Abstract
During adolescence, functional and structural changes in the brain facilitate the transition from childhood to adulthood. Because the cortex and the striatum mature at different rates, temporary imbalances in the frontostriatal network occur. Here, we investigate the development of the subcortical and cortical components of the frontostriatal network from early adolescence to early adulthood in 60 subjects in a cross‐sectional design, using functional MRI and a stop‐signal task measuring two forms of inhibitory control: reactive inhibition (outright stopping) and proactive inhibition (anticipation of stopping). During development, reactive inhibition improved: older subjects were faster in reactive inhibition. In the brain, this was paralleled by an increase in motor cortex suppression. The level of proactive inhibition increased, with older subjects slowing down responding more than younger subjects when anticipating a stop‐signal. Activation increased in the right striatum, right ventral and dorsal inferior frontal gyrus, and supplementary motor area. Moreover, functional connectivity during proactive inhibition increased between striatum and frontal regions with age. In conclusion, we demonstrate that developmental improvements in proactive inhibition are paralleled by increases in activation and functional connectivity of the frontostriatal network. These data serve as a stepping stone to investigate abnormal development of the frontostriatal network in disorders such as schizophrenia and attention‐deficit hyperactivity disorder. Hum Brain Mapp 35:4415–4427, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: response inhibition, adolescence, development, frontostriatal network, connectivity, cognitive control
INTRODUCTION
Adolescence is a transitional stage from childhood to adulthood that marks a period of considerable change. In the brain, structural as well as functional changes occur that parallel the development of higher‐order cognitive functions [Crone and Dahl, 2012]. It has been suggested, primarily based on animal data, that the rate of maturation differs across the brain: subcortical regions such as the amygdala and striatum are thought to be among the first areas to fully mature, but the frontal cortex continues to develop until early adulthood [Casey et al., 1997, 2008]. This may result in a temporary functional imbalance within frontostriatal and frontolimbic circuits, which may give rise to impulsivity and risk‐taking behavior typical for adolescents [Casey and Caudle, 2013]. We have previously studied the frontostriatal network in the context of inhibitory control, which is the ability to suppress prepotent responses or impulses [Vink et al., 2005; Zandbelt and Vink, 2010; Zandbelt et al., 2013a,b]. Inhibitory control can be subdivided into reactive and proactive inhibition [Aron, 2011; Vink et al., 2005]. Reactive inhibition refers to outright inhibition triggered by an external event, acting as a stop‐signal. Reactive inhibition is associated with suppression of activation in the motor cortex [Aron and Poldrack, 2006; Coxon et al., 2006; Li et al., 2008; Robbins, 2007; van den Wildenberg et al., 2010; Vink et al., 2005, 2006; Zandbelt and Vink, 2010; Zandbelt et al., 2008, 2011], and this suppression is thought to be achieved via the interplay of the right inferior frontal gyrus (rIFG), striatum, subthalamic nucleus (STN), and supplementary motor area (SMA) [Aron, 2011; Zandbelt et al., 2013a].
Proactive inhibition, on the other hand, involves the restraint of actions in preparation for stopping, driven by environmental contexts. In general, subjects slow down their responses to go‐stimuli when they anticipate a stop‐signal [Chikazoe et al., 2009a,b; Jahfari et al., 2010; Logan and Burkell, 1986; Verbruggen and Logan, 2009; Vink et al., 2005, 2006; Zandbelt and Vink, 2010; Zandbelt et al., 2011], allowing more time to cancel their response when the stop‐signal really appears. The higher the stop‐signal probability, the longer it takes for activity in the primary motor cortex to reach the threshold for response initiation [Jahfari et al., 2010]. This possibly indicates that the state of the motor system before the onset of a stop‐signal determines whether or not a response can be stopped [Lo et al., 2009; van den Wildenberg et al., 2010]. Proactive inhibition relies on the integrated actions of the frontal cortex and the striatum [Chikazoe et al., 2009a,b; Jahfari et al., 2010; Vink et al., 2005, 2006; Zandbelt and Vink, 2010; Zandbelt et al., 2011]. Specifically, adult subjects show increased activation in the striatum and SMA in response to cues indicating a high stop‐signal probability [Zandbelt et al., 2013b]. However, to date, the development of proactive inhibitory control during adolescence has not been studied. Although several studies have been performed on inhibition that likely include some form of proactive control [Casey et al., 1997; Luna et al., 2001; Rubia et al., 2007; Somerville et al., 2011], these did not include a baseline go‐signal condition, making it impossible to disentangle reactive and proactive inhibition components.
