Abstract
In this study we demonstrate that the pattern of an amygdala‐centric network contributes to individual differences in trait anxiety. Individual differences in trait anxiety were predicted using maximum likelihood estimates of amygdala structural connectivity to multiple brain targets derived from diffusion‐tensor imaging (DTI) and probabilistic tractography on 72 participants. The prediction was performed using a stratified sixfold cross validation procedure using a regularized least square regression model. The analysis revealed a reliable network of regions predicting individual differences in trait anxiety. Higher trait anxiety was associated with stronger connections between the amygdala and dorsal anterior cingulate cortex, an area implicated in the generation of emotional reactions, and inferior temporal gyrus and paracentral lobule, areas associated with perceptual and sensory processing. In contrast, higher trait anxiety was associated with weaker connections between amygdala and regions implicated in extinction learning such as medial orbitofrontal cortex, and memory encoding and environmental context recognition, including posterior cingulate cortex and parahippocampal gyrus. Thus, trait anxiety is not only associated with reduced amygdala connectivity with prefrontal areas associated with emotion modulation, but also enhanced connectivity with sensory areas. This work provides novel anatomical insight into potential mechanisms behind information processing biases observed in disorders of emotion. Hum Brain Mapp 36:4819–4830, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: amygdala, trait anxiety, diffusion tensor imaging, emotion regulation, probabilistic tractography, medial prefrontal cortex, extinction
INTRODUCTION
Anxiety‐related disorders are the most prevalent mental illnesses [Kessler et al., 2005a, 2005b], and high trait anxiety is associated with increased risk for numerous mental disorders, including depression and bipolar disorder [Bruckl et al., 2007; Reinherz et al., 2000]. Neurocognitive models of anxiety highlight the importance of the amygdala [Davis, 1992; Rauch et al., 2003], and interactions with regions important for cognitive control, as well as emotion generation, regulation, and perception [Bishop, 2007; Milad and Quirk, 2012]. Despite its relevance to affective disorders, little is known about the relationship between trait anxiety and the integrity of structural connections between the amygdala and these systems.
Functionally, individual differences in trait anxiety are negatively correlated with aspects of the ventromedial prefrontal cortex, including mOFC activity, and positively correlated with amygdala activity during fear modulation [Indovina et al., 2011]. Furthermore, functional connectivity between the amygdala and mOFC is negatively related to temperamental anxiety [Kim et al., 2011; Pezawas et al., 2005]. Thus, robust amygdala‐mOFC connectivity may be protective for anxiety. Conversely, activation of dACC appears involved in the acquisition of threat‐related learning [Phelps et al., 2004] and anxiety [Kim et al., 2011; Milad et al., 2009]. In addition to connections between amygdala and prefrontal cortex, research has found that enhanced functional connectivity between amygdala and perceptual regions during fear‐generalization is positively correlated with trait anxiety [Dunsmoor et al., 2011]. One possibility is that high trait anxiety is associated with enhanced structural connectivity between amygdala and regions involved in perceptual and semantic processing.
The connective architecture of the brain plays a key role in determining a region's functional attributes, and functional connectivity is often assumed to reflect, at least in part, the underlying anatomical connectivity. While there is evidence suggesting that a correspondence often exists between the strength of functional connectivity and structural pathways in a number of networks [Greicius et al., 2009; Hermundstad et al., 2013, 2014], evidence of functional connectivity can be observed in the absence of anatomical connectivity [Honey et al., 2009]. Although there are studies relating anxiety to functional connectivity, far less in known about the relationship between measures of structural white matter and individual differences in anxiety. Kim and Whalen [2009] demonstrated using DTI that fractional anisotropy (FA; a measure of white‐matter microstructure) in a region of putative uncinate fasciculus is negatively correlated with trait anxiety. More recently, in a large multi‐cohort study, Westlye et al. [2011] found that FA throughout much of the white‐matter skeleton was negatively correlated with harm avoidance (a personality trait characterized by heightened worrying and anxiety). Despite these contributions, it remains unknown whether a more distributed pattern of structural connections between the amygdala in particular and other neural regions combine to contribute to individual differences in trait anxiety. Moreover, no studies to date have examined which amygdala structural connections make positive contributions to anxious traits.
To address these unknowns, the present study combined a multiple regression analysis using regularized least square regression model, ridge regression [Hoerl and Kennard, 1970], with maximum likelihood estimates of structural connectivity of the amygdala using seed‐based probabilistic tractography [Behrens et al., 2007, 2003]. This powerful approach allows for the identification of structural connections between the amygdala and multiple brain regions in a whole‐brain manner [Saygin et al., 2011, 2012]. After first ensuring that the pattern of amygdala structural connectivity could be used to predict individual differences in trait anxiety significantly above chance, we sought to determine which connections were most reliably included in the prediction model. Thus, we tested the intriguing possibility that trait anxiety would not only be associated with reduced connectivity between the amygdala and emotion control regions like mOFC, but that it would also be associated with both enhanced structural connectivity between amygdala and dACC, as well as between the amygdala and sensory cortical pathways responsible for driving perceptual and semantic representations of emotional reactivity and sensation.
