Abstract
Objectives
Functional neuroimaging and voxel‐based morphometry studies have confirmed the important role of the cerebellum in motor behavior. However, little is known about the relationship between cerebellar gray (GMv) and white matter (WMv) volume and manual motor performance in aging individuals. This study aims to quantify the relationship between cerebellar tissue volume and manual motor performance.
Experimental design
To gain more insight into cerebellar function and how it relates to the role of the primary motor cortex (M1), we related cerebellar GMv, WMv, and M1v to manual motor performance in 217 healthy older individuals. Left and right cerebellar GMv and WMv, and M1v were obtained using FreeSurfer. The following motor measures were obtained: grip force, tapping speed, bimanual visuomotor coordination, and manual dexterity.
Principal observations
Significant positive relationships were observed between cerebellar GMv and WMv and grip strength, right cerebellar WMv and right‐hand tapping speed, right cerebellar WMv and dexterity, M1v and grip strength, and right M1v and left‐hand dexterity, though effect sizes were small.
Conclusions
Our results show that cerebellar GMv and WMv are differently associated with manual motor performance. These associations partly overlap with the brain‐behavior associations between M1 and manual motor performance. Not all observed associations were lateralized (i.e., ipsilateral cerebellar and contralateral M1v associations with motor performance), which could point to age‐related neural dedifferentiation. The current study provides new insights in the role of the cerebellum in manual motor performance. In consideration of the small effect sizes replication studies are needed to validate these results. Hum Brain Mapp 36:2352–2363, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: cerebellum, MRI, volume, motor function, gray matter, white matter
INTRODUCTION
Studies that have investigated the association between aging and motor performance in healthy adults have reported that age‐related atrophy of motor cortical regions and the cerebellum is associated with balance and gait deficits, timing, coordination problems, and slowing of movement [Bernard and Seidler, 2013b; Manto et al., 2012; Seidler et al., 2010].
However, neuroimaging studies that have reported how cerebellar volume in healthy adults correlates with behavior [Allin et al., 2001; Bauer et al., 2009; Hellwig et al., 2013; Hogan et al., 2011; Lojkowska et al., 2013] and how it is affected by aging [Bernard and Seidler, 2014; Hoogendam et al., 2012; Luft et al., 1999; Raz et al., 1998; Sullivan et al., 2000] have mainly focused on the volume of relatively large regions of interest such as hemispheres, lobes, and lobules, and often did not differentiate between volume of gray and white matter [Allin et al., 2001; Balsters et al., 2010; Bauer et al., 2009; Bernard and Seidler, 2013b; Diedrichsen et al., 2009; Hellwig et al., 2013; Luft et al., 1999; Raz et al., 1998; Richter et al., 2005].
The few studies that have examined cerebellar gray and white matter volume almost exclusively looked at the association between volume and age [Hoogendam et al., 2012; Jernigan et al., 2001; Walhovd et al., 2005], cognition [Hogan et al., 2011], or differences in tissue class volume between healthy control subjects and patients with cerebellar disorders [de Zeeuw et al., 2012; Dennis et al., 2004; Hong et al., 2002; Keuthen et al., 2007; Sullivan et al., 2000]. To date, only three studies have looked at the association between cerebellar gray matter volume and motor performance [Bernard and Seidler, 2013a; Hoogendam et al., 2014; Nadkarni et al., 2014]. These studies ‐that investigated different populations with different sets of motor tasks‐ have not provided conclusive evidence on the association of cerebellar gray and white matter volume on motor performance. Two of these studies reported relationships between cerebellar gray matter volume, manual sensorimotor functioning [Bernard and Seidler, 2013b], and gait speed [Nadkarni et al., 2014], while the third did not observe associations between measures of fine motor performance required to draw an Archimedes spiral and either cerebellar gray or white matter volume [Hoogendam et al., 2014]. Except for the latter, no studies have investigated how cerebellar white matter volume is associated with motor functioning, although associations have been reported on cerebellar white matter microstructural integrity and motor coordination [Roberts et al., 2013] and motor learning [Della‐Maggiore et al., 2009]. In addition, volume of white matter lesions in cerebral locations (i.e., around the ventricles) has been associated with motor measures such as gait speed [Soumare et al., 2009]. Distinguishing between gray and white matter volume in the association between cerebellar volume and motor function could yield new insights into the role of the cerebellum in motor control.
To investigate the association between cerebellar morphology, age, and manual motor performance we related left and right cerebellar gray and white matter volume with performance on several unimanual and bimanual tasks in a group of 217 healthy older adults. The cerebellum and motor cortex play different roles in motor behavior. Whereas the cerebellum regulates coordination and timing [Koziol et al., 2014] the primary motor cortex is involved in the planning and execution of voluntary movement [Scott, 2008]. Furthermore, the association between motor performance and region can be affected by aging, because cerebral and cerebellar gray and white matter volume show differential decline with accumulating age [Hoogendam et al., 2012]. To gain a better understanding of the role of the cerebellum in manual motor performance in old age and how it relates to that of the primary motor cortex, we investigated both cerebellar gray and white matter tissue volume and primary motor cortex volume. By investigating cerebellar tissue volume in a large group of healthy older adults our measurements will not be confounded by atrophy that results from neurological or psychiatric diseases. We predicted that cerebellar and motor cortex volumes are negatively associated with age, and that both gray and white matter cerebellar volume and motor cortex volume are positively associated with motor performance.
METHOD
Participants
For the current study, we used complete data of 217 subjects from the first time point of the longitudinal healthy aging brain (LHAB) database, a population‐based study conducted at the International Normal Aging and Plasticity Imaging Center in Zürich, Switzerland [Zöllig et al., 2011]. All subjects were recruited through advertisements in local newspapers and at the end of public lectures by L.J. Subjects were included if they were 65 years of age or older, right‐handed, fluent in German, if they had a mini mental state examination score >26 to exclude any potential demented subjects, and passed magnetic resonance imaging (MRI) safety standards. Exclusion criteria that were not specific for the current analyses, but were set for the LHAB study in general encompassed neurological and psychiatric diseases, diseases of the hematopoietic system, traumatic brain injury in the two years preceding participation, migraine, diabetes, and tinnitus.
The current study was approved by the ethical committee of the canton of Zurich. Written informed consent was obtained from all participants.
