Abstract
The ability to encode information into long‐term memory is not a passive process but can be influenced by motivational factors. While the mesolimbic system has long been associated with reward‐driven memory enhancement, the precise neurobiology of processing aversive events and their effects on declarative learning remain unclear. To address this issue, human subjects encoded a series of scene images, which was combined with cues predicting an aversive electric shock with different probabilities (0.2, 0.5, 0.8). Subsequently, recognition memory for the scenes was tested using a remember/know procedure. In a behavioral experiment, shock probability had linear effects on familiarity and inverted u‐shaped effects on recollection. While the behavioral effect was absent in experiment 2 (fMRI), at the neural level encoding‐related activity in the hippocampus mimicked the recollection specific quadratic effect, whereas activity in the anterior parahippocampal gyrus mirrored the familiarity specific linear relationship that was evident in experiment 1. Importantly, the probability of upcoming shocks was linearly coded in the substantia nigra / ventral tegmental area, and pain associated brain regions, such as the insula, responded to shock delivery. Our results demonstrate that anticipating primary aversive events recruits the human mesolimbic system and differentially modulates declarative memory functions via medial temporal lobe structures. Hum Brain Mapp 35:4594–4606, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: fMRI, mesolimbic system, aversive learning, recollection, familiarity
INTRODUCTION
The dopaminergic mesolimbic system, including substantia nigra / ventral tegmental area (SN/VTA), has long been associated with different forms of learning. Particularly, neurons in the SN/VTA and interconnected ventral striatum respond to rewards and to cues that predict their occurrence. Furthermore, they signal the difference between anticipated and received outcome (i.e. reward prediction errors) (Guitart‐Masip et al., 2010; Haber and Knutson, 2009; O'Doherty, 2004; Schultz et al., 1997; Tobler et al., 2005). Although these studies have led to the notion of a mesolimbic reward system, there is other work (mainly in animals) that questions the functional specificity of these neural structures (Horvitz, 2000; Lammel et al., 2012; Redgrave et al., 1999; Ungless, 2004; Zweifel et al., 2011). For instance, activity in the human SN/VTA signals the anticipation of painful heat stimuli (Fairhurst et al., 2007) and, in monkeys, SN/VTA activity to both appetitive stimuli and aversive air‐puff to the eye linearly scales as a function of outcome probability (Matsumoto and Hikosaka, 2009). Together with the fact that the ventral striatum also responds to noxious stimuli, such as thermal heat (Baliki et al., 2010; Becerra et al., 2001), this points towards common functional characteristics in processing aversive and appetitive events at the level of the dopaminergic mesolimbic system (Bromberg‐Martin et al., 2010; Wang and Tsien, 2011).
Behaviorally, the prospect of appetitive outcomes, such as monetary reward, can facilitate declarative memory formation. For instance, recognition memory for objects or photographs of scenes is facilitated when encoding is linked with the concurrent anticipation of a monetary win. This effect is mediated by activity changes in the SN/VTA, ventral striatum, and medial temporal lobe (MTL, including hippocampus and surrounding parahippocampal gyrus) (Adcock et al., 2006; Bunzeck et al., 2012; Wittmann et al., 2005), which conform to an established model claiming that the dopaminergic midbrain forms a functional loop with memory‐related MTL regions to support long‐term memory (Lisman and Grace, 2005; Lisman et al., 2011).
Although pain has long been known to impact on cognitive functions (Eccleston and Crombez, 1999; Kuhajda et al., 2002; Patil et al., 1995; Wiech, 2013) there is only little research on the neural mechanisms underlying the interaction between anticipating aversive events and long‐term memory formation. Studies in humans could show that pain typically impairs learning by reducing neural activity in the MTL when a painful stimulus is presented simultaneously with the studied event (Bingel et al., 2007; Forkmann et al., 2013). However, when presented shortly after encoding, aversive events can enhance recognition possibly as a result of increased arousal (Schwarze et al., 2012) (see also McCullough and Yonelinas, 2013). In the same vein, a recent behavioral study showed evidence that images that belonged to an object category that was conditioned with an electrical shock resulted in better recognition memory after a 24‐hour delay relatively to images that belonged to an object category that was not conditioned with an electrical shock (Dunsmoor et al., 2013). Thus, this study shows initial evidence that the anticipation of aversive events may have beneficial effects on recognition memory.
To our knowledge, there is only one recent fMRI study (Murty et al., 2012) investigating the link between aversive stimulation, declarative learning and the SN/VTA. Here, participants were motivated to successfully encode scene images to avoid subsequent electrical shocks during unsuccessful retrieval (i.e. avoidance learning). Cues that predicted possible punishments in case of subsequent retrieval failure were only associated with increased encoding‐related activity of the amygdala but not midbrain. As these and other studies (Mackiewicz et al., 2006; Murty et al., 2011) did not provide any evidence for the involvement of the human SN/VTA, it remains unclear whether it codes probability during the anticipation of aversive events as in animals, (Matsumoto and Hikosaka, 2009) and concurrently modulates recognition‐related encoding activity in downstream brain regions of the MTL.
The present study addressed these open questions in one behavioral (Experiment 1) and one combined behavioral / functional magnetic resonance imaging (fMRI) study (Experiment 2). Volunteers initially learned the association between three different visual cues and their probabilities (0.2, 0.5, or 0.8) to predict an electrical shock to the hand. Subsequently, subjects made indoor/outdoor judgments to a series of scene images that were surrounded by these visual cues and followed by an electric shock according to the cue's probability. Around 15–30 min after encoding, memory for the scenes was tested incidentally using a remember/know recognition task (Fig. 1). Various manipulations and neuroimaging findings provide evidence that remember and know judgments are associated with distinct processes (Diana et al., 2007; Eichenbaum et al., 2007; for caveats see Rotello et al., 2004). According to dual process models, information can be remembered based on recollection and familiarity (for review see Yonelinas, 2002). While recollection refers to the retrieval of contextual information of the episode, familiarity is based on recognition in absence of any contextual information (Yonelinas, 1995).
Figure 1.