METHODS
Participants were seventy‐two healthy right‐handed participants [mean age = 25.5 ± 6.5 (SD), 41 females and 31 males; no significant difference in age between genders (P > 0.5)]. Trait anxiety scores were obtained for each participant via the State‐Trait Anxiety Inventory (STAI) [Spielberger, 1983] [mean = 30.6 ± 7.7 (SD), range 21–63; M Women = 29.8 ± 6.4 (SD), M Men = 31.6 ± 9.2 (SD); no significant difference in trait anxiety between genders (P > 0.3)]. Each subject completed a diffusion‐tensor imaging (DTI) scan. All participants were in good health, and had no history of psychiatric illness, neurological disease, or head injury as determined by screening and interviews using the Structured Clinical Interview of the DSM‐IV [First et al., 2002]. All participants provided informed written consent, and the study was approved by the research ethics board of the University of Western Ontario.
Data Acquisition
Diffusion‐tensor and T1‐weighted imaging was performed on a 3‐Tesla Siemens MRI scanner with a 32‐channel head coil at Robarts Research Institute, University of Western Ontario. DTI images were acquired in the axial plane with echo‐planar imaging consisting of 55 slices, 2.1 × 2.1 × 2 mm voxels, 200 × 200 mm field of view, 96 × 96 mm base resolution, 65 isotropically weighted diffusion directions, b‐value = 700 s/mm2, repetition time 6 s, and echo time 75 ms. The high‐resolution T1‐weighted anatomical scan covered the whole brain (repetition time = 2,300 ms, echo time = 4.25 ms; field of view = 25.6 cm; 192 slices; 1 mm3 isovoxels; 256 × 256 matrix).
Segmentation and Probabilistic Tractography
Relevant to the current study and our focus on the amygdala and individual differences, the validity and utility of probabilistic tractography has recently been demonstrated in two studies of amygdala segmentation [Bach et al., 2011; Saygin et al., 2011], and another study predicting individual differences in functional activation in fusiform gyrus [Saygin et al., 2012]. The primary analysis was performed after combining the ipsilateral connectivity estimates for both the right and left amygdala. The method for determining the connectivity estimates is described below and summarized in Figure 1. Seed and target masks were defined using the cortical and subcortical automated segmentation tools in FreeSurfer [Fischl et al., 2002, 2004] using the T1‐weighted anatomical images of each participant. This produced an individually derived right and left amygdala seed mask and 85 target masks, which included 84 unilateral cortical and subcortical regions and one bilateral region (brainstem), all of which were visually inspected for quality control. Prior to probabilistic tractography, the DTI data were eddy current corrected, skull stripped, and the principal diffusion directions of each voxel were determined [Behrens et al., 2007]. Probabilistic tractography was carried out in each participant's native diffusion space using FSL‐FDT [Behrens et al., 2007] in which 25,000 sample tracts were drawn from each voxel in the amygdala seed region, which produced a frequency distribution of connecting tracts to each ipsilateral target plus the brainstem while avoiding a mask of the ventricles for each amygdala for each subject [Saygin et al., 2011, 2012]. Each resulting amygdala image was transformed to MNI space to determine the group‐wise probability density for each amygdala‐target pair. In this manner we identified the group‐wise peak voxel for each amygdala‐target pair, and placed a 6 mm radius spherical mask centered on the peak voxel (see Supporting Information Table I for MNI coordinates of the peak voxel for each amygdala‐target pairing). Once each spherical group mask was obtained they were reverse transformed to each individual's native space using nearest‐neighbor registration. In this manner we could determine the maximum likelihood of amygdala‐target connectivity by taking the value of the amygdala voxel with the largest number of sample tracts connecting to the respective target and dividing by 25,000 (producing a scaled value between 0 and 1). Thus, the maximum likelihood estimate was determined for each of the 86 amygdala‐target combinations, 43 pathways per hemisphere, for each of the 72 participants (observations). These values from both right and left amygdala were combined, which produced a matrix of 72 observations by 86 features for performing multiple regression. We elected to focus exclusively on ipsilateral connections in order to reduce the number of features included in a single full model and because tracer studies using non‐human primates find that first order amygdala connection are largely ipsilateral [Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007]. Gender was included as an additional feature in the model given evidence from meta‐analyses of functional imaging data suggesting possible gender differences in amygdala activity to emotional stimuli [Sergerie et al., 2008; Wager et al., 2003]. Notably, the exclusion of gender in the analysis described below had no effect on the prediction accuracy of the model, nor did the feature corresponding to gender make a reliable contribution to a full model.
Figure 1.