Previous developmental research has focused predominantly on reactive inhibition. These studies showed that young children make more inhibition errors on the antisaccade task [Velanova et al., 2009], and are slower [Tamm et al., 2002] and less accurate in inhibiting responses than adults [Durston et al., 2002]. Therefore, it is thought that reactive inhibition reaches its optimum only in early adulthood [Williams et al., 1999; van de Laar et al., 2011].
We hypothesize that this improvement in reactive inhibition throughout adolescence and into early adulthood is linked to the process of learning to proactively exert control, implemented within frontostriatal networks [Cools, 2011; Gladwin et al., 2011].
Here, we investigate age‐related changes in activation and connectivity in the frontostriatal network in a cohort of 60 healthy subjects aged 10–25 years. Participants performed the stop‐signal anticipation task (SSAT) [Zandbelt and Vink, 2010] while being scanned with fMRI. We examine age‐related changes on performance, frontostriatal activation, and frontostriatal functional connectivity in two ways: (a) regression analyses with age as a continuous factor, and (b) analyses across three age‐groups, representing early adolescence (age: 10–15 years), late adolescence (age: 15–20 years), and early adulthood (age: 20–25 years). We use predefined regions of interest (ROI) taken from an independent sample [Zandbelt and Vink, 2010].
First, we investigate basic response execution, by examining reaction times on go‐trials and activation in the primary motor cortex in a baseline context in which stop‐signals never occur. Given the fact that the SSAT is a timed response task, we do not hypothesize age‐related effects on baseline reaction times and motor cortex activation.
Second, we examine reactive inhibition by measuring the latency of inhibition [stop‐signal reaction time (SSRT)] and by contrasting brain activation during successful inhibition versus unsuccessful inhibition and successful inhibition versus baseline responding in the frontostriatal network and motor cortex. On the basis of the studies of inhibitory control across the lifespan [Williams et al., 1999; van de Laar et al., 2011], we hypothesize that reactive inhibition performance improves with age. Furthermore, on the basis of our studies in adults [Zandbelt and Vink, 2010; Zandbelt et al., 2013a], we hypothesize that this improvement during development is paralleled by increased activation and functional connectivity in the frontostriatal network and stronger motor cortex deactivation.
Third, we investigate proactive inhibition by measuring the effect of the probability of having to stop on reaction times as well as on brain activation in the frontostriatal network [Vink et al., 2005; Zandbelt and Vink, 2010]. As the frontal cortex and its anatomical and functional connections with the striatum are still underdeveloped in early adolescence [Casey and Caudle, 2013; Casey et al., 2008; Crone and Dahl, 2012], we hypothesize to find the lowest levels of proactive inhibition, both in terms of performance and brain activation, in the youngest subjects. In line with this, we expect that proactive inhibition increases with age, thus older subjects show more response slowing and more frontostriatal activation as stop‐signal probability increases. Finally, we hypothesize that, with age, the functional connectivity between the frontal cortex and striatum increases as responding becomes more proactive with development.
METHODS
Participants
We obtained written informed consent from all subjects (and their parents if applicable) after they received a complete description of the study, in accordance with the procedures approved by the University Medical Center Utrecht (UMCU) ethics committee. Data from a total of 71 subjects were obtained. Seven subjects were excluded due to excessive head movement following the procedures described by van Dijk et al. [2012] (>3 mm scan‐to‐scan as reflected by the realignment parameters obtained during image preprocessing). Rosner's test for outliers revealed three subjects to be outliers on the measure of proactive inhibition and one on the level of motor cortex activation (contrast successful versus failed stop trials). Moreover, these subjects were also identified as highly influential observations (Cook's distance > 1) in the regression analyses. Consequently, we excluded data from these 11 subjects, leaving a total of 60 healthy volunteers aged 10–25 years (mean age: 16.97 ± 4.6 years, 25 males) in the analyses. There was no significant relationship between head movement during scanning and age [F(1,59) = 1.02; P = 0.36]. For explorative purposes, we divided this group into three age groups of 5 years each, representing early adolescence (10–15 years: n = 24, mean age 12.5 ± 1.6, 12 males), late adolescence (15–20 years: n = 12, mean age 17.1 ± 1.8, 5 males), and early adulthood (20–25 years: n = 24, mean age 22 ± 1.6, 13 males).