Image Acquisition
Multisequence MRI was performed on a 3T Philips Ingenia MRI scanner with a 15‐channel head coil, located at the University Hospital Zurich, Switzerland. We collected two T1‐weighted Ultrafast Gradient Echo 3D (TFE; turbo field echo) images (TR = 8.18 MS, TE = 3.799 MS, flip angle = 8°; FOV = 240 × 240 mm; slice thickness = 1.0 mm; 160 sagittal slices; matrix = 256 × 256; voxel size = 0.94 × 0.94 × 1.0 = 0.88 mm3; duration = ∼7:30 min), and a T2‐weighted multislice Turbo Spin Echo image (TR = 3,000 MS; TE = 80 MS; flip angle = 90°; FOV = 230 × 182.87 mm; slice thickness = 5.0 mm; 28 axial slices; matrix = 488 × 390; voxel size = 0.45 × 0.45 × 5.0 = 1.0 mm3; duration = ∼90 s).
Image Preprocessing
The following software packages were used for analyses: FMRIB Software Library (FSL), version 5.0.1; FreeSurfer v5.3.0. All data were transferred onto a computer cluster running Red Hat Enterprise Linux release 6.4.
The portion below the cerebellum, at Z = 80, was automatically stripped from all of the T1 weighted images. Results were inspected to verify that no brain was stripped off. Stripping was done to improve averaging of the T1 weighted image pairs that was performed using FSL's AnatomicalAverage. Inhomogeneity correction was applied to the averaged T1 weighted and the T2 weighted images using N4ITK [Tustison et al., 2010]. The correction was applied within a subject and sequence‐specific brain mask created using FSL's Brain Extraction Tool [Smith, 2002] with robust brain center estimation and a fractional intensity threshold of 0.15.
Cerebellar and Motor Cortex Segmentation
Cerebellar left and right gray and white matter volume and left and right motor cortex volumes were obtained using FreeSurfer. Figure 1 shows the cerebellar segmentation of one random subject. Both the average T1 image and the T2 image were fed into FreeSurfer's default pipeline: this fully automated procedure assigns a neuroanatomical label to all voxels of the T1‐weighted image based on information from a manually labeled training set [Fischl et al., 2002; Fischl et al., 2004]. FreeSurfer uses the T2 image for refinement of the pial surface. For intracranial volume (ICV), we used FreeSurfer's estimated total ICV (eTIV). eTIV is an estimation of a subject's total ICV that is derived by calculating the volume expansion/contraction (the “shrink factor”) required to register the subject's brain into an atlas template, and subsequently dividing the volume of the atlas ICV by the shrink factor [Buckner et al., 2004]. eTIV has been shown previously to be highly correlated (r = 0.93) with manual measures of total ICV [Buckner et al., 2004].
Figure 1.
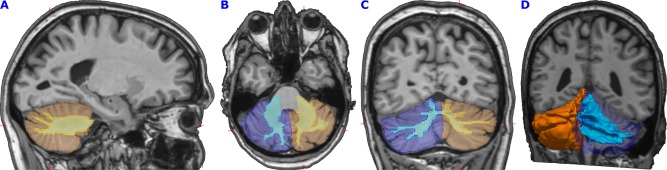
Cerebellar segmentation of one random subject. The first three images show sagittal (A), axial (B), and coronal (C) sections through the cerebellum of a random study subject. FreeSurfer segmentations are transformed back to native (i.e., subject) space. Orange and dark blue colors show left and right cerebellar gray matter. Yellow and light blue colors show the cerebellar white matter. Red markers show the slices' intersections. (D) shows a three‐dimensional representation of the cerebellar gray in orange semitransparent dark blue, and cerebellar white matter in light blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Motor Behavioral Tasks
Motor performance was measured with four tasks that are sensitive to cerebellar function [Meindl et al., 2012; Miall and Christensen, 2004; Miall et al., 2001; Witt et al., 2008]. These tasks yielded the following outcome measures: (a) grip force, (b) maximum tapping speed, (c) bimanual visuomotor coordination, and (d) manual dexterity and motor speed.
Grip force was measured in kilograms using a hydraulic hand dynamometer (Model SH5001, Saehan Corporation, Korea) [Podell, 2010]. Participants were instructed to sit upright, with their feet positioned flat on the ground, their shoulders in a neutral position, their elbow in a 90° flexion, their forearm in a neutral position and the wrist in an extension of 0°–30°. Data were collected for both the right and left hand with a 30 s break between measurements. Participants were asked to apply their maximum grip force and to keep it steady for 4 s. Both left and right hand was measured three times. If the third measurement was the largest of the three, data collection continued until the final measurement was smaller than the previous one. Mean grip force was calculated for the three highest measurements.
Left‐ and right‐hand motor tapping was measured using the digital tapping test from the Vienna Test System [Schatz, 2010; Schoppe and Hamster, 2011]. Participants were asked to tap as many times as possible on a 4 × 4 cm metal plate within 32 s using a pen‐like device held in their right hand and subsequently to repeat this with their left hand.
Bimanual visuomotor coordination was measured using the two‐hand coordination test from the Vienna Test System [Schufried, 2011]. The test requires participants to navigate a red dot on a computer screen along a track using two joysticks simultaneously: one joystick controls up–down movement, the other joystick controls left–right movement. Participants completed two practice trials and subsequently four actual trials during which the following information was obtained: mean time used to finish the track (in seconds), and total error duration (time that participants were off track) as percentage of the total duration.
Manual dexterity and motor speed were measured with the grooved pegboard test (Lafayette Instruments, Lafayette, IN) [Merker and Podell, 2010]. For the grooved pegboard test, participants had to put metal keyhole shaped pegs in a board with keyhole shaped holes (5 columns × 5 rows) in various orientations, as fast as possible. Participants completed the test twice: once with their left hand, starting from right to left, and once with their right hand starting from right to left. Total time needed to fill all 25 holes in the board was recorded.
Confounding Factors
The following factors that have been associated with brain volume and aging were collected for all participants: sex [Luders and Toga, 2010], diastolic and systolic blood pressure [Beauchet et al., 2013; Ikram et al., 2008; Pinto, 2007], smoker status [Debette et al., 2011; Ikram et al., 2008], and years of education [Coffey et al., 1999]. Blood pressure was measured using an electric hemodynamometer (Model M6, HEM‐7211‐E; Omron Corporation, Kyoto, Japan). Participants were asked to sit quietly with their left forearm held stable on a table. A cuff was attached to the participants' left arm and the measurement was conducted automatically. Blood pressure was measured three times over the day of the behavioral testing session. Self‐reported smoker status (current smoker/nonsmoker) and years of education were obtained from questionnaires.
