Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by muscular atrophy, spasticity, and bulbar signs caused by loss of upper and lower motor neurons. Evidence suggests that ALS additionally affects other brain areas including premotor cortex and supplementary motor area. Here, we studied movement execution and inhibition in ALS patients using a stop‐signal paradigm and functional magnetic resonance imaging. Seventeen ALS patients and 17 age‐matched healthy controls performed a stop‐signal task that required responding with a button press to a right‐ or left‐pointing black arrow (go‐stimuli). In stop‐trials, a red arrow (stop‐stimulus) was presented shortly after the black arrow indicating to withhold the prepared movement. Patients had by trend higher reaction times in go‐trials but did not differ significantly in their inhibition performance. Patients showed stronger inhibition‐related activity in inferior, superior, and middle frontal gyri as well as in putamen and pallidum. Error‐related activity, conversely, was found to be stronger in healthy controls, particularly in the insula bilaterally. Patients also showed increased activity in the motor cortex during button presses. The results provide evidence for altered prefrontal and subcortical networks underlying motor execution, motor inhibition, and error monitoring in ALS. Hum Brain Mapp 36:2878–2889, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: stop‐signal task, error monitoring, ALS, fMRI, prefrontal cortex
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder which is characterized by muscular atrophy, spasticity, and bulbar signs attributable to the loss of upper and lower motor neurons. Although changes in the motor system are clinically most prominent, more widespread changes in the central nervous system have been suggested by structural (Cosottini et al., 2013; Lillo et al., 2012; Thorns et al., 2013) and functional neuroimaging (Cosottini et al., 2012; Mohammadi et al., 2009a, 2009b) and pathological (Maekawa et al., 2004) and neuropsychological findings (Phukan et al., 2007). For instance, reports of reduced gray matter in sensorimotor cortical areas as well as premotor cortex (PMC), middle and inferior frontal gyri, and parietal cortex point to system‐wide effects of ALS (Cosottini et al., 2012, 2013; Lillo et al., 2012). Moreover, these structural changes are accompanied by altered functional activity in prefrontal and motor areas as shown by positron emission tomography (PET) (Abrahams et al., 1996) and functional magnetic resonance imaging (fMRI) studies (Cosottini et al., 2012; Mohammadi et al., 2009a). Specifically, ALS patients have been repeatedly found to show increased activity in (pre)motor cortical areas during manual movements, supposedly reflecting reduced intracortical inhibition (Konrad et al., 2002, 2006; Mohammadi et al., 2011; Schoenfeld et al., 2005).
Although ALS has traditionally been deemed a pure motor disease, nonmotor symptoms have long been recognized and suggested to be associated with frontotemporal dementia (Phukan et al., 2007). Most consistent neuropsychological findings are impairments in executive functions such as in verbal fluency or attention, whereas memory or language functions are often (Phukan et al., 2007) but not always (Frank et al., 1997) reported to be spared. One central aspect of executive functions is the inhibition of prepotent responses (Miyake et al., 2000), which is typically studied with the Stroop task, Go/Nogo‐task or Stop‐signal‐task. Reports about performance of ALS patients in the Stroop task are inconsistent, with most studies finding no impairments (Kew et al., 1993; Moretti et al., 2002; Phukan et al., 2007). However, Go/Nogo‐ and Stop‐signal paradigms might be more sensitive to detect subtle deficits, and behavioral measures alone cannot reveal possibly altered underlying neural activity.
In a stop‐signal task (SST), a go‐stimulus is presented in each trial asking for a simple motor response. The go‐stimulus is infrequently followed by a second stimulus, the stop signal, which requires withholding the already prepared response (Logan et al., 1984). The SST allows computing a behavioral measure of response inhibition, the stop‐signal reaction time (SSRT), which estimates the latency of the inhibitory process (Logan et al., 1984). Neuroimaging studies using the Go/Nogo‐ or Stop‐signal paradigm report middle and inferior frontal gyri (MFG, IFG), presupplementary motor area, intraparietal sulcus, and subcortical structures as striatum and subthalamic nucleus activated in association with response inhibition (Aron et al., 2014; Chikazoe, 2010; Swick et al., 2011). As ALS also affects prefrontal structures, including middle and inferior frontal gyri, it seems likely that not only basic motor functions are impaired but also cognitive control of motor functions including motor inhibition and action monitoring.
Indeed, this was found in an electroencephalography (EEG) study which investigated response preparation and inhibition in ALS patients using the SST and event‐related potentials (ERPs; Thorns et al., 2010). Patients presented with slower reaction times in go‐trials and diminished lateralized readiness potentials as neurophysiological markers of movement initiation. Patients also showed diminished inhibitory control behaviorally and reduced stop‐signal‐related frontolateral activity (Thorns et al., 2010). Moreover, error‐related electrophysiological activity was reduced indicating compromised action monitoring functions in ALS patients. Together, the authors interpreted the findings as indices of prefrontal dysfunction in patients. This was further supported by worse performance in several neuropsychological tests of executive functions including verbal fluency and the Stroop task (Thorns et al., 2010). Another recent study investigated oculomotor inhibitory control in ALS patients using an antisaccade paradigm and fMRI (Witiuk et al., 2014). ALS patients made more antisaccade errors and showed reduced dorsolateral prefrontal (dlPFC) and increased insula activity in error trials compared to healthy controls. When preparing for antisaccades, however, ALS patients showed increased activity in a number of regions of the saccade preparation network including the dlPFC, the supplementary eye fields, and frontal eye fields. This activity was negatively correlated with saccade reaction times. The authors interpreted this as evidence for impaired oculomotor inhibitory control in ALS related to dlPFC dysfunction. The data also pointed to possible prefrontal compensatory mechanisms that helped to counteract these impairments (Witiuk et al., 2014).
