.
Table 1.
Demographics and clinical details of the subjects (mean ± SD).
| Patients | Controls | |
|---|---|---|
| Age (years) | 58.96 ± 4.97 (49–67) | 58.92 ± 5.29 (49–68) |
| Sex | 10 Female, 16 Male | 10 Female, 16 Male |
| Handedness | 97.31 ± 3.53 (90–100) | 96.92 ± 3.76 (90–100) |
| Mini‐Mental State Examination | 29.04 ± 0.92 | 29.15 ± 1.01 |
| Disease Duration (months) | 12.15 ± 2.98 | |
| UPDRS motor score | 13.27 ± 3.19 | |
| Hoehn and Yahr staging | 1.23 ± 0.25 |
Motor Task
The subjects were instructed to briskly tap their right index finger with amplitude of about 2.5 cm. It has been demonstrated that the force of right hand movement has significant effect on the appearance of mirror movement in the left hand. Sehm et al. [2010] found mirror EMG in left flexor carpi radialis (FCR) when using more than 20% of right isometric wrist flexion force; in contrast, during 10% of right hand force, there was no mirror EMG in left FCR. Therefore, we used an electronic pressure gauge to quantitatively measure the force of right index finger tapping, and asked the subjects to tap with about 10% of maximal tapping force. We gave the subjects enough practice until they could tap at the required amplitude and force. EMG was used to monitor left hand mirror movements. Surface electrodes were positioned on the bilateral flexor digitorum superficialis II, extensor digitorum II, and biceps brachii muscles. Because of the irregularity and small amplitude, it is difficult to reliably detect and quantify mirror activity on the basis of kinematic data or conventional EMG analysis. Therefore, we used frequency and phase locking between EMG from homologous muscles to detect mirror movement [Ridderikhoff et al., 2005; Bank et al., 2014]. Only the subjects without mirror EMG during right hand tapping with 10% of force were included in this study. In total, 33 PD patients and 30 healthy controls were screened. Seven patients and four controls were excluded because of mirror movements (Supporting Information Fig. 1). The remaining 26 patients and 26 controls were involved in this study.
Figure 1.
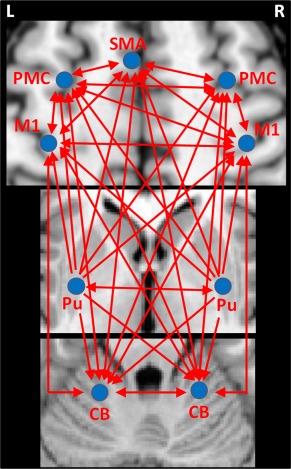
Illustration of the effective connectivities measured in the current study. Abbreviations: L, left; R, right; CB, cerebellum; M1, primary motor cortex; PMC, premotor cortex; Pu, putamen; SMA, supplementary motor area.
fMRI Procedure
fMRIs were performed on a 3T MR scanner (Trio system; Siemens Magnetom scanner, Erlangen, Germany). A standard head coil was used with foam padding to restrict head motion. High‐resolution axial T1‐ and T2‐weighted images were obtained in every participant to detect clinically silent lesions. High‐resolution anatomical images were acquired with 3D‐MPRAGE sequence (repetition time (TR) = 2,530 ms, echo time (TE) = 3.39 ms, 128 axial slices, 1.33‐mm thickness, field of view [FOV] = 256 mm). Blood‐oxygen level dependent data were acquired with gradient‐echo echo‐planar sequences (TR = 2,000 ms, TE = 30 ms, 33 axial slices, 3.5‐mm thickness, Flip angle = 90°, FOV = 220 mm, matrix size = 64 × 64).
Each fMRI scan lasted 8 min, was block‐designed and contained two conditions, which were defined as the “rest” and “active” condition, respectively. Each condition lasted 30 s and was repeated eight times. In the rest condition, subjects were asked to relax and focus on the screen. Subjects' arms were positioned alongside the body and kept relaxed. During the active condition, the subjects were instructed to perform the practiced right index finger tapping task as described above, following visual signals on a screen through a mirror built into the head coil. The visual signal was a red square and was presented for 300 ms each time. Intervals between the visual signals were irregular (from 2 to 3s). A MRI compatible electrical response button was fixed to their right hand to record finger movements during fMRI scanning.
DATA ANALYSIS
Behavioral Data Analysis
The reaction times during fMRI scanning were recorded for each subject. The results were compared between PD patients and healthy controls (two‐sample t‐test, P level = 0.05).
Imaging Data Analysis
Data Preprocessing
Image analysis was performed with SPM8 software (Wellcome Institute of Cognitive Neurology, London, UK). fMRI data were slice‐time corrected and aligned to the first image of each run for motion correction, and coregistered to high‐resolution anatomical images. After spatial normalization, all images were resampled into voxels that were 3 × 3 × 3 mm in size, and smoothed with a 6‐mm Gaussian smoothing kernel. Each participant's movement parameters were examined. No subject had more than 1.5 mm maximum translation in x, y, or z, or 1.5° of maximum rotation about the three axes.
Brain Activity Analysis
Data were analyzed for each single participant separately on a voxel‐by‐voxel basis using the general linear model approach for the time series. We defined a model using a fixed effect boxcar design convolved with a hemodynamic response function for analysis of task‐dependent activation. We added the six head motion parameters as regressors to optimally control for the motion effects. A contrast representing the effect of the active condition compared with the rest condition was calculated in each participant. These contrast images were used in the second level for random effects analyses. A one‐sample t‐test model was used to identify the brain activity in each group (P < 0.05, familywise error [FWE] corrected). Then, a two‐sample t‐test was applied to measure between‐group difference (P < 0.05, FWE corrected). Extent threshold was 10 voxels.
Analysis of Laterality
The laterality of brain activity was measured using LI toolbox 1.2.1 [Wilke and Lidzba, 2007; Wilke and Schmithorst, 2006]. The laterality index (LI) was calculated as (L − R)/(L + R), in which the L and R represents the number of activated voxels at P < 0.001, uncorrected at left and right side, respectively. A positive LI indicates lateralization to the left hemisphere, whereas a negative LI indicates right hemisphere lateralization. In healthy subjects, unilateral right hand simple movement is typically accompanied by left motor cortical and right cerebellar activation. Recent studies using fMRI and transcranial magnetic stimulation (TMS) have proved that the cerebellum has significantly stronger functional correlation with the contralateral M1 than that with the ipsilateral motor cortex [Buckner et al., 2011; Schlerf et al., in press]. To reflect the typical laterality of motor system, we converted the activity in the left and right cerebellum in calculation of the LI, that is, the activity in the right cerebellum was used as L, and the activity in the left cerebellum was used as R in the above equation. We first calculated the laterality in each subject, then a one‐sample t‐test was used to measure the laterality in each group, while a two‐sample t‐test was applied to compare the laterality between the two groups. In addition, a Pearson correlation analysis between LI and UPDRS motor scores was performed in the patients to examine whether the laterality related with the disease severity.