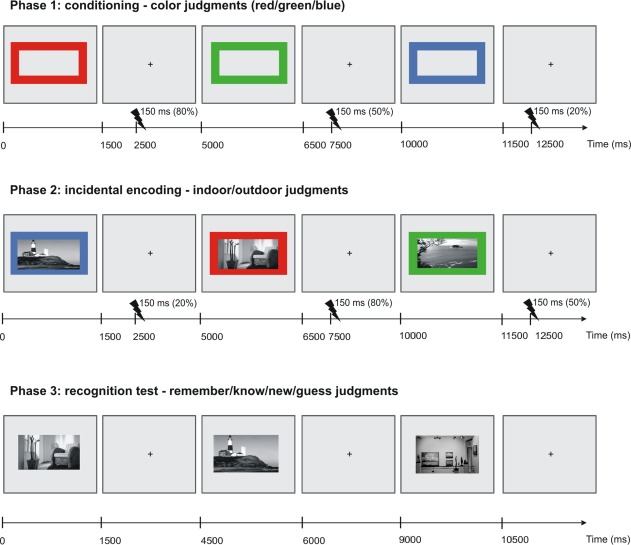
Experimental design. During the conditioning phase, three different colored picture frames (red, blue, and green) were presented for 1500 ms, followed by a fixation cross for 3500 ms (±500 ms). 1000 ms after the image offset an electrical shock was applied to the right hand according to the frame's probability (0.2, 0.5, 0.8). In a second phase, participants incidentally encoded images of scenes that were surrounded by these colored cues while making an indoor/outdoor judgment. Again an electrical shock was applied according to the frame's probability. After a delay, memory for the scene images was tested in a modified remember/know recognition task. In experiment 2, conditioning and encoding phase took place in the MRI scanner (see experimental procedures for details). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We predicted SN/VTA activity to the cues to be scaled as a function of shock probability (Matsumoto and Hikosaka, 2009) and differential effects of pain anticipation on recollection and familiarity (Tulving, 2002). According to dual process models of recognition, recollection closely relates to the integrity of the hippocampus and familiarity to the surrounding anterior parahippocampal gyrus (Diana et al., 2007). As a result of the fact that both brain regions receive dopaminergic afferents from the SN/VTA, but with different strengths (Fields et al., 2007), the anticipation of aversive events may modulate recollection and familiarity and the underlying activation patterns differently.
MATERIALS AND METHODS
All participants were healthy, right‐handed and had normal or corrected‐to‐normal vision. None of them reported a history of neurological, psychiatric, or medical disorders or any current medical problems. Each subject gave written informed consent according to the approval of the local ethics committee (medical association Hamburg).
Experiment 1
Participants
Sixteen participants took part in Experiment 1 (mean age: 26; age range: 20–33; nine women).
Task
The experiment consisted of a conditioning phase, an encoding phase, and an incidental recognition task (see Fig. 1). During the conditioning phase, 40 green, 40 red, and 40 blue colored, rectangular picture frames served as cues and were randomly intermixed. In each trial, a cue (i.e. frame) was presented for 1.5 s in the center of the computer screen and followed by an electric shock to the hand with different probabilities (0.2, 0.5, and 0.8). The contingencies between color and shock probability were randomly assigned for each subject. A colored cue was always followed by a fixation cross in central vision for 3.5 s. At 1 s after the offset of the picture frame, an electrical shock containing a train of three pulses of 2 ms duration each intermittent with a 50 ms interval was applied to the back of the right hand according to the frame's probability. At encoding and conditioning, the duration of each trial was 5 s. The participants were asked to respond as quickly and accurately as possible.
For each subject, the individual pain threshold was determined before the experiment. An electrical shock that was rated with an intensity of seven on a rating scale ranging from 0 (i.e. electrical stimulation is not perceptible) to 10 (i.e. electrical stimulation is intolerable) was used as nociceptive stimulus throughout the experiment. During the conditioning phase, volunteers were asked to make a color judgment by pressing one of three buttons according to the response assignment, which was counterbalanced across participants. The conditioning consisted of two blocks with 60 items and took in total approximately 12 min.
During encoding, the trial structure and timing was identical to the conditioning phase but this time each colored frame surrounded a trial unique photograph of a scene. Volunteers were asked to make indoor/outdoor judgments as quickly and as accurately as possible. The response buttons for indoor and outdoor judgments were counterbalanced across participants. The encoding phase was split into three blocks of 50 scenes resulting in 150 images being encoded (i.e., 25 indoor and 25 outdoor scenes for each of the three shock probability conditions). The images were gray‐scaled and normalized to a mean value of 127 (SD = 75, RGB‐space 0–255).
Around 15 min after encoding ended, participants saw all studied items intermixed with 50 unstudied (new) items (25 indoor and 25 outdoor scenes) and judged their old/new status in a modified version of the remember/know task. To dissociate between recognition based on recollection and familiarity, subjects were asked to make a remember/know judgment by pressing one of four buttons: if subjects were confident that they have seen the item before and could recollect any specific details about the encoding episode, they were instructed to give a “remember” response. If the item was familiar and participants recognized the picture without recollecting specific details, they were asked to press the “know” button. If they were confident that the image has not been studied, they had to give a “new” response. To minimize contamination by successful guesses, participants were asked to press the “guess” button when they were not confident about whether they had seen the scene previously or not. The mapping of the responding fingers (i.e. index finger, middle finger, ring finger, and little finger of the right hand) and corresponding decisions (i.e. remember, know, guess, new) was randomized across subjects. Again, both accuracy and speed were stressed. The recognition test consisted of four blocks of 50 scenes. All items were presented in central vision on a grey background for 1.5 s.
Behavioral data analyses
The analysis of recognition memory performance was based on the assumption that recollection and familiarity are functionally distinct processes (Yonelinas, 1995). Recollection for each shock probability was calculated as the probability of pressing the “remember” button (R) corrected for the probability of incorrectly making a “remember” response to new items (False alarm [Fa R]). In other words, recollection was estimated by the following equation: recollection = R – Fa R. Familiarity was calculated as the probability of “know” responses (K) corrected for the probability of making “know” responses to new items (Fa K) and corrected for the fact that “know” responses were given in the absence of recollection: familiarity = (K‐Fa K)/(1‐R) (Yonelinas, 2002). Greenhouse‐Geisser corrected degrees of freedom (df) and P values are reported whenever a factor had more than two levels (Keselman and Rogan, 1980).