Data Analysis
Measures of visuomotor coordination, performance, and error were square‐root transformed because of skewness of the untransformed measures. Outliers of both dependent and independent variables were defined as values three standard deviations below or above the mean. Subjects with outliers in outcome measures or predictor variables were excluded from analysis. Multivariate analysis of covariance (MANCOVA) adjusted for age and sex was used as an omnibus test to test the relationship between either left or right cerebellar gray and white matter and the combined set of motor performance measures. General multivariate linear models were used to explore the associations between cerebellar volume and age, gender, and motor performance. All models were adjusted for ICV, sex, age, smoker status, and diastolic blood pressure. The analysis of the association between cerebellar volume and motor performance was subsequently adjusted for years of education. To test if there was a quadratic relationship between cerebellar or motor cortex volume with motor performance, we additionally ran models in which we included both volume and volume‐squared. Eta‐squared (η 2) is reported as an effect‐size measure [Levine and Hullett, 2002; Olejnik and Algina, 2003; Richardson, 2011]. Alpha levels were set at P = 0.05 for all analyses. To prevent Type‐II errors [Perneger, 1998], we report P‐values unadjusted for multiple comparisons, although we additionally discuss the impact of multiple comparisons correction on our work in the discussion section. Stata Statistical Software: Release 13 was used for data analysis (StataCorp LP, College Station, TX: StataCorp. 2013).
RESULTS
Population characteristics are presented in Table 1. The mean age of the participants was 70.7 years and ranged from 64 to 87.
Table 1.
Population characteristics, N = 217
| #missing | |||
|---|---|---|---|
| Gender: malea | 104 | (47.9) | 0 |
| Smoker status: non (vs. current)a | 176 | (81.1) | 12 |
| Age (years) | 70.7 | (5.0) | 0 |
| Diastolic blood pressure (mm Hg) | 81.4 | (9.7) | 5 |
| Systolic blood pressure (mm Hg) | 142.6 | (17.1) | 5 |
| Years of education | 14.6 | (3.3) | 0 |
| Grooved pegboard right hand (s) | 73.7 | (12.5) | |
| Grooved pegboard left hand (s) | 81.1 | (14.6) | |
| Tapping right hand (taps/32 s) | 189.9 | (20.7) | |
| Tapping left hand (taps/32 s) | 170.9 | (22.3) | |
| Visuomotor coordination (in seconds)b | 45.0 | (32.7–59.4) | |
| Visuomotor coordination error %b | 2.4 | (1.4–4.5) | |
| Right‐hand grip force (in Kg) | 33.0 | (10.5) | |
| Left‐hand grip force (in Kg) | 30.8 | (9.9) | |
| Left cerebellar gray matter (ml) | 46.0 | (4.7) | |
| Right cerebellar gray matter (ml) | 47.8 | (5.2) | |
| Left cerebellar white matter (ml) | 15.5 | (2.3) | |
| Right cerebellar white matter (ml) | 15.7 | (2.4) | |
| Left motor cortex (ml) | 11.4 | (1.2) | |
| Right motor cortex (ml) | 11.5 | (1.3) |
Values indicate means and (standard deviations) unless specified otherwise.
Number and (% of nonmissing).
median and (interquartile range).
Cerebellar left and right gray and white matter volume as well as left and right primary motor cortex volume significantly linearly decreased with accumulating age (see Table 2 and Fig. 2). MANCOVAs multivariate test statistic (i.e., Wilks' lambda) revealed that there were significant associations between left cerebellar gray matter volume and motor performance λ = 0.89, F(1, 161) = 2.20, P = 0.025 and right cerebellar gray matter volume and motor performance λ = 0.88, F(1, 162) = 2.37, P = 0.015. The omnibus test did not show significant associations between the combined motor performance measures and right or left cerebellar white matter. Larger cerebellar and motor cortex volumes were linearly associated with better performance on several motor tasks: Left‐ and right‐hand grooved pegboard performance was associated with right cerebellar white matter volume (see Table 3 and Fig. 3). Left‐hand grooved pegboard performance was furthermore linearly associated with right motor cortex volume. Right‐hand tapping rate was significantly linearly associated with right cerebellar white matter volume. Right‐hand grip strength was significantly predicted by all volumetric outcome measures, except for right cerebellar white matter volume. Left‐hand grip strength was only significantly associated with right cerebellar gray matter volume.
Table 2.
Association between age and cerebellar and motor cortex volume in ml
| 95% CI for β | ||||||
|---|---|---|---|---|---|---|
| β age | Low | High | P a | η 2 | ||
| Cerebellar | Left | −0.221 | −0.324 | −0.119 | <0.001 | 0.052 |
| Gray matter | Right | −0.239 | −0.351 | −0.127 | <0.001 | 0.051 |
| Total | −0.450 | −0.660 | −0.240 | <0.001 | 0.051 | |
| Cerebellar | Left | −0.121 | −0.180 | −0.062 | <0.001 | 0.064 |
| White matter | Right | −0.96 | −0.158 | −0.034 | 0.003 | 0.037 |
| Total | −0.217 | −0.334 | −0.100 | <0.001 | 0.052 | |
| Motor cortex | Left | −0.066 | −0.097 | −0.036 | <0.001 | 0.068 |
| Right | −0.076 | −0.107 | −0.045 | <0.001 | 0.086 | |
All analyses are adjusted for FreeSurfer's estimated total ICV (eTIV), diastolic blood pressure, and smoker status.
CI, confidence interval; βage = increase in cerebellar tissue class volume per one year increase in age.
Figure 2.
