Abstract
Response inhibition is commonly thought to rely on voluntary, reactive, selective, and relatively slow prefrontal mechanisms. In contrast, we suggest here that response inhibition is achieved automatically, nonselectively, within very short delays in uncertain environments. We modified a classical go/nogo protocol to probe context‐dependent inhibitory mechanisms. Because no single neuroimaging method can definitely disentangle neural excitation and inhibition, we combined fMRI and EEG recordings in healthy humans. Any stimulus (go or nogo) presented in an uncertain context requiring action restraint was found to evoke activity changes in the supplementary motor complex (SMC) with respect to a control condition in which no response inhibition was required. These changes included: (1) An increase in event‐related BOLD activity, (2) an attenuation of the early (170 ms) event related potential generated by a single, consistent source isolated by advanced blind source separation, and (3) an increase in the evoked‐EEG Alpha power of this source. Considered together, these results suggest that the BOLD signal evoked by any stimulus in the SMC when the situation is unpredictable can be driven by automatic, nonselective, context‐dependent inhibitory activities. This finding reveals the paradoxical mechanisms by which voluntary control of action may be achieved. The ability to provide controlled responses in unpredictable environments would require setting‐up the automatic self‐inhibitory circuitry within the SMC. Conversely, enabling automatic behavior when the environment becomes predictable would require top‐down control to deactivate anticipatorily and temporarily the inhibitory set. Hum Brain Mapp 35:5517–5531, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: executive control, response inhibition, go/nogo, fMRI, EEG, Alpha oscillations, automaticity, task setting
INTRODUCTION
Inhibition of prepotent behavioral impulses is a key component of executive functions [Hofmann et al., 2012]. However, it is a significant challenge to assess brain–behavior relationships when the function under scrutiny is precisely intended to suppress overt measurable behaviors. The challenge is all the more complex that brain imaging techniques are not very powerful at unravelling the time course of concurrent excitatory and inhibitory mechanisms. This limitation relates to the physiological nature of the signal [e.g., Logothetis, 2008], the necessary compromise for spatio‐temporal resolution [e.g., Babiloni et al., 2009], and the technical aspects of data processing [e.g., Lio and Boulinguez, 2013].
Response inhibition is usually tested by means of reaction time (RT) tasks in which subjects are asked to provide a motor response to one stimulus and to withhold their response to another, like in the classical go/nogo paradigm [Chambers et al., 2009]. Although standard chronometric paradigms do not provide behavioral markers for identifying successfully inhibited responses, functional neuroimaging studies have reported a large distributed network of cortical and subcortical regions activated by nogo stimuli [Swick et al., 2011]. However, these nogo activations are by no means direct markers of response inhibition mechanisms. As recently demonstrated in a series of meta‐analyses, most of the regions forming the “nogo” network are inconsistently activated across studies and most of the BOLD modulations typically elicited by nogo signals are actually driven by the engagement of high attentional resources, not by inhibitory processes per se [Criaud and Boulinguez, 2013]. Based on the high temporal resolution of electroencephalography (EEG), numerous studies [recently reviewed by Huster et al., 2013] used psychophysiological measures to try to identify the specific processes underlying behavioral inhibition. Unfortunately, it seems that none of the standard EEG‐derived measures can be considered an unambiguous indicator of a proper inhibitory process either [Huster et al., 2013].
From a theoretical point of view, this high level of inconclusiveness is not totally surprising for at least two reasons. First, neuroimaging studies that rely on blood oxygenation level‐dependant (BOLD) signals are unable to distinguish between neural excitation and inhibition [Buzsáki et al., 2007; Logothetis, 2008], whereas most EEG studies on behavioral inhibition have not convincingly solved the problem of source signal mixing at scalp electrodes to identify unequivocally the EEG components contributing to the averaged event‐related potentials (ERPs) [Huster et al., 2013]. Second, the psychological models that have guided neuroimaging protocols and analyses rely on the disputed assumption that inhibitory processes are selectively1 triggered by the external stimulus one must refrain from reacting to [see Criaud and Boulinguez, 2013 for critical review]. Converging evidence now indicates that: (1) nonselective inhibitory mechanisms may operate to prevent actions from being emitted prematurely [Duque and Ivry, 2009; Duque et al., 2012, 2010; Frank, 2006; Frank et al., 2007; Jaffard et al., 2007], and (2) action restraint may apply by default, before any stimulus is presented in an uncertain environment. This type of processing is commonly designated “proactive control” [Boulinguez et al., 2009, 2008; Boy et al., 2010c; Chen et al., 2010; Criaud et al., 2012; Forstmann et al., 2010, 2008; Jaffard et al., 2007, 2008; Lo et al., 2009; Stuphorn et al., 2010; Zandbelt et al., 2013]. It is thus possible that, when facing potential conflict, inhibition of response applies early on to any stimulus rather than specifically to the inappropriate stimulus after it has been identified. Evaluating this possibility cannot be done with standard go/nogo protocols which do not allow to disentangle between the hypothesis of a late, selective, inhibition of the erroneous response and the existence of an early, nonselective, inhibition of all possible responses (Fig. 1).
Figure 1.
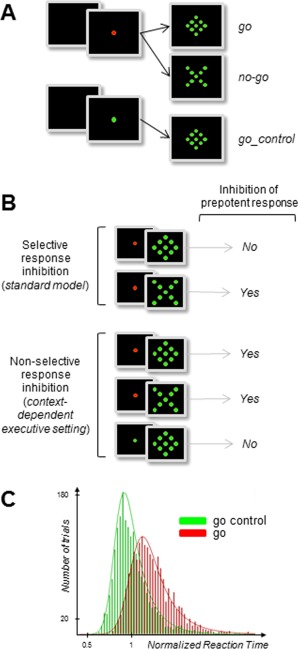
Protocol (A), models' predictions (B), and behavioral results (C). (A) Subjects were asked to react as fast as possible to a go stimulus (diamond) by means of a button press with the right thumb, and to withhold responses to an equiprobable nogo stimulus (X). In a control condition requiring no hypothetical inhibitory setting, only go stimuli were presented (go_control). In classical go/nogo tasks, go and nogo signals are scrambled within the same block of trials (standard mixed block design), assuming classically that inhibition is triggered by the nogo but not by the go stimulus. In contrast, an alternative view suggests that both stimuli induce automatic response inhibition in order to prevent premature responding. In other words, the usual nogo vs. go contrast would be incomplete to evidence all response inhibition mechanisms. To this aim, a control condition in which response inhibition is definitely not involved would be necessary (go trials for which subjects know in advance that there is no need to refrain from reacting). In the present experiment, this control condition was set by manipulating the color of the central fixation point (FP) of the display. A green FP indicated that not any nogo stimulus would be presented, enabling subjects to react automatically to any upcoming event (go_control condition). Conversely, a red FP was not informative of the identity of the upcoming target. (B) Strong, specific, predictions are attached to each hypothesis. The late, selective, account predicts that stimuli that have to be withheld (nogo) should induce specific brain activations with respect to stimuli that require a motor response (go). Conversely, the early, nonselective, account predicts that all stimuli presented in a context of uncertainty (both nogo and go) should induce inhibition‐related brain activations with respect to stimuli presented in a predictable environment (go_control). At the behavioral level, the standard model does not predict RT differences between go and go_control conditions. Conversely, the alternative model predicts that inhibition of automatic responses to any visual stimulus should lengthen RT in the red FP with respect to the green FP condition [e.g., Criaud et al., 2012]. (C) Normalized RT for go and go_control trials, pooled for all subjects. Distributions are best fitted by ex‐Gaussian functions. The RT difference between go and go_control trials reflects the effect of fast automatic response inhibition, a prerequisite for giving appropriate slow volitional response. Consistent with recent investigations using comparable methods and providing similar data and conclusions [Chiu and Aron, 2014], this major behavioral result fits the predictions of the automatic, nonselective, account of response inhibition.