Experiment 2
The identical experimental paradigm and behavioral data analyses as in Experiment 1 were applied in the fMRI study. Differences between both experiments are highlighted.
Participants
Twenty four participants took part in the fMRI experiment (mean age: 26; age range: 20–31; 11 women).
Task
Only the conditioning and encoding phase took place and were scanned inside the MRI scanner where all stimuli were presented via a mirror system attached to the head coil of the scanner. Anatomical scans were acquired after the encoding phase. After a delay of approximately 30 min, memory for the indoor and outdoor scenes was tested outside of the scanner.
fMRI data acquisition
fMRI acquisition was performed on a 3 tesla system (Siemens Trio) with echo planar imaging. During functional imaging, 48 T2*‐weighted images per volume (i.e., covering whole head) with BOLD contrast were obtained (matrix, 64 × 64; 48 oblique axial slices per volume angled at −30° along the anteroposterior axis; spatial resolution, 2 × 2 × 2 mm; TR = 2870 ms; TE = 25 ms). For each subject, functional MRI data at encoding were acquired in three scanning sessions consisting of 114 volumes per session. Six additional volumes were recorded at the beginning of each block to allow for steady‐state magnetization; these were excluded from the analyses.
At the end of the experiment, anatomical images of each subject's brain were collected using multi‐echo three‐dimensional FLASH (fast low angle shot) acquisition for mapping proton density, T1, and magnetization transfer (MT) at 1‐mm3 resolution (Weiskopf and Helms, 2008).
fMRI data analysis
Pre‐processing procedures included realignment of all fMRI images to the first volume, unwarping, spatial normalizing to the Montreal Neurology Institute space, and smoothing with a 4 mm Gaussian kernel using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The fMRI time series data were high‐pass filtered (cutoff = 128 s) and whitened using an AR(1) model.
For each subject, two first‐level analyses were computed by applying a canonical hemodynamic response function combined with time and dispersion derivatives (Friston et al., 1998). In the first model, we defined five regressors: pain anticipation for each shock probability (i.e., three regressors: 0.2, 0.5, and 0.8), one regressor for shock delivery and one regressor for trials with incorrect responses (i.e. errors). Note that the order of the trials was fully randomized, and that the contingency between the cues and the shock delivery was not 100%, which allowed us to disentangle the hemodynamic responses associated with different conditions.
The second first‐level model aimed to capture neural activity predictive of subsequent memory performance separate for “remember” and “know” responses (i.e. so called “DM‐effect”: difference because of later memory (Paller et al., 1987)). Here, 11 regressors were defined: subsequent “remember” hits for each probability (i.e. three regressors), subsequent “know” hits for each probability (i.e. three regressors), subsequent misses (i.e. “new” or “guess” responses) for each probability condition (i.e. three regressors), one regressor for shock delivery and one regressor for error trials (i.e. incorrect indoor/outdoor decision or no response during encoding). To capture residual movement‐related artifacts, six covariates were included (the three rigid‐body translation and three rotations resulting from realignment) as regressors of no interest in both models.
The contrast images resulting from both first‐level analyses were entered into four separate second‐level random‐effects analyses. To test for pain anticipation effects, in one model the hemodynamic effects of each probability condition were entered into a one‐way ANOVA with the factor shock probability (0.2, 0.5, and 0.8) allowing us to test for linear and inverted u‐shaped effects (based on behavioral results, experiment 1). A one‐sample t‐test was used to assess brain regions involved in outcome activity (i.e. when the shock was presented vs. implicit baseline).
Further two second‐level analyses were also based on one‐way ANOVAs with the factor shock probability (0.2, 0.5 and 0.8) and included the DM‐effect contrast (hits vs. misses) for each probability condition either for “remember” or “know” responses, respectively. These ANOVAs allowed us to identify brain regions showing either an inverted u‐shaped or linear effect (see results). Note that the ANOVA for “know” responses was limited to only seven subjects, which contained at least 14 trials per probability condition.
All contrasts were initially thresholded at P < 0.001 (uncorrected) and corrected for multiple comparisons using small volume correction (P < 0.05, family‐wise error [FWE]‐correction) for a priori regions of interest where we hypothesized the effects (i.e. bilateral SN/VTA, bilateral MTL [i.e. bilateral hippocampus and bilateral parahippocampal cortex], bilateral amygdala and one mask for brain regions that are involved in processing nociceptive and/or salient sensory events including bilateral insula, anterior and middle cingulate cortex, post central gyrus (Iannetti and Mouraux, 2010). The masks for the regions of interest were defined using the WFU‐Pickatlas (Maldjian et al., 2003). FWE was used as implemented in SPM8 (Brett et al., 2004).
The sources of the effects were localized by overlaying the SPMs on T1‐ and MT‐weighted group images, which were generated by averaging all normalized T1 or MT images, respectively (spatial resolution of 1 × 1 × 1 mm). The MT‐images allow a more precise distinction between the SN/VTA, which appears as bright stripe, and surrounding brain structures (Bunzeck and Duzel, 2006; Bunzeck et al., 2007; Eckert et al., 2004). The T1‐weighted template was used for the localization of effects outside of the midbrain.
RESULTS
Experiment 1
Behavioral results
At encoding, discrimination performance between indoor and outdoor scenes for shock probabilities of 0.2, 0.5, and 0.8 was high [mean accuracy: 0.97 (SD = 0.03), 0.97 (SD = 0.02), 0.96 (SD = 0.04)]. A 3 × 1 ANOVA with shock probability as within‐subject factor revealed no difference between conditions (P = 0.422). Mean reaction times (RT) for shock probabilities of 0.2, 0.5, and 0.8 were 835 ms (SD = 222 ms), 854 ms (SD = 261 ms) and 851 ms (SD = 224 ms). Mean RTs are listed in Table 1. A 3 × 1 ANOVA with the within‐subject factor shock probability was not significant (P = 0.371).
Table 1.