Effective Connectivity Analysis
We used the Granger causality analysis (GCA) method [Granger, 1969] to measure effective connectivity in the form of signed path coefficients, following Chen's model [Chen et al., 2009; Hamilton et al., 2011; Palaniyappan et al., 2013; Wu et al., in press], to explore the neural reasons contributing to the laterality in PD. The GCA was performed using the REST‐GCA toolkit (http://www.restfmri.net). Only the data in active conditions was chosen for connectivity analysis. Nuisance covariates including the three translational and three rotational head‐motion estimates, the white matter signal, the cerebrospinal fluid signal, and global mean signal were regressed.
According to brain activity results, the left M1, PMC, SMA, and posterior putamen, and right cerebellum were activated in both groups, while the right M1, PMC, and left cerebellum were additionally activated in PD patients during right index finger tapping. These regions were chosen as ROIs (regions of interest) for GCA analysis. All our patients only had right side motor symptoms, thus, the function of left posterior putamen should be diminished, while the right posterior putamen should be relatively spared. We also chose the right posterior putamen as a ROI to examine whether the relatively intact putamen has compensatory effects. The coordinate for the right posterior putamen was the mirror position of the left posterior putamen.
The ROIs were centered at the voxels showing the maximum magnitude of activation within the selected areas, with a radius of 5 mm. To avoid the ROI extending to the adjacent regions (e.g., globus pallidus), a radius of 3 mm was used for the ROIs of the bilateral posterior putamen [Wu et al., 2011]. As some regions (e.g., the right M1, PMC, and left cerebellum) were not activated in each subject, the ROIs were chosen based on the grouped results across the subjects.
The putamen modulates the excitability of motor cortex, for example, the M1, PMC, and SMA, through the corticobasal ganglia‐thalamo‐cortical circuits [DeLong and Wichmann, 2007]. Two sides of basal ganglia have mutual influence on each other [Brun et al., 2012]. Studies on monkeys and humans found that the subthalamic nucleus (STN) has a projection to the cerebellum [Bostan et al., 2010; Pelzer et al., 2013]. The putamen projects to the external globus pallidus, and then to the STN, which is a part of the so called “indirect” pathway [DeLong and Wichmann, 2007]. Thus, the putamen may project to the cerebellum by the way of STN. A diffusion tensor imaging (DTI) study proved that there are projections between the putamen and cerebellum [Leh et al., 2007]. The advantage of GCA method is, it can estimate the directionality of modulation from recorded time series across all nodes of a network without a priori assumption. Therefore, we could estimate the influence from the putamen to the cerebellum, without a priori assumption of the exact pathway. As we focused on the consequences of dopaminergic impairment on motor network, only the influences from the putamen to other areas were measured, the influences from other motor regions to the putamen were not calculated.
The connections between the SMA and bilateral M1 [Rouiller et al., 1994] and PMC [Boussaoud et al., 2005], between the PMC and bilateral M1 [Jenny, 1979; Rouiller et al., 1994], between the cerebellum and bilateral M1, PMC, and SMA [Akkal et al., 2007; Buckner et al., 2011; Middleton and Strick, 2000; O'Reilly et al., 2010], as well as reciprocal transcallosal connections between bilateral M1 [Jenny, 1979; Leichnetz, 1986; Rouiller et al., 1994], and bilateral PMC [Boussaoud et al., 2005; Marconi et al., 2003] have been identified in monkey, and have been supported by neuroimaging studies on human [Gao et al., 2011; Grefkes et al., 2008; Pool et al., 2013]. All of these above connectivities were calculated in this study. The effective connections we measured are illustrated in Figure 1.
The effective connectivity between these ROIs was calculated in each subject. The resulting path coefficients characterized the strength and direction of the connectivity. A two‐sample t‐test was used to evaluate whether the path coefficients between the ROIs and other regions were significantly different from zero (P < 0.001) [Hamilton et al., 2011; Wu et al., in press]. Then, a two‐sample t‐test was applied to analyze between‐group difference (P < 0.001). In this study, positive and negative connectivity indicate excitatory and inhibitory influence, respectively.
RESULTS
Task Performance
There were no significant differences in age, gender, or MMSE between the groups. PD patients had longer reaction times compared with controls (327.2 ± 31.3 vs. 314.1 ± 29.8 ms), but the difference was not significant (two‐sample t‐test, P = 0.128). Thus, the motor performance had no significant effect on our imaging results.
Brain Activity
In controls, performance of right finger tapping task was associated with activations in the left primary sensorimotor area (SM1), left PMC, left SMA, left putamen, and right cerebellum (one‐sample t‐test, P < 0.05, FWE corrected; Fig. 2a). In PD patients, besides these areas, the right M1, right PMC, and left cerebellum were additionally activated (one‐sample t‐test, P < 0.05, FWE corrected; Fig. 2b). The patients had more activity in the left postcentral gyrus, right M1, right PMC, and bilateral cerebellum, and had less activity in the left SMA and left putamen compared with the controls (two‐sample t‐test, P < 0.05, FWE corrected; Fig. 3 and Table 2).
Figure 2.
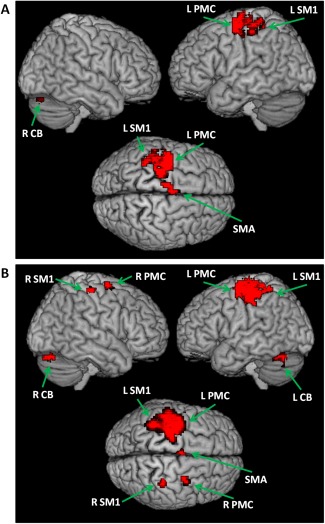
Brain activity during performance of right hand movement in controls (A), as well as in PD patients (B). Abbreviations: L, left; R, right; L CB, left cerebellum; R CB, right cerebellum; L PMC, left premotor cortex; R PMC, right premotor cortex; L SM1, left sensorimotor cortex; R SM1, right sensorimotor cortex; SMA, supplementary motor area.
Figure 3.
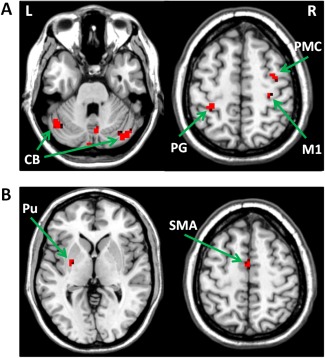
Differences of brain activity between PD patients and controls during performance of unilateral right hand motor task. (A) brain regions more activated in PD patients than in controls; (B) brain regions more activated in controls than in PD patients. Abbreviations: L, left; R, right; CB, cerebellum; M1, primary motor cortex; PMC, premotor cortex; PG, postcentral gyrus; Pu, putamen; SMA, supplementary motor area.