Two elements would be necessary to address the shortcomings above. (i) The inclusion in the experimental design of a control condition for which response inhibition is definitely absent. In this case, for instance, on some trials, the subjects would be provided with advance information that there will be no conflict for the upcoming stimulation and hence no need to refrain from reacting (Fig. 1). This idea is reminiscent of recent behavioral findings suggesting that an executive setting is required for the manifestation of automatic response inhibition [Chiu and Aron, 2014]. (ii) The use, in addition of fMRI, of EEG techniques able to probe inhibitory neural response with high temporal resolution. Indeed, provided that proper separation of the different sources of interest from a set of mixed signals contributing to the overall electrical activity recorded on the scalp has been performed [Makeig and Onton, 2009; Lio and Boulinguez, 2013], EEG frequency‐specific signals may generate more detailed information than corresponding measures based on the BOLD fMRI signal [Huster et al., 2013; Siegel et al., 2012]. In particular, it has been suggested that Alpha and Beta oscillations might play a substantial role in response inhibition. Starting with Alpha oscillations, it has been hypothesized that they would partly stem from rhythmic fluctuations of GABAergic inhibitory interneurons [see Jones et al., 2000 and Lorincz et al., 2009 for physiological and computational accounts] and would drive neuronal spike timing and firing rate [see Haegens et al., 2011 for demonstration that Alpha power is negatively correlated with spiking rate in the monkey premotor and motor cortex during sensorimotor tasks]. As a consequence, it has been proposed that Alpha oscillations might index an active inhibitory mechanism that modulates cortical excitability or contributes to information gating within a given region [Hindriks and Van Putten, 2013; Jensen and Mazaheri, 2010; Klimesch et al., 2007; Klimesch, 2012; Mathewson et al., 2011]. Beta band oscillations have also been associated with GABAergic activity and sensorimotor processing, and with the idea that these oscillations could be associated with the functional inhibition of sensorimotor cortical regions [Jensen et al., 2005; Gaetz et al., 2011]. However, the exact role of Beta oscillations in sensorimotor transmission still needs to be specified. It is assumed to relate to the maintenance of the current sensorimotor state [Engel and Fries, 2010] and, more generally, to large‐scale communication between sensorimotor and nonsensorimotor areas [Kilavik et al., 2013].
In the present study, we combined fMRI and high‐resolution EEG recordings (with spectral analyses performed at the source level) in a go/nogo paradigm that was amended in accordance with the methodological principles described above (Fig. 1).
MATERIALS AND METHODS
Subjects
fMRI experiment
Twenty naïve right‐handed subjects (ages: 25 ± 5.1, 7 females) with normal or corrected‐to‐normal vision, and without history of psychiatric or neurological disease, participated in the experiment.
EEG experiment
Twenty naïve right‐handed subjects (whole group mean age: 26 ± 5.3, 12 females) with normal or corrected‐to‐normal vision and without history of psychiatric or neurological disease, participated in the EEG experiment. Thirteen of the twenty subjects who participated in the fMRI experiment also participated in the EEG experiment. For these subjects, the two experiments were performed within a single day. Seven subjects participated in the fMRI experiment in the morning while the EEG experiment was performed in the afternoon, and vice versa for the other six subjects. Both experiments were performed in compliance with the code of ethics of the World Medical Association (Declaration of Helsinki) and the protocol was preapproved by the appropriate ethics committee in Biomedical Research (CPP sud‐est IV, N°11/025). All subjects gave written informed consent and were paid 50€ for their participation in each experiment.
Behavioral Testing Procedures
We used a go/nogo task inspired by our recent work [Criaud et al., 2012] (Fig. 1A). Subjects were asked to react as fast as possible to go stimuli by pressing a button with the right thumb while refraining from reacting to nogo stimuli. At the beginning of a trial, the visual fixation point could turn either red or green, randomly. A red fixation point indicated that a go stimulus, a nogo stimulus or no stimulus at all could occur, go and nogo events being equiprobable. In a control condition, no inhibition was required: A green fixation point indicated that no nogo stimulus would be presented. This condition enabled subjects to react automatically to any upcoming event.
Apparatus
A panel equipped with light‐emitting diodes (LEDs –Ø5 mm, 8,800 mcd) was used to present the visual stimuli. One LED was placed in the centre of the panel and set at the subject's eye level. It served as a fixation point for the eyes. The target stimulus (go) was composed of eight other LEDs surrounding the central fixation point and forming a diamond (3.44° of visual angle). Stimuli were presented and behavioral data were acquired using a real‐time acquisition system (ADwin‐Pro, Keithley Instruments, Cleveland, OH) controlled by laboratory‐made software (Docometre) by courtesy of Franck Buloup (Institut des Sciences du Mouvement, Marseille).
The appearance of the fixation point indicated the beginning of a trial and lasted until the end of the trial. Prestimulus delays (time between the beginning of a trial and stimulus presentation) varied randomly from 2 to 6 s in steps of 500 ms. The inter‐trial interval was fixed to 1 s in the EEG experiment, but varied randomly and exponentially from two to six seconds in the fMRI experiment. Subjects were asked to react as fast as possible to target presentation (100 ms duration) by pressing a button with their right thumb. In a control condition (go_control trials), the visual fixation point was green indicating that only targets could be presented. Subjects were then able to react automatically to any upcoming event. In another condition (go and nogo trials), the fixation point turned red, indicating that a go stimulus or a nogo stimulus could occur with equal probability. The nogo stimulus was composed of eight LEDs forming a X of 3.44° of visual angle centered on the fixation point (100 ms duration). Subjects were asked to refrain from reacting to these nogo signals. Catch trials (no stimulus after the appearance of the fixation point) were added (25% of all trials). Subjects were instructed to comply with a maximum error rate (false alarms and omissions) of 10% of all trials.
Procedure
In the fMRI experiment, the visual display was projected onto a screen located 56 cm from the subject's eyes (the screen was viewed through a mirror). Subjects were holding an amagnetic handle mounted with a highly sensitive button in the right hand positioned below the sternum. The experiment was divided into four acquisition sessions. Each session was composed of 20 go trials, 20 nogo trials, 20 go_control trials, and 20 catch trials, randomly presented, for a total of 80 trials/condition of interest.
The EEG experiment took place in a dedicated room within the fMRI center. Subjects were seated in a darkened, shielded room in front of the panel set at 50 cm from their eyes. Ten blocks of forty trials each were performed by each subject. Each block was composed of 10 go trials, 10 nogo trials, 10 go_control trials and 10 catch trials, randomly presented, for a total of 100 trials/condition of interest.
Behavioral analyses
Typically, inhibitory performance in go/nogo tasks is estimated using the percent of responses to nogo stimuli. However, provided that suitable executive setting conditions are controlled in the experimental design, RT of trials for which an appropriate motor response was given might offer a reliable dependent variable indexing the involvement of response inhibition mechanisms [e.g., Boulinguez et al., 2008; Criaud et al., 2012; Jaffard et al., 2007; see Fig. 1 legend for detailed description]. Indeed, although RT differences between go trials in the uncertain versus the control conditions are multifactorial (involving especially different requirements with respect to visual identification mechanisms), they are conditioned on the implementation of an inhibitory setting [Chiu and Aron, 2014; Marini et al., 2013]. For each subject and each trial, RT was normalized with respect to the subject's mean value of the control condition (go_control) [Boulinguez et al., 2008]. Through this computation, all individual RTs are distributed around the value 1, which represents the individual mean RT of the control condition. The mode of each individual distribution was used for group statistical analysis. This was intended to avoid potential biases due to interindividual variability and non‐Gaussian distributions of individual RT. A Wilcoxon's test was applied to compare go and go_control conditions.
Event‐Related fMRI
Data acquisition
Images were acquired on a 3‐T MEDSPEC 30/80 AVANCE whole‐body imager (Bruker, Ettlingen, Germany), equipped with a circular polarized head coil. For each participant, we acquired a high‐resolution structural T1‐weighted image (MPRAGE sequence, resolution 1 × 0.75 × 1.22 mm) in sagittal orientation, covering the whole brain. For functional imaging, we used a T2*‐weighted echoplanar sequence, covering the whole brain with 36 interleaved 3‐mm‐thick/0‐mm‐gap axial slices (repetition time = 1,867 ms, echo time = 30 ms, flip angle = 77°, field of view = 19.2 × 19.2 cm, 64 × 64 matrix of 3 × 3 mm voxels). We acquired 337 functional volumes per session during four sessions, for a total of 1,348 volumes per subject.
fMRI preprocessing
Data were processed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/), according to the general linear model [Friston et al., 1995]. The first six functional volumes of each session were removed to eliminate nonequilibrium effects of magnetization. The remaining 331 images were corrected for differences in slice acquisition time. The images were then corrected for head movements by realigning all the images with the first image using rigid body transformations, and unwrapped according to the fieldmap recording. Spatial normalization was improved using the DARTEL toolbox on an MNI template. Data were spatially smoothed with an isotropic Gaussian filter (8 mm full width at half maximum).