RT (ms) in recognition test for indoor and outdoor scenes associated with a shock probability of 0.2, 0.5, and 0.8 in experiment 1 (n = 16)
| Probability | Item type | Remember | Know | New | Guess |
|---|---|---|---|---|---|
| 0.8 | Old | 1259 (208) | 1559 (416) | 1490 (408) | 1818 (480) |
| 0.5 | Old | 1307 (273) | 1552 (343) | 1453 (226) | 1854 (498) |
| 0.2 | Old | 1191 (264) | 1489 (343) | 1453 (226) | 1823 (524) |
| New | 449 (723) | 1077 (778) | 1044 (423) | 1668 (350) |
Values are across‐subject means (SD).
Recognition memory performance is listed in Table 2; all analyses are based on corrected hit rates (see methods). In a first statistical analysis, we performed a 3 × 2 ANOVA with the within‐subject factors shock probability (0.2/0.5/0.8) and memory type (recollection/familiarity). It revealed a significant main effect of shock probability (F(1.87, 27,97) = 8.10; P = 0.002) and a significant interaction between shock probability and memory type (F(1.98, 29.68) = 3.49; P = 0.044). Two separate 3 × 1 ANOVAs, including memory performance either on recollection or familiarity, were conducted to understand the nature of the interaction (see Fig. 2). The analysis on recollection revealed a significant shock probability effect (F(1.49, 22.30) = 9.12; P = 0.003) that was driven by a quadratic relationship (F(1,15)=12.27; P = 0.003; linear effect: P = 0.481). Follow‐up paired t‐tests revealed that recollection for a shock probability of 0.5 differed significantly from a shock probability of 0.2 (t(15) = 3.17; P = 0.006) and 0.8 (t(15) = −3.55; P = 0.003), but there was no difference between 0.2 vs. 0.8 (P > 0.05). The ANOVA on familiarity‐based recognition resulted in a significant effect of shock probability (F(1.85, 27.72) = 3.87; P = 0.036) that was driven by a statistically significant linear increase in recognition memory performance as a function of shock probability (Fig. 2) (F(1,15) = 6.48; P = 0.022; quadratic effect: P = 0.282). Follow‐up paired sample t‐tests on familiarity showed a higher rate for a shock probability of 0.5 and 0.8 relatively to 0.2 (t(15) = 2.59; P = 0.021; t(15) = 2.55; P = 0.022) but no difference between 0.5 vs. 0.8 (P > 0.05).
Table 2.
Recognition performance for indoor and outdoor scenes associated with a shock probability of 0.2, 0.5 and 0.8 in experiment 1 (n = 16)
| Probability | Item type | Remember | Know | New | Guess |
|---|---|---|---|---|---|
| 0.8 | Old | 0.24 (0.14) | 0.25 (0.15) | 0.18 (0.14) | 0.33 (0.13) |
| 0.5 | Old | 0.29 (0.18) | 0.24 (0.13) | 0.18 (0.12) | 0.29 (0.14) |
| 0.2 | Old | 0.23 (0.12) | 0.22 (0.12) | 0.20 (0.15) | 0.35 (0.14) |
| New | 0.02 (0.03) | 0.07 (0.07) | 0.59 (0.25) | 0.32 (0.17) |
Values represent proportions of remember, know, new and guess responses across‐subject means (SD).
Figure 2.
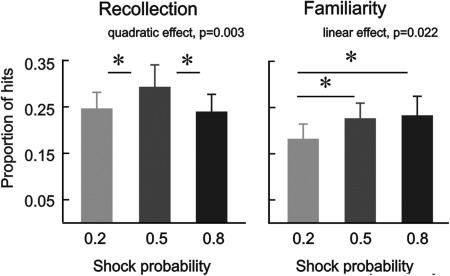
Recognition performance associated with recollection (i.e., R – Fa R) and familiarity (i.e., (K‐Fa K)/(1‐R)) in experiment 1. Pain anticipation modulated recollection following an inverted u‐shape function and subsequent familiarity increased linearly as a function of shock probability. Error‐bars denote one standard error of the mean; asterisks indicate statistically significant differences (P < 0.05).
A 3 × 2 ANOVA on RTs consisting of shock probability and memory as within‐subject factors revealed neither a shock probability effect nor an interaction with memory (P's > 0.126).
A subsequent analysis on the effect of shock outcome on recognition memory revealed no significant results. The ANOVA consisting of the within‐subject factors shock outcome (shock trials/no‐shock trials) and memory type (recollection/familiarity) did not result in significant effects of shock outcome (P = 0.422). In the same vein, a three‐way ANOVA with shock probability (0.2/0.5/0.8) as additional within‐subject factor revealed no interaction with shock outcome (P's > 0.470) indicating that the effects of shock probability on recollection and familiarity are associated with pain anticipation rather than with the actual shock outcome.
Experiment 2—fMRI
Behavioral results
Subjects discriminated between indoor and outdoor scenes with an average hit rate of 0.96 for all three shock probabilities (SD for 0.2 = 0.07; SD for 0.5 = 0.06; SD for 0.8 = 0.8) and there was no significant difference between conditions (P = 0.991). RT during encoding for the shock probability of 0.2, 0.5, and 0.8 was 787 ms (SD = 126 ms), 777 ms (SD = 100 ms) and 779 ms (SD = 122 ms), respectively; there was no significant difference between conditions (P = 0.441). Both analyses were based on 3 × 1 ANOVA's.
Recognition performance is shown in Table 3. A 3 × 2 ANOVA with the within‐subject factors shock probability (0.2/0.5/0.8) and memory type (recollection/familiarity) did not result in any significant effects (all P's > 0.303). Separate analyses for recollection and familiarity also did not reveal any significant effects of shock probability (all P's > 0.451). The ANOVA on RTs at retrieval with the within‐subject factors probability and memory type did not reveal any significant effect (P > 0.05); RT's are listed in Table 4.
Table 3.
Recognition performance for indoor and outdoor scenes associated with a shock probability of 0.2, 0.5, and 0.8 in experiment 2 (n = 24)
| Probability | Item type | Remember | Know | New | Guess |
|---|---|---|---|---|---|
| 0.8 | Old | 0.27(0.18) | 0.27 (0.14) | 0.26 (0.15) | 0.20 (0.11) |
| 0.5 | Old | 0.29 (0.19) | 0.25 (0.14) | 0.23 (0.13) | 0.23 (0.12) |
| 0.2 | Old | 0.28 (0.18) | 0.28 (0.15) | 0.22 (0.14) | 0.22 (0.11) |
| New | 0.02 (0.02) | 0.07 (0.07) | 0.65 (0.19) | 0.26 (0.17) |
Values represent proportions of remember, know, new and guess responses across‐subject means (SD).