Although the two studies agreed on impaired inhibitory control in ALS, the reported neural changes associated with the impairments and compensatory mechanisms are less consistent. This is obviously partly due to differences in the tasks (manual vs. oculomotor inhibition) and the methods (fMRI vs. EEG). With this study, we, therefore, aimed to expand on this research with an fMRI study using a standard visual SST that required the inhibition of manual responses (Aron et al., 2007). Based on previous findings (Mohammadi et al., 2011; Thorns et al., 2010; Witiuk et al., 2014), we expected ALS patients to show (i) increased and more widespread activity in motor cortex during motor execution, (ii) impaired response inhibition behaviorally, and (iii) reduced neural activity related to motor inhibition and error monitoring.
METHODS
Participants
The study was approved by the local ethics committee. All participants gave their written consent prior to their inclusion in the study. Two groups of participants were investigated using BOLD‐fMRI. The first group comprised 17 healthy volunteers (seven women) aged from 49 to 70 years (mean age 53 years). The second group comprised 17 ALS patients (five women) aged from 44 to 69 years (mean age 55 years), who, during the course of the disease, fulfilled the diagnostic criteria for probable or definite ALS according to the revised El Escorial criteria of the World Federation of Neurology. The mean (± SD) age at disease onset was 54 (± 6) years. According to the initial site of symptoms, ALS patients were divided into bulbar‐onset (n = 5) and limb‐onset (n = 12) groups. The mean ALSFRS‐R score was 40 (± 4). The interval between the diagnosis and the study was 10 (±8) months. None of the patients had other neurological diseases. None of the ALS patients needed noninvasive ventilation or percutaneous endoscopic gastrostomy at time of the investigation.
Grip Strength
Grip strength is considered to be a good indicator of upper limb strength. We, therefore, used the Martin‐Type Squeeze Dynamometer (Vigorimeter, Martin, Tüttlingen, Germany) to assess grip strength of the right hand to supplement our clinical assessment. The medium‐sized bulb was positioned in the palm of the participant's hand with the air tube extending out between the individual's thumb and index finger and with the fingers wrapped around the bulb such that the fingers touched the surface of the bulb as much as possible. Three measurements with maximum voluntary force were taken and averaged to obtain the grip strength score. Pauses of 30 s were taken in between trials. This method is known to be precise and reliable (Merkies et al., 2000, 2003). The mean (±SD) grip strength of the right hand was 104 (±19) kPa in the group of healthy volunteers and 87 (±8.6) kPa in the ALS group. Grip strength of the ALS group was thus reduced compared to that of the healthy volunteers (P = 0.02).
Neuropsychological Testing
Patients and healthy controls underwent neuropsychological testing. To assess verbal fluency, we applied the Regensburger Wortflüssigkeitstest with its subtests for lexical and semantic fluency and flexibility (Aschenbrenner et al., 2000). Verbal intelligence was assessed using the Mehrfach‐Wortschatztest (MWT‐B) (Lehrl, 2005). Verbal learning and memory were assessed using the German version of the auditory verbal learning and memory test (Verbaler Lern‐ und Merkfähigkeitstest [VLMT]) testing learning and delayed recollection of a 15‐item word list (Helmstaedter et al., 2001). Additionally, we administered the attention network task (ANT) in the version presented by Fan et al. (2002). The ANT is an established choice reaction time task which provides measures for spatial attention, alertness, and executive functions. It was programmed and presented using Presentation® (Neurobehavioral Systems).
Experimental Design
We applied a SST that required the participants to respond with a right button press to a right‐pointing black arrow and with a left button press to a left‐pointing black arrow (go‐stimuli). In stop‐trials, a red arrow (stop‐stimulus) was presented shortly after the black arrow indicating to withhold the prepared movement (see Fig. 1 for trial outline). We presented 75% of go‐trials and 25% of stop‐trials. Each stimulus array was presented in the middle of the screen. Stimulus duration was set to 700 ms. Trial duration was jittered between 1 and 4 s. Trial duration and order of go‐and stop‐trials were optimized to yield a better estimation of the condition‐specific hemodynamic response. The delay of the stop‐signal was adapted on a trial‐by‐trial basis by means of a staircase‐tracking algorithm to yield a rate of 50% of successful inhibitions. The signal delay was set to 200 ms initially. After a successful inhibition, the signal delay was increased by 50 ms (making the inhibition harder), after an unsuccessful inhibition the signal delay was reduced by 50 ms. The SSRT was calculated by first sorting the participant's reaction times to go‐stimuli and then subtracting the participant's average stop‐signal delay from the nth reaction time with n being the percentage of stop‐errors (Band et al., 2003).
Figure 1.
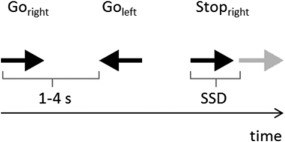
Trial outline. In each trial, an arrow pointing to the right or left was presented for 700 ms, asking for a right or left hand button press (go‐trials). Trial duration was jittered between 1 and 4 seconds. In stop‐trials, the black arrow was followed by a second arrow (depicted in gray here but red in the experiment) after a variable interval (stop‐signal delay, SSD). The SSD was adapted trial‐by‐trial to yield an inhibition rate of 50% (see text for details.).
Participants received two training blocks of 40 trials each to get acquainted with the task. In the first block, only go‐trials were presented; in the second block, go‐ and stop‐trials were presented. The experiment proper was divided into four runs, each comprising 128 trials, resulting in a total of 512 trials. Participants were informed that the experiment was programmed in a way that made it impossible for them to perform correctly every stop‐trial. This was explained to prevent them from slowing down in the primary task. They were instructed to perform as fast and accurately as possible. After the practice and every run, participants were given feedback about the percentage of correct inhibitions and their average reaction time.
Behavioral Data Analysis
Reaction times and percentage of errors in go‐trials were computed for each participant and submitted to independent sample t‐tests. The SSRT, percentage of inhibitions, and average stop‐signal delay were compared with independent sample t‐tests. All behavioral data stems from the testing within the MRI machine.