Event‐related analysis of BOLD signal changes
In the statistical analysis, 12 event types were defined at the first level, including 10 effects of interest (2 periods—Prestimulus and poststimulus—For 5 types of trial ‐go_control, go, nogo, catch_control, catch) and two effects of no interest (intertrial interval, short prestimulus delays). The events were timelocked to the onset of the target, modeled according to their onset and their duration, and convolved with a canonical hemodynamic response function (HRF). Poststimulus regressors were built on the basis of the onset of the stimulus convolved with the standard canonical HRF. Prestimulus regressors were built on the basis of the onset of the cue, and the variable duration of the cue‐stimulus period was used to define the duration of the event for convolution with the HRF. Since pre‐ and poststimulus were disjointed by a jittered delay comprised between 2 and 6 s, the different regressors were easily defined and separated. Data were highpass filtered at 128 s and summarized into one contrast per subject.
We performed two contrasts to assess, respectively, the activity specifically triggered by nogo stimuli with respect to go stimuli, and the activity evoked by any stimulus when the context is uncertain with respect to when the context is predictable. For each participant, the difference in stimulus evoked activity between the nogo and go conditions was assessed by a one sample t test applied to the contrast [(nogo)–(go)]. The difference in stimulus evoked activity between the two conditions of uncertainty was assessed by a one sample t test applied to the contrast [(nogo + go) – go_control)]. The contrast was balanced by weighting the go_control condition (×2) to compensate for the unequal number of trials in the red fixation point and green fixation point conditions. The SPM group maps were generated with a random‐effects model. The resulting individual statistical maps were entered into one sample t tests. Clusters displayed on statistical parametric maps were thresholded at a corrected cluster level of P < 0.05 using a voxel level threshold of P < 0.0001 uncorrected for multiple comparisons (and a cluster extent of more than 30 contiguous voxels).
We used a region of interest (ROI)‐based analysis approach, with the label function of the WFU pickAtlas program [Maldjian et al., 2004, 2003] to include all the regions which might play a role in response inhibition in go/nogo tasks [according to the review and meta‐analysis from Swick et al. 2011]. The selected regions were the Anterior Cingulate Cortex, the Inferior Frontal Gyrus, the Superior Frontal Gyrus, the Medial Frontal Gyrus, the Middle Frontal Gyrus, the Inferior Parietal Lobule, and the Insula.
EEG
Data acquisition
The Biosemi™ ActiveTwo Mk2 system (31.25 nV resolution) was used to record EEG data from 128 electrodes mounted in an elastic cap at Biosemi™ ABC system standard locations. Six additional external electrodes were added: Four temporal electrodes (Biosemi spherical coordinates: Phi −103.5 Theta −18 −36, and Phi 103.5 Theta 18 36), and two electrodes attached to the outer canthi of the left and right eyes (Phi 103.5 −103.5 Theta 81 −81). The CMS active electrode and the DRL passive electrode of the ActiveTwo system were used instead of classical ground electrodes of conventional systems (these two electrodes form a feedback loop driving the average potential of the subject—The common mode voltage—As close as possible to the analogue‐to‐digital reference voltage in the AD box). All electrode offsets were kept below 20 mV. EEG data were recorded at a sampling rate of 2,048 Hz. Offline, data were high‐pass filtered above 1 Hz, low‐pass filtered at 95 Hz, notch filtered at 50 Hz, and downsampled to 1,024 Hz. Then, data were epoched from 200 ms before stimulus onset to 1,000 ms after stimulus onset.
EEG preprocessing
For each subject, corrupted epochs and artifacts (blinks, eye movements, ballistocardiac noise, and other electrical noises) were automatically detected and rejected using a first ICA dedicated only to EEG preprocessing. We used an higher order statistics (HOS)‐based blind source separation (BSS) algorithm [Infomax ICA; Bell and Sejnowski, 1995] with EEGLAB [Delorme and Makeig, 2004] and the FASTER toolbox [Nolan et al., 2010]. A total of 5,834 trials (corresponding to the concatenation of individual trials obtained from our 20 participants) were acquired after trials rejection. Only trials with RT distant from no more than the distribution mode ±2 standard deviations were included in the group independent component analysis (gICA) (4,251 trials).
Advanced processing was performed on the basis of recent methodological developments. First, we applied BSS. Indeed, as recently reviewed by Huster et al. [2013], classical ERPs (N2/P3) are not reliable markers of inhibitory processes because they likely involve several mechanisms confounded with proper inhibitory processes, like conflict‐related and evaluative processing stages. One likely reason is that the overall electrical activity recorded on the scalp is composed of a set of mixed signals. To address this problem, we first used a robust second‐order statistics (SOS)‐based algorithm to unmix this set of signals. To optimize separation, UW‐SOBI [Belouchrani et al., 1997; Yeredor, 2000] was privileged over more popular but less robust higher order statistics‐based algorithms [Lio and Boulinguez, 2013]. Then, in a second step, we applied group independent component analysis (gICA). This approach offers a straightforward and computationally tractable solution to the problem of multisubject analysis by creating aggregate data containing observations from all subjects. By providing a single estimation of the mixing and the demixing matrices for the whole group, gICA allows direct estimation of the components that are consistently expressed in the population [see Eichele et al., 2011 for discussion of the broad interest of using this method] and, hence, more efficient source separation and localization of these components [Lio and Boulinguez, 2013].
The UW‐SOBI algorithm is an adaptation of the well known SOBI algorithm [Tang et al., 2005] reformulated as an uniformly weighted nonlinear least squares problem to avoid the common “whitening” phase which is known to limit the performance of BSS/ICA algorithms in noisy conditions [Cardoso, 1994, 1998]. One hundred time‐delayed covariance matrices, with time delays from 1/1,024s to 100/1,024s were calculated on each of the 4,251 remaining epochs. Then the 100 averaged time‐delayed covariance matrices were approximately joint diagonalized with the UWEDGE algorithm [Tichavsky and Yeredor, 2009], leading to the identification of 134 independent components (ICs).
Methodological principals of event‐related analyses of EEG signal changes
We took advantage of our unique design offering a psychophysical marker of the motor output (RT) both in the condition requiring inhibition of automatic responses and in the control condition. Indeed, the introduction of a go_control condition not only allows comparison of inhibition‐related brain activations generated in nogo and go trials with an appropriate reference condition, it also gives the opportunity to assess quantitative behavioral markers of automatic response inhibition by contrasting go with go_control RTs (Fig. 1C). This alternate approach is based on the following rationale: The more powerful the inhibitory activity, the more the response to go with respect to go_control trials should be delayed, bearing in mind that attentional modulations are also likely to contribute to the overall effect [Marini et al., 2013]. Primarily, we selected the relevant sources among the 134 ICs for further analysis by tracking over time, for each component, the significant activity changes between the go and go_control conditions. The critical (i.e., earliest) sources coming out from this blind test were further submitted to a first coarse‐grained analysis consisting in the comparison of the ERPs evoked within each single source by the nogo, the go and the go_control stimuli, respectively. Then, a more fine‐grained assay was performed, based on supplementary analyses guided by the behavioral data on a single‐trial basis.
Selection of relevant sources/detection of the earliest electrophysiological indices of response inhibition
In order to track the earliest activity evoked by reactive inhibitory processes, a multiple hypothesis testing procedure was designed. For each IC, the mean evoked activity was estimated both for the go and the go_control conditions in height time‐periods of 25 ms, from 50 to 250 ms after stimulus onset. A Wilcoxon's test (P < 0.05, Bonferroni's corrected) was used to test differences between go and the go_control conditions for each IC and each time period. Since we found only one IC showing significant early differences (Supporting Information Fig. S1), the next processing steps were applied only to this component (Fig. 2).