Table 2.
The difference of brain activity between PD patients and controls during performing unilateral right hand motor task
| Group | Brain region | Brodmann area | MNI coordinates | t Value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Patients > Controls | L postcentral gyrus | 3 | −34 | −33 | 57 | 7.66 | 945 |
| R M1 | 4 | 31 | −23 | 59 | 7.49 | 675 | |
| R PMC | 6 | 38 | −6 | 56 | 7.24 | 937 | |
| L cerebellum, posterior lobe, tuber | −38 | −72 | −35 | 8.33 | 3375 | ||
| R cerebellum, anterior lobe, culmen | 33 | −75 | −39 | 8.12 | 3294 | ||
| Controls > Patients | L SMA | 6 | −3 | −4 | 56 | 8.02 | 621 |
| L putamen | −32 | −3 | −4 | 7.37 | 675 | ||
Abbreviations: L, left; R, right; M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area.
Results of Laterality
The LIs of neural activity during performance of unilateral right hand motor task in healthy controls and PD patients were 0.84 ± 0.09 and 0.41 ± 0.14, respectively. There was a significant between group difference (two‐sample t‐test, P < 0.0001). Correlation analysis showed that there was a negative correlation between LI and UPDRS motor scores (r = −0.63, P = 0.001; Fig. 4).
Figure 4.
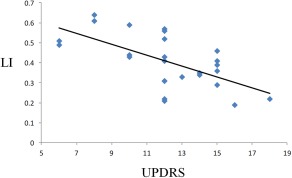
The results of correlation analysis between the laterality index (LI) and Unified Parkinson's Disease Rating Scale (UPDRS) motor score in PD patients. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Network Connectivity Results
In healthy subjects, the left posterior putamen had significant positive effective connectivity to the left M1, PMC, SMA, right putamen, right PMC, and bilateral cerebellum, while the right posterior putamen had positive connectivity to the left SMA, left putamen and right cerebellum. The right M1 received negative connectivity from the left M1, PMC, and SMA. In addition, there were several reciprocal positive connections between the cortical motor regions, between the cortical regions and cerebellum, as well as between the bilateral cerebellum (Fig. 5A and Table 3).
Figure 5.
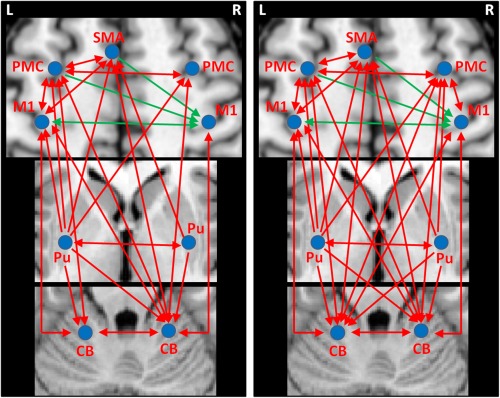
The effective connections in healthy controls (A) and PD patients (B) during performance of unilateral right hand movement. The results shown are the path coefficients between the ROIs and other regions that are significantly different from zero (two‐sample t‐test, P < 0.001, Table 3). The arrows indicate the direction of connectivity. The red/blue lines indicate positive/negative connectivity, respectively. Abbreviations: L, left; R, right; CB, cerebellum; M1, primary motor cortex; PMC, premotor cortex; Pu, putamen; SMA, supplementary motor area.
Table 3.
Network connectivity in healthy controls and PD patients during performance of unilateral right hand movement
| Brain Regions | Controls | Patients | |
|---|---|---|---|
| Origin | Target | ||
| L putamen | L M1 | 0.236 ± 0.078 | 0.113 ± 0.046* |
| L PMC | 0.212 ± 0.060 | 0.087 ± 0.028* | |
| L SMA | 0.248 ± 0.052 | 0.078 ± 0.035* | |
| R PMC | 0.122 ± 0.028 | 0.062 ± 0.019* | |
| L cerebellum | 0.118 ± 0.031 | 0.070 ± 0.026* | |
| R cerebellum | 0.192 ± 0.058 | 0.096 ± 0.046* | |
| R putamen | 0.164 ± 0.038 | 0.118 ± 0.041* | |
| R putamen | R PMC | 0.069 ± 0.017* | |
| L SMA | 0.052 ± 0.024 | 0.087 ± 0.035* | |
| L cerebellum | 0.072 ± 0.024* | ||
| R cerebellum | 0.048 ± 0.018 | 0.083 ± 0.029* | |
| L putamen | 0.050 ± 0.022 | 0.074 ± 0.020* | |
| L M1 | L PMC | 0.118 ± 0.026 | 0.166 ± 0.069* |
| L SMA | 0.132 ± 0.049 | 0.198 ± 0.091* | |
| R M1 | −0.232 ± 0.089 | −0.093 ± 0.053* | |
| L cerebellum | 0.088 ± 0.035 | 0.180 ± 0.103* | |
| R cerebellum | 0.165 ± 0.077 | 0.229 ± 0.070* | |
| R M1 | L M1 | 0.0549 ± 0.023 | 0.072 ± 0.031 |
| R PMC | 0.109 ± 0.036* | ||
| L cerebellum | 0.098 ± 0.041* | ||
| R cerebellum | 0.068 ± 0.030 | 0.094 ± 0.032* | |
| L SMA | L M1 | 0.187 ± 0.091 | 0.235 ± 0.077* |
| L PMC | 0.154 ± 0.052 | 0.2027 ± 0.061* | |
| R M1 | −0.176 ± 0.078 | −0.087 ± 0.064* | |
| L cerebellum | 0.112 ± 0.029* | ||
| R cerebellum | 0.082 ± 0.031 | 0.137 ± 0.055* | |
| L PMC | L M1 | 0.117 ± 0.028 | 0.166 ± 0.089* |
| L SMA | 0.130 ± 0.054 | 0.179 ± 0.071* | |
| R M1 | −0.149 ± 0.057 | −0.75 ± 0.033* | |
| R PMC | 0.108 ± 0.041 | 0.122 ± 0.037 | |
| L cerebellum | 0.079 ± 0.043 | 0.110 ± 0.051* | |
| R cerebellum | 0.114 ± 0.033 | 0.179 ± 0.062* | |
| R PMC | R M1 | 0.131 ± 0.042* | |
| L PMC | 0.075 ± 0.021 | 0.091 ± 0.027 | |
| L cerebellum | 0.109 ± 0.038* | ||
| R cerebellum | 0.068 ± 0.037 | 0.105 ± 0.026* | |
| L cerebellum | L M1 | 0.092 ± 0.033 | 0.151 ± 0.062* |
| L PMC | 0.068 ± 0.025 | 0.113 ± 0.044* | |
| L SMA | 0.109 ± 0.037* | ||
| R M1 | 0.123 ± 0.057* | ||
| R PMC | 0.099 ± 0.035* | ||
| R cerebellum | 0.083 ± 0.041 | 0.120 ± 0.043* | |
| R cerebellum | L M1 | 0.204 ± 0.108 | 0.267 ± 0.128* |
| L PMC | 0.127 ± 0.041 | 0.179 ± 0.088* | |
| L SMA | 0.119 ± 0.036 | 0.167 ± 0.063* | |
| R M1 | 0.073 ± 0.031 | 0.121 ± 0.057* | |
| R PMC | 0.062 ± 0.022 | 0.101 ± 0.040* | |
| L cerebellum | 0.125 ± 0.055 | 0.171 ± 0.077* | |
Abbreviations: L, left; R, right; M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area.