Table 4.
RT (ms) in recognition test for indoor and outdoor scenes associated with a shock probability of 0.2, 0.5, and 0.8 in experiment 2 (n = 24)
| Probability | Item type | Remember | Know | New | Guess |
|---|---|---|---|---|---|
| 0.8 | Old | 1236 (365) | 1643 (380) | 1653 (367) | 1880 (405) |
| 0.5 | Old | 1360 (338) | 1653 (376) | 1638 (432) | 1846 (371) |
| 0.2 | Old | 1344 (291) | 1661 (488) | 1660 (446) | 1903 (500) |
| New | 706 (722) | 1329 (838) | 1563 (324) | 1718 (521) |
Values are across‐subject means (SD).
The ANOVA including the within‐subject factors shock outcome (shock trials/no‐shock trials) and memory type (recollection/familiarity) did not reveal significant effects of shock outcome (all P's > 0.246). Similarly, a three‐way ANOVA with shock probability (0.2/0.5/0.8) as additional within‐subject factor revealed no interaction with shock outcome (P's > 0.181).
Imaging results
In a first analysis, we were interested in those brain regions expressing a linear increase (t‐contrast: −1 0 1) in activity as a function of shock probability. Within our a priori defined regions of interest (SN/VTA, MTL, and pain associated brain regions—see Methods), such a response pattern was observed in the SN/VTA complex (2, −20, −14; SVC‐FWE P = 0.039, Fig. 3A) and right post‐central gyrus (62, −18, 32; SVC‐FWE P = 0.022, Fig. 3B). In contrast, we did not observe a probability effect following an inverted u‐shaped function (t‐contrast: −1 2 −1) at a liberal threshold of P = 0.001 (uncorrected). Although there was no effect of shock probability in the amygdala (linear P = 0.333; quadratic P = 0.535), we observed a significant main effect of pain anticipation across all three shock probabilities in the left amygdala (−22, 0, −12; SVC‐FWE P < 0.001; Fig. 3C).
Figure 3.
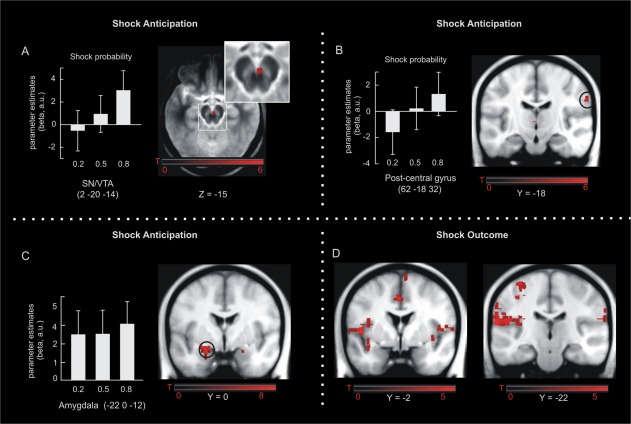
fMRI results. Shock anticipation was associated with linear activity increases in the SN/VTA (A) and right post‐central gyrus (B). The amygdala responded to all three cues but its activity was not scaled according to their probability (C). Activity changes in response to shock delivery were observed in bilateral insula, anterior/middle cingulate cortex, and left post‐central gyrus (D). Maps of activations are superimposed on an MT group template (A) and on a T1 group template in (B‐D). Error‐bars denote one standard error of the mean. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
At outcome, significant activations were found in the anterior/middle cingulate cortex (−2, 18, 34; SVC‐FWE P < 0.001), bilateral insula (−36, −18, 16; SVC‐FWE P < 0.001) and left post‐central gyrus (−64, −22, 16; SVC‐FWE P < 0.001, Fig. 3D). These effects concord with previous studies on painful or otherwise salient somatosensory perception (Iannetti and Mouraux, 2010; see Discussion).
In a final step, we assessed those brain regions associated with successful memory encoding. A DM‐effect for “remember” responses that followed an inverted u‐shaped response (i.e. stronger DM‐effects in the 0.5 vs. 0.2 and 0.8 probability condition) was observed in the right posterior hippocampus (20, −34, 6; SVC‐FWE P = 0.04, Fig. 4A) and bilateral post‐central gyrus (−34, −34, 64; SVC‐FWE P = 0.004). There were no significant linear remember DM‐effects in our regions of interest. For subsequent “know” responses, there were linear effects in the left anterior parahippocampal gyrus (−20, 2, −24; SVC‐FWE P = 0.023, Fig. 4B) but there were no significant quadratic effects.
Figure 4.
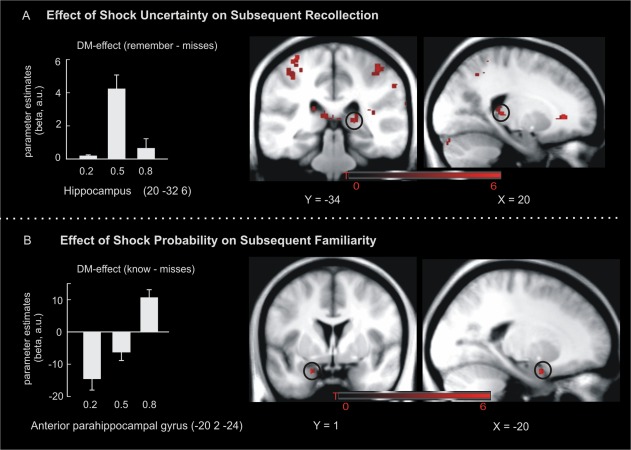
fMRI activations during encoding predict subsequent memory. DM‐effects for subsequent recollection were evident in the right posterior hippocampus and this activity pattern followed an inverted u‐shape (A). DM‐effects for subsequent familiarity (i.e., know‐misses) were observed in the left anterior parahippocampal gyrus and this activity increased linearly with shock probability (B). Activation maps are superimposed on a T1 group template. Error‐bars denote one standard error of the mean. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The present study sought to elucidate the neural mechanisms of pain anticipation and its effect on long‐term memory formation. Behaviorally, the anticipation of an electric shock to the hand differentially modulated subsequent recollection‐ and familiarity‐based recognition in experiment 1, which was paralleled by distinct neural correlates in the MTL in experiment 2. More precisely, familiarity scores (experiment 1) and the DM‐effect in the anterior parahippocampal gyrus (experiment 2) increased linearly with shock probability. In contrast, subsequent recollection was modulated by shock probability following an inverted u‐shaped function (i.e. highest scores for 0.5 probability) and this effect was mimicked by activity in the posterior part of the hippocampus in experiment 2.