Figure 2.
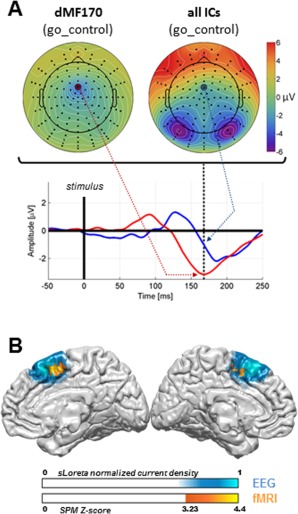
(A) Topographic mapping of dMF170 activity at peak time, back‐projected on the scalp after source separation (upper left side), as compared with topographic mapping of all mixed components before source separation (upper right side). Time‐series of the net activity at C23 are presented for each condition (lower part; red: dMF170; blue: overall activity). The whole topography is strongly influenced by powerful visual activity around 170 ms to the extent that the dMF170 component remains invisible without filtering all interferent sources by means of advanced source separation. (B) Source localization of the dMF170 component with sLoreta. The probability map is presented on the MNI atlas. It extends across the SMC. Combined fMRI results are superimposed. BOLD imaging reveals an overlapping region that is more activated by the stimulus when the situation requires response inhibition (go and nogo conditions pooled together for analysis) than when it does not (go_control condition).
Analyses of relevant sources activity
IC source localization. The 3D distribution of the selected source current densities was estimated by means of the sLoreta software [Pascual‐Marqui, 2002]. The head model used for this analysis was obtained by applying the BEM method to the MNI152 template [Mazziotta et al., 2001]. The 3D solution space was restricted to cortical gray matter and was partitioned into 6,239 voxels with a spatial resolution of 5 mm. Then, the sLoreta solution of the inverse problem was computed using an amount of Tikhonov regularization optimized for an estimated Signal/Noise Ratio of 100. Four sets of analyses where performed.
Coarse‐grained analysis: Event‐related potentials. The ERPs respectively evoked by the nogo, the go and the go_control stimuli were assessed. For each trial the voltage at peak amplitude (t = 168 ms) was recorded. Comparisons between the three conditions were performed by means of Wilcoxon's tests.
Fine‐grained analysis: Psychophysiological correlations. In order to assess the relation between the behavioral and electrophysiological markers of response inhibition at a more detailed level, all trials for which an appropriate motor response was provided (go and go_control distributions) were merged for refined single‐trial analyses. The relation between RT and the amplitude of the evoked component of the selected source was assessed by means of Pearson correlations. These calculations were applied after Vincentization of RT data. The 3,926 artifact free trials were partitioned in 9 classes: RT < 5th < 10th < 20th < 40th < 60th < 80th < 90th < 95th < 100th percentile of the RT distribution.
Fine‐grained analysis: Event‐related spectral power analysis. We assessed how the different frequency bands contribute to the ERPs by means of fast Fourier transforms. Then, we calculated stimulus‐induced (time locked) power changes of ongoing oscillations of the selected source [see Nikulin et al., 2007; Van Dijk et al., 2010 for discussion] in order to estimate how these changes modulate the evoked response. These modulations were assessed by means of single‐trial analyses. Further data preprocessing was performed in order to get optimal time/frequency resolution within delta/theta (1.5–7.5 Hz), Alpha (7.5–13.5 Hz), Beta 1–2 (13.5–19.5 Hz), Beta 3 (19.5–30.5 Hz), low Gamma (30.5–44.5 Hz), and high Gamma (57.5–77.5) band activities. Six elliptic infinite impulse response (IIR) bandpass filters were designed with the Matlab™ signal processing toolbox. Relatively large pass band widths were set to get optimal time resolution, i.e., optimal estimation of the temporal dynamics of the frequency bands of interest at the single‐trial level. The filters used the following specifications:
Filter 1 (Delta/Theta power): High pass frequency: 1.5 Hz; Low pass frequency: 7.5 Hz; Transition band width: 1 Hz; Attenuation: 80 dB; Order: 18; sections: 9.
Filter 2 (Alpha power): High pass frequency: 7.5 Hz; Low pass frequency: 13.5 Hz; Transition band width: 1 Hz; Attenuation: 80 dB; Order: 16; sections: 8.
Filter 3 (Beta 1–2 power): High pass frequency: 13.5 Hz; Low pass frequency : 19.5 Hz; Transition band width: 1 Hz; Attenuation: 80 dB; Order: 16; sections: 8.
Filter 4 (Beta 3 power): High pass frequency: 19.5 Hz; Low pass frequency: 30.5 Hz; Transition band width: 1 Hz; Attenuation: 80 dB; Order: 20; sections: 10.
Filter 5 (Low Gamma power): High pass frequency: 30.5 Hz; Low pass frequency: 44.5 Hz; Transition band width: 1 Hz; Attenuation: 80 dB; Order: 20; sections: 10.
Filter 6 (High Gamma power): high pass frequency: 57.5 Hz ; low pass frequency: 77.5 Hz ; transition band width: 5 Hz ; attenuation: 80 dB; order: 14; sections: 7.
Then, the activity of the selected component was extracted from the recorded scalp activity of the 3,926 artifact free trials (re‐epoched from 1,500 ms before to 1,000 ms after the stimulus onset). In order to quantify the power (the scalp contribution) of the component, the source activity was back transformed to the electrode space, and displayed on the electrode that mostly contributes to the source variance (C23/Fcz for the dmf170 component). Then, to analyze the temporal dynamics of the signal within each frequency band of interest, the following method was implemented: First, each trial was filtered with the corresponding filter in both forward and reverse directions to insure zero‐phase distortion. Second, the complex analytic signal of each filtered trial was derived by the Hilbert transform (Matlab™ Hilbert function). Third, the instantaneous amplitude envelopes of the filtered trials were computed by taking the absolute magnitude of the complex waveform. Then, the studied time range was restricted to 200 ms pre‐ to 800 ms poststimulus in order to avoid edge effects/transient responses of digital filters. Finally, for visualization only, a trial moving average smoothing was applied (windows length: 400).
Fine‐grained analysis: ERP/evoked power correlations. The relationship between the amplitude of the evoked component and the power within each frequency band at component peak time was assessed by means of Pearson's correlations, as described above (psychophysiological correlations).
RESULTS
Behavioral Data
The false alarm rate (number of responses to nogo signals/number of nogo signals) was low both in the EEG (0.082 ± 0.076) and in the fMRI (0.12 ± 0.089) experiments, indicating good inhibitory performance.
Go RT normalized with respect to the mean go_control RT was found to be significantly longer than go_control normalized RT in both the EEG (1.24 vs. 1, P < 0.001) and the fMRI (1.3 vs. 1, P < 0.001) experiments. Considering that the go_control condition involves no inhibitory control, this result is consistent with the hypothesis that a certain level of inhibitory control is involved in the go condition (Fig. 1).
fMRI Data
We first contrasted the nogo and go conditions to assess the specificity of response inhibition activations in uncertain environments. We found no significant difference, suggesting that response inhibition is not triggered differently by nogo and go signals. Thus, both conditions were collapsed for further analysis.
We then contrasted the condition with uncertainty (red fixation point condition, nogo and go conditions merged) with the condition without uncertainty (green fixation point condition, go_control condition). This contrast is prone to reveal the brain regions supporting nonselective response inhibition that are more activated by any stimulus when the context is uncertain. Only one region (cluster size: 99 voxels) returned a significant difference. This area was localized in the medial frontal gyrus (BA 6). Response peaks were observed both in the SMA‐proper ([−6, −6, 63], z score: 4.45; [3, −3, 54], z score: 4.65) and in the pre‐SMA ([6, 9, 57], z score: 4.97).