Values are given as mean path coefficients ± SD that are significantly different from zero (two‐sample t‐test, P < 0.001).
indicates significant between‐group difference (two‐sample t‐test, P < 0.001).
In PD patients, besides these connectivities, the right posterior putamen additionally had positive connectivity to the right PMC and left cerebellum. The left SMA had positive connectivity with the left cerebellum. The right M1, right PMC, and left cerebellum had reciprocal positive connections with each other. In addition, the left cerebellum had positive connectivity with left SMA (Fig. 5B and Table 3).
The comparison between PD patients and healthy controls showed that the positive connectivities from the left posterior putamen to the left M1, PMC, SMA, right PMC, right putamen, and bilateral cerebellum, and the negative connectivities from the left M1, left PMC, and left SMA to the right M1 were significantly weakened in the patients (two‐sample t‐test, P < 0.001). At the same time for the patients, there were strengthened connections from the right putamen to the right PMC, left SMA, left putamen, and left cerebellum. In addition, the connectivities between several cortical motor regions, between the cortical regions and cerebellum, as well as between the bilateral cerebellum were increased in PD patients (Fig. 6 and Table 3).
Figure 6.
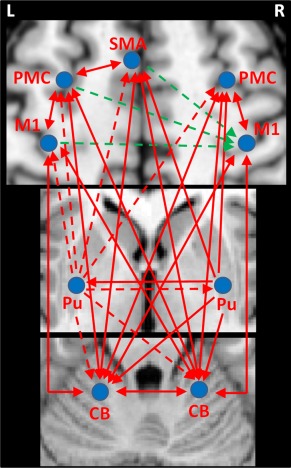
The differences of network connectivity between PD patients and healthy controls during performance of unilateral right hand movement. The results shown are the path coefficients that are significantly different between patients and controls (two‐sample t‐test, P < 0.001, Table 3). The arrows indicate the direction of connectivity. The red/blue lines indicate positive/negative connectivity, respectively. The dotted/full lines indicate decreased/increased connectivity in PD patients compared to controls, respectively. Abbreviations: L, left; R, right; CB, cerebellum; M1, primary motor cortex; PMC, premotor cortex; Pu, putamen; SMA, supplementary motor area.
DISCUSSION
In this study, we investigated the lateralization of neural activity during performance of unilateral movement in PD patients. The novel finding is that the laterality of motor‐related neural activity is diminished in PD. Disrupted motor network interactions secondary to basal ganglia dysfunction is a likely reason contributing to this phenomenon.
We used a very simple tapping task to examine laterality, because it has been shown that such a task usually activates contralateral motor areas; while the activations in the ipsilateral motor regions become more obvious when the complexity of motor tasks increased [Catalan et al., 1998; Sadato et al., 1996]. In addition, finger movement rate has a significant effect on brain activity. It has been shown that brain activation in several areas (i.e., M1, cerebellum, and SMA) is related to movement frequency [Deiber et al., 1999; Sadato et al., 1997], while the activation of the ipsilateral motor areas (i.e., PMC) increased with increasing movement rates [Tanaka et al., 2009]. Thus, a simple and slow motor task, like used in the present study, is appropriate for the investigation of motor laterality.
Activation in the ipsilateral motor areas during performance of unilateral movements has been frequently reported [Chen et al., 1997; Cramer et al., 1999; Hanakawa et al., 2005; Horenstein et al., 2009]. As discussed above, the presentation of ipsilateral activation is usually in accordance with motor complexity or frequency. Besides these reasons, involuntary contralateral movement (mirror movement) may also induce ipsilateral neural activity. Thus, we used EMG monitor to ensure that only subjects without mirror movements were investigated, and our results truly reflect strictly unilateral movement‐related motor lateralization.
Reduced Laterality in PD
In healthy controls, unilateral right hand movement activated a lateralized motor network, including the left SM1, PMC, SMA, putamen, and right cerebellum. The LI in controls was 0.84 ± 0.09, which indicates a strong left dominance. Compared with controls, although PD patients had more activity in the right cerebellum and left postcentral gyrus, which is within this lateralized motor network, they also had decreased activity in regions within this network, that is, the left putamen and SMA. Moreover, the patients recruited some motor regions mirror to this network, that is, the right M1 and PMC and left cerebellum (Fig. 2 and Table 2). These changes resulted in the significantly reduced laterality in PD patients (0.41 ± 0.14) compared with controls.
It has been suggested that when a LI is close to 0, there is a lack of dominance of lateralization, or is so called “bilateral dominance” [Seghier, 2008]. The LI value used to determine the lack of dominance is usually set to 0.2 [Deblaere et al., 2004; Springer et al., 1999], but up to 0.3 has also been used [Benbadis et al., 1998]. Thus, when the |LI| ≤ 0.2 (or 0.3), the motor lateralization is no longer significant. Our results indicate that in early PD, the motor lateralization during unilateral right hand movement remains left dominant; however, the lateralization is clearly weaker than that in healthy controls, and shows a tendency toward lack of dominance. The negative correlation between LI values and UPDRS motor scores (Fig. 4) suggests that as the disorder progresses, the lateralized brain activity pattern is gradually lost. As introduced previously, this lateralized brain activity pattern (or nonmirror transformation network) is critical in facilitation of intended movements, and inhibition of unnecessary movements of the other hand [Chan and Ross, 1988; Cincotta and Ziemann, 2008]. The disruption of this nonmirror transformation network enhances the tendency toward symmetrical bimanual movement. Therefore, the loss of this lateralized brain activity pattern might be a reason underlying mirror movements or deficits in motor coordination in PD.