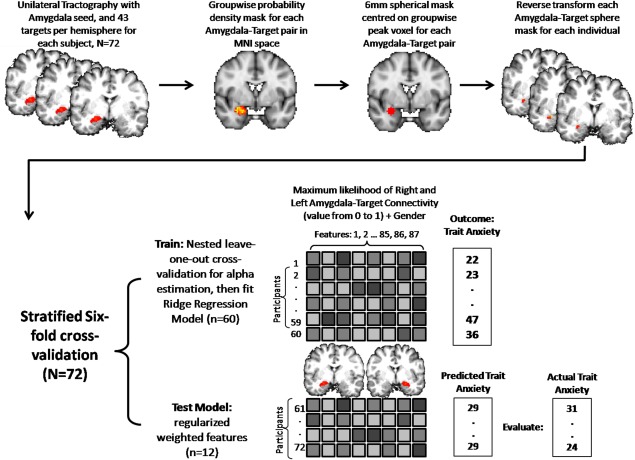
Schematic of the multiple regression approach: After deriving a group‐wise peak voxel for each amygdala‐target pair (TOP), participants were split using a stratified sixfold cross validation approach (BOTTOM), producing six independent training (n = 60) and testing (n = 12) sets. The regression model was trained on the scaled values of the maximum likelihood of amygdala‐target connectivity derived from probabilistic tractography (between 0 and 1, represented as the gray‐scaled boxes). Testing the model at each fold produced predicted trait‐anxiety scores for 12 participants. The accuracy of the model was derived by computing the MSE between the 72 predicted trait‐anxiety scores and actual trait‐anxiety scores provided by self‐report. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Multiple Regression analysis: Multiple linear regression was implemented using the ridge regression model from the scikit‐learn toolbox in python [Pedregosa et al., 2011]. Ridge regression is a regularized regression model and was selected for use in the current study because it is robust to instances in which the number of features is greater than the number of observations and can be suitable for dealing with instances of collinearity between features [Hoerl and Kennard, 1970]. Thus, regularization identifies a subset of features important to the model and enhances the magnitude of their respective weights while lowering the weights of unimportant features. While other sparse regression techniques like LASSO are suitable for dealing with high dimensional datasets, it does not perform as well with collinearty between features [Zou and Hastie, 2005]. The regularization parameter, alpha, was determined using nested cross‐validation with an automated internal cross‐validation function, ridgeCV, which performs leave‐one‐out cross validation using only the training set for determining alpha. We performed the regularization parameter estimate by considering a vector of alpha values from 0.01 to 10 in steps of 0.01. The resulting mean alpha was 6.22, which was also used in the permutation testing. In order to assess whether the regression model could significantly predict trait anxiety scores we used a stratified six‐fold cross‐validation approach with nested‐cross‐validation for regularization parameter estimation (see Fig. 1 for a schematic). Similar to previous application of sparse regression in neuroimaging [Wager et al., 2011], a stratified sixfold approach was selected as it tends to have a prediction accuracy biased towards zero (i.e., is more conservative), and produces more consistent (i.e., less variable) results relative to leave‐one‐out cross‐validation [Hastie et al., 2009; Kohavi, 1995]. We performed six iterations in which we split the data, trained the regression model on 60 participants and tested on the remaining 12 participants (see Fig. 1). The data was split such that no participant was ever included in the training and testing set simultaneously, no participant was in more than one of the six test sets, and there was a similarly distributed range of trait anxiety values at each fold. Concatenating the 12 predicted anxiety scores from each fold produced a vector of 72 predicted anxiety scores, one predicted score for each participant. The accuracy of the predictions was measured by computing the mean squared error (MSE) between the predicted and actual trait anxiety scores. To determine the statistical significance of the connectivity model accuracy for predicting anxiety we used random permutation testing. For this we performed 1,000 permutations samples, each time randomly pairing observations with a trait anxiety score. The P value was determined as the proportion of iterations in which a model generated on the randomized data outperformed or was equal to the model generated on the real data. We also assess how well the predicted anxiety scores correlated with participant's self‐report trait anxiety using Pearson's correlation (r), and the proportion of variance accounted for by the regression model (r 2). Indeed, estimation of correlation effect sizes in this manner is known to produce more robust estimates [Hastie et al., 2009].
Full‐Model Estimation: In order to determine which features (i.e., which amygdala‐target anatomical connections) made a reliable contribution to a full‐model for predicting individual differences in anxiety we adapted the bootstrap procedure used by Wager et al. [2011]. We performed 1,000 bootstrapped samples with replacement, which produced training sets of 60 observations. In this fashion, we derived 1,000 independent models and their respective weights. To assess the reliability of each amygdala‐target connection to predicting trait anxiety we determine which features had 95% confidence intervals which were either entirely above or below zero. The median coefficient and confidence intervals for each reliable amygdala‐target pathway and the confidence intervals are reported in Table 1. An estimation of which amygdala‐target connections made the most reliable contribution to the full‐model is extremely relevant to the neuroscience of anxiety, though it must be noted that predictions are necessarily made from the combination of all weights and are therefore not strictly independent [Wager et al., 2011].
Table 1.