EEG Data
Selection of relevant sources/detection of the earliest electrophysiological indices of response inhibition
We searched for the components that significantly accounted for the RT difference between the conditions with and without uncertainty. In order to focus on relevant inhibitory activity, we performed this analysis within an early 0–250 ms poststimulus time window. We found only one component showing early significant difference in activity between the conditions with and without uncertainty (within an early 125–175 ms time window with respect to stimulus onset, Supporting Information Fig. S1). Consistent with the FMRI data, this component was localized in the dorsal medial frontal cortex (Fig. 2), with a probability map covering the supplementary and presupplementary motor areas (supplementary motor complex, SMC). The other EEG components for which significant differences were found between the conditions with and without uncertainty reported late, nonoverlapping changes in activity, starting around 200 ms and lasting beyond 250 ms (Supporting Information Fig. S1). These late components were generated within the cuneus, the precuneus and the anterior cingulate cortex (ACC), whose combined activities more likely account for the classical ERPs N2 and P3 (Supporting Information Fig. S2). As a consequence, only the relevant early dorsomedial frontal component was selected for further analysis.
Coarse‐grained analysis
The corresponding event‐related potential peaked negatively at 168 ms. Its amplitude was not different between go and nogo trials (P > 0.82). Yet, it was greater in the condition without uncertainty (go_control) than in the conditions with uncertainty (go or nogo trials) (−3.12 vs. −2.58 and −2.63 µV, respectively, Ps < 0.01, Fig. 3).
Figure 3.
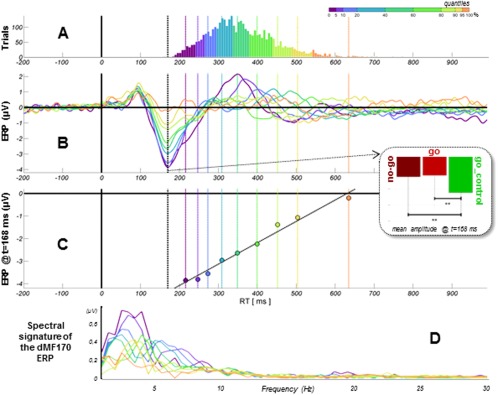
Psychophysiological characteristics of the dMF170 component. (A) Cumulated distributions of RT (go and go_control trials merged for analysis). Data are Vincentized with unequal‐sized subsets to compensate for RT distribution inhomogeneity in order to better assess what accounts for these differences in RT (quantiles are displayed with color code). (B) Time series of dMF170 activity (back‐projected on electrode C23/Fcz) (t0 = target presentation). The component peaks negatively approximately 170 ms after stimulus presentation, identically for go and nogo trials (means), but shows larger amplitude for the go_control condition. The mean evoked potential is displayed for each quantile. (C) The mean evoked potential for each quantile is referred to the corresponding mean RT. The amplitude of the dMF170 closely predicts RT. (D) Fast Fourier transform of the dMF170 ERP.
Fine‐grained analyses
Refined analyses showed that the amplitude of the event‐related potential is a linear function of RT (Pearson's correlation = 0.99; P < 0.001). Spectral analyses performed at the source level revealed that delta/theta and Alpha bands show a burst of activity evoked by the stimulus. However, only the Alpha band power evoked modulations are consistent with the BOLD increase observed within the same region (more powerful evoked activity for longer RT) (Fig. 4A). Consistently, the amplitude of the ERP is negatively correlated with Alpha power measured at ERP peak time (Pearson's correlation = 0.90; P < 0.01) (Fig. 4B).
Figure 4.
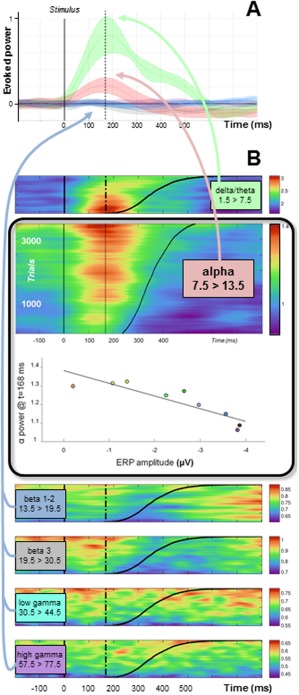
Spectral analyses of the “dMF170” component. (A) Mean evoked activity within each frequency band of interest (power is normalized with respect to the prestimulus period). Only delta/theta and Alpha bands show evoked activity. (B) Single‐trial modulations in spectral power within each frequency band of interest. Trials are sorted according to RT (black line). Only the Alpha band shows power evoked modulations consistent with the BOLD increase observed within the same region (more powerful evoked activity for longer RT). Correlation analysis shows that the higher the Alpha power at peak time, the smaller the amplitude of the dMF170. In contrast, the delta/theta band shows less powerful evoked activity for longer RT, reflecting possibly the evoked excitatory activity driving SMC efference (more powerful evoked activity for shorter RT, i.e., for noninhibited responses). No activity evoked by the stimulus is observed in upper frequency bands.
Theoretically, groupBSS estimates sources that are group/task related and maximally similar across space/time and subjects [Lio and Boulinguez, 2013]. As a consequence, to observe the full effect size across the whole group RT distribution, the ERPs for each class of RT have been calculated directly from the single trial analysis. In this case it cannot be precisely determined whether each subject contributes equally to each class. To control for this possible source of inaccuracy, we performed complementary, more conventional group level (second level) analyses on the basis of single subject (first level) analyses. These supplementary analyses are presented in the Supporting information file (2. Supplementary analyses). They provide similar results than the groupBSS analyses, strengthening the validity of our initial approach and demonstrating the contribution of most subjects to the overall group result.
DISCUSSION
It is problematical to distinguish critical brain activity from redundant brain activity in inhibitory tasks on the basis of BOLD analyses [e.g., Chambers et al., 2009], especially when the methods rely on complex designs [Criaud and Boulinguez, 2013]. Consequently, while fMRI studies have repeatedly found a large number of structures, the interpretation of brain activations in go/nogo tasks is often questionable. In the present fMRI study, we found no evidence for specific activations induced by the stimuli that had to be withheld with respect to those requiring a motor response. In other words, we found no evidence for the involvement of selective response inhibition in the present go/nogo task. The difference between the classical reports and ours likely relies on the fact that we used a simple, refined, task design preventing from potential confounds with the numerous cognitive processes involved in the complex tasks typically used to probe response inhibition. Consistent with this interpretation, a critical review of fMRI investigations of response inhibition based on repeated meta‐analyses of typical go/nogo experiments has recently suggested that most of the activity specifically elicited by stimuli requiring action restraint is actually driven by the engagement of high attentional or working memory resources, not by inhibitory processes per se [Criaud and Boulinguez, 2013]. Obviously, there is too much evidence from clinical [e.g., Nachev et al., 2008; Picton et al., 2007; Sumner et al., 2007], stimulation [e.g., Chen et al., 2009; Duque et al., 2013; Juan and Muggleton, 2012; Obeso et al., 2013], and animal [e.g., Chen et al., 2010; Isoda and Hikosaka, 2007] studies to infer from this result that the most expected brain region, the SMC, does not play a direct role in response inhibition. This result rather suggests that, in this type of task which does not require selection between alternative responses, the critical inhibitory process is not selective, i.e., is not specific to the processing of nogo signals. As discussed below, the critical inhibitory process would rather be context‐dependent. It would consist in blindly suppressing any automatic response when the situation is potentially conflicting in order to allow deliberate, long latency responses.
Bursts in Alpha Power Evoked by Unpredictable Stimuli are Related to Increases in BOLD and Suppression of Early ERP in the SMA
Using an approach that manipulated stimulus uncertainty, we show that, in unpredictable contexts, any stimulus triggers an increase in BOLD activity in the supplementary motor complex (SMC) (Fig. 2B). Theoretically, this enhancement is not straightforward to interpret. It can reflect early inhibition of automatic responses, preparation of long latency volitional responses, conflict resolution or action selection, reminding how complex the functional attributes of this region can be [Nachev et al., 2008; Rushworth et al., 2005]. EEG signals provide relevant data to evaluate the validity of each of these possibilities. As reported in the results, advanced blind source separation revealed that the only source showing early amplitude differences between the conditions with and without uncertainty was consistently located in the SMC (Fig. 2).2 Consistent with the model assuming context dependent executive setting, the amplitude of this single source (hereinafter referred to as the “dMF170”) was identical for the nogo and go trials, but different in the go_control condition (Fig. 3). Interestingly, spectral analyses of the dMF170 uncovered only one frequency band showing power evoked modulations consistent with the BOLD increase observed within the same region (Fig. 4). The fact that this activity was observed in the Alpha band strongly suggests, according to recent studies, that the BOLD signal evoked by any stimulus when the situation was unpredictable was driven by inhibitory activity [Haegens et al., 2011; Hindriks and Van Putten, 2013; Jensen and Bonnefond, 2013; Jensen and Mazaheri, 2010; Klimesch et al., 2007; Klimesch, 2012; Mazaheri et al., 2009; Mathewson et al., 2011]. Supporting this interpretation, the efficiency of automatic inhibitory mechanisms has been related to GABA concentration in the SMA as measured with magnetic resonance spectroscopy, and attributed to the involvement of local inhibitory interneurons [Boy et al., 2010a].