The SN/VTA is the origin of the dopaminergic mesolimbic system and has often been associated with reward‐based learning. Recent work, however, questioned the reward specific role of the SN/VTA by demonstrating its responsiveness to aversive or otherwise salient stimuli (Bromberg‐Martin et al., 2010; Bunzeck and Duzel, 2006; Fairhurst et al., 2007; Lammel et al., 2012; Redgrave et al., 1999; Ungless, 2004; Wang and Tsien, 2011; Zweifel et al., 2011). Here, we can show in humans that SN/VTA activity increases to cues predicting the delivery of electric shocks. Importantly, this activity increased as a function of shock probability (Fig. 3A), which mirrors well‐known coding strategies of midbrain dopamine neurons during reward anticipation (Fiorillo et al., 2003; Schultz et al., 1997; Tobler et al., 2005). Moreover, our findings are coherent with recent cell recordings in monkeys, reporting similar activity patterns in the SN/VTA in response to the anticipation of aversive stimuli and appetitive events (Matsumoto and Hikosaka, 2009). Hence, these and our current findings provide compelling evidence that the SN/VTA signals the anticipation of appetitive and aversive events following similar coding parameters, which, in turn, argues in favor of a much more general role of the mesolimbic system in salience processing across species. As our design did not include reward conditions, our data cannot address the question whether the same or different neural populations signal both appetitive and aversive values (for a review, see e.g. Lammel et al., 2013).
It should be noted that the role of dopamine neurons in processing aversive events is an open debate. Particularly, on the basis of cell recordings in animals Fiorillo (2013) recently claimed that dopaminergic midbrain neurons do not signal aversiveness. Although fMRI recordings are less sensitive to allow clear conclusions regarding specific cell types, our findings of linear increases to shock probability in the SN/VTA concords with others arguing in favor of a dopaminergic mechanism (e.g. Matsumoto and Hikosaka, 2009).
Additionally to the SN/VTA, a small portion of the right post‐central gyrus was activated in response to pain anticipation and scaled as a function of shock probability. In contrast, a relatively larger activation pattern, consisting of left post‐central gyrus, bilateral insular, and anterior/midcingulate cortex, was only evident during shock delivery. Among others, these regions are part of a complex brain network (called “pain matrix” or “neuromatrix”), which is known to be activated in response to nociceptive or otherwise salient somatosensory stimuli (Apkarian et al., 2005; Iannetti and Mouraux, 2010; Legrain et al., 2011; Tracey and Mantyh, 2007; Tracey, 2008). These findings are important for two reasons: first, they indicate the preference of this functional network to signal outcome rather than anticipation of electric shocks with respect to their probability. And second, the differential activation pattern across brain regions (i.e. SN/VTA vs. “pain matrix”) demonstrates that we were able to disentangle the effects of pain anticipation and shock delivery.
Consistent with our second prediction, shock anticipation affected subsequent recognition memory and encoding‐related activity in the MTL. More specifically, in experiment 1, the relationship between shock probability and subsequent recollection followed an inverted u‐shaped function, whereby recollection scores were highest in the 0.5 as compared to the 0.2 and 0.8 probability conditions. At the neural level (experiment 2), this effect was mimicked by activity in the posterior hippocampus, whereby encoding‐related DM‐effects (i.e., remember vs. misses) increased for subsequently remembered images that were encoded in the context of maximal shock uncertainty (i.e. P = 0.5). In contrast, familiarity scores increased linearly as a function of shock probability (experiment 1) and this effect was paralleled by linear changes in the strength of probability dependent DM‐effects in the left anterior parahippocampal gyrus (experiment 2). While previous studies have also reported beneficial effects of aversive events on long‐term memory (Murty et al., 2012; Schwarze et al., 2012), to our knowledge, this is the first study to demonstrate differential effects of pain anticipation on recollection and familiarity‐based recognition.
In anatomical terms, the behavioral effects of shock anticipation can be reconciled on the basis of dual‐process models. They suggest that the MTL supports memory for events and that sub‐regions can be linked with different psychological functions (Diana et al., 2007; Eichenbaum et al., 2007). Accordingly, the hippocampus integrates item with contextual information giving rise to recollection; the surrounding anterior parahippocampal structures (including the ento‐ and perirhinal cortex), on the other hand, seem to support item memory independent of specific contextual associations, i.e. recognition based on familiarity. Both brain regions (i.e. hippocampus and anterior parahippocampal gyrus) receive afferents from midbrain dopamine neurons with richer dopamine projections to the entorhinal cortex (46%) as compared to the hippocampus (6–18%) (Fields et al., 2007), which might be one physiological reason for the differential effects. However, given the absence of a clear link between SN/VTA and MTL activity in our data, such a relationship remains highly speculative.
The quadratic effect of shock probability on recollection could also be explained on the basis of studies showing an association between emotional arousal and hippocampus‐dependent memory dysfunctions (e.g. Murray and Kensinger, 2013; Murty et al., 2011). They suggest that in a state of high emotional arousal (i.e. high shock probability) memory formation can be impaired because of the inhibition of hippocampal responses. Although the neural response pattern in the hippocampus is partly compatible with such a conclusion, there was no differential effect of shock probability in the amygdala (a brain region often associated with emotional processing) arguing against such an account.