Targets of structural connectivity with the amygdala that make a reliable contribution to the full‐model for predicting trait‐anxiety
| Target region | Coefficient | C.I. | Peak sphere coordinate | ||
|---|---|---|---|---|---|
| Targets making a reliably positive contribution to predicting anxiety | |||||
| L | Paracentral lobule | 577.40 | 141.75 | 1550.36 | −30 −4 −20 |
| R | Dorsal anterior cingulate cortex | 42.58 | 4.57 | 146.12 | 30 0 −18 |
| R | Caudate nucleus | 3.38 | 0.43 | 7.45 | 30 0 −20 |
| R | Inferior temporal gyrus | 0.94 | 0.07 | 2.46 | 28 −8 −14 |
| R | Entorhinal cortex | 0.74 | 0.14 | 1.24 | 28 0 −22 |
| R | Temporal pole | 1.00 | 0.35 | 1.87 | 30 0 −20 |
| Targets making a reliably negative contribution to predicting anxiety | |||||
| L | Posterior cingulate cortex | −2.64 | −6.79 | −0.86 | −26 −4 −20 |
| R | Medial orbital frontal cortex | −1.11 | −2.25 | −0.06 | 30 0 −18 |
| L | Parahippocampal gyrus | −0.70 | −1.40 | −0.003 | −22 −2 −30 |
RESULTS
Model Testing
The ridge model (mean α = 6.22) trained on the maximum likelihood of amygdala‐target connectivity for both right and left amygdala, as well as the gender of participants, performed significantly better than chance at predicting trait anxiety (P < 0.01, one‐tailed, Fig. 2). While the MSE of trait anxiety estimates was 55.01, the mean MSE from the randomized permutation samples was 60.88. The Pearson's correlation between the predicted trait anxiety and participants self‐reported trait anxiety was also significant (r = 0.246, P < 0.05), indicating that our model of amygdala connectivity to a network of regions accounted for 6% of the variance in trait anxiety (see Fig. 2).
Figure 2.

Left, permutation testing with 1,000 iterations revealed that the regression model predicts trait anxiety significantly better than chance. The dashed line represents the prediction accuracy of the model across the sixfolds reported as MSE. The solid gray bars represent the MSE permutation scores. The number of permutations within a given bin of the histogram is found on the y‐axis, while the MSE for the bins is presented along the x‐axis. Right, scatter plot of the predicted trait anxiety scores (x‐axis) versus participants' self‐report trait anxiety (y‐axis). The regression demonstrates that an amygdala‐centric network accounts for 6% of the variance in trait anxiety. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Full‐Model Estimation
Given that the model could predict trait anxiety significantly better than chance from probabilistic connectivity profiles of amygdala‐target pathways of right and left amygdala, a critical question was which pathways make the most reliable contribution to a full‐model (see Table 1 for full results and Fig. 3). Whereas those connections resulting in reliably positive weights suggest that stronger anatomical connectivity between the amygdala and those regions is associated with elevated trait anxiety, reliably negative weights indicates that stronger anatomical connectivity is associated with reduced trait anxiety. In line with our hypotheses, our full‐model estimation revealed that the reliable amygdala pathways with positive weights included neural regions associated with the expression of anxious behavior, right dACC, as well as those associated with perception, semantic representation, and sensation, including right ITG, right temporal pole, right entorhinal cortex, and left paracentral lobule. Those reliable amygdala pathways with negative weights included regions associated with the extinction learning, right mOFC, as well as those regions associated with memory encoding and environmental context recognition, left isthumus of the cingulate cortex and left parahippocampal gyrus.
Figure 3.
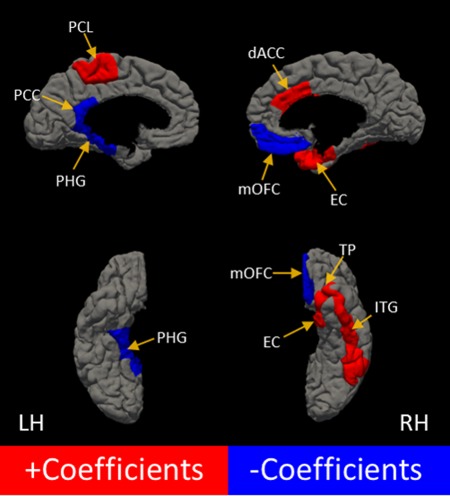
Features making the most reliable contribution to a generalized full regression model for predicting trait anxiety. Higher trait anxiety was associated with greater connectivity between the amygdala and regions with positive coefficients (in red). Higher trait anxiety was also associated with weaker connectivity between the amygdala and regions with negative coefficients (in blue). Images on the left correspond to the left hemisphere and ipsilateral connections with left amygdala. Images on the right correspond to the right hemisphere and ipsilateral connections with the right amygdala. The estimated weight for each target region implicated can be found in Table I. Regions displayed are: dorsal anterior cingulate cortex (dACC), entorhinal cortex (EC), posterior cingulate cortex (PCC), inferior temporal gyrus (ITG), medial orbitofrontal cortex (mOFC), paracentral lobule (PCL), parahippocampal gyrus (PHG), temporal pole (TP). NB: right caudate is not displayed.
DISCUSSION
Using maximum likelihood structural connectivity estimates derived from probabilistic tractography, the current study is the first to demonstrate that the pattern of amygdala structural connectivity is predictive of a significant portion of the variance associated with individual differences in trait anxiety. Furthermore, consistent with our prediction and relevant to the understanding of neurocognitive models of anxiety and emotions more generally, the current results revealed that a reliable network of multiple structural pathways connected with the amygdala contributes to individual differences in trait anxiety. Higher trait anxiety was related to stronger connections between the amygdala and target regions implicated in affect generation, dACC [Milad and Quirk, 2012], and target regions involved in perceptual and semantic processing, including right ITG, right temporal pole, right entorhinal cortex, and left paracentral gyrus [Murray, 2007; Murray et al., 2007]. Conversely, higher trait anxiety was associated with weaker connections between the amygdala and target regions implicated in extinction learning, such as right mOFC [Milad and Quirk, 2012], and those involved in memory and visuospatial processing, including right posterior cingulate cortex [Parvizi et al., 2006; Vogt et al., 1992, 2006] and parahippocampal gyrus [Epstein and Kanwisher, 1998; Epstein and Higgins, 2007]. Although prior studies have identified pathways that show a negative relationship with trait anxiety, our study is the first to identify pathways wherein stronger connectivity is predictive of higher individual differences in trait anxiety.