Dysfunction of Basal Ganglia Motor Circuit
We found decreased activity in the left putamen and SMA in PD compared with controls (Fig. 2 and Table 2). These two regions are within the basal ganglia motor circuit, which projects somatotopically from the M1, PMC, and SMA to the striatum, then projects back to these cortical motor areas throughout the thalamus [DeLong and Wichmann, 2007]. The decreased activity in the putamen has been commonly reported in PD patients during performance of motor tasks [Holden et al., 2006; Playford et al., 1992; Prodoehl et al., 2010; Wu et al., in press]. In PD, the impairment of dopaminergic neurons in the substantia nigra causes dopamine depletion in the basal ganglia, especially in the posterior putamen [Brooks et al., 1990; Kish et al., 1988]. The posterior putamen is a sensorimotor area. In our study the impaired posterior putamen had decreased influences more on ipsilateral cortical motor areas (Fig. 6), that is consistent with findings from DTI studies showing that the putamen mainly projects to the ipsilateral motor cortices [Leh et al., 2007; Lehéricy et al., 2004]. In addition, PD patients had decreased connectivity from more affected (left) putamen to the cerebellum and right putamen. The impaired striatum–cerebellar connection is likely a reflection of abnormal signals from the basal ganglia to influence cerebellar function [Bostan et al., 2010]. A deep brain stimulation study demonstrated that one side of the basal ganglia can influence the functioning of the other [Brun et al., 2012]. Our findings demonstrate that the dysfunction of sensorimotor putamen results in disconnection of striatum–cortical and striatum–cerebellar loops during unilateral hand movements in PD [Wu et al., 2011].
Among those cortical motor regions receiving less excitatory influences from the left posterior putamen, only the SMA had decreased activity; while the activations in other motor regions were not decreased (left M1 and left PMC), or even increased (right PMC; Figs. 2 and 6; Tables 2 and 3). We suppose a very likely reason for this phenomenon is that the compensatory effects from the cortical and cerebellar motor networks and the less affected (right) putamen can help to maintain the activity of these regions (see Discussion section). However, the connectivity in these regions was modulated, which was shown as changed excitatory or inhibitory influences from these areas to other motor regions. This finding indicates that the function of these motor areas has been changed secondary to the dysfunction of basal ganglia motor circuit; even their activations were within the normal range. It is likely that the connectivity analysis may be more sensitive to detect neural changes in PD than activity [Palmer et al., 2010]. In contrast, as the SMA receives strong projections from the basal ganglia [Hoover and Strick, 1993], this compensatory effort may not be strong enough to overcome the dysfunction of striatum–SMA pathway, which results in the hypoactivation of the SMA.
The SMA is important in motor initiation and coordination [Jenkins et al., 2000; Serrien et al., 2002]. Decreased activity in the SMA has been extensively reported in PD patients and is related to the difficulty in performing self‐initiated or bimanually coordinated movements [Buhmann et al., 2003; Haslinger et al., 2001; Jahanshani et al., 1995; Jenkins et al., 1992; Playford et al., 1992; Rascol et al., 1994; Samuel et al., 1997; Wu et al., 2010]. Animal studies have shown that the SMA has extensive projections to the M1 [Dum and Strick, 2005]. A majority of SMA neurons are selective for the use of either the ipsilateral or contralateral arm [Hoshi and Tanji, 2004]. As a large proportion of SMA neurons solely respond to contralateral hand movements, SMA may have a specific role for lateralized hand movements [Kazennikov et al., 1999]. Unilateral damage of the SMA in human subjects or monkeys can induce disturbances in bimanual coordination that include mirror movements [Brinkman, 1984; Chan and Ross, 1988]. Thus, the SMA may have a role in the nonmirroring transformation network, and the dysfunction of SMA may contribute to weakened motor lateralization in PD.
Weakened Transcallosal Inhibition on the Right M1
The execution of strictly unilateral motor tasks requires restriction of motor output to the contralateral M1 and suppression of motor activation of the mirror hand [Carson, 2005; Cincotta and Ziemann, 2008; Leocani et al., 2000]. When preparing to execute a movement, there is a temporary inhibition of the M1 controlling the mirror finger in the passive hand [Leocani et al., 2000; Sohn et al., 2002]. Extensive studies have showed that both M1 exhibit a mutual inhibitory influence (interhemispheric inhibition, IHI) on each other [Cincotta and Ziemann, 2008; Ferbert et al., 1992; Grefkes et al., 2008; Ni et al., 2009; Pool et al., 2013; Wassermann et al., 1991; Ziemann and Hallett, 2001]. The IHI has been related to the activity of inhibitory GABA‐ergic interneurons [Daskalakis et al., 2002] and is probably mediated by transcallosal fibres crossing the posterior part of the corpus callosum [Meyer et al., 1998]. The IHI is thought to responsible for the inhibition of mirror movements [Hubers et al., 2008; Mayston et al., 1999], thus, is crucial for performing strictly unilateral movements [Duque et al., 2005; Leocani et al., 2000]. Our observation that there was reciprocal negative connectivity between both M1, and the left M1 had stronger inhibitory influence on the right M1 than that from the right M1 to the left M1 during right hand movement (Table 3) is consistent with these previous reports.
We additionally found that the left PMC and SMA had a negative connectivity to the right M1 during right hand movement, which is in agreement with previous fMRI reports analyzed with dynamic causal modeling method [Grefkes et al., 2008; Pool et al., 2013]. Studies in animals have confirmed the existence of direct commissural fibers from dorsal PMC to contralateral M1 [Jenny, 1979; Marconi et al., 2003; Rouiller et al., 1994]. TMS studies found that a conditioning TMS over PMC can suppress motor‐evoked potentials evoked by stimulation of the contralateral M1 [Mochizuki et al., 2004a, 2004b]. It has been suggested that the PMC is part of the nonmirror transformation network that contribute in restricting the motor output to the hemisphere contralateral to the intended movement [Cincotta et al., 2004; Giovannelli et al., 2006]. Our findings demonstrate that, besides the inhibition from the contralateral M1, there is a transcallosal inhibition from the contralateral PMC and SMA to the ipsilateral M1 during unilateral hand movements. All of these inhibitory efforts should be for suppression of ipsilateral M1 activity, and thus is important for performing strictly unilateral movements.
In PD patients, the decreased transcallosal inhibition from the left M1, PMC, and SMA should weaken the suppression on right M1, and results in the excessive activation in the right M1 during unilateral right hand movements. TMS studies have proved that IHI is decreased in PD [Li et al., 2007; Spagnolo et al., 2013], while more obvious in patients with mirror movements [Li et al., 2007]. It has been suggested that the appearance of mirror movements in PD do not depend on unmasking of ipsilateral projections but are due to motor output along the crossed corticospinal projection from the mirror M1 [Cincotta et al., 2006]. Thus, the increased ipsilateral M1 activity contributes to the reduced motor lateralization, and presumably, might be a reason for some motor deficits in PD, like mirror movements.