Task and Procedure
Subjects performed the SSAT to measure reactive and proactive inhibitory control. The task and experimental procedures were identical to those described in detail earlier [Zandbelt and Vink, 2010] and are briefly explained in Figure 1. In short, participants are instructed to make timed responses in response to a moving bar (referred to as go trials). In some trials, the bar stops moving (referred to as the stop‐signal) and subjects have to refrain from responding. A cue presented at the start of each trial indicates the probability that the bar will stop (stop‐signal probability: 0, 17, 20, 25, and 33%). In total, 414 go trials (0%, n = 234; 17%, n = 30; 20%, n = 48; 25%, n = 54; 33%, n = 48) and 60 stop trials (17%, n = 6; 20%, n = 12; 25%, =18; 33%, n = 24) were presented in a single run in pseudorandom order. Stop‐signal delay, the interval between trial onset and presentation of the stop‐signal, was initially 550 ms and varied from one stop trial to the next according to a staircase procedure: if stopping was successful, then stopping was made more difficult on the next stop trial by increasing stop‐signal delay by 25 ms. The process was reversed when stopping failed. Each trial lasted 1,000 ms, and the intertrial interval was also 1,000 ms. For more details on the SSAT, see Zandbelt and Vink [2010].
Figure 1.
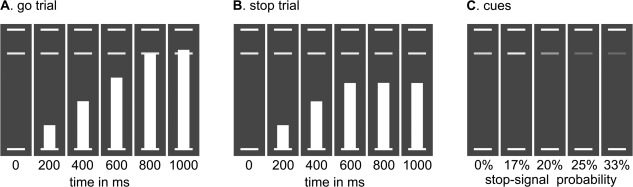
Stop‐signal anticipation task. Three horizontal lines formed the background displayed continuously during the task. A: In each trial, a bar moved at constant speed from the bottom up, reaching the middle line in 800 ms. The main task was to stop the bar as close to the middle line as possible by pressing a button with the right thumb. In other words, the target response time was 800 ms. These trials are referred to as go trials. B: In a minority of trials, the bar stopped moving automatically before reaching the middle line (i.e., the stop‐signal), indicating that a response had to be withheld. These trials are referred to as stop trials. C: The probability that a stop‐signal would occur was manipulated across trials and was indicated by the color of the target response line. There were five stop‐signal probability levels: 0% (green); 17% (yellow); 20% (amber); 25% (orange); and 33% (red).
Prior to scanning, subjects were trained extensively on the task to ensure that they understood the task and the meaning of the cues. In brief, subjects were first presented with 30 trials with a 0% stop‐signal probability (green cue), and subjects were told that they had to respond on each trial. In this way, subjects could familiarize themselves with the general task procedure. Subjects who failed to respond in time were excluded from further participation. Next, 30 trials were presented with a yellow cue (17% stop‐signal probability), and subjects were told that in some trials the bar would stop moving. The purpose of this setup was to practice stopping. Then, all cues were presented (green to red) and subjects were asked to explain their meaning to the experimenter. All subjects were able to properly indicate the meaning of the cues. After a final standardized instruction on the task, the complete task (different sequence from the scanner sequence) was practiced in the presence of the experimenter.
Functional MRI Acquisition
The experiment was performed on a Philips Achieva 3.0 T MRI scanner at the UMCU. We collected 622 whole‐brain, T2*‐weighted echo planar images with blood oxygen‐dependent contrast [repetition time = 1,600 ms, echo time = 23.5 ms, flip angle = 72.5°, 4 mm × 4 mm in‐plane resolution, 4 mm slice thickness, 30 slices per volume, SENSE factor, 2.4 (anterior–posterior)] in a single run, and a T1‐weighted image for within‐subject registration purposes [for details, see Zandbelt and Vink, 2010].
Data Analysis
Performance
Basic response execution was measured by the latency and variability (standard deviation) of correct responses during go trials with a 0% stop‐signal probability.
Reactive inhibition was measured by the latency (SSRT) and success of stopping on stop trials. The SSRT was computed according to the integration method [Logan and Cowan, 1984] and pooled across all stop‐signal probability levels.
Proactive inhibition was measured as the effect of stop‐signal probability on go‐signal response time. Typically, adult subjects tend to slow down responding as the probability increases that they have to stop their response [Vink et al., 2005]. For all measures, the effect of age was estimated using a regression analysis with age as a continuous regressor. Additional analyses (ANOVA) were performed over the three age groups (early adolescence: 10–15 years; late adolescence: 15–20 years; and early adulthood: 20–25 years).
Activation
Image data were analyzed using SPM (http://www.fil.ion.ucl.ac.uk/spm). Preprocessing and first‐level statistical analysis were performed as described before [Zandbelt and Vink, 2010]. In brief, preprocessing involved correction for slice timing differences, realignment to correct for head motion, spatial normalization, and spatial smoothing to accommodate interindividual differences in neuroanatomy.