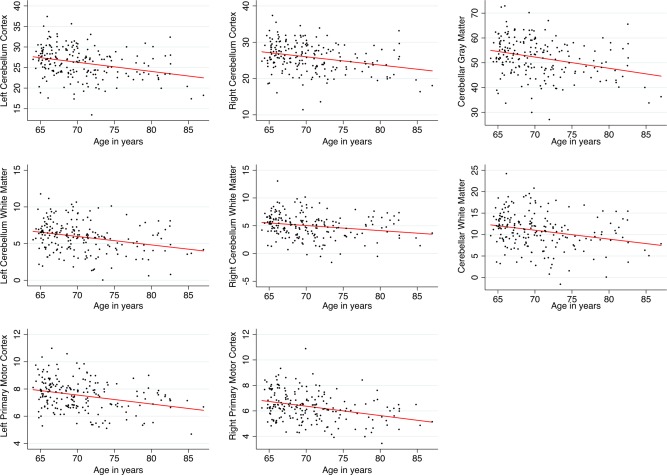
Scatterplots with linear fit lines of relationships between age and cerebellar volume/motor cortex volume. Volumes of the cerebellar and motor cortex regions were obtained by regressing out the effects of total ICV, sex, smoker status, and diastolic blood pressure from the raw volumes. Volumes are expressed in milliliter. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Linear association between cerebellar and motor cortex volume and motor behavior
| Left hemisphere | Right hemisphere | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||||
| β vol | Low | High | P b | η 2 | β vol | Low | High | P b | η 2 | ||
| GPB | cGM | −0.190 | −0.642 | 0.262 | 0.41 | 0.003 | −0.196 | −0.608 | 0.217 | 0.35 | 0.004 |
| Right | cWM | −0.561 | −1.340 | 0.217 | 0.16 | 0.008 | −0.870 | −1.606 | −0.134 | 0.021 | 0.022 |
| (time in sec) | M1 | −0.207 | −1.747 | 1.333 | 0.79 | <0.001 | −0.509 | −2.034 | 1.015 | 0.51 | 0.002 |
| GPB | cGM | −0.284 | −0.820 | 0.252 | 0.30 | 0.005 | −0.328 | −0.819 | 0.163 | 0.19 | 0.008 |
| Left | cWM | −0.525 | −1.462 | 0.411 | 0.27 | 0.005 | −0.935 | −1.814 | −0.057 | 0.037 | 0.019 |
| (time in sec) | M1 | −1.760 | −3.573 | 0.053 | 0.057 | 0.016 | −1.898 | −3.705 | −0.091 | 0.040 | 0.018 |
| Tapping | cGM | 0.163 | −0.617 | 0.944 | 0.68 | 0.001 | 0.189 | −0.523 | 0.900 | 0.60 | 0.001 |
| Right | cWM | 1.150 | −0.176 | 2.477 | 0.09 | 0.012 | 1.724 | 0.468 | 2.981 | 0.007 | 0.031 |
| (taps/32 s) | M1 | −0.080 | −2.608 | 2.447 | 0.95 | <0.001 | −0.788 | −3.351 | 1.774 | 0.55 | 0.002 |
| Tapping | cGM | 0.491 | −0.377 | 1.358 | 0.27 | 0.006 | 0.350 | −0.441 | 1.140 | 0.38 | 0.004 |
| Left | cWM | 0.418 | −1.068 | 1.903 | 0.58 | 0.001 | 1.265 | −0.148 | 2.677 | 0.08 | 0.014 |
| (taps/32 s) | M1 | 0.170 | −2.653 | 2.992 | 0.91 | <0.001 | −0.337 | −3.194 | 2.519 | 0.82 | <0.001 |
| VMC | cGM | −0.009 | −0.066 | 0.047 | 0.74 | <0.001 | −0.015 | −0.066 | 0.037 | 0.57 | 0.001 |
| Durationa | cWM | −0.058 | −0.156 | 0.040 | 0.24 | 0.006 | −0.035 | −0.131 | 0.061 | 0.47 | 0.002 |
| M1 | −0.077 | −0.269 | 0.114 | 0.43 | 0.003 | −0.060 | −0.250 | 0.130 | 0.53 | 0.002 | |
| VMC | cGM | −0.003 | −0.043 | 0.036 | 0.87 | <0.001 | 0.011 | −0.025 | 0.048 | 0.56 | 0.002 |
| Error %a | cWM | 0.021 | −0.048 | 0.090 | 0.55 | 0.002 | 0.035 | −0.031 | 0.102 | 0.30 | 0.005 |
| M1 | −0.053 | −0.186 | 0.080 | 0.43 | 0.003 | −0.043 | −0.175 | 0.090 | 0.53 | 0.002 | |
| Grip | cGM | 0.278 | 0.029 | 0.526 | 0.029 | 0.009 | 0.323 | 0.104 | 0.542 | 0.004 | 0.014 |
| Right | cWM | 0.440 | 0.011 | 0.869 | 0.011 | 0.007 | 0.209 | −0.202 | 0.620 | 0.32 | 0.002 |
| (in Kg) | M1 | 0.967 | 0.147 | 1.788 | 0.021 | 0.010 | 0.954 | 0.126 | 1.782 | 0.024 | 0.009 |
| Grip | cGM | 0.228 | −0.006 | 0.462 | 0.06 | 0.007 | 0.271 | 0.059 | 0.482 | 0.013 | 0.011 |
| Left | cWM | 0.382 | −0.022 | 0.787 | 0.06 | 0.006 | 0.250 | −0.137 | 0.636 | 0.20 | 0.003 |
| (in Kg) | M1 | 0.509 | −0.271 | 1.289 | 0.20 | 0.003 | 0.554 | −0.227 | 1.336 | 0.16 | 0.003 |
These measures were square root transformed to accommodate for the skewness of the untransformed measure.
All analyses are adjusted for FreeSurfer's estimated total ICV (eTIV), age, gender, diastolic blood pressure, smoker status, and years of education.
CI, confidence interval; β vol = increase in 1 unit of the task outcome per 1 ml increase in volume.
GPB, Grooved pegboard test; VMC, visuomotor coordination test; cGM, cerebellar Gray Matter; cWM, cerebellar white matter; M1, primary motor cortex.
Figure 3.
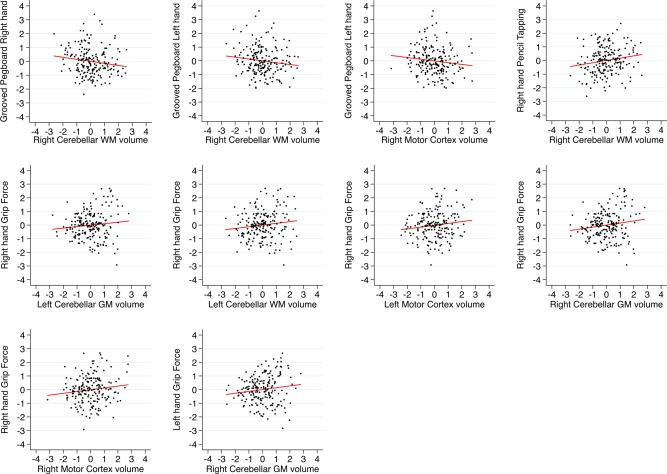
Scatterplots with linear fit lines of relationships between gray matter cerebellar volume/motor cortex volume and manual motor performance. Only significant associations are plotted (see Table 3). The y‐axis shows normalized (z‐scores) residual scores of manual motor performance. Residual scores were obtained by regressing out the effect of age, sex, education, diastolic blood pressure, and ICV. The x‐axis shows normalized (z‐score) values of the volumetric measures. Red lines depict linear trend lines. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
There was a significant negative quadratic (inverted U) relationship between left motor cortex volume and left‐hand grooved pegboard score (see Table 4). Both left and right cerebellar white matter volume showed a significant negative quadratic relationship with left‐hand pencil tapping. Right cerebellar white matter volume had a significant positive quadratic relationship with visuomotor coordination test duration and a significant negative quadratic relationship with left‐hand grip force.