Image Acquisition
Magnetic‐resonance images were acquired on a 3‐T Siemens Magnetom Scanner (Erlangen, Germany) equipped with a standard quadrature head coil. A total of 113 T2*‐weighted volumes of the whole brain (echo planar imaging (EPI)‐sequence; TR 2000 ms, TE 30 ms, flip angle 80°, FOV 192 mm, 34 slices, slice thickness 3 mm, and interslice gap 0.75 mm) parallel to the AC‐PC line were recorded for functional imaging for each run. Each participant underwent four runs. A T1‐weighted high resolution data set was acquired using a three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D‐MPRAGE) sequence for anatomical information (1 mm isovoxel). The subject's head was fixed during the entire measurement to avoid head movements.
fMRI Data Analyses
fMRI data processing was carried out using fMRI Expert Analysis Tool Version 5.98, part of FSL (functional MRI of the brain (FMRIB's) Software Library, available at: http://www.fmrib.ox.ac.uk/fsl). The following preprocessing was applied for all functional data: motion correction using MCFLIRT (Jenkinson et al., 2002); nonbrain removal using BET (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand‐mean intensity normalisation of the entire 4D dataset by a single‐multiplicative factor; highpass temporal filtering (Gaussian‐weighted least‐squares straight line fitting, with sigma = 50.0 s). For all functional data, registration to high resolution structural and standard space images was carried out using affine brain image registration implemented in FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Registration from high resolution structural to standard space was then further refined using FNIRT nonlinear registration (Smith et al., 2004).
The four different runs in each participant were then used to calculate one statistical map for each person using general linear model (GLM) analysis with fixed effects. At the next step, these individual maps were entered into a group GLM analysis using a mixed‐effects approach in FMRIB's local analysis of mixed effects. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P < 0.05 (Worsley, 2001). The resulting images show the Z‐scores for the group, condition or interaction effects.
It is debated which is the best comparison in the SST to identify inhibition‐specific neural activity (Aron et al., 2007; Boehler et al., 2010; Swick et al., 2011). As the stop‐signal is infrequent, a comparison between go‐ and inhibited stop‐trials might be confounded with a salience response to the infrequent stop‐signal. Comparing successfully inhibited and noninhibited stop‐trials, conversely, might be overly conservative as inhibitory activity might be merely delayed or weaker but not absent in noninhibited stop‐trials. Here, we chose the following approach to study neural activity reflecting motor execution, motor inhibition, and action monitoring. The comparison “go‐trials > successful stop‐trials (SS)” was taken as measure of movement execution; both contrasts “successful stop‐trials (SS) > unsuccessful stop‐trials (US)” and “SS > go‐trials” were examined as measures of motor inhibition; “US > go‐trials” and “US > SS” were examined as measures of error‐monitoring.
RESULTS
Neuropsychological Test Results
Patients showed slightly reduced verbal fluency reflected in significantly less words produced in the semantic flexibility and by trend less words in the semantic fluency and lexical fluency tasks. Patients did neither differ from controls in verbal intelligence nor measures of verbal learning and memory. In the ANT, patients showed a marginally reduced spatial attention effect but did not differ in the alertness or executive functions parameters, neither for reaction time effects nor accuracy (Table 1).
Table 1.
Results of neuropsychological tests
| Test | ALS | HC | P‐value |
|---|---|---|---|
| Lexical fluency (M‐words) | 15.2 | 20 | 0.080* |
| Lexical fluency (S‐words) | 21 | 19.6 | 0.687 |
| Lexical flexibility (G‐R‐words) | 18.9 | 21.5 | 0.381 |
| Semantic fluency (animals) | 29 | 35 | 0.089* |
| Semantic flexibility (fruits/sports) | 18.9 | 23 | 0.035** |
| MWT‐B | 29.2 | 31 | 0.206 |
| VLMT (runs 1–5) | 44.5 | 48 | 0.259 |
| VLMT (run 7) | 8.6 | 8.1 | 0.429 |
| ANT – Alertness (s) | 38 | 41 | 0.658 |
| ANT – Spatial (s) | 7 | 18 | 0.095* |
| ANT – Executive (s) | 170 | 155 | 0.676 |
| ANT – Executive (% errors) | 16 | 18 | 0.798 |
HC: healthy controls; MWT‐B: vocabulary test; VLMT: verbal learning and memory test (runs 1–5 are 1st–5th give number of correct answers in five repetitions of word list; run 7 gives number of correctly recollected words after a delay of 30 min); ANT: attention network task.
*P < 0.1.
**P < 0.05.
Behavioral Data SST
During the first‐practice run (go‐trials only), ALS patients did not differ from controls in their reaction time (t 32 = −1.4, P = 0.135). They were slower though in the first practice session with stop‐trials (t 32 = −2.2, P = 0.033), indicating that patients slowed down more to account for the stop‐trials. During the fMRI sessions, ALS patients remained nominally slower than controls in go‐trials, but this effect marginally failed to reach significance (ALS: 585 ms, HC: 533 ms; t 32 = 1.9, P = 0.06). Patients and controls were equally accurate in go‐trials (percentage of errors in ALS: 4.6%, in HC: 4.5%; P > 0.9). The stair‐case tracking algorithm in stop‐trials was successful in that patients and controls made on average 51% (ALS) and 53% (HC) of errors in stop‐trials (P > 0.5). Patients and controls did neither differ in the average stop‐signal delay (ALS: 204 ms, HC: 185 ms; P = 0.52) nor their SSRT (SSRT in ALS: 375 ms, in HC: 345 ms; P > 0.2).
fMRI Results Related to Motor Execution
When comparing go‐ to inhibited stop‐trials, healthy controls and ALS patients showed a similar pattern of activity in primary motor and somatosensory cortex, cingulate gyrus, cerebellum, and thalamus among other areas (Table 2). The exact lists of activations for the different main effects of condition (go‐ vs. stop‐inhibited vs. stop‐error) separately for both groups are provided in the Supporting Information (Supporting Information, Tables I—III). In Figure 2, the contrast of go‐ vs. inhibited stop‐trials is shown for right hand responses only yielding left‐sided M1 activity in controls but bilateral activations in the ALS patients. When comparing the two groups across right and left hand responses, healthy participants showed increased activity in the left central operculum cortex, whereas ALS patients showed increased activity bilaterally in primary motor and somatosensory cortex, posterior cingulate gyrus, parietal operculum (Fig. 2B), and cerebellum (Table 2 and Supporting Information, Table I).