The DMF170 Component as a Physiological Marker of Both Automatic Response Activation and Concurrent Automatic Inhibition
The evoked potential of the dMF170 component was found to peak around 170 ms poststimulus (Figs. 2, 3, 4), well before the standard N2/P3 ERP markers of response inhibition (Supporting Information Fig. S1 and Fig. S2). This supports the idea that early, automatic, inhibitory mechanisms within the SMC are involved in action control [Boy et al., 2010a–2010c; Sumner et al., 2007]. Importantly, the amplitude of the dMF170 was predictive of RT in trials for which a response was required: large negative amplitudes were observed for fast responses in predictable situations, but peak amplitude was gradually suppressed as RT increased (Fig. 3). In other words, the amplitude of the evoked potential of the dMF170 was attenuated when the response had to be withheld. Importantly, this attenuation of the ERP is unequivocally attributable to the burst in Alpha power. Indeed, the burst of Alpha power is time locked to the ERP but evolves in the opposite direction (Fig. 4). Thus, the burst of Alpha power cannot be just the spectral representation of the ERP, which is rather accounted for by delta/theta activity (Fig. 3D; see also Supporting Information analyses). In this respect, the pattern of activity observed within the lowest frequency band in the present study (Fig. 4) is partly reminiscent of previous reports associating the low‐theta burst preceding rapid motor responses to the disinhibition of impulsive motor responses [Delorme et al., 2007]. Taken together, these findings suggest that: (i) automatic inhibition is self‐generated within the supplementary motor system and (ii) automatic inhibition develops concurrently with any automatic motor activation, not selectively in response to undesired automatic motor activation.
These findings could not have been derived from standard psychophysical, fMRI, or ERP approaches. Although some clues about the involvement of nonselective inhibitory mechanisms have been provided separately by former fMRI [Jaffard et al., 2008] and EEG [Boulinguez et al., 2009] studies, only the use of advanced methods allowing proper separation of the numerous sources mixed in the EEG signal allowed identification of the involvement of the early dMF170 component (Fig. 2). This masked component likely provides the missing link in the framework of the electroencephalography of response inhibition, supporting the idea that none of the standard EEG‐derived measures (see Supporting Information Fig. S2) can provide an unambiguous indicator of functional inhibition [Huster et al., 2013]. Nevertheless, due to the intimate nature of the EEG signal, some caution should be exerted when interpreting these data. Indeed, the basis of the EEG signal is formed of spatiotemporally summed postsynaptic potentials, and strongly relates to the input a region generates, not necessarily to its output. As a consequence, if the dMF170 ERP evoked by any stimulus likely reflects transient automatic activation of motor processes, it does not necessarily reflect the output of the SMC. Assuming, in light of the existing literature (see “Introduction”), that Alpha oscillations actually index an active inhibitory mechanism that gates information processing within a given region [Hindriks and Van Putten, 2013; Jensen and Mazaheri, 2010; Klimesch et al., 2007; Klimesch, 2012; Mathewson et al., 2011], what can be inferred from the present data is that integrative processes in the SMC are likely suppressed when action restraint is required, with consequent suppression of short latency responses at the behavioral level. Yet, recent evidence that Alpha power is negatively correlated with spiking rate in monkeys premotor cortex [Haegens et al., 2011] makes it tempting to further speculate about the possible role of Alpha oscillations in driving neuronal spiking in human SMC as well.
Based on these observations, we propose that the dMF170 component likely provides a reliable biomarker of both automatic response activation and concurrent automatic inhibition. While the ERP would index the strength of automatic motor activations, the burst of Alpha power time‐locked to the stimulus might provide a direct, real time, physiological correlate of activation of local, automatic, self‐inhibitory networks that gates information within the SMC.
Controlling Automatic Inhibition of Automatic Responses as a Basic Executive Mechanism?
As suggested by its flexible functioning (whether or not the situation is potentially conflicting), the SMC appears to play a central role in task setting [Forstmann et al., 2010, 2008; Nachev et al., 2008; Rushworth et al., 2004; Vallesi et al., 2009]. Yet, dissociating the nearly indistinguishable roles of the SMC in response inhibition and switching during executive control is far from trivial [e.g., Kenner et al., 2010]. Previous findings support the hypothesis that a contextual activation of inhibitory networks within the SMC via proactive control might play a critical role in executive functions [e.g., Obeso et al., 2013]. Depending on their expectations of upcoming events, subjects could switch anticipatorily from one mode of control to another, i.e., from automatic inhibition of response to automatic processing of sensorimotor information, and reciprocally. This mechanism could be achieved by activating/deactivating local inhibitory circuitry within SMC with consequent attenuation/enhancement of SMC early, reactive, automatic activity. These findings are partly reminiscent of pioneer electrophysiological studies in monkeys [Isoda and Hikosaka, 2007]. They reveal, in humans, the complex, flexible, and paradoxical mechanisms by which voluntary control of action may be achieved [Braver et al., 2009; Haggard, 2008; McBride et al., 2012; Verbruggen and Logan, 2008, 2009]. Yet controversy remains regarding which part of the SMC precisely supports this elementary form of functional inhibition [Wardak, 2011], and which brain regions provide the control signals for the generation of the inhibitory set within the SMC [Brass and Haggard, 2007; Braver et al., 2009; Filevich et al., 2012; Jaffard et al., 2007, 2008; Kuhn et al., 2009]. The concept of proactive control [effects of foreknowledge on inhibition‐related neurocognitive processes: Aron, 2011; Braver et al., 2009; Chen et al., 2010; Criaud et al., 2012; Criaud and Boulinguez, 2013; Jaffard et al., 2007, 2008; Jahfari et al., 2010, 2012; Lo et al., 2009; Zandbelt and Vink, 2010; Zandbelt et al., 2013] combined with the methodological advances provided by EEG‐based spectral analyses [e.g., Bengson et al., 2012; Mazaheri et al., 2009] provide a powerful framework for further investigation.
Relevance to More General Models of Action Control
The control mechanism proposed above is reminiscent of some processes described in former models. Our model resembles the “dual‐route” model of interference [e.g., Forstmann et al., 2008; Eimer, 1995; Kornblum et al., 1990; Ridderinkhof, 2002] in the sense that the controlled process of response activation is paralleled by a direct response capture route that requires inhibition to prevent erroneous responses. Yet, the dual route model assumes a selective inhibition of inappropriate automatic activations, while our model assumes inhibition of any automatic response whether inappropriate or not. In this respect, our model resembles more closely the “hold your horses” model of decision making [Franck, 2006; Franck et al., 2007] which assumes that preventing premature responding is achieved by the generation of a “global nogo” signal in the cortico‐basal ganglia loops, acting on suppression of all responses rather than modulating the execution of any particular response. Yet, the “hold your horses” model is assumed to apply to high‐conflict win/win decisions in complex choice tasks involving concurrent responses (i.e., decision about which action to execute). The present data do not only identify a physiological marker of the “global nogo” signal, they also extend the concept to simple situations for which only the decision whether to execute an action or not is concerned. At a more general level, the basic control mechanism inferred from the present results is also partly reminiscent of some processes described in the impulse control model of movement preparation [Duque and Ivry, 2009; Duque et al., 2012, 2010], in the sense that a nonselective inhibitory mechanism can be directed at an already selected response in order to control when this response is executed. Of particular interest for that purpose is certainly the integrative “What, When, Whether” model of intentional action proposed by Brass and Haggard [2008]. This model clearly separates the mechanisms related to the decision about which action to execute from the mechanisms related to the decision about whether to execute an action or not, and when to initiate it. Although this issue remains to be further explored, the elementary inhibitory mechanism inferred from the present findings might represent a basic function common to all these actions whether simple or complex.