Table 4.
Significant quadratic associations between cerebellar and motor cortex volume and motor behavior
| Volume | Volume‐squared | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | combined | ||||||||||
| Volume | Task | β vol | Low | High | P a | η 2 | β vol^2 | Low | High | P a | η 2 | η 2 |
| Left M1 | Left GPB | 19.283 | −1.797 | 40.364 | 0.073 | 0.014 | −0.920 | −1.838 | −0.002 | 0.050 | 0.017 | 0.030 |
| Left cWM | Left Tap | 16.647 | 4.001 | 29.293 | 0.010 | 0.031 | −0.519 | −0.920 | −0.117 | 0.012 | 0.030 | 0.061 |
| Right cWM | Left Tap | 14.056 | 2.492 | 25.620 | 0.017 | 0.026 | −0.404 | −0.766 | −0.041 | 0.029 | 0.022 | 0.048 |
| Right cWM | VMC | −1.016 | −1.866 | −0.166 | 0.019 | 0.024 | 0.030 | 0.004 | 0.057 | 0.023 | 0.022 | 0.046 |
| Right cWM | Left Grip | 3.647 | 0.453 | 6.481 | 0.025 | 0.009 | −0.107 | −0.207 | −0.007 | 0.036 | 0.008 | 0.016 |
All analyses are adjusted for FreeSurfer's estimated total ICV (eTIV), age, gender, diastolic blood pressure, smoker status, and years of education.
CI, confidence interval; β vol = increase in 1 unit of the task outcome per 1 ml increase in volume.
GPB, Grooved pegboard test (time in seconds); Tap, tapping test (number of taps per 32 s); Grip, Grip force strength (in Kg); VMC, visuomotor coordination test (time in seconds); cGM, cerebellar Gray Matter; cWM, cerebellar White Matter; M1, primary motor cortex.
DISCUSSION
In line with our hypothesis, we observed significantly smaller left and right cerebellar gray and white matter and primary motor cortex volumes with older age in a sample of healthy older adults. The size of the effect of age on volume was medium [Richardson, 2011], although the effect of age on primary motor cortex volume was somewhat larger than the effect of age on the cerebellar gray and white matter. In these elderly participants, larger cerebellar gray and white matter volume and primary motor cortex volume was associated with better manual motor performance, though the effects were small in size [Richardson, 2011]. The magnitude of effect of cerebellar gray and white matter volume and motor cortex volume on motor behavior was similar.
The observed association between cerebellar gray and white matter volume and age [Hoogendam et al., 2012; Jernigan et al., 2001; Walhovd et al., 2005], and motor cortex volume and age [Salat et al., 2004] are in line with previous imaging studies that reported similar findings. In addition, our results correspond with a stereological study on cerebellar cortical and white matter volume. This study used postmortem brains from 19 subjects aged 19–84 years and calculated tissue class volumes by summing up the tissue class volume in consecutive coronal cerebellar slices using the Cavalieri method and point counting [Andersen et al., 2003]. The results showed a decline in global cerebellar volume with age that was due to white matter loss and volume loss of the Purkinje cells and granule cells.
We observed linear and quadratic associations between cerebellar gray and white matter volume and primary motor cortex volume with several measures of manual motor performance. Interestingly, although the motor cortex controls the contralateral side of the body, and the cerebellum controls the ipsilateral side of the body, not all associations between volume and motor function that we observed followed this pattern. For example, we found associations between right cerebellar gray matter volume and left‐hand grip strength and vice versa. It could be that because of age‐related neural dedifferentiation [Carp et al., 2011] the laterality was less pronounced in this elderly group of subjects, although we could not formally test this because we did not include a group of younger adults. Carp et al. showed that motor distinctiveness is reduced in older adults in several motor control areas, including the primary motor cortex, the supplementary motor area, the insula, and the cerebellum. This corroborates with our results showing both contralateral as well as ipsilateral (marginally) significant associations between motor cortex and cerebellar gray and white matter volumes and grip strength [Bernard and Seidler, 2012]. Future studies that investigate cerebellar volume in relation to motor function should include adult participants over the whole age range to be able to verify potential age related neural dedifferentiation in cerebellar structural–functional relationships. Although no studies have related motor functioning to the volume of the cerebellar tissue class volume per hemisphere, or whole primary motor cortex volume, several of our results are supported by fMRI, positron emission tomography (PET), and voxel‐based morphometry (VBM) studies.
The positive association between cerebellar gray matter volume and grip force that we observed is in consonance with previous functional MRI studies that reported widespread activation in the cerebellum specific to force amplitude and force rate [Spraker et al., 2012], and increases in activation during the production of increased grip force [Keisker et al., 2009; Noble et al., 2011; Pope et al., 2005; Sulzer et al., 2011]. The fact that grip force was associated with gray matter volume of the bilateral cerebellum and motor cortex is in line with an fMRI study that showed revealed activation in the bilateral cerebellum and primary motor cortex that was related to force in a precision grip task [Sulzer et al., 2011]. Although, lesion studies have linked grip force to the cerebellum [Anens et al., 2010; Muller and Dichgans, 1994], no quantitative structural MRI studies have explored the association between grip force and cerebellar tissue volume. In the current study, grip strength was also associated with cerebellar white matter volume and motor cortex volume, suggesting that grip strength is a complex behavior that is mediated by multiple interconnected brain regions, which corroborates with fMRI studies that showed activation in cortical and cerebellar regions during generation of grip force [Ehrsson et al., 2000; Spraker et al., 2012]. Furthermore, an fMRI study revealed that compared to younger adults, older adults recruit more brain regions, including the cerebellum, to control grip force magnitude [Noble et al., 2011].
Our observed association between right cerebellar white matter volume and left and right‐hand motor speed and dexterity measured with the grooved pegboard test has not been described before. It could be that less white matter cerebellar volume represents lower qualitative connections with other brain regions resulting in worse performance [Hugenschmidt et al., 2008]. This idea is further supported by a recent study that showed myelin levels, albeit in frontal regions, was strongly correlated with maximum manual motor speed [Bartzokis et al., 2010]. Whether myelin levels in other brain regions such as the cerebellum were also associated with manual motor performance was not evaluated. Previous studies did observe an association between local cerebellar gray matter volume and manual dexterity. A VBM study in 14‐year olds revealed a significant positive association between gray matter density in the cerebellum and manual dexterity measured using the Purdue pegboard test [Kühn et al., 2012]. Male, but not female professional keyboard players showed to have a larger cerebellum than nonmusician control subjects [Hutchinson et al., 2003]. PET imaging in boys suffering from muscular dystrophy showed impaired manual dexterity and regional brain glucose hypometabolism in regions including the bilateral cerebellum [Lee et al., 2002]. Research from our labs demonstrated a significant positive relationship between performance on the grooved pegboard test and Crus I volume [Bernard and Seidler, 2013b], and activation in the intermediate and lateral portions of the anterior cerebellum during bimanual and unimanual movements [Jäncke et al., 1999; Koeneke et al., 2004]. The fact that we did not observe an association between hemispheric cerebellar gray matter volume and pegboard performance might be because our whole‐hemisphere approach may not be sufficiently sensitive to pick up small local volume‐behavior associations.