Table 2.
Group differences in movement execution (go > successful inhibition)
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions of activation | Hemisphere | voxels | X | Y | Z | z‐score |
| Healthy group > ALS patients | ||||||
| Central Operculum Cortex | L | 51 | −42 | −2 | 16 | 4.7 |
| ALS patients > healthy group | ||||||
| Primary motor cortex | L | 220 | −30 | −18 | 60 | 4.5 |
| Primary motor cortex | R | 180 | 36 | −22 | 62 | 5.2 |
| Primary somatosensory cortex | L | 59 | −42 | −28 | 50 | 4.4 |
| Primary somatosensory cortex | R | 61 | 36 | −32 | 58 | 4.4 |
| Cingulate gyrus, posterior | L | 145 | −2 | −48 | 20 | 4.5 |
| Cingulate gyrus, posterior | R | 149 | 6 | −44 | 20 | 4.4 |
| Parietal operculum cortex | R | 46 | 56 | −24 | 18 | 4.9 |
| Cerebellum | L | 210 | −16 | −48 | −30 | 4.5 |
| Cerebellum | R | 264 | 28 | −42 | −32 | 4.7 |
Figure 2.
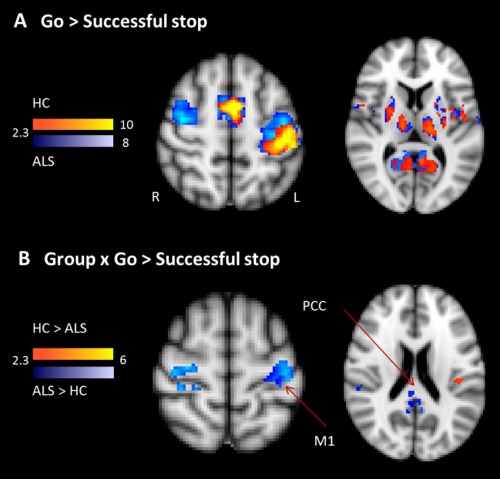
Movement execution. A shows the contrast in right‐handed Go versus SS for the healthy controls (yellow–red) and ALS patients (blue). B shows the interaction of Group (ALS patients compared to healthy controls) and the contrast of go versus SS‐trials. ALS patients show increased activity in M1 and posterior cingulate cortex (PCC; shown in blue), but reduced activity in the left frontal operculum (shown in red). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI Results Related to Motor Inhibition
Neural activity related to motor inhibition was reflected in the contrasts comparing SS‐ relative to US‐ or go‐trials (Table 3 and Supporting Information, Table II). Both healthy controls and ALS patients showed in SS‐trials relative to go‐trials activity in the typical network of response inhibition including prefrontal activity in inferior, superior and middle frontal gyri, anterior cingulate cortex (ACC), and supplementary motor area (SMA) (Fig. 3A; Supporting Information, Table II). Prefrontal activity was observed bilaterally but more extended on the right hemisphere. When comparing SS‐trials to US‐trials, a largely similar pattern of activity was detected in controls and patients (Fig. 3C; Supporting Information, Table II). In addition to bilateral IFG, superior frontal gyri (SFG), and MFG, subcortical regions were found including pallidum, putamen, and amygdala (Fig. 3B,D; Supporting Information, Table II). Importantly, group comparison revealed increased activity for ALS patients in the IFG and MFG for both comparisons (SS > go: Fig. 3B; and SS > US: Fig. 3D; Table 3). For the contrast SS > US, ALS patients additionally showed stronger subcortical activity in putamen, pallidum (Fig. 3D; Table 3), and amygdala.
Table 3.
Group differences in motor inhibition
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions of activation | Hemisphere | voxels | X | Y | Z | z‐score |
| Successful > unsuccessful inhibition | ||||||
| ALS patients > healthy group | ||||||
| Inferior frontal gyrus | R | 45 | 52 | 32 | 18 | 8.6 |
| Superior frontal gyrus | L | 32 | −16 | 8 | 62 | 5.8 |
| Superior frontal gyrus | R | 40 | 26 | 8 | 60 | 4.6 |
| Paracingulate gyrus | L | 39 | −6 | 32 | 34 | 4.5 |
| Middle frontal gyrus | L | 275 | −40 | 14 | 32 | 5.3 |
| Precuneus cortex | L | 49 | −4 | −48 | 62 | 5.5 |
| Precuneus cortex | R | 39 | 4 | −46 | 64 | 4.5 |
| Putamen | R | 67 | 28 | 4 | −4 | 4.7 |
| Pallidum | R | 73 | 24 | −12 | 2 | 4.8 |
| Amygdala | R | 84 | 30 | −2 | −22 | 4.6 |
| Successful inhibition > go‐trials | ||||||
| Healthy group > ALS patients | ||||||
| Lateral Occipital Cortex | R | 25 | 24 | ‐62 | 48 | 4.8 |
| ALS patients > healthy group | ||||||
| Inferior Frontal Gyrus | R | 57 | 48 | 32 | 16 | 4.7 |
| Middle Frontal Gyrus | L | 52 | −46 | 32 | 24 | 4.7 |
Figure 3.