The fMRI data were modeled voxelwise using a general linear model, in which the following events were included as regressors: successful stop trials, failed stop trials, and go trials with stop‐signal probability >0% (i.e., 17, 20, 25, and 33%). Rest blocks were also modeled so that go trials with a 0% stop‐signal probability served as baseline. For go trials with a stop‐signal probability > 0%, we also included two parametric regressors modeling response time and stop‐signal probability level. The response time regressor was included to control for variation in response speed independent from stop‐signal probability effects. The realignment parameters were included to account for residual effects of head motion during scanning. A high‐pass filter was included to correct for low‐frequency drifts. For each participant, we computed four contrast images: (1) activation in the motor cortex during go trials with a 0% stop‐signal probability (to assess basic response execution), (2) activation during successful stop trials versus failed stop trials (to assess reactive inhibition), (3) activation during successful stop trials versus go trials in the 0% stop‐signal probability context (also to assess reactive inhibition), and (4) the parametric effect of stop‐signal probability on go‐signal activation (to assess proactive inhibition). We computed two contrasts for reactive inhibition because there is no consensus on which contrast is most appropriate for investigating reactive inhibition, and the contrasts may provide complementary information.
Next, we examined the effect of age on brain activation in predefined ROI. ROI were defined using data from a previous experiment [Zandbelt and Vink, 2010], in which a sample of 24 healthy volunteers performed the same task. These ROI were defined using a cluster‐level threshold (cluster‐defining threshold P < 0.001, cluster probability of P < 0.05, family‐wise error corrected for multiple comparisons). These included regions covering the frontostriatal network and the motor cortex during the proactive inhibition condition and the two reactive inhibition conditions (see Supporting Information Table S1). In addition, we defined bilateral STN ROI as 6‐mm spheres around the MNI coordinates [±12, −16, −4], in accordance with a human basal ganglia template [Prodoehl et al., 2008] and a previous study investigating STN activation during inhibitory control [Aron and Poldrack, 2006]. From these ROI, we extracted for each participant the mean activation level (i.e., parameter estimate) for the four contrasts of interest. Mean activation levels of all ROI were analyzed using a regression analysis with age as predictor. Additional analyses were performed with the three age groups. Finally, to investigate potential age‐related effects in regions outside the predefined ROI, whole‐brain analyses with age as covariate were performed. Maps resulting from this analysis were tested for significance using cluster‐level inference (cluster‐defining threshold, P < 0.001, cluster probability of P < 0.05, family‐wise error corrected for multiple comparisons). These parameters were determined using SPM and a script (CorrClusTh.m, http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm), which uses estimated smoothness (estimated full width at half maximum: 3.56 × 3.65 × 3.46 voxels) and random field theory to find these corrected thresholds.
Functional connectivity
Functional connectivity analyses were performed using psychophysiological interaction (PPI) analyses [Friston et al., 1997] to investigate the effect of age on the coupling between ROI of the frontostriatal network. For reactive inhibition, the seed (i.e., volume of interest) was defined as a 6‐mm‐radius sphere in the right striatum (MNI coordinates [28, 8, −4]). This location was obtained from our previous study [Zandbelt and Vink, 2010] and showed increased connectivity with the right IFG, SMA, and motor cortex during successful versus unsuccessful inhibition. Using this seed, a PPI analysis was conducted to investigate the functional coupling during successful stop trials versus unsuccessful stop trials (i.e., psychological factor) between the striatum, the right IFG, and SMA. For proactive inhibition, the seed was defined as a 6‐mm‐radius sphere around the center‐of‐mass of the right striatum (MNI coordinates [20, 12, 0]). A PPI analysis was conducted to investigate the functional coupling during go trials with stop‐signal probability >0% between the striatum and the right IFG and SMA.
For each subject, the first eigenvariate of the BOLD signal within the seed region was calculated and adjusted for average activation during the task (i.e., F‐contrast showing effects of task) and head motion. The interaction between activity within the seed region and the psychological factor (i.e., PPI regressor) was then calculated, and activity positively as well as negatively related to each interaction was investigated. Subsequently, these individual contrast images of the PPI analyses were entered into the second‐level analyses to test for the effect of age on functional connectivity within the frontostriatal network.