Connections Making a Positive Contribution to Trait Anxiety
The present study is the first to demonstrate that stronger structural connections between amygdala and dACC are related to higher levels of trait anxiety. This supports findings from both animal and human research implicating dACC and its direct projections to the amygdala in the generation and persistence of fear‐related learning [Milad and Quirk, 2012; Quirk et al., 2006]. Using human fMRI, Phelps et al. [2004] found greater dACC during the acquisition of fear conditioning, while Milad et al. [2009] found greater activation of dACC and impaired extinction recall in individuals with post‐traumatic stress disorder. Additionally, Milad et al. [2007] found that the cortical thickness of dACC was positively correlated with emotional reactivity, as measured using skin conductance activity to fear‐conditioned versus safe stimuli. Indeed, a pathway involving the dACC and the amygdala has been referred to as an “aversive amplification circuit,” that drives harm‐avoidant behaviors [Robinson et al., 2014]. Thus, together with previous functional and anatomical research, the present findings indicate that stronger connections between dACC and amygdala contribute to increased trait anxiety.
We also observed that the strength of amygdala connectivity to regions associated with perception were positively associated with trait anxiety. The target regions for these pathways included ITG, temporal pole, entorhinal cortex, and paracentral lobule. Human lesion studies have demonstrated that the temporal pole and parts of the anterior temporal lobe are necessary for facets of emotion perception, such as emotional prosody recognition [Adolphs et al., 2002]. Along with such findings, the current results suggest that enhanced connectivity between the amygdala and perceptual and sensory regions is related to increased sensitivity to threat‐related information [Murray, 2007]. This is also consistent with functional imaging studies demonstrating that emotional face processing is associated with enhanced functional connectivity between amygdala and regions of the occipitotemporal cortex [Morris et al., 1999; Pessoa et al., 2002]. Furthermore, our findings are also in accordance with clinical studies that find that interactions between the amygdala and perceptual cortices are positively associated with anxiety disorders [Ahs et al., 2009; Gilboa et al., 2004]. In addition to visual perception regions, expression of feelings during an emotion induction paradigm recruits activity in somatosensory regions, including aspects of the paracentral lobule [Saxbe et al., 2013]. Stronger connections between the amygdala and these regions were also associated with increased trait anxiety in the current study.
It is interesting to consider the above results in the context of recent models of emotion perception, and cognitive biases in affective disorders. It is thought that excitatory interactions between the amygdala and sensory regions enhance representations of emotional stimuli [Anderson and Phelps, 2001; Mitchell and Greening, 2012; Vuilleumier and Driver, 2007]. In affective disorders, however, emotional thoughts or stimuli disproportionately affect cognition, producing “efficient but maladaptive” [Beck, 2008; p 971] information processing biases [e.g., Greening et al., 2013, 2014]. Thus, one possibility is that in highly anxious individuals persistent functional connectivity strengthens anatomical connections between the amygdala and brain regions involved in both affect production and emotion perception, possibly via Hebbian mechanisms. In this manner, emotions exert an even stronger influence on sensory processes, cognition, and behavior. Alternatively, the strength of such connections may represent an inherited trait, predisposing affected individuals to increased risk of psychopathology. These strengthened connections may pose a particular challenge for attempts to regulate unwanted emotional reactions. For example, it was recently demonstrated that, despite attempts to down‐regulate negative effect, depressed patients had persistently elevated activity in the amygdala and sensory areas, despite activating regions associated with emotional control [Greening et al., 2014]. Thus, our results, and recent experimental evidence, provide novel insight into the importance of enhanced amygdala connectivity to parts of the prefrontal cortex and the temporal and somatosensory regions, the latter of which are pathways that have been overlooked in current neurocognitive models of psychopathology.
The current findings also have potentially important implications for theories related to emotion regulation and the management of affective disorders. For example, previous research has demonstrated that affective encoding of emotional stimuli can be down‐regulated indirectly through attentional mechanisms [Mitchell et al., 2007; Pessoa et al., 2002]. It is thought that this occurs because attention augments the representation of non‐emotional task‐relevant information in occipitotemporal cortices, thereby leading, through competitive interactions, to the suppression of unwanted, possibly pathological, emotional representations [Blair and Mitchell, 2009; Mitchell, 2011; Ochsner et al., 2012]. In such models, top‐down attentional mechanisms are thought to compete with pathways associated with emotional attention (amygdala‐sensory cortex connections) to determine the relative impact of affective stimuli and representations. In line with this idea, our finding show that increased anatomical connectivity in this latter pathway is associated with higher levels of trait anxiety.