Alternatively, the quadratic effect of shock probability on recollection may be explained by computational frameworks suggesting a tight link between expected uncertainty and cholinergic neuromodulation (Yu and Dayan, 2002, 2005). Accordingly, acetylcholine varies monotonically with the uncertainty induced by the validity of a cue (i.e. it is highest in the 0.5 condition) (Yu and Dayan, 2005) and as a result potentially influences plasticity in the hippocampus, which receives prominent input from the cholinergic basal forebrain (for a review see Hasselmo, 2006). Therefore, one could speculate that the quadratic effect on recollection could be driven by uncertainty dependent cholinergic mechanisms.
Our results are only partly in line with a recent fMRI study investigating the involvement of the dopaminergic midbrain and amygdala in avoidance learning (Murty et al., 2012). Specifically, Murty and colleagues found increased anticipatory activation of the right amygdala but not SN/VTA, indicating that emotional arousal may be the driving mechanism in aversive learning. In contrast, our data indicate an important role of the SN/VTA rather than amygdala in these processes. However, there are at least three experimental differences between both studies that can account for these diverging results. First, we manipulated shock probabilities while there was no such condition in the study by Murty et al. Second, while there was actually no shock delivery in Murty et al., the opposite is true for our design. Third, in Murty et al. participants were motivated to successfully encode scene images to avoid subsequent punishment. Our study employed a Pavlovian conditioning paradigm similar to animal studies investigating the involvement of the dopaminergic midbrain in appetitive and aversive learning (Bromberg‐Martin et al., 2010).
Although one might argue that our paradigm did not involve a direct manipulation of motivational states during encoding, the memory effects in experiment 1 and probability effects in the SN/VTA (experiment 2) point towards a motivational mechanism rather than effects of emotional arousal (Murty et al., 2012). This interpretation also conforms to the observation that the amygdala responded to shock predicting cues (Fig. 3) but did not scale according to their probability. Furthermore, the amygdala activity did not mimic the behavioral effects of shock probability on recollection or familiarity (in experiment 1) nor did it express DM‐effects. Together, our findings are not mutually exclusive with the study by Murty et al. and they demonstrate that the SN/VTA signals probability during the anticipation of acute aversive events possibly in support of motivation‐related memory formation.
Finally, there was no modulation of recognition memory by shock probability in experiment 2 (i.e. when encoding took place in the fMRI scanner). The most likely explanation here is that the scanner itself induced arousal and/or anxiety. This, in turn, increases the level of adrenal stress hormones, which could have interfered with the effects of shock anticipation on subsequent recognition (Eatough et al., 2009; Muehlhan et al., 2011; Peters et al., 2011; Tessner et al., 2006; Tornqvist et al., 2006). Alternatively, in experiment 1, the encoding and the retrieval context were identical (subjects learned and were tested in the same room), whereas for experiment 2 they were different (subjects encoded in the fMRI and were tested outside). Such changes in context can influence memory performance (Godden and Baddeley, 1975) and might also affect shock probability effects on recognition memory. On a similar note, we acknowledge that the analysis of familiarity‐related activity in experiment 2 was restricted to a relatively small number of participants with a sufficient number of “know” trials. However, those effects survived FWE corrections (P < 0.05) strongly supporting their validity. Nonetheless, future studies are needed to confirm and support our findings and to address open questions.
Taken together, our findings demonstrate that the probability of upcoming aversive events is linearly coded in the SN/VTA indicating common functional properties of the mesolimbic system in aversive and appetitive learning. Behaviorally, shock probability had linear effects on familiarity and inverted u‐shaped effects on recollection. Although absent in the MRI scanner, these specific effects were paralleled by encoding‐related hemodynamic activity in the anterior parahippocampal gyrus and posterior hippocampus, respectively, suggesting a possible link. As such, our results point towards a previously unreported mechanism, whereby pain anticipation differentially modulates recollection and familiarity‐based recognition memory via MTL activity. From a more general point of view, these findings underline that pain not only has detrimental consequences but instead can facilitate cognitive functions as a result of enhanced motivational states.
ACKNOWLEDGMENTS
We thank Dr. Tobias Sommer‐Blöchl for helpful discussions and comments on a previous version of the manuscript.
REFERENCES
- Adcock RA, Thangavel A, Whitfield‐Gabrieli S, Knutson B, Gabrieli JDE (2006): Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron 50:507–517. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R‐D, Zubieta J‐K (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–463. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV (2010): Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D (2001): Reward circuitry activation by noxious thermal stimuli. Neuron 32:927–946. [DOI] [PubMed] [Google Scholar]
- Bingel U, Rose M, Gläscher J, Büchel C (2007): fMRI Reveals How Pain Modulates Visual Object Processing in the Ventral Visual Stream. Neuron 55:157–167. [DOI] [PubMed] [Google Scholar]
- Brett M, Penny W, Kiebel S (2004): Introduction to random field theory In: Human Brain Function. Oxford: Elsevier; pp 867–880. [Google Scholar]
- Bromberg‐Martin ESMatsumoto M, Hikosaka O (2010):Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Doeller CF, Dolan RJ, Duzel E (2012): Contextual interaction between novelty and reward processing within the mesolimbic system. Hum Brain Mapp 33:1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E (2006): Absolute coding of stimulus novelty in the human substantia Nigra/VTA. Neuron 51:369–379. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Schütze H, Stallforth S, Kaufmann J, Düzel S, Heinze H‐J, Düzel E (2007): Mesolimbic novelty processing in older adults. Cereb Cortex 17:2940–2948. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart‐Masip M, Bunzeck N, Dolan RJ, Düzel E (2012): Dopamine modulates episodic memory persistence in old age. J Neurosci 32:14193–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M (2011): Inverted‐U‐shaped dopamine actions on human working memory and cognitive control. Biol Psychiatr 69, e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C (2007): Imaging recollection and familiarity in the medial temporal lobe: a three‐component model. Trends Cogn Sci 11:379–386. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Martin A, LaBar KS (2012): Role of conceptual knowledge in learning and retention of conditioned fear. Biol Psychol 89:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD (2009): Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology 34:1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Crombez G (1999): Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychol Bull 125:356–366. [DOI] [PubMed] [Google Scholar]