Compensation from the Right Putamen
In healthy controls, the right posterior putamen only had connectivity to the left SMA, right cerebellum, and left putamen while performing unilateral right hand movement. In PD patients, the right putamen had additional connectivity to the right PMC and left cerebellum (Fig. 5 and Table 3). Although receiving less connectivity from the left putamen, the right putamen had more connectivity to the right PMC, left SMA, left cerebellum, and left putamen in patients compared to controls (Fig. 6). As our patients only had right‐sided symptoms, the dopaminergic system in the right putamen should be much less affected, and the function of the right putamen would be relatively preserved. It has been shown that loss of dopaminergic neurons can trigger sprouting of residual neurons [Finkelstein et al., 2000; Song and Haber, 2000], and spared dopaminergic fibers in the putamen may compensate for severe dopamine depletion [Mounayar et al., 2007]. Our findings that the regions receiving strengthened connections from the right (preserved) putamen were overlapped with the brain areas disconnected with the left (damaged) putamen (Fig. 6) provide support to the compensatory role of the right putamen. Presumably, at early stage of PD, spared dopaminergic neurons in the less affected putamen may compensate for impaired putamen helping to execute desired movements. However, the compensatory efforts from the right putamen might be a reason inducing overactivation in the right PMC and left cerebellum, thus, also contributing to the weakened motor lateralization.
Compensation from the Cortical Motor and Cerebellar Networks
PD patients showed increased activity in cortical motor regions and bilateral cerebellum, as well as enhances connectivity between these regions compared to healthy controls (Figs. 2 and 6; Tables 2 and 3). Similar patterns of hyperactivation or strengthened connectivity have been extensively reported in PD [Buhmann et al., 2003; Catalan et al., 1999; Rascol et al., 1997; Sabatini et al., 2000; Wu and Hallett, 2005a; Wu et al., 2010, 2011]. The nature of the hyperactivation or strengthened connectivity of motor networks remains unclear. One likely explanation is that this phenomenon represents a compensatory effect [Catalan et al., 1999; Haslinger et al., 2001; Rascol et al., 1997; Wu and Hallett, 2005a; Wu et al., 2011]. In our patients, accompanying the hyperactivation or strengthened connectivity were hypoactivations in the left putamen and SMA, and weakened striatum–cortical and striatum–cerebellar connections (Figs. 2 and 6; Tables 2 and 3). The dysfunction of striatum–cortical motor pathway secondary to dopamine depletion is likely to be an important reason inducing akinesia in PD, which is expected to result in deteriorated motor performance in our patients. In contrast, our patients executed the task at the same level as the healthy subjects. Therefore, we speculate that these increased activity or connectivity is to compensate for the dysfunction of the striatum–cortical and striatum–cerebellar circuits to perform motor task properly. However, although this compensatory effort may benefit motor performance, it may also reduce the spatial segregation between different motor circuits [Helmich et al., 2010]. In this study, recruitment of mirror homologous motor areas (the right M1, right PMC, and left cerebellum) disrupted the normal pattern of the nonmirror transformation network during unilateral movements, which should be a reason underlying abnormal motor lateralization in PD.
Our observation that the most affected (left) motor cortical regions had weakened inhibitory effects but had enhanced excitatory connectivities (Fig. 6) indicates that dopaminergic depletion in the striatum has different influences on motor excitatory and inhibitory circuits. This finding is in agreement with a recent TMS study showing that motor cortical inhibition is decreased and facilitation is increased in PD patients compared to controls [Ni et al., 2013]. The authors suggested that the decreased motor cortical inhibition may partly due to increased cortical facilitation, but may also be caused by impaired GABA‐mediated inhibition. They also considered the increased cortical facilitation as a compensatory effect in PD [Ni et al., 2013].
Although the motor lateralization was significantly decreased, our patients did not present mirror movements. We assume that because the lateralization remained left dominant, mirror movements were still largely suppressed. It is possible when the lateralization further weakens or is lost, mirror movements might frequently appear. This assumption, however, cannot be examined in the current study and needs to be considered in future studies. How to design such a study is not clear since bilateral movement would certainly be expected to have relatively symmetric activation in the two hemispheres.
In conclusion, our study demonstrates that in early PD patients, the lateralization of brain activity pattern during unilateral right hand movement is weakened. The left dominance of lateralization is significantly reduced, but shows a tendency to bilateral dominance. The impaired striatum, decreased transcallosal inhibition secondary to the dysfunction of striatum–cortical circuit, and compensatory efforts from cortical motor regions, cerebellum, and preserved striatum are likely critical reasons contributing to the reduced motor lateralization in PD. The disruption of the nonmirror transformation network might be a reason underlying some motor deficits in PD, like mirror movements or impaired motor coordination, which needs to be examined in future studies.
Supporting information
Supporting Information Figure 1.
ACKNOWLEDGMENT
Dr. Hallett is supported by the NINDS Intramural Program.
REFERENCES
- Akkal D, Dum RP, Strick PL (2007): Supplementary motor area and presupplementary motor area: Targets of basal ganglia and cerebellar output. J Neurosci 27:10659–10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida QJ, Wishart LR, Lee TD (2002): Bimanual coordination deficits with Parkinson's disease: The influence of movement speed and external cueing. Mov Disord 17:30–37. [DOI] [PubMed] [Google Scholar]
- Baliz Y, Armatas C, Farrow M, Hoy KE, Fitzgerald PB, Bradshaw JL, Georgiou‐Karistianis N (2005): The influence of attention and age on the occurrence of mirror movements. J Int Neuropsychol Soc 11:855–862. [DOI] [PubMed] [Google Scholar]
- Bank PJ, Peper CL, Marinus J, Beek PJ, van Hilten JJ (2014): Evaluation of mirrored muscle activity in patients with complex regional pain syndrome. Clin Neurophysiol 125:2100–2108. [DOI] [PubMed] [Google Scholar]
- Beaulé V, Tremblay S, Théoret H (2012): Interhemispheric control of unilateral movement. Neural Plast 2012:627816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbadis SR, Binder JR, Swanson SJ, Fischer M, Hammeke TA, Morris GL, Frost JA, Springer JA (1998): Is speech arrest during Wada testing a valid method for determining hemispheric representation of language? Brain Lang 65:441–446. [DOI] [PubMed] [Google Scholar]
- Borgheresi A, Espay AJ, Giovannelli F, Vanni P, Zaccara G, Cincotta M (2010): Congenital mirror movements in Parkinson's disease: Clinical and neurophysiological observations. Mov Disord 25:1520–1523. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2010): The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Tanne‐Gariepy J, Wannier T, Rouiller EM (2005): Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: A multiple retrograde tracing study. BMC Neurosci 6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman C (1984): Supplementary motor area of the monkey's cerebral cortex: Short‐ and long‐term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS (1990): Differing patterns of striatal 18F‐dopa uptake in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol 28:547–555. [DOI] [PubMed] [Google Scholar]
- Brun Y, Karachi C, Fernandez‐Vidal S, Jodoin N, Grabli D, Bardinet E, Mallet L, Agid Y, Yelnik J, Welter ML (2012): Does unilateral basal ganglia activity functionally influence the contralateral side? What we can learn from STN stimulation in patients with Parkinson's disease. J Neurophysiol 108:1575–1583. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C (2003): Pharmacologically modulated fMRI—Cortical responsiveness to levodopa in drug‐naïve hemiparkinsonian patients. Brain 126:451–461. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA (1993): Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain 116:1223–1247. [DOI] [PubMed] [Google Scholar]