CONCLUSION
In summary, the present data provide empirical support for the claim that response inhibition is not necessarily a control process intended to override a prepotent response tendency, but can also be itself a prepotent response tendency which has to be temporarily controlled [Jasinska, 2013]. We suggest that an early, automatic, nonselective self‐inhibitory mechanism of the SMC is involved in response control when the context is uncertain, and is released when the situation becomes predictable. It is not too speculative to suggest that automatic inhibition of automatic motor responses probably has a pivotal role in the numerous functions supported by the SMC [Nachev et al., 2008]. These results open‐up new clinical perspectives since impairments in the ability to implement or release this form of inhibitory setting would be devastating in different psychiatric and neurologic conditions. Such executive dysfunctions may account for various motor and cognitive disorders, as might obviously be the case for impulsivity [Ballanger et al., 2009] but also for opposing symptoms like akinesia [e.g., Favre et al., 2013].
Supporting information
Supplementary Information Figures.
ACKNOWLEDGMENTS
The authors declare no competing financial interests. We express our thanks to the Centre Hospitalier St Jean de Dieu for promoting this research program, and to Antoine Boulinguez for constructive discussion during the revision of the manuscript.
Footnotes
Here, selectivity does not refer to the selection between alternative movements (as it often does in studies using choice RT tasks). It rather refers to the perceptual decision mechanisms that involve the detection, discrimination, or identification of sensory stimuli [Gold and Ding, 2013]. This point is central because studies interested in response inhibition have, in compliance with the implicit dynamics of the dominant models assuming selective, reactive processing, focused on the cascade of events specifically launched by information derived from nogo stimuli with respect to go stimuli.
Longer latency effects were found in the ACC and the visual system, starting about 200 ms (Supporting Information Fig. S1), which is also the timing of the fastest automatic responses of the control condition (Fig. 3). These activity changes are consistent with a different level of involvement of visual attention in the two conditions, but do not seem critical for response inhibition with regard to their timing since inhibitory processes are expected to be active before the temporal window during which fast automatic motor responses are triggered (Supporting Information Fig. S1). This activity of the cingulate/visual regions might rather be associated with subsequent processes critical for producing long latency responses (Supporting Information Fig. S2), like mismatch and conflict detection, perceptual decision or response program updating (e.g., Gonzalez‐Rosa et al., 2013; Huster et al., 2011, 2013; Kropotov et al., 2011).
REFERENCES
- Aron AR (2011): From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Pizzella V, Gratta CD, Ferretti A, Romani GL (2009): Fundamentals of electroencefalography, magnetoencefalography, and functional magnetic resonance imaging. Int Rev Neurobiol 86:67–80. [DOI] [PubMed] [Google Scholar]
- Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, Pellecchia G, Houle S, Poon YY, Lang AE, Strafella AP (2009): Stimulation of the subthalamic nucleus and impulsivity: Release your horses. Ann Neurol 66:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159. [DOI] [PubMed] [Google Scholar]
- Bengson JJ, Mangun GR, Mazaheri A (2012): The neural markers of an imminent failure of response inhibition. NeuroImage 59:1534–1539. [DOI] [PubMed] [Google Scholar]
- Belouchrani A, A‐Meraim K, Cardoso JF, Moulines E (1997): A blind source separation technique using second order statistics. IEEE Trans Signal Process 45:434–444. [Google Scholar]
- Boulinguez P, Ballanger B, Granjon L, Benraiss A (2009): The paradoxical effect of warning on reaction time: Demonstrating proactive response inhibition with event‐related potentials. Clin Neurophysiol 120:730–737. [DOI] [PubMed] [Google Scholar]
- Boulinguez P, Jaffard M, Granjon L, Benraiss A (2008): Warning signals induce automatic EMG activations and proactive volitional inhibition: Evidence from analysis of error distribution in simple RT. J Neurophysiol 99:1572–1578. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Singh KD, Husain M, Sumner P (2010a): Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 20:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Husain M, Singh KD, Sumner P (2010b): Supplementary motor area activations in unconscious inhibition of voluntary action. Exp Brain Res 206:441–448. [DOI] [PubMed] [Google Scholar]
- Boy F, Husain M, Sumner P (2010c): Unconscious inhibition separates two forms of cognitive control. Proc Natl Acad Sci USA 107:11134–11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Haggard P (2007): To do or not to do: The neural signature of self‐control. J Neurosci 27:9141–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Haggard P (2008): The what, when, whether model of intentional action. Neuroscientist 14:319–325. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM (2009): Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci USA 106:7351–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Kaila K, Raichle M (2007): Inhibition and brain work. Neuron 56:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso JF (1994): On the performance of orthogonal source separation algorithms. In: Proceedings of EUSIPCO‐94, VII European Signal Processing Conference, Edinburgh, UK, September 13–16, 1994. pp 776–779.
- Cardoso JF (1998): Blind signal separation: Statistical principles. Proc IEEE 86:2009–2025. [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA (2009): Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33:631–646. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJL, Hung DL, Juan CH (2009): Control of prepotent responses by the superior medial frontal cortex. NeuroImage 44:537–545. [DOI] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V (2010): Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci 30:14657–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Aron AR (2014): Unconsciously triggered response inhibition requires an executive setting. J Exp Psychol 143:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P (2013): Have we been asking the right questions when assessing response inhibition in go/nogo tasks with fMRI? A meta‐analysis and critical review. Neurosci Biobehav Rev 37:11–23. [DOI] [PubMed] [Google Scholar]
- Criaud M, Wardak C, Ben Hamed S, Ballanger B, Boulinguez P (2012): Proactive inhibitory control of response as the default state of executive control. Front Psychol 3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S (2004): EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. [DOI] [PubMed] [Google Scholar]
- Delorme A, Westerfield M, Makeig S (2007): Medial prefrontal theta bursts precede rapid motor responses during visual selective attention. J Neurosci 27:11949–11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB (2009): Role of corticospinal suppression during motor preparation. Cereb Cortex 19:2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB (2012): Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci 32:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB (2010): Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci 30:3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Olivier E, Rushworth M (2013): Top‐down inhibitory control exerted by the medial frontal cortex during action selection under conflict. J Cogn Neurosci 25:1634–1648. [DOI] [PubMed] [Google Scholar]
- Eichele T, Rachakonda S, Brakedal B, Eikeland R, Calhoun VD (2011): EEGIFT: Group independent component analysis for event‐related EEG data. Comput Intell Neurosci 2011:129365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M (1995): Stimulus–response compatibility and automatic response activation: Evidence from psychophysiological studies. J Exp Psychol Hum Percept Perform 21:837–854. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P (2010): Beta‐band oscillations–signalling the status quo? Curr Opin Neurobiol 20:156–165. [DOI] [PubMed] [Google Scholar]
- Favre E, Ballanger B, Thobois S, Broussolle E, Boulinguez P (2013): Deep brain stimulation of the subthalamic nucleus, but not dopaminergic medication, improves proactive inhibitory control of movement initiation in Parkinson's disease. Neurotherapeutics 10:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich E, Kühn S, Haggard P (2012): Intentional inhibition in human action: The power of “no”. Neurosci Biobehav Rev 36:1107–1118. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers E‐J, Bogacz R, Turner R (2010): Cortico‐striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA 107:15916–15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ (2008): Striatum and pre‐SMA facilitate decision‐making under time pressure. Proc Natl Acad Sci USA 105:17538–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ (2006): Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Netw 19:1120–1136. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007): Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318:1309–1312. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. NeuroImage 2:45–53. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TPL (2011): Relating MEG measured motor cortical oscillations to resting γ‐aminobutyric acid (GABA) concentration. NeuroImage 55:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Ding L (2013): How mechanisms of perceptual decision‐making affect the psychometric function. Prog Neurobiol 103:98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Rosa JJ, Inuggi A, Blasi V, Cursi M, Annovazzi P, Comi G, Falini A, Leocani L (2013): Response competition and response inhibition during different choice‐discrimination tasks: Evidence from ERP measured inside MRI scanner. Int J Psychophysiol 89:37–47. [DOI] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O (2011): α‐Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci USA 108:19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P (2008): Human volition: Towards a neuroscience of will. Nat Rev Neurosci 9:934–946. [DOI] [PubMed] [Google Scholar]