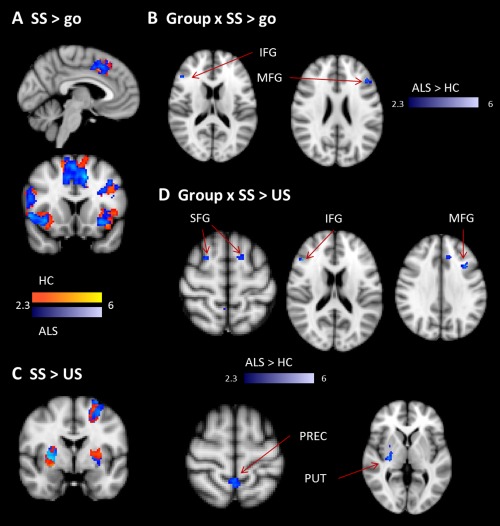
Motor inhibition. A shows the contrast of SS versus Go for the healthy (yellow‐red) and ALS group (blue). B shows the results for the interaction of group and SS > go‐trials. There was increased activity in ALS group compared to the controls in right IFG and left MFG. C shows the contrast of SS relative to US, again separately for controls (in red) and ALS patients (in blue). D shows the interaction of group and successful relative to US. ALS patients showed increased activity in bilateral SFG, right IFG and MFG, bilateral precuneus (PREC), right pallidum, and putamen (PUT). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI Results Related to Error Monitoring
Neural activity related to error monitoring was evaluated with the comparison of US‐trials relative to SS‐trials and relative to go‐trials (Fig. 4; Table 4 and Supporting Information, Table III). US‐trials relative to SS‐trials yielded activity in bilateral insula, frontal operculum, and primary motor and somatosensory cortex in both groups (Fig. 4A; Supporting Information, Table III). When comparing US‐trials to correct go‐trials, controls and patients showed activity in the typical error monitoring network including bilateral insula, frontal operculum, IFG, ACC, and SMA (Fig. 4C; Supporting Information, Table III). Critically, healthy controls showed increased activity in the error monitoring network including ACC (Fig. 4D; Table 4), orbitofrontal cortex, inferior frontal cortex, and insula (Fig. 4B).
Figure 4.
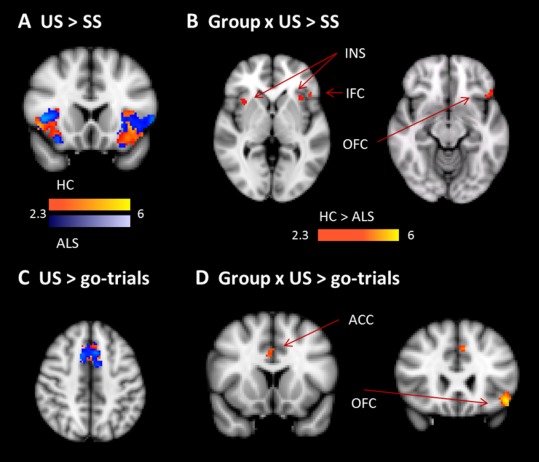
Error monitoring. A shows the contrast of unsuccessful (US) relative to SS, separately for healthy controls (yellow–red) and ALS patients (blue). B shows the interaction of group and the contrast of US relative to SS. We found decreased activity in ALS patients in left inferior frontal cortex (IFC), bilateral insula (INS) and left orbitofrontal cortex (OFC). C shows the contrast of US relative to go‐trials, separately for controls (red) and patients (blue). D shows the interaction of group and the contrast of US relative to go‐trials. ALS patients showed reduced activity in right ACC and left OFC. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 4.
Group differences in error monitoring
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions of activation | Hemisphere | voxels | X | Y | Z | z‐score |
| Unsuccessful > successful inhibition | ||||||
| Healthy group > ALS patients | ||||||
| Frontal Operculum Cortex | L | 42 | −44 | 24 | −2 | 6.3 |
| Frontal Orbital Cortex | L | 101 | −40 | 24 | −8 | 5.3 |
| Insular Cortex | L | 48 | −34 | 18 | −2 | 4.8 |
| Insular Cortex | R | 59 | 36 | 16 | −4 | 5.1 |
| Unsuccessful inhibition > go‐trials | ||||||
| Healthy group > ALS patients | ||||||
| Cingulate Gyrus, anterior | R | 32 | 2 | 16 | 36 | 5.2 |
| Frontal Orbital Cortex | L | 85 | −46 | 22 | −12 | 5.1 |
| Paracingulate Gyrus | L | 21 | −6 | 24 | 38 | 5.5 |
DISCUSSION
We investigated behavioral and neural changes related to response inhibition and error monitoring in ALS patients compared to age‐matched, healthy controls. ALS patients showed by trend slower response times but did not differ in their inhibition performance. On the neural level, patients as hypothesized showed increased activity in cortical and cerebellar nodes of the motor network and decreased activity in the error‐monitoring network. In contrast to previous ERP data (Thorns et al., 2010) which had found an amplitude reduction of a right frontal negative component about 200 ms after the stop‐signal in ALS patients, increased inhibition‐related neural activity was observed in ALS patients. To the extent to which both effects index inhibition processes and their amplitudes index the extent of inhibition, these effects are at odds. One possibility for the discrepancy could be that ALS patients were investigated at different stages of the disease. Whereas disease duration was 2.8 years in Thorns et al. (2010), it was just 1 year in this study. The patients of this study were thus at an earlier stage of the disease and the increased recruitment of the inhibitory network might point to a compensatory mechanism that allowed them to perform on a level comparable to controls. Moreover, the precise relationship between the fMRI and ERP indices of inhibition is not fully known (Huster et al., 2010).