- Eckert T, Sailer M, Kaufmann J, Schrader C, Peschel T, Bodammer N, Heinze HJ, Schoenfeld MA (2004): Differentiation of idiopathic Parkinson's disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 21:229–235. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C (2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P, Tracey I (2007): Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 128:101–110. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM (2007): Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci 30:289–316. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD (2013): Two dimensions of value: Dopamine neurons represent reward but not aversiveness. Science 341:546–549. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W (2003): Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299:1898–1902. [DOI] [PubMed] [Google Scholar]
- Forkmann K, Wiech K, Ritter C, Sommer T, Rose M, Bingel U (2013): Pain‐specific modulation of hippocampal activity and functional connectivity during visual encoding. J Neurosci 33:2571–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998): Event‐related fMRI: Characterizing differential responses. NeuroImage 7:30–40. [DOI] [PubMed] [Google Scholar]
- Godden DR, Baddeley AD (1975): Context‐dependent memory in two natural environments: On land and underwater. Br J Psychol 66:325–331. [Google Scholar]
- Goldman‐Rakic P., Muly I, Williams G (2000): D1 receptors in prefrontal cells and circuits. Brain Res Rev 31:295–301. [DOI] [PubMed] [Google Scholar]
- Guitart‐Masip M, Bunzeck N, Stephan KE, Dolan RJ, Düzel E (2010): Contextual novelty changes reward representations in the striatum. J Neurosci 30:1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2009): The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME (2006): The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16:710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz J (2000): Mesolimbocortical and nigrostriatal dopamine responses to salient non‐reward events. Neuroscience 96:651–656. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A (2010): From the neuromatrix to the pain matrix (and back). Experimental Brain Research 205:1–12. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Rogan JC (1980): Repeated measures F tests and psychophysiological research: controlling the number of false positives. Psychophysiology 17:499–503. [DOI] [PubMed] [Google Scholar]
- Kuhajda MC, Thorn BE, Klinger MR, Rubin NJ (2002): The effect of headache pain on attention (encoding) and memory (recognition). Pain 97:213–221. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Wolf OT, Fink GR (2008): Increased cortisol levels in cognitively challenging situations are beneficial in young but not older subjects. Psychopharmacology 201:293–304. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC (2013): Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC (2012): Input‐specific control of reward and aversion in the ventral tegmental area. Nature 491:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011): The pain matrix reloaded. Prog Neurobiol 93:111–124. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E (2011): A neoHebbian framework for episodic memory; role of dopamine‐dependent late LTP. Trends Neurosci 34:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA (2005): The Hippocampal‐VTA loop: Controlling the entry of information into long‐term memory. Neuron 46:703–713. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB (2006): The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. PNAS 103:14200–14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2009): Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459:837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Yonelinas AP (2013): Cold‐pressor stress after learning enhances familiarity‐based recognition memory in men. Neurobiol Learn Mem 106:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen H‐U, Kirschbaum C (2011): The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int J Psychophysiol 79118–126. [DOI] [PubMed] [Google Scholar]
- Murray BD, Kensinger EA (2013): A review of the neural and behavioral consequences for unitizing emotional and neutral information. Front Behav Neurosci 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, LaBar KS, Adcock RA (2012): Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. J Neurosci 32:8969–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, LaBar KS, Hamilton DA, Adcock RA (2011): Is all motivation good for learning? Dissociable influences of approach and avoidance motivation in declarative memory. Learn Mem 18:712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP (2004): Reward representations and reward‐related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol 14:769–76. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR (1987): Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol 67:360–71. [DOI] [PubMed] [Google Scholar]
- Patil PG, Apfelbaum JL, Zacny JP (1995): Effects of a cold‐water stressor on psychomotor and cognitive functioning in humans. Physiol Behav 58:1281–1286. [DOI] [PubMed] [Google Scholar]
- Peters S, Cleare AJ, Papadopoulos A, Fu CHY (2011): Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoneuroendocrinology 36:737–741. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K (1999): Is the short‐latency dopamine response too short to signal reward error? Trends Neurosci 22:146–51. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Schwarze U, Bingel U, Sommer T (2012): Event‐related nociceptive arousal enhances memory consolidation for neutral scenes. J Neurosci 32:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S (2006): Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp 27:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W (2005): Adaptive coding of reward value by dopamine neurons. Science 307:1642–1645. [DOI] [PubMed] [Google Scholar]
- Tornqvist E, Mansson A, Larsson E‐M, Hallstrom I (2006): It's like being in another world—patients' lived experience of magnetic resonance imaging. J Clin Nurs 15:954–961. [DOI] [PubMed] [Google Scholar]
- Tracey I (2008): Imaging pain. Br J Anaesth 101:32–39. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW (2007): The cerebral signature for pain perception and its modulation. Neuron 55:377–391. [DOI] [PubMed] [Google Scholar]
- Tulving E (2002): EPISODIC MEMORY: From mind to brain. Annu Rev Psychol 53:1–25. [DOI] [PubMed] [Google Scholar]
- Ungless MA (2004): Dopamine: the salient issue. Trends Neurosci 27:702–706. [DOI] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ (2011): Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS ONE 6, e17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Helms G (2008): Multi‐parameter mapping of the human brain at 1 mm resolution in less than 20 minutes, in: ISMRM 16. Toronto, Canada.
- Wiech K (2013): Pain, decisions, and actions: a motivational perspective. Front Neurosci 7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman‐Rakic PS (1995): Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H‐J, Düzel E (2005): Reward‐related fMRI activation of dopaminergic midbrain is associated with enhanced hippocampus‐ dependent long‐term memory formation. Neuron 45:459–467. [DOI] [PubMed] [Google Scholar]
- Yonelinas A (1995). The relation between remembering and knowing as bases for recognition: effects of size congruency. J Mem Lang 34:622–643. [Google Scholar]
- Yonelinas AP (2002): The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang 46:441–517. [Google Scholar]
- Yu AJ, Dayan P (2002): Acetylcholine in cortical inference. Neural Netw 15:719–730. [DOI] [PubMed] [Google Scholar]
- Yu,AJ , Dayan P (2005): Uncertainty, neuromodulation, and attention. Neuron 46:681–692. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TMK, Allen JM, Mizumori SJY, Bonci A, Palmiter RD (2011): Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci 14:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]