- Carson RG (2005): Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev 49:641–662. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998): The functional neuroanatomy of simple and complex sequential finger movements: A PET study. Brain 121:253–264. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M (1999): A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain 122:483–495. [DOI] [PubMed] [Google Scholar]
- Chan JL, Ross ED (1988): Left‐handed mirror writing following right anterior cerebral artery infarction: Evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology 38:59–63. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG (1997): Involvement of the ipsilateral motor cortex in fine finger movements of different complexities. Ann Neurol 41:247–254. [DOI] [PubMed] [Google Scholar]
- Chen G, Hamilton JP, Thomason ME, Gotlib IH, Saad ZS, Cox RW (2009): Granger causality via vector auto‐regression tuned for fMRI data analysis. Proc Intl Soc Mag Reson Med 17:1718. [Google Scholar]
- Cincotta M, Ziemann U (2008): Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol 119:744–762. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Borgheresi A, Balestrieri F, Giovannelli F, Rossi S, Ragazzoni A, Zaccara G, Ziemann U (2004): Involvement of the human dorsal premotor cortex in unimanual motor control: An interference approach using transcranial magnetic stimulation. Neurosci Lett 367:189–193. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Borgheresi A, Balestrieri F, Giovannelli F, Ragazzoni A, Vanni P, Benvenuti F, Zaccara G, Ziemann U (2006): Mechanisms underlying mirror movements in Parkinson's disease: A transcranial magnetic stimulation study. Mov Disord 21:1019–1025. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS (1991): Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol 65:1392–1401. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR (1999): Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol 81:383–387. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R (2002): The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere K, Boon PA, Vandemaele P, Tieleman A, Vonck K, Vingerhoets G, Backes W, Defreyne L, Achten E (2004): MRI language dominance assessment in epilepsy patients at 1.0 T: Region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology 46:413–420. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, and Hallett M (1999): Mesial motor areas in self‐initiated versus externally triggered movements examined with fMRI: Effect of movement type and rate. J Neurophysiol 81:3065–3077. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T (2007): Circuits and circuit disorders of the basal ganglia. Arch Neurol 64:20–24. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2005): Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci 25:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG (2005): Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex 15:588–593. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Li JY, Johnston L, Chen R, Lang AE (2005): Mirror movements in parkinsonism: Evaluation of a new clinical sign. J Neurol Neurosurg Psychiatry 76:1355–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992): Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK (2000): Axonal sprouting following lesions of the rat substantia nigra. Neuroscience 97:99–112. [DOI] [PubMed] [Google Scholar]
- Gao Q, Duan X, Chen H (2011): Evaluation of effective connectivity of motor areas during motor imagery and execution using conditional Granger causality. Neuroimage 54:1280–1288. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Ragazzoni A, Zaccara G, Cincotta M, Ziemann U (2006): Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp Brain Res 175:633–640. [DOI] [PubMed] [Google Scholar]
- Granger CWJ (1969): Investigating causal relations by economic models and cross‐spectral methods. Econometrica 37:424–438. [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR (2008): Dynamic intra‐ and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage 41:1382–1394. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Bonanni P, Biagi L, Tosetti M, Montanaro D, Guerrini R, Cioni G (2007): Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol 118:1110–1121. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH (2011): Investigating neural primacy in Major Depressive Disorder: Multivariate Granger causality analysis of resting‐state fMRI time‐series data. Mol Psychiatry 16:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Parikh S, Bruno MK, Hallett M (2005): Finger and face representations in the ipsilateral precentral motor areas in humans. J Neurophysiol 93:2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos‐Baumann AO (2001): Event‐related Functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain 124:558–570. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 20:1175–1186. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD (1967): Parkinsonism: Onset, progression and mortality. Neurology 17:427–442. [DOI] [PubMed] [Google Scholar]
- Holden A, Wilman A, Wieler M, Martin WR (2006): Basal ganglia activation in Parkinson's disease. Parkinsonism Relat Disord 12:73–77. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL (1993): Multiple output channels in the basal ganglia. Science 259:819–821. [DOI] [PubMed] [Google Scholar]
- Horenstein C, Lowe MJ, Koenig KA, Phillips MD (2009): Comparison of unilateral and bilateral complex finger tapping‐related activation in premotor and primary motor cortex. Hum Brain Mapp 30:1397–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J (2004): Differential roles of neuronal activity in the supplementary and presupplementary motor areas: From information retrieval to motor planning and execution. J Neurophysiol 92:3482–3499. [DOI] [PubMed] [Google Scholar]
- Hubers A, Orekhov Y, Ziemann U (2008): Interhemispheric motor inhibition: Its role in controlling electromyographic mirror activity. Eur J Neurosci 28:364–371. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992): Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshani M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995): Self‐initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement‐related potentials in normal and Parkinson's disease subjects. Brain 118:913–933. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, Brooks DJ (1992): Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol 32:749–757. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000): Self‐initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123:1216–1228. [DOI] [PubMed] [Google Scholar]
- Jenny AB (1979): Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol 188:137–146. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Cunnington R, Bradshaw JL, Phillips JG, Iansek R, Rogers MA (1998): Bimanual co‐ordination in Parkinson's disease. Brain 121:743–753. [DOI] [PubMed] [Google Scholar]
- Kazennikov O, Hyland B, Corboz M, Babalian A, Rouiller EM, Wiesendanger M (1999): Neural activity of supplementary and primary motor areas in monkeys and its relation to bimanual and unimanual movement sequences. Neuroscience 89:661–674. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O (1988): Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 318:876–880. [DOI] [PubMed] [Google Scholar]
- Lang AE, Fahn S (1989): Assessment of Parkinson's disease In: Munsat TL, editor. Quantification of Neurological Deficit. Boston: Butterworths; pp 285–309. [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP (2007): Fronto‐striatal connections in the human brain: A probabilistic diffusion tractography study. Neurosci Lett 419:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (2004): Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55:522–529. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR (1986): Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol 254:460–492. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000): Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123:1161–1173. [DOI] [PubMed] [Google Scholar]
- Li JY, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE, Chen R (2007): Interhemispheric and ipsilateral connections in Parkinson's disease: Relation to mirror movements. Mov Disord 22:813–821. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R (2003): Callosal connections of dorso‐lateral premotor cortex. Eur J Neurosci 18:775–788. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Santha AKS, Van Horn JD, Tallent KA, Frank JA, Weinberger DR (1998): Hemispheric control of motor function: A whole brain echo planar fMRI study. Psychiatry Res 83:7–22. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA (1999): A neurophysiological study of mirror movements in adults and children. Ann Neurol 45:583–594. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Woiciechowsky C (1998): Topography of fibres in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43:360–369. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev 31:236–250. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang Y, Rothwell J (2004a): Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol 561:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Terao Y, Okabe S, Furubayashi T, Arai N, Iwata NK, Hanajima R, Kamakura K, Motoyoshi K, Ugawa Y (2004b): Effects of motor cortical stimulation on the excitability of contralateral motor and sensory cortices. Exp Brain Res 158:519–526. [DOI] [PubMed] [Google Scholar]
- Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, Féger J, Savasta M, François C, Tremblay L (2007): A new model to study compensatory mechanisms in MPTP‐treated monkeys exhibiting recovery. Brain 130:2898–2914. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC (2006): Does healthy aging affect the hemispheric activation balance during paced index‐to‐thumb opposition task? An fMRI study. NeuroImage 32:1250–1256. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R (2009): Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex 19:1654–1665. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R (2013): Increased motor cortical facilitation and decreased inhibition in Parkinson's disease. Neurology 80:1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, Tomaiuolo F, Caltagirone C (1999): Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain 122:1731–1739. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen‐Berg H (2010): Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani D, Tiple D, Suppa A, Colosimo C, Fabbrini G, Cincotta M, Defazio G, Berardelli A (2008): Mirror movements in patients with Parkinson's disease. Mov Disord 23:253–258. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF (2013): Neural primacy of the salience processing system in schizophrenia. Neuron 79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SJ, Li J, Wang ZJ, McKeown MJ (2010): Joint amplitude and connectivity compensatory mechanisms in Parkinson's disease. Neuroscience 166:1110–1118. [DOI] [PubMed] [Google Scholar]
- Pelzer EA, Hintzen A, Goldau M, von Cramon DY, Timmermann L, Tittgemeyer M (2013): Cerebellar networks with basal ganglia: Feasibility for tracking cerebello‐pallidal and subthalamo‐cerebellar projections in the human brain. Eur J Neurosci 38:3106–3114. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ (1992): Impaired mesial frontal and putamen activation in Parkinson's disease: A positron emission tomography study. Ann Neurol 32:151–161. [DOI] [PubMed] [Google Scholar]
- Pool EM, Rehme AK, Fink GR, Eickhoff SB, Grefkes C (2013): Network dynamics engaged in the modulation of motor behavior in healthy subjects. Neuroimage 82:68–76. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Spraker M, Corcos D, Comella C, Vaillancourt D (2010): Blood oxygenation level‐dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson's disease. Mov Disord 25:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Fabre N, Senard JM, Montastruc JL, Celsis P, Marc‐Vergnes JP, Rascol A (1994): Normal activation of the supplementary motor area in patients with Parkinson's disease undergoing long‐term treatment with levodopa. J Neurol Neurosurg Psychiatry 57:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F (1997): The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 120:103–110. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Fink GR, Von Cramon DY, Grefkes C (2011): The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex 21:756–768. [DOI] [PubMed] [Google Scholar]
- Ridderikhoff A, Daffertshofer A, Peper CL, Beek PJ (2005): Mirrored EMG activity during unimanual rhythmic movements. Neurosci Lett 381:228–233. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M (1994): Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res 102:227–243. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O (2000): Cortical motor reorganization in akinetic patients with Parkinson's disease: A functional MRI study. Brain 123:394–403. [DOI] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibanez V, Deiber MP, Hallett M (1996): Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16:2693–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Campbell G, Deiber MP, Le Bihan D, Hallett M (1997): Frequency‐dependent changes of regional cerebral blood flow during finger movements: Functional MRI compared to PET. J Cereb Blood Flow Metab 17:670–679. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos‐Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ (1997): Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain 120:963–976. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Spampinato D, Celnik PA (in press): Laterality differences in cerebellar‐motor cortex connectivity. Cereb Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML (2008): Laterality index in functional MRI: Methodological issues. Magn Reson Imaging 26:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG (2010): Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien D, Strens LHA, Oliviero A, Brown P (2002): Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci Lett 328:89–92. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M (2006): What is the Bereitschaftspotential? Clin Neurophysiol. 117:2341–2356. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM (2002): Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125:1896–1907. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M (2002): Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol 88:333–338. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Noll DC, Small SL (2001): Lateralization of motor circuits and handedness during finger movements. Eur J Neurol 8:425–434. [DOI] [PubMed] [Google Scholar]
- Song DD, Haber SN (2000): Striatal responses to partial dopaminergic lesion: Evidence for compensatory sprouting. J Neurosci 20:5102–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo F, Coppi E, Chieffo R, Straffi L, Fichera M, Nuara A, Gonzalez‐Rosa J, Martinelli V, Comi G, Volontè MA, Leocani L (2013): Interhemispheric balance in Parkinson's disease: A transcranial magnetic stimulation study. Brain Stimul 6:892–897. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM (1999): Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 122:2033–2046. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Fujimura N, Tsuji T, Maruishi M, Muranaka H, Kasai T (2009): Functional interactions between the cerebellum and the premotor cortex for error correction during the slow rate force production task: An fMRI study. Exp Brain Res 193:143–150. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Beek PJ, Wagenaar RC, van Wieringen PC (2000): Coordination disorders in patients with Parkinson's disease: A study of paced rhythmic forearm movements. Exp Brain Res 134:174–186. [DOI] [PubMed] [Google Scholar]
- Vidal JS, Derkinderen P, Vidailhet M, Thobois S, Broussolle E (2003): Mirror movements of the non‐affected hand in hemiparkinsonian patients: A reflection of ipsilateral motor overactivity. J Neurol Neurosurg Psychiatry 74:1352–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, Hallett M (1991): Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology 41:1795–1799. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ (2006): A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. NeuroImage 33:522–530. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K (2007): LI‐tool: A new toolbox to assess lateralization in functional MR‐data. J Neurosci Methods 163:128–136. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M (2005a): A functional MRI study of automatic movements in patients with Parkinson's disease. Brain 128:2250–2259. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M (2005b): The influence of normal human ageing on automatic movements. J Physiol 562:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M (2008): Neural correlates of dual task performance in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 79:760–766. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P (2010): Neural correlates of bimanual anti‐phase and in‐phase movements in Parkinson's disease. Brain 133:2394–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P (2011): Effective connectivity of brain networks during self‐initiated movement in Parkinson's disease. NeuroImage 55:204–215. [DOI] [PubMed] [Google Scholar]
- Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P (in press): Attention to automatic movements in Parkinson's disease: Modified automatic mode in the striatum. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M (2001): Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: Further evidence for motor dominance. Clin Neurophysiol 112:107–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.