Motor Network Activity
ALS patients showed increased activity in bilateral primary motor and somatosensory cortex as well as in the anterior cingulate gyrus and bilateral cerebellum during go‐trials compared to healthy controls. Increased and/or more widespread movement‐related activity in regions of the motor network has repeatedly been reported for ALS patients (Kollewe et al., 2011; Konrad et al., 2002, 2006; Mohammadi et al., 2011; Schoenfeld et al., 2005), although reduced movement‐related activity in ALS has been found (Cosottini et al., 2012). Moreover, the increased size of the activated area has been found to be unrelated to interindividual differences in hand weakness of the patients (Mohammadi et al., 2011), and increased cortical activity during hand movements was evident in both limb and bulbar onset patients (Kollewe et al., 2011). The finding of more widespread activity and coactivation of the ipsilateral motor cortex is typically explained with a loss of intracortical inhibition in ALS (Konrad et al., 2002; Mohammadi et al., 2011; Schoenfeld et al., 2005). Reduced intracortical inhibition has been demonstrated by transcranial magnetic stimulation (TMS) studies (Ziemann et al., 1997) and could be related to a loss of inhibitory interneurons in motor but also prefrontal cortex as revealed by postmortem morphometric analyses (Maekawa et al., 2004).
Regarding motor performance in this study, ALS patients showed slower response times only during the practice block and by trend during the actual experiment. In the first‐practice block without stop‐trials, ALS patients did not differ from controls in the response times suggesting that ALS patients traded speed in the go‐trials off against accuracy in the stop‐trials. It has previously been argued that the increased motor activity in ALS reflects the higher motor task difficulty for the patients rather than any neural reorganization (Schoenfeld et al., 2005). As task performance was largely comparable between patients and controls in the present study, a mere difficulty effect can be ruled out as explanation for the neural activity differences. However, recent work also demonstrated short‐term changes in motor‐related cortical activity during the progression of ALS (Stoppel et al., 2014). The authors examined ALS patients in two sessions separated by a 3‐months interval and found increased motor‐related activity relative to controls only in the first session but not 3 months later. These results were explained with compensatory mechanisms at earlier stages of the disease which break down with progressive loss of motor neurons (Stoppel et al., 2014). A three‐month interval might be too short, though, to reveal clinically relevant disease changes and a different picture emerged when directly comparing patients at different stages of motor weakness (none, mild, or marked weakness; Mohammadi et al., 2011). Here, ALS patients showed a larger area of activation in M1 contralateral to the response hand independent of the disease stage, whereas at the same time the activation strength as indicated by percent signal change decreased with progressing motor weakness. It was argued that the increased activation size reflects reduced intracortical inhibition which is at ceiling already at early disease stages, whereas the reduced M1 activity strength reflects loss of motor neurons directly related to the motor weakness (Mohammadi et al., 2011). In fact, another study using a maximal force hand grip task reported reduced movement‐related activity in sensorimotor cortex in ALS patients (Cosottini et al., 2012) but hyperactivity in prefrontal and parietal regions. Here, we did not separately assess activation size and strength, but the observed group differences might be rather caused by an increased area of activation in bilateral M1 in the ALS patients. Moreover, our paradigm was a minimal force task requiring only button presses which limits the direct comparison of our results with studies using simple and maximal force motor tasks.
Inhibitory Motor Control
Both healthy controls and ALS patients showed the typical pattern of activations when contrasting inhibited stop‐trials with go‐trials or failed inhibitions, namely activity in inferior, middle and SFG, medial prefrontal cortex (PFC), and basal ganglia (Swick et al., 2011). Within this network, ALS patients showed increased prefrontal (IFG, MFG) and basal ganglia activity (pallidum, putamen), whereas healthy controls showed increased activity only in lateral occipital cortex. Although ALS patients showed a nominally increased stopping latency (SSRT) on the behavioral level, this group difference was not significant. As alluded to above, the marginally slower response times of patients in the stop‐signal paradigm but not in the practice block without stop‐trials suggest a more pronounced speed–accuracy trade‐off in patients compared to controls.
A previous EEG‐study using the SST reported a reduced inhibition‐related neurophysiological response (stop‐N2) in ALS patients (Thorns et al., 2010). Although EEG and fMRI have different temporal and spatial resolution and measure different physiological signals, the present result of increased inhibition‐related activity in IFG and MFG is difficult to reconcile with a reduced stop‐N2. Moreover, whereas the previous study also found impaired inhibitory control on a behavioral level, we did not detect significant group differences in the SSRT, although patients had a nominally larger SSRT. Notably, as the previous study used a stop‐signal paradigm with fixed stop‐signal delays, the authors did not compute the SSRT, but rather inhibition probability, as a behavioral measure. The two behavioral variables are thus not completely comparable. It might be the case, however, that the patient group of Thorns et al. (2010) was already more affected by the disease, resulting in impaired behavior and reduced stopping‐related neural activity. The present patient group was relatively less affected and is also suggested by the neuropsychological test results, which showed only mild deficits, as well as by the rather short disease duration since diagnosis. It might be that an increase of inhibition‐related neural activity is seen during earlier stages of the disease which is followed by a reduction of inhibition‐related PFC activity in later stages. Longitudinal studies examining changes in inhibitory motor control are needed to test this directly. In addition, combined EEG/fMRI recordings should reveal important information on the relationship between electrophysiological and hemodynamic indices of inhibition (Donamayor et al., 2012; Huster et al., 2010) to reconcile the differences between this study and Thorns et al. (2010).
Our results of increased inhibition‐related prefrontal activity fit with the recent study on antisaccades in ALS patients (Witiuk et al., 2014). Correct performance in the antisaccade task requires the inhibition of the automatic (prosaccadic) response and generation of the more controlled saccade to the opposite side of the target stimulus. The authors reported increased activity in the frontal eye fields, the dlPFC, and the supplementary eye fields when patients prepared for an antisaccade relative to a prosaccade (Witiuk et al., 2014). Although our paradigm did not allow disentangling preparation for and execution of the response inhibition, the results converge in increased prefrontal activity related to inhibitory action control in ALS patients. Interestingly, a recent study comparing cortical activity during a maximal force hand task also reported hyperactivation in prefrontal areas in ALS together with hypoactivation in sensorimotor areas (Cosottini et al., 2012). The authors argued that the increased activity in premotor activity reflected an over‐recruitment of frontoparietal networks during the motor task (Cosottini et al., 2012). Although their task was a simple motor task and the reported activation cluster more posterior than in this study, enhanced prefrontal activity in ALS patients might be generalizable to demanding motor tasks.