We observed that right cerebellar white matter volume was associated with tapping performance of the left and right hand, and that left cerebellar white matter volume was associated with tapping performance of the left hand. Previous studies have reported associations between motor tapping and cerebellar gray matter, but not white matter volume. A meta‐analysis of 38 fMRI studies reported activation in the anterior cerebellum during finger tapping [Witt et al., 2008]. A recent study conducted in our lab revealed an association between tapping variability and volume of the vermis and anterior cerebellum in healthy younger and older adults [Bernard and Seidler, 2013b].
Most studies that revealed associations between the cerebellum and motor performance focused on the regions of interest that mainly included gray matter, used VBM, or used fMRI that focuses on the activity in the gray matter, and did not focus on macrostructural cerebellar white matter changes. Besides associations between cerebellar hemispheric gray matter volume and motor performance, we observed several associations between cerebellar hemispheric white matter and motor performance, suggesting that in addition to gray matter volume, the structural connectivity between the cerebellum and other brain regions plays a major role in dexterity, grip strength, and tapping performance. Previous studies have observed a decline in microstructural integrity of the cerebellar white matter with aging, while others reported associations between white matter microstructural integrity of the cerebellum and gait and balance [Bernard and Seidler, 2014]. Whether microstructural white matter integrity in the cerebellum is also related to fine motor performance has yet to be determined, but is likely, considering the association between white matter macrostructure and motor behavior that we observed, and the previously reported association between microstructural white matter integrity and white matter atrophy [Hugenschmidt et al., 2008; Vernooij et al., 2008]. Furthermore, only cerebellar white matter, but not gray matter, showed quadratic relationships with motor performance. This could indicate that a larger white matter volume does not necessary result in better function, but rather that there is an optimum, potentially reflecting a more efficient neural organization [Hanggi et al., 2014]. This is difficult to validate as other studies that investigated the relation between regional brain volume and performance have used linear models. Future studies, preferably in healthy adults are warranted to test this hypothesis.
We are aware that our study has some limitations that need to be addressed. The analyses of the association between manual motor performance and cerebellar tissue class volume have not been corrected for multiple comparisons. However, all significant effects pointed in the expected directions (i.e., larger volumes relate to better performance), and even though these effects are small to moderate, it is striking to see that after controlling for the effect of age, gender, diastolic blood pressure, smoker status, and years of education, cerebellar volume still predicts motor performance. Correction for multiple comparisons could prevent Type I errors, while at the same time result in Type II errors [Perneger, 1998]. Because of paucity of studies investigating the association between cerebellar volume and manual motor performance we believe it is important to report these unadjusted results because they could aid in our understanding of cerebellar functioning. Additionally, use of suboptimal algorithms segmentation for cerebellar tissue might have weakened the associations between cerebellar tissue volume and manual motor performance. The cerebellum has narrow gyri or “folia.” The white matter branches at the end of the folia can be smaller than 0.5 mm [Diedrichsen et al., 2009]. The T1 MRI sequence that we used has a voxel size of ∼1 mm3. Because of the subvoxel resolution of cerebellar tissue, it is not possible to separate the gray and white matter in the cerebellum at the voxel level [Marques et al., 2010]. The FreeSurfer software that we used uses a binary cerebellar tissue class segmentation that only takes into account the partial volume effect of bordering gray/white matter voxels. Differential misclassification of gray and white matter may have acted as bias in the association between cerebellar tissue volume and manual motor performance. Replication studies are necessary to validate our results. Furthermore, this study is cross‐sectional, and therefore, we cannot investigate causal relationships between cerebellar tissue class volume, aging, and manual motor performance. The current study is embedded in an ongoing prospective longitudinal study with yearly follow up [Zöllig et al., 2011], and therefore, we will have longitudinal volumetric and behavioral data at our disposal in the near future that will help us answering questions about causality. A strength of our study is the combination of volumetric cerebellar tissue class volumes and motor behavioral measures of a large number of healthy older subjects. Up until now, most studies that related cerebellar volume to manual motor performance have been conducted in patient populations [Allin et al., 2001; Dennis et al., 2004; Horská et al., 2010; Richter et al., 2005] and not in large samples of the general or healthy elderly population. The two studies that did combine cerebellar volumetrics and manual motor performance data either had a much smaller sample size than the current study [Bernard and Seidler, 2013b], or only investigated one test of fine motor performance [Hoogendam et al., 2014]. The unique combination of cerebellar volumetry and multiple measures of manual motor performance in a relatively large sample of healthy older adults provides new insights in the association between cerebellar tissue class volume and manual motor performance. An additional strength is that we specifically looked at associations between white matter volume and motor behavior, as most studies focus on focal and global gray matter cerebellar volume, or activation in the gray matter of the cerebellum using fMRI.
Conclusion
Gray and white matter cerebellar tissue volume and motor cortex volume is very strongly related to age in a group of healthy older adults, suggesting degeneration of the cerebellum and motor cortex with accumulating age. In addition, cerebellar gray matter and white matter are differently associated with manual motor function, and these associations partly overlap with the brain‐behavior associations of the primary motor cortex and motor function. Regardless of age, left and right cerebellar gray matter volume and motor cortex volume is associated with grip force and manual dexterity in healthy older adults, which may indicate that widespread cerebellar regions are involved in these motor functions. Furthermore, left and right cerebellar white matter volume was associated with grip force, manual dexterity, visuomotor coordination, and hand tapping, validating the importance of corticocerebellar connections in the performance of these motor functions. Not all associations that we observed in this group of older adults showed ipsilateral cerebellar‐performance or contralateral motor cortex‐performance relationships, which may be the result of previously described neural dedifferentiation with aging.
ACKNOWLEDGMENTS
The current analysis incorporates data from the Longitudinal Healthy Aging Brain (LHAB) database project, which is performed as one of the core projects at the International Normal Aging and Plasticity Imaging Center/INAPIC and the URPP “Dynamics of Healthy Aging.” The following members of the core INAPIC team were involved in the design, setup, maintenance, and support of the LHAB database: Anne Eschen, Lutz Jäncke, Mike Martin, Susan Mérillat, Christina Röcke, and Jacqueline Zöllig. RS and LF are faculty members of the LIFE Course: Evolutionary and Ontogenetic Dynamics.