Connections Making Negative Contribution to Trait Anxiety
We observed that the strength of connectivity between the amygdala and mOFC was negatively related to trait anxiety. This is consistent with findings in both rodents and humans suggesting that the mOFC regulates amygdala output during extinction learning [Milad and Quirk, 2012]. Recent neuroimaging studies suggest an inverse relationship between activity in the amygdala and medial prefrontal cortex, including mOFC, during the modulation of fear‐related stimuli [Amting et al., 2010; Linnman et al., 2012]. This pattern is impaired in patients with anxiety disorders [Etkin et al., 2010; Shin et al., 2005]. Intriguingly, a recent resting state connectivity study demonstrated that individuals with low (but not high) trait anxiety displayed positive resting state functional connectivity between the amygdala and mOFC [Kim et al., 2011]. Positive resting state activity between regions is interpreted here as reflecting an increased capacity for communication and mutual influence. Interestingly, however, negative resting state mPFC‐amygdala resting state activity has been shown in rats, which is suggested to specifically reflect an inhibitory interaction [Liang et al., 2012]. Although the specific functional significance and direction of the functional connectivity may require additional consideration, the existing functional data also highlights the importance of cross‐talk between mPFC and amygdala in anxiety. Complementing these functional effects, both Westlye et al. [2011] and Kim and Whalen [2009] similarly identify that white‐matter in mOFC areas was negatively related to anxious traits. In sum, the present results together with other related findings support the conclusion that strong functional and structural connectivity between amygdala and mOFC is protective against anxiety, possibly by way of mechanisms associated with extinction learning.
We also made the novel observation that weaker amygdala connectivity with PHG and the posterior cingulate cortex was related to higher levels of trait anxiety. The functional significance of this finding is less clear. Nevertheless, both the PHG [Epstein and Kanwisher, 1998; Epstein and Higgins, 2007] and posterior cingulate cortex [Burianova and Grady, 2007; Maddock et al., 2001] have been implicated in memory as well as in spatial and contextual encoding. In addition, functional studies have revealed that both PHG and posterior cingulate cortex are also sensitive to emotional information [Maddock et al., 2001; Robinson et al., 2013]. Importantly, connections between the PHG and amygdala have been implicated in the context‐dependent regulation of fear memory [see Maren et al., 2013]. Given the complimentary function of these regions, one possibility is that reduced amygdala connectivity with the PHG and PCC may be associated with a reduced capacity to restrict threat‐related associations to specific contexts, resulting in greater susceptibility to generalized (i.e., trait) anxiety, as was observed in the current study. This remains speculative, however, and further work clarifying the role of these pathways in emotional learning and anxiety is warranted.
Implications for Clinical Studies of Anxiety
Although the current work involved an examination of anxiety in a nonclinical sample, our findings appear consistent with previous studies of patients with anxiety disorders. Consistent with our finding that amygdala‐mOFC connectivity was negatively related to anxiety, the most frequently reported finding in anxiety disorders is reduced FA in regions of the uncinate fasciculus [Baur et al., 2011; Hettema et al., 2012; Phan et al., 2009; Tromp et al., 2012]. On the other hand, whereas we found that greater connectivity between the amgydala and dACC was associated with higher anxiety, studies of patients with post‐traumatic stress disorder find reductions in FA around the anterior cingulate cortex [Kim et al., 2005; Schuff et al., 2011]. However, the whole‐brain approach used in those studies does not allow for inferences regarding amygdala‐dACC connectivity per se. The current approach provides important information regarding the specificity of structural connections to the amygdala and their role in anxiety and affective disorders more specifically.
Limitations and Future Directions
It is important to note that probabilistic tractography is agnostic to whether connections are first‐order or higher. It is therefore possible that the connections described between the amygdala and the given target regions are indirect. Recent models of emotion control do indeed implicate such indirect pathways in the modulation of amygdala function [Blair and Mitchell, 2009; Delgado et al., 2008; Mitchell, 2011]. It is also likely that multiple anatomical risk factors contribute to individual differences in anxiety and emotional reactivity. The current study demonstrated that an amygdala‐centric network may represent one such risk factor, accounting for a small but significant proportion of the variance in trait anxiety in our sample of participants. However, it is possible that the use of alternative seed regions could yield other important pathways, as suggested by previous whole‐brain DTI studies [Baur et al., 2011; Hettema et al., 2012]. The approach used in the present study could be adapted in the future to examine the pattern of connectivity of other structures previously implicated in anxiety [e.g, middle frontal gyrus, Bishop, 2009], which did not factor into the current model. In addition, the current evidence regarding laterality is equivocal and requires future research, as our findings differentially implicated both right and left amygdala, while previous research has emphasized either left [Kim and Whalen, 2009], or bilateral [Westlye et al., 2011] amygdala structural connections in trait anxiety. Furthermore, it is noteworthy that the amygdala, rather than acting as a homogenous unit, is made up of multiple functionally heterogeneous nuclei that can make different or even opposing functional contributions [Amaral, 2002; Janak and Tye, 2015]. In the present study, we do not speculate on which nuclei within the amygdala might be driving the particular observed effects due to concerns about reliably distinguishing between the boundaries of nuclei vis‐a‐vis the DTI targets, and the desire to limit the number of independent variables to avoid over‐fitting the data (however, see Supporting Information Table I for the coordinates of the amygdala maxima associated with each region identified). Future work involving larger samples may further refine the existing model, and potentially increase predictive power by separately examining the connectivity patterns of individual nuclei.