Different explanations can be conceived for the increased prefrontal activity in ALS patients. Postmortem morphometric analyses showed a reduction of inhibitory interneurons not only in the precentral gyrus but also in the PMC, dlPFC, and ACC (Maekawa et al., 2004). This indicates that increased prefrontal activity might be a byproduct of reduced intracortical inhibition rather than a functionally relevant compensatory mechanism (Witiuk et al., 2014). We cannot exclude this explanation as we did not observe any relationship between neural activity and behavioral performance, which could provide evidence for a functionally relevant mechanism. ALS patients showed reduced prefrontal activity related to error monitoring, however (see below), which rather argues against this point.
Alternatively, changes downstream in the motor inhibition pathway might lead to increased activity in regions upstream. Specifically, a dysfunctional motor and PMC in ALS might be compensated for by increased activity in the prefrontal cortex. A TMS‐study using the SST, for instance, demonstrated the relevance of intracortical inhibition in primary motor cortex by showing that the GABAB‐mediated silent period was prolonged in inhibited stop‐trials (van den Wildenberg et al., 2009). Earlier work had shown that nogo‐trials in a go/nogo‐paradigm are associated with increased GABAA‐mediated short intracortical inhibition (SICI) (Sohn et al., 2002). As pointed out above, ALS patients were found to have reduced GABAA‐mediated intracortical inhibition, but the cortical silent period was unaffected (Ziemann et al., 1997). It is thus possible that reduced intracortical inhibition in motor cortex might be compensated for by increased activity in the “source” of inhibitory motor control, namely inferior and middle frontal cortex as well as in the basal ganglia nuclei. TMS studies probing intracortical inhibition in motor cortex in ALS during SSTs could test this hypothesis and distinguish between the alternative explanations of increased prefrontal activity in ALS patients. In this study, we did not have enough patients with bulbar‐onset to investigate group differences between bulbar‐onset and limb‐onset patients in inhibition‐related cortical activity. However, as a previous study reported group differences only in cortical activity during tongue movement but not during hand movement (Kollewe et al., 2011), we would hypothesize that groups should not differ in neural activity underlying inhibitory control of hand movements either.
Error Monitoring
While both patients and controls showed activity in the typical error monitoring network when comparing unsuccessful inhibitions to successful inhibitions or go‐trials (Aron et al., 2007; Rubia et al., 2003), ALS patients showed reduced activity in several nodes of this network, namely the anterior cingulate cortex, insula, and inferior frontal cortex. We know from studies using structural MRI and diffusion tensor imaging that these regions are affected by ALS, which might explain the reduced error‐related activity (Agosta et al., 2012; Cosottini et al., 2012; Thivard et al., 2007). Our finding is inline with a reduced neurophysiological response to stop‐errors in ALS patients, which presumably emanated from medial PFC (Thorns et al., 2010). These results provide further support for early involvement of prefrontal structures in ALS. Notably, neural activity changes in PFC were evident in this study although patients did not (yet) have any strong neuropsychological deficits.
CONCLUSIONS
ALS patients at a relatively early stage of the disease and with only mild neuropsychological deficits showed altered neural activity in PFC regions and insula with regard to inhibitory motor control and error monitoring. Increased inhibition‐related PFC activity might reflect compensatory activity for dysfunctional intracortical inhibition in motor cortex, although it cannot be excluded that reduced intracortical inhibition in PFC itself leads to increased BOLD responses. However, we also observed reduced medial PFC activity after errors, speaking against a general and unspecific effect of ALS on PFC activity. Future studies examining longitudinal changes in the progression of the disease will be helpful to delineate the compensatory effects and deficits in PFC functions caused by ALS.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors have no conflict of interest to declare.
REFERENCES
- Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, Leigh PN (1996): Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119 (Pt 6):2105–2120. [DOI] [PubMed] [Google Scholar]
- Agosta F, Valsasina P, Riva N, Copetti M, Messina MJ, Prelle A, Comi G, Filippi M (2012): The cortical signature of amyotrophic lateral sclerosis. PLoS One 7:e42816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007): Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner S, Tucha O, Lange KW (2000): Regensburger Wortflüssigkeits‐Test. Handanweisung. Göttingen: Hogrefe Publisher. [Google Scholar]
- Band GP, van der Molen MW, Logan GD (2003): Horse‐race model simulations of the stop‐signal procedure. Acta Psychologica 112:105−142. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG (2010): Pinning down response inhibition in the brain–conjunction analyses of the Stop‐signal task. Neuroimage 52:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J (2010): Localizing performance of go/no‐go tasks to prefrontal cortical subregions. Curr Opin Psychiatry 23:267–272. [DOI] [PubMed] [Google Scholar]
- Cosottini M, Pesaresi I, Piazza S, Diciotti S, Cecchi P, Fabbri S, Carlesi C, Mascalchi M, Siciliano G (2012): Structural and functional evaluation of cortical motor areas in amyotrophic lateral sclerosis. Exp Neurol 234:169–180. [DOI] [PubMed] [Google Scholar]
- Cosottini M Cecchi P Piazza S Pesaresi I Fabbri S Diciotti S Mascalchi M Siciliano G Bonuccelli U (2013): Mapping cortical degeneration in ALS with magnetization transfer ratio and voxel‐based morphometry. PloS One 8:e68279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donamayor N, Heilbronner U, Munte TF (2012): Coupling electrophysiological and hemodynamic responses to errors. Hum Brain Mapp 33:1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002): Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347. [DOI] [PubMed] [Google Scholar]
- Frank B, Haas J, Heinze HJ, Stark E, Munte TF (1997): Relation of neuropsychological and magnetic resonance findings in amyotrophic lateral sclerosis: Evidence for subgroups. Clin Neurol Neurosurg 99:79–86. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Lendt M, Lux S. (2001): Verbaler Lern‐ und Merkfähigkeitstest. Göttingen: Hogrefe. [Google Scholar]