REFERENCES
- Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MH, Stewart AL, Rifkin L, Murray RM (2001): Cognitive and motor function and the size of the cerebellum in adolescents born very pre‐term. Brain 124:60–66. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJ, Pakkenberg B (2003): Aging of the human cerebellum: A stereological study. J Comp Neurol 466:356–365. [DOI] [PubMed] [Google Scholar]
- Anens E, Kristensen B, Hager‐Ross C (2010): Reactive grip force control in persons with cerebellar stroke: Effects on ipsilateral and contralateral hand. Exp Brain Res 203:21–30. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK, Ramnani N (2010): Evolution of the cerebellar cortex: The selective expansion of prefrontal‐projecting cerebellar lobules. Neuroimage 49:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J (2010): Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging 31:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD (2009): Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol Psychiatry 66:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Celle S, Roche F, Bartha R, Montero‐Odasso M, Allali G, Annweiler C (2013): Blood pressure levels and brain volume reduction: A systematic review and meta‐analysis. J Hypertens 31:1502–1516. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2012): Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging 33:1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2013a): Cerebellar contributions to visuomotor adaptation and motor sequence learning: An ALE meta‐analysis. Front Hum Neurosci 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2013b): Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum 12:721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2014): Moving forward: Age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev 42C:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ (2004): A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas‐based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 23:724–38. [DOI] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk TA (2011): Age‐related neural dedifferentiation in the motor system. PLoS One 6:e29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF (1999): Relation of education to brain size in normal aging: Implications for the reserve hypothesis. Neurology 53:189–96. [DOI] [PubMed] [Google Scholar]
- de Zeeuw P, van Belle J, van Dijk S, Weusten J, Koeleman B, Janson E, van Engeland H, Durston S (2012): Imaging gene and environmental effects on cerebellum in Attention‐Deficit/Hyperactivity Disorder and typical development. Neuroimage Clin 2:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C (2011): Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della‐Maggiore V, Scholz J, Johansen‐Berg H, Paus T (2009): The rate of visuomotor adaptation correlates with cerebellar white‐matter microstructure. Hum Brain Mapp 30:4048–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Hetherington R, Copeland K, Frederick J, Blaser SE, Kramer LA, Drake JM, Brandt M, Fletcher JM (2004): Neurobiology of perceptual and motor timing in children with spina bifida in relation to cerebellar volume. Brain 127:1292–1301. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009): A probabilistic MR atlas of the human cerebellum. Neuroimage 46:39–46. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H (2000): Cortical activity in precision‐ versus power‐grip tasks: An fMRI study. J Neurophysiol 83:528–36. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Hanggi J, Fovenyi L, Liem F, Meyer M, Jancke L (2014): The hypothesis of neuronal interconnectivity as a function of brain size‐a general organization principle of the human connectome. Front Hum Neurosci 8:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig S, Gutmann V, Trimble MR, van Elst LT (2013): Cerebellar volume is linked to cognitive function in temporal lobe epilepsy: A quantitative MRI study. Epilepsy Behav 28:156–162. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Murray AD, Ahearn TS, Deary IJ, Whalley LJ (2011): Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex 47:441–450. [DOI] [PubMed] [Google Scholar]
- Hong KE, Ock SM, Kang MH, Kim CE, Bae JN, Lim MK, Suh CH, Chung SJ, Cho SC, Lee JS (2002): The segmented regional volumes of the cerebrum and cerebellum in boys with Tourette syndrome. J Korean Med Sci 17:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam YY, van der Geest JN, van der Lijn F, van der Lugt A, Niessen WJ, Krestin GP, Hofman A, Vernooij MW, Breteler MM, Ikram MA (2012): Determinants of cerebellar and cerebral volume in the general elderly population. Neurobiol Aging 33:2774–2781. [DOI] [PubMed] [Google Scholar]
- Hoogendam YY, van der Lijn F, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Ikram MA, van der Geest JN (2014): Older age relates to worsening of fine motor skills: A population‐based study of middle‐aged and elderly persons. Front Aging Neurosci 6:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horská A, Laclair A, Mohamed M, Wells CT, McNutt T, Cohen KJ, Wharam M, Mahone EM, Kates W (2010): Low cerebellar vermis volumes and impaired neuropsychologic performance in children treated for brain tumors and leukemia. AJNR Am J Neuroradiol 31:1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ (2008): Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex 18:433–442. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Lee LH, Gaab N, Schlaug G (2003): Cerebellar volume of musicians. Cereb Cortex 13:943–949. [DOI] [PubMed] [Google Scholar]
- Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, Niessen WJ, Breteler MM (2008): Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol Aging 29:882–890. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Specht K, Mirzazade S, Peters M (1999): The effect of finger‐movement speed of the dominant and the subdominant hand on cerebellar activation: A functional magnetic resonance imaging study. Neuroimage 9:497–507. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594. [DOI] [PubMed] [Google Scholar]
- Keisker B, Hepp‐Reymond MC, Blickenstorfer A, Meyer M, Kollias SS (2009): Differential force scaling of fine‐graded power grip force in the sensorimotor network. Hum Brain Mapp 30:2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuthen NJ, Makris N, Schlerf JE, Martis B, Savage CR, McMullin K, Seidman LJ, Schmahmann JD, Kennedy DN, Hodge SM, Rauch SL (2007): Evidence for reduced cerebellar volumes in trichotillomania. Biol Psychiatry 61:374–381. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Wustenberg T, Jäncke L (2004): Long‐term training affects cerebellar processing in skilled keyboard players. Neuroreport 15:1279–1282. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, Yamazaki T (2014): Consensus paper: The cerebellum's role in movement and cognition. Cerebellum 13:151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Romanowski A, Schilling C, Banaschewski T, Barbot A, Barker GJ, Bruhl R, Buchel C, Conrod PJ, Czech K, Dalley JW, Flor H, Garavan H, Hake I, Ittermann B, Ivanov N, Mann K, Lathrop M, Loth E, Lüdemann K, Mallik C, Martinot JL, Palafox C, Poline JB, Reuter J, Rietschel M, Robbins TW, Smolka MN, Nees F, Walaszek B, Schumann G, Heinz A, Gallinat J, Consortium I (2012): Manual dexterity correlating with right lobule VI volume in right‐handed 14‐year‐olds. Neuroimage 59:1615–1621. [DOI] [PubMed] [Google Scholar]