Also of importance is the fact that the current study focused exclusively on trait anxiety in a non‐clinical sample. Because trait anxiety has been described as a general risk factor for emotional disorders including anxiety and depression [Grupe and Nitschke, 2013], and some of the pathways identified in the current study have also been implicated in affective disorders [Milad and Quirk, 2012], it is tempting to speculate on the significance of the findings for clinical populations. Nevertheless, it will be important to conduct similar work in patients suffering from affective disorders to better understand the relationship between individual differences in anatomical connectivity and psychopathology or symptomatology. Related to this issue, although the current study focused on trait anxiety, it would also be valuable to use similar techniques to predict other important affective characteristics. For example, mPFC‐amygdala functional connectivity is inversely related to trait anger [Fulwiler et al., 2012]. Work examining other affective characteristics and psychopathology would be helpful in determining the extent to which this circuit plays a more general role in regulating various forms of affective responding.
CONCLUSION
The current findings demonstrate that an amygdala‐centric network of structural connections accounts for a significant proportion of individual differences in trait anxiety. For the first time, we show that trait anxiety is positively related to the strength of structural connectivity between the amygdala and a distributed set of brain regions implicated in affect generation and perception, dACC, ITG, and temporal pole. These findings provide evidence that individual differences in structural connections may contribute to the information processing biases observed in dysregulated affect. Critically, consistent with previous functional and structural literature, we found that whereas amygdala‐dACC connectivity was positively related to anxiety, amygdala‐mOFC connectivity was negatively related to trait anxiety, emphasizing the importance of interactions between these structures in modulating anxiety and fear. We also found that greater connections between amygdala and regions implicated in memory and environmental context representation were protective against anxiety. Future work is needed to determine whether individual differences in the identified network arise during development (e.g., via Hebbian mechanisms), represent a congenital risk factor, or both. The present study also provides a novel approach for estimating individual differences in personality traits from patterns of structural connectivity, which can be applied in a multitude of domains within social‐affective neuroscience.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the members of the Brain and Mind Institute who provided helpful discussions during the development and completion of this project. The authors also thank Kescha Kazmi for her help with data collection, and the Centre for Metabolic Mapping for help with scanning.
REFERENCES
- Adolphs R, Damasio H, Tranel D (2002): Neural systems for recognition of emotional prosody: A 3‐D lesion study. Emotion 2:23–51. [DOI] [PubMed] [Google Scholar]
- Ahs F, Pissiota A, Michelgard A, Frans O, Furmark T, Appel L, Fredrikson M (2009): Disentangling the web of fear: amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Res, 172:103–108. [DOI] [PubMed] [Google Scholar]
- Amaral DG (2002): The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry 51:11–17. [DOI] [PubMed] [Google Scholar]
- Amting JM, Greening SG, Mitchell DG (2010): Multiple mechanisms of consciousness: The neural correlates of emotional awareness. J Neurosci 30:10039–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA (2001): Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411:305–309. [DOI] [PubMed] [Google Scholar]
- Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ (2011): Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. J Neurosci 31:618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Rufer M, Delsignore A, Jancke L, Herwig U, Beatrix Bruhl A (2011): White matter alterations in social anxiety disorder. J Psychiatr Res, 45:1366–1372. [DOI] [PubMed] [Google Scholar]
- Beck AT (2008): The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry 165:969–977. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2007): Neurocognitive mechanisms of anxiety: An integrative account. Trends Cogn Sci 11:307–316. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2009): Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci 12:92–98. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DG (2009): Psychopathy, attention and emotion. Psychol Med 39:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckl TM, Wittchen HU, Hofler M, Pfister H, Schneider S, Lieb R (2007): Childhood separation anxiety and the risk of subsequent psychopathology: Results from a community study. Psychother Psychosom 76:47–56. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL (2007): Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cogn Neurosci 19:1520–1534. [DOI] [PubMed] [Google Scholar]
- Davis M (1992): The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15:353–375. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA (2008): Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS (2011): Neurobehavioral mechanisms of human fear generalization. Neuroimage 55:1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N (1998): A cortical representation of the local visual environment. Nature 392:598–601. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS (2007): Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb Cortex 17:1680–1693. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF (2010): Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 167:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, MB , Spitzer, RL , Gibbon, M , Williams, JBW (2002). Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. (SCID‐I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, King JA, Zhang N (2012): Amygdala‐orbitofrontal resting‐state functional connectivity is associated with trait anger. Neuroreport 23:606–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H (2002): Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. [Research Support, U.S. Gov't, P.H.S.]. Neuroscience 115:1261–1279. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H (2007): Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. [Research Support. N.I.H., Extramural]. Neuroimage 34:905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O (2004): Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 55:263–272. [DOI] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DG (2013): Emotion‐related brain activity to conflicting socio‐emotional cues in unmedicated depression. J Affect Disord 150:1136–1141. [DOI] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DG (2014): The neural correlates of regulating positive and negative emotions in medication‐free major depression. Soc Cogn Affect Neurosci 9:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013): Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Mining D, Friedman J (2009). Chapter 7: Model Assessment and Selection The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed New York, NY: Springer; pp 219–259. [Google Scholar]