- Hindriks R, Van Putten MJAM (2013): Thalamo‐cortical mechanisms underlying changes in amplitude and frequency of human alpha oscillations. NeuroImage 70:150–163. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD (2012): Executive functions and self‐regulation. Trends Cogn Sci 16:174–180. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez‐Geppert S, Lavallee CF, Falkenstein M, Herrmann CS (2013): Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int J Psychophysiol 87:217–233. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Eichele T, Enriquez‐Geppert S, Wollbrink A, Kugel H, Konrad C, Pantev C (2011): Multimodal imaging of functional networks and event‐related potentials in performance monitoring. NeuroImage 56:1588–1597. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O (2007): Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10:240–248. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Benraiss A, Longcamp M, Velay J‐L, Boulinguez P (2007): Cueing method biases in visual detection studies. Brain Res 1179:106–118. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Longcamp M, Velay J‐L, Anton J‐L, Roth M, Nazarian B, Boulinguez P (2008): Proactive inhibitory control of movement assessed by event‐related fMRI. NeuroImage 42:1196–1206. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR (2010): Responding with restraint: What are the neurocognitive mechanisms? J Cogn Neurosci 22:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Verbruggen F, Frank MJ, Waldorp LJ, Colzato L, Ridderinkhof KR, Forstmann BU (2012): How preparation changes the need for top‐down control of the basal ganglia when inhibiting premature actions. J Neurosci 32:10870–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ (2013): Automatic inhibition and habitual control: Alternative views in neuroscience research on response inhibition and inhibitory control. Front Behav Neurosci 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B (2005): On the human sensorimotor‐cortex beta rhythm: Sources and modeling. NeuroImage 26:347–355. [DOI] [PubMed] [Google Scholar]
- Jensen O, Bonnefond M (2013): Prefrontal alpha‐ and beta‐band oscillations are involved in rule selection. Trends Cogn Sci 17:10–12. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010): Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front Hum Neurosci 4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Pinto DJ, Kaper TJ, Kopell N (2000): Alpha‐frequency rhythms desynchronize over long cortical distances: A modeling study. J Comput Neurosci 9: 271–291. [DOI] [PubMed] [Google Scholar]
- Juan CH, Muggleton NG (2012): Brain stimulation and inhibitory control. Brain Stimul 5:63–69. [DOI] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leiben‐luft E, Poldrack RA (2010): Inhibitory motor control in response stopping and response switching. J Neurosci 30:8512–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A (2013): The ups and downs of β oscillations in sensorimotor cortex. Exp Neurol 245:15–26. [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012): α‐band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53:63–88. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A (1990): Dimensional overlap: Cognitive basis for stimulus–response compatibility—A model and taxonomy. Psychol Rev 97:253–270. [DOI] [PubMed] [Google Scholar]
- Kropotov JD, Ponomarev VA, Hollup S, Mueller A (2011): Dissociating action inhibition, conflict monitoring and sensory mismatch into independent components of event related potentials in GO/NOGO task. NeuroImage 57:565–575. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Haggard P, Brass M (2009): Intentional inhibition: How the “veto‐area” exerts control. Hum Brain Mapp 30:2834–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio G, Boulinguez P (2013): Greater robustness of second order statistics than higher order statistics algorithms to distortions of the mixing matrix in blind source separation of human EEG: Implications for single‐subject and group analyses. NeuroImage 67:137–152. [DOI] [PubMed] [Google Scholar]
- Lo CC, Boucher L, Paré M, Schall JD, Wang XJ (2009): Proactive inhibitory control and attractor dynamics in countermanding action: A spiking neural circuit model. J Neurosci 29:9059–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2008): What we can do and what we cannot do with fMRI. Nature 453:869–878. [DOI] [PubMed] [Google Scholar]
- Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW (2009): Temporal framing of thalamic relaymode firing by phasic inhibition during the alpha rhythm. Neuron 63:683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Onton J (2009): ERP features and EEG dynamics: An ICA perspective In: Luck S, Kappenman E, editors. Oxford Handbook of Event‐Related Potential Components. New York, NY: Oxford University Press; pp 51–87. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Marini F, Chelazzi L, Maravita A (2013): The costly filtering of potential distraction: Evidence for a supramodal mechanism. J Exp Psychol Gen 142:906–922. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G (2011): Pulsed out of awareness: EEG alpha oscillations represent a pulsed‐inhibition of ongoing cortical processing. Front Psychol 19:2–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis ILC, Van Dijk H, Jensen O (2009): Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp 30:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero‐Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B (2001): A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 356:1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J, Boy F, Husain M, Sumner P (2012): Automatic motor activation in the executive control of action. Front Hum Neurosci 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008): Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9:856–869. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Linkenkaer‐Hansen K, Nolte G, Lemm S, Müller KR, Ilmoniemi RJ, Curio G (2007): A novel mechanism for evoked responses in the human brain. Eur J Neurosci 25:3146–3154. [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB (2010): FASTER: Fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods 192:152–162. [DOI] [PubMed] [Google Scholar]
- Obeso I, Robles N, Marrón EM, Redolar‐Ripoll D (2013): Dissociating the role of the pre‐SMA in response inhibition and switching: A combined online and offline TMS approach. Front Hum Neurosci 7:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Marqui RD (2002): Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin Pharmacol 24:5–12. [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S (2007): Effects of focal frontal lesions on response inhibition. Cereb Cortex 17:826–838. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR (2002): Micro‐ and macro‐adjustments of task set: Activation and suppression in conflict tasks. Psychol Res 66:312–323. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Kennerley SW, Walton ME (2005): Cognitive neuroscience: Resolving conflict in and over the medial frontal cortex. Curr Biol 15:54–56. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM (2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012): Spectral fingerprints of large‐scale neuronal interactions. Nat Rev Neurosci 13:121–134. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Brown JW, Schall JD (2010): Role of supplementary eye field in saccade initiation: Executive, not direct, control. J Neurophysiol 103:801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M (2007): Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U (2011): Are the neural correlates of stopping and not going identical? Quantitative meta‐analysis of two response inhibition tasks. NeuroImage 56:1655–1665. [DOI] [PubMed] [Google Scholar]
- Tang AC, Sutherland MT, McKinney CJ (2005): Validation of SOBI components from high‐density EEG. NeuroImage 25:539–553. [DOI] [PubMed] [Google Scholar]
- Tichavsky P, Yeredor A (2009): Fast approximate joint diagonalization incorporating weight matrices. Trans Sig Proc 57:878–891. [Google Scholar]
- Vallesi A, McIntosh AR, Alexander MP, Stuss DT (2009): FMRI evidence of a functional network setting the criteria for withholding a response. NeuroImage 45:537–548. [DOI] [PubMed] [Google Scholar]
- Van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O (2010): Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event‐related responses. Proc Natl Acad Sci USA 107:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2008): Automatic and controlled response inhibition: Associative learning in the go/nogo and stop‐signal paradigms. J Exp Psychol Gen 137:649–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2009): Automaticity of cognitive control: Goal priming in response‐inhibition paradigms. J Exp Psychol Learn Mem Cogn 35:1381–1388. [DOI] [PubMed] [Google Scholar]
- Wardak C (2011): The role of the supplementary motor area in inhibitory control in monkeys and humans. J Neurosci 31:5181–5183. [Google Scholar]
- Yeredor A (2000): Blind separation of Gaussian sources via second‐order statistics with asymptotically optimal weighting. IEEE Signal Process Lett 7:197–200. [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SF, Kahn RS, Vink M (2013): Expectations and violations: Delineating the neural network of proactive inhibitory control. Hum Brain Mapp 34:2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M (2010): On the role of the striatum in response inhibition. PLoS One 5:e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figures.