- Lee JS, Pfund Z, Juhasz C, Behen ME, Muzik O, Chugani DC, Nigro MA, Chugani HT (2002): Altered regional brain glucose metabolism in Duchenne muscular dystrophy: A pet study. Muscle Nerve 26:506–512. [DOI] [PubMed] [Google Scholar]
- Levine TR, Hullett CR (2002): Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum Commun Res 28:612–625. [Google Scholar]
- Lojkowska W, Witkowski G, Bednarska‐Makaruk M, Wehr H, Sienkiewicz‐Jarosz H, Graban A, Bochynska A, Wisniewska A, Gugala M, Slawinska K, Sawicka B, Poniatowska R, Ryglewicz D (2013): Correlations between cerebellar and brain volumes, cognitive impairments, ApoE levels, and APOE genotypes in patients with AD and MCI. Curr Alzheimer Res 10:964–972. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW (2010): Sex differences in brain anatomy. Prog Brain Res 186:3–12. [DOI] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voight K (1999): Patterns of age‐related shrinkage in cerebellum and brainstem observed in vivo using three‐dimensional MRI volumetry. Cereb Cortex 9:712–721. [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado‐Garcia JM, da Guarda SN, Gerwig M, Habas C, Hagura N, Ivry RB, Marien P, Molinari M, Naito E, Nowak DA, Oulad Ben Taib N, Pelisson D, Tesche CD, Tilikete C, Timmann D (2012): Consensus paper: Roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum 11:457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, van der Zwaag W, Granziera C, Krueger G, Gruetter R (2010): Cerebellar cortical layers: In vivo visualization with structural high‐field‐strength MR imaging. Radiology 254:942–948. [DOI] [PubMed] [Google Scholar]
- Meindl T, Schmid BC, Timmann D, Kolb FP, Kutz DF (2012): Contribution of the cerebellum to the coupling of grip force and pull force during an isometric precision grip task. Cerebellum 11:167–180. [DOI] [PubMed] [Google Scholar]
- Merker B, Podell K (2010): Grooved pegboard test. In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer; pp 1176–1178. [Google Scholar]
- Miall RC, Christensen LO (2004): The effect of rTMS over the cerebellum in normal human volunteers on peg‐board movement performance. Neurosci Lett 371:185–189. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H (2001): The cerebellum coordinates eye and hand tracking movements. Nat Neurosci 4:638–644. [DOI] [PubMed] [Google Scholar]
- Muller F, Dichgans J (1994): Impairments of precision grip in two patients with acute unilateral cerebellar lesions: A simple parametric test for clinical use. Neuropsychologia 32:265–269. [DOI] [PubMed] [Google Scholar]
- Nadkarni NK, Nunley KA, Aizenstein H, Harris TB, Yaffe K, Satterfield S, Newman AB, Rosano C, Health ABCS (2014): Association between cerebellar gray matter volumes, gait speed, and information‐processing ability in older adults enrolled in the Health ABC study. J Gerontol A Biol Sci Med Sci 69:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JW, Eng JJ, Kokotilo KJ, Boyd LA (2011): Aging effects on the control of grip force magnitude: An fMRI study. Exp Gerontol 46:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik S, Algina J (2003): Generalized eta and omega squared statistics: Measures of effect size for some common research designs. Psychol Methods 8:434–447. [DOI] [PubMed] [Google Scholar]
- Perneger TV (1998): What's wrong with Bonferroni adjustments. BMJ 316:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto E (2007): Blood pressure and ageing. Postgrad Med J 83:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podell K (2010): Hand dynamometer In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer; pp 1208–1209. [Google Scholar]
- Pope P, Wing AM, Praamstra P, Miall RC (2005): Force related activations in rhythmic sequence production. Neuroimage 27:909–918. [DOI] [PubMed] [Google Scholar]
- Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD (1998): Differential effects of age and sex on the cerebellar hemispheres and the vermis: A prospective MR study. AJNR Am J Neuroradiol 19:65–71. [PMC free article] [PubMed] [Google Scholar]
- Richardson JTE (2011): Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev 6:135–147. [Google Scholar]
- Richter S, Dimitrova A, Maschke M, Gizewski E, Beck A, Aurich V, Timmann D (2005): Degree of cerebellar ataxia correlates with three‐dimensional mri‐based cerebellar volume in pure cerebellar degeneration. Eur Neurol 54:23–27. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Bain PG, Day BL, Husain M (2013): Individual differences in expert motor coordination associated with white matter microstructure in the cerebellum. Cereb Cortex 23:2282–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. [DOI] [PubMed] [Google Scholar]
- Schatz P (2010): Finger tapping test In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer; pp 1050–1051. [Google Scholar]
- Schoppe KJ, Hamster W (2011): Motor Performance Series; Comprehensive fine motor abilities test battery with special norms for Morbus Parkinson patients. Vienne Test System—Psychological assessment. Moedling, Austria: Schuhfried GmbH. p 70.
- Schufried G (2011): Special Ability Tests; Two‐hand coordination. Vienne Test System—Psychological assessment. Moedling, Austria: Schuhfried GmbH. p 53.
- Scott SH (2008): Inconvenient truths about neural processing in primary motor cortex. J Physiol 586:1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010): Motor control and aging: Links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumare A, Elbaz A, Zhu Y, Maillard P, Crivello F, Tavernier B, Dufouil C, Mazoyer B, Tzourio C (2009): White matter lesions volume and motor performances in the elderly. Ann Neurol 65:706–715. [DOI] [PubMed] [Google Scholar]
- Spraker MB, Corcos DM, Kurani AS, Prodoehl J, Swinnen SP, Vaillancourt DE (2012): Specific cerebellar regions are related to force amplitude and rate of force development. Neuroimage 59:1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000): Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology 14:341–352. [DOI] [PubMed] [Google Scholar]
- Sulzer JS, Chib VS, Hepp‐Reymond MC, Kollias S, Gassert R (2011): BOLD correlations to force in precision grip: An event‐related study. Conf Proc IEEE Eng Med Biol Soc 2011:2342–2346. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC (2010): N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM (2008): White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage 43:470–477. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B (2005): Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26:1261–1270; discussion 1275‐8. [DOI] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME (2008): Functional neuroimaging correlates of finger‐tapping task variations: An ALE meta‐analysis. Neuroimage 42:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllig J, Mérillat S, Eschen A, Röcke C, Martin M, Jäncke L (2011): Plasticity and imaging research in healthy aging: Core ideas and profile of the International Normal Aging and Plasticity Imaging Center (INAPIC). Gerontology 57:190–192. [DOI] [PubMed] [Google Scholar]