- Hermundstad AM, Bassett DS, Brown KS, Aminoff EM, Clewett D, Freeman S, Frithsen A, Johnson A, Tipper CM, Miller MB, Grafton ST, Carlson JM (2013): Structural foundations of resting‐state and task‐based functional connectivity in the human brain. Proc Natl Acad Sci USA 110:6169–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermundstad AM, Brown KS, Bassett DS, Aminoff EM, Frithsen A, Johnson A, et al (2014): Structurally‐constrained relationships between cognitive states in the human brain. PLoS Comput Biol 10:e1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Kettenmann B, Ahluwalia V, McCarthy C, Kates WR, Schmitt JE, Silberg JL, Neale MC, Kendler KS, Fatouros P (2012): Pilot multimodal twin imaging study of generalized anxiety disorder. Depress Anxiety 29:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerl AE, Kennard RW (1970): Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 12:55. [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009): Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez‐Elizalde AO, Dunn BD, Bishop SJ (2011): Fear‐conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM (2015): From circuits to behavior in the amygdala. Nature 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005a): Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005b): Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011): Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 21:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lyoo IK, Kim SJ, Sim M, Kim N, Choi N, Jeong DU, Covell J, Renshaw PF (2005): Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport 16:1049–1053. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ (2009): The structural integrity of an amygdala‐prefrontal pathway predicts trait anxiety. [Research Support, N.I.H., Extramural]. J Neurosci 29:11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohavi R (1995). A study of cross‐validation and bootstrap for accuracy estimation and model selection. In: Proceedings of the 14th International Joint Conference on Artificial Intelligence, Vol. 2 pp 1137–1143. Morgan Kaufmann Publishers Inc., San Francisco, CA, USA. [Google Scholar]
- Liang Z, King J, Zhang N (2012): Anticorrelated resting‐state functional connectivity in awake rat brain. Neuroimage 59:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR (2012): Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry 169:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2001): Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013): The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009): Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2012): Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol 63:129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL (2007): A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 62:1191–1194. [DOI] [PubMed] [Google Scholar]
- Mitchell DG (2011): The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behav Brain Res 217:215–231. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Greening SG (2012): Conscious perception of emotional stimuli: Brain mechanisms. Neuroscientist 18:386–398. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ (2007): The impact of processing load on emotion. Neuroimage 34:1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA (2007): The amygdala, reward and emotion. Trends Cogn Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM (2007): Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci 30:99–122. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT (2012): Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann NY Acad Sci 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A (2006): Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA 103:1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay E (2011): Scikit‐learn: Machine learning in python. J Mach Learn Res 12:2825–2830. [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG (2002): Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99:11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer‐Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K (2009): Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry 66:691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez‐Lima F (2006): Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI (2003): Neuroimaging studies of amygdala function in anxiety disorders. [Review]. Ann NY Acad Sci 985:389–410. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Hauf AM, Wasserman MS, Paradis AD (2000): General and specific childhood risk factors for depression and drug disorders by early adulthood. [Research Support, U.S. Gov't, P.H.S.]. J Am Acad Child Adolesc Psychiatry 39:223–231. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C (2014): Towards a mechanistic understanding of pathological anxiety: The dorsal medial prefrontal‐amygdala 'aversive amplification' circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry 1:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Charney DR, Vytal K, Grillon C (2013): Stress increases aversive prediction error signal in the ventral striatum. Proc Natl Acad Sci USA 110:4129–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Yang XF, Borofsky LA, Immordino‐Yang MH (2013): The embodiment of emotion: language use during the feeling of social emotions predicts cortical somatosensory activity. Soc Cogn Affect Neurosci 8:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Augustinack J, Fischl B, Gabrieli JD (2011): Connectivity‐based segmentation of human amygdala nuclei using probabilistic tractography. Neuroimage 56:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Koldewyn K, Reynolds G, Gabrieli JD, Saxe RR (2012): Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci 15:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, Mueller SG, Wang Z, Marmar CR, Weiner MW, Neylan TC (2011): Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage 54(Suppl 1):S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL (2008): The role of the amygdala in emotional processing: A quantitative meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev, 32:811–830. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL (2005): A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273–281. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tromp DPM, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, Kral TR, Lee JE, Adams M, Alexander AL, Nitschke JB (2012): Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry 69:925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb Cortex 2:435–443. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S (2006): Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29:452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J (2007): Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci 362:837–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011): Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 31:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF (2003): Valence, gender, and lateralization of functional brain anatomy in emotion: a meta‐analysis of findings from neuroimaging. [Meta‐Analysis]. Neuroimage 19:513–531. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Bjornebekk A, Grydeland H, Fjell AM, Walhovd KB (2011): Linking an anxiety‐related personality trait to brain white matter microstructure: Diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry 68:369–377. [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T (2005): Regularization and variable selection via the elastic net. J R Stat Soc Ser B Stat Methodol 67:301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