- Huster RJ, Westerhausen R, Pantev C, Konrad C (2010): The role of the cingulate cortex as neural generator of the n200 and p300 in a tactile response inhibition task. Hum Brain Mapp 31:1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Jenkinson, P Bannister, JM Brady, SM Smith (2002): Improved Optimisation for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- M Jenkinson, SM Smith (2001): A global optimisation method for robust affine registration of brain images. Medical Image Analysis 5:143–156. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, Frackowiak RS, Brooks DJ (1993): The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain 116(Pt 6):1399–1423. [DOI] [PubMed] [Google Scholar]
- Kollewe K, Munte TF, Samii A, Dengler R, Petri S, Mohammadi B (2011): Patterns of cortical activity differ in ALS patients with limb and/or bulbar involvement depending on motor tasks. J Neurol 258:804–810. [DOI] [PubMed] [Google Scholar]
- Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks B (2002): Pattern of cortical reorganization in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Exp Brain Res 143:51–56. [DOI] [PubMed] [Google Scholar]
- Konrad C, Jansen A, Henningsen H, Sommer J, Turski PA, Brooks BR, Knecht S (2006): Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172:361–369. [DOI] [PubMed] [Google Scholar]
- Lehrl S (2005): Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Balingen: Spitta Verlag. [Google Scholar]
- Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M (2012): Grey and white matter changes across the amyotrophic lateral sclerosis‐frontotemporal dementia continuum. PLoS One 7:e43993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA (1984): On the ability to inhibit simple and choice reaction time responses: A model and a method. J Exp Psychol Hum Percept Perform 10:276–291. [DOI] [PubMed] [Google Scholar]
- Maekawa S, Al‐Sarraj S, Kibble M, Landau S, Parnavelas J, Cotter D, Everall I, Leigh PN (2004): Cortical selective vulnerability in motor neuron disease: A morphometric study. Brain 127(Pt 6):1237–1251. [DOI] [PubMed] [Google Scholar]
- IS Merkies, PI Schmitz, JP Samijn, FG Meche, KV Toyka, PA Van Doorn (2000): Assessing grip strength in healthy individuals and patients with immune‐mediated polyneuropathies. Muscle Nerve 23:1393–1401. [DOI] [PubMed] [Google Scholar]
- IS Merkies, PI Schmitz, FG Van Der Meche, PA Van Doorn (2003): Comparison between impairment and disability scales in immune‐mediated polyneuropathies. Muscle Nerve 28:93–100. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: A latent variable analysis. Cogn Psychol 41:49–100. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Münte TF (2009a): Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp Neurol 217:147–153. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Münte TF (2009b): Decreased brain activation to tongue movements in amyotrophic lateral sclerosis with bulbar involvement but not kennedy syndrome. J Neurol 256:1263–1269. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Dengler R, Munte TF (2011): Functional neuroimaging at different disease stages reveals distinct phases of neuroplastic changes in amyotrophic lateral sclerosis. Hum Brain Mapp 32:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Carraro N, Cazzato G, Bava A (2002): Complex cognitive disruption in motor neuron disease. Dement Geriatr Cogn Disord 14:141–150. [DOI] [PubMed] [Google Scholar]
- Phukan J, Pender NP, Hardiman O (2007): Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 6:994–1003. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E (2003): Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20:351–358. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Tempelmann C, Gaul C, Kuhnel GR, Duzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S (2005): Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252:944–952. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M (2002): Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol 88:333–338. [DOI] [PubMed] [Google Scholar]
- SM Smith (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SM Smith, M Jenkinson, MW Woolrich, CF Beckmann, TEJ Behrens, H Johansen‐Berg, PR Bannister, M De Luca, I Drobnjak, DE Flitney, R Niazy, J Saunders, J Vickers, Y Zhang, N De Stefano, JM Brady, PM Matthews (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:208–219. [DOI] [PubMed] [Google Scholar]
- Stoppel CM, Vielhaber S, Eckart C, Machts J, Kaufmann J, Heinze HJ, Kollewe K, Petri S, Dengler R, JM Hopf, AM Schoenfeld, Hopf JM (2014): Structural and functional hallmarks of amyotrophic lateral sclerosis progression in motor‐ and memory‐related brain regions. Neuroimage Clin 5:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U (2011): Are the neural correlates of stopping and not going identical? Quantitative meta‐analysis of two response inhibition tasks. Neuroimage 56:1655–1665. [DOI] [PubMed] [Google Scholar]
- Thivard L, Pradat PF, Lehericy S, Lacomblez L, Dormont D, Chiras J, Benali H, Meininger V (2007): Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: Relationships with motor disability. J Neurol Neurosurg Psychiatry 78:889–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorns J, Wieringa BM, Mohammadi B, Hammer A, Dengler R, Munte TF (2010): Movement initiation and inhibition are impaired in amyotrophic lateral sclerosis. Exp Neurol 224:389–394. [DOI] [PubMed] [Google Scholar]
- Thorns J, Jansma H, Peschel T, Grosskreutz J, Mohammadi B, Dengler R, Munte TF (2013): Extent of cortical involvement in amyotrophic lateral sclerosis–An analysis based on cortical thickness. BMC Neurol 13:148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WP, Burle B, Vidal F, van der Molen MW, Ridderinkhof RK, Hasbroucq T (2009): Mechanisms and dynamics of cortical motor inhibition in the Stop‐signal paradigm: A TMS study. J Cogn Neurosci 22:225–239. [DOI] [PubMed] [Google Scholar]
- Witiuk K, Fernandez‐Ruiz J, McKee R, Alahyane N, Coe BC, Melanson M, Munoz DP (2014): Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. J Neurosci 34:14260–14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Ch 14, in Functional MRI: An Introduction to Methods, eds. P. Jezzard, P.M. Matthews and S.M. Smith. OUP, 2001.
- Ziemann U, Winter M, Reimers CD, Reimers K, Tergau F, Paulus W (1997): Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology 49:1292–1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