RESULTS
Basic Response Execution
Performance
Basic response execution data are presented in Figure 2. As hypothesized, baseline response latency (reaction times on go trials with a 0% stop‐signal probability) did not change with age [F(1,59) = 1.22; r = −0.14; P = 0.27]. This is probably due to the fact that our task requires timed rather than speeded responses. However, the variability in response latency on go trials with a 0% stop‐signal probability decreased across development [F(1,59) = 29.1, P < 0.001], indicating that older subjects were more consistent in timing their responses.
Figure 2.
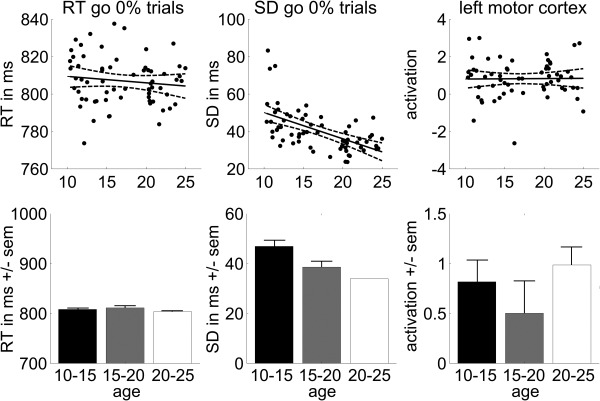
Basic response execution. Scatter plots of reaction times (RT), standard deviations (SD), and left motor cortex activation for go trials with a 0% stop‐signal probability as a function of age (with linear trend line and 95% confidence interval), and bar plots with averages for the three age groups (± standard error of the mean).
Activation
As expected, activation in the primary motor cortex during baseline go trials with a 0% stop‐signal probability did not change with age [F(1,59) = 0.007, P = 0.93; see Fig. 2]. Together with the behavioral data, we take this result to indicate that all subjects performed the task at an adequate level.
Reactive Inhibition
Performance
Reactive inhibition data are presented in Figure 3. As hypothesized, there was a negative relationship between reactive inhibition latency (SSRT) and age [F(1,59) = 8.89; r = −0.36; P = 0.004], with older subjects showing shorter latencies. Post hoc t‐tests for the three age groups revealed that young adults (20–25 years) were significantly faster in inhibition than young adolescents (10–15 years) [t(46) = 2.47; P = 0.02]. Inhibition accuracy also improved with age [F(1,59) = 3.84; r = 0.25; P = 0.05], confirming earlier reports [Bedard et al., 2002; van de Laar et al., 2011; Williams et al., 1999]. There was a trend toward an improvement between the youngest group (10–15 years) and the oldest group (20–25 years: t(46) = −1.45; P = 0.154). Finally, an ANOVA across the three age groups did not reveal a significant effect [F(2,57) = 1.25; P = 0.29].
Figure 3.
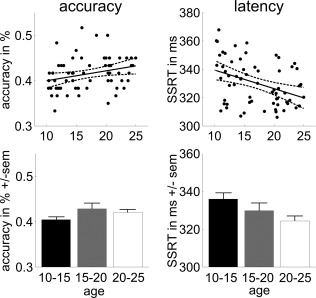
Reactive inhibition performance data. Scatter plots of inhibition accuracy (in percent) and stop‐signal reaction time (SSRT in milliseconds) as a function of age (with linear trend line and 95% confidence interval), and bar plots with averages for the three age groups (± standard error of the mean).
Activation
Reactive inhibition activation data are presented in Figure 4. We calculated brain activation related to reactive inhibition using two contrasts (successful stop trials versus failed stop trials, and successful stop trials versus go trials in the 0% stop‐signal probability context). Both contrasts revealed a negative relationship between age and activity in the left primary motor cortex indicating more motor cortex suppression during successful inhibition in older subjects [F(1,59) = 4.15; r = −0.26; P = 0.047 and F(1,59) = 7.64, P = 0.008, respectively]. Moreover, the level of stop accuracy was negatively correlated with the level of deactivation of the motor cortex for the latter contrast [F(1,59) = 7.47; r = −0.34; P = 0.008].
Figure 4.
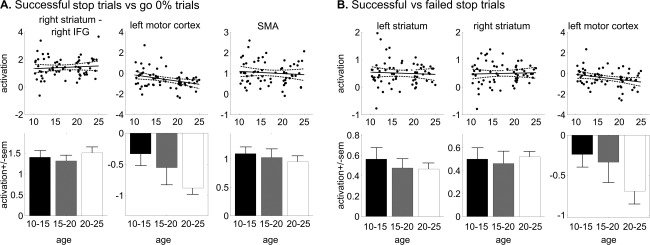
Reactive inhibition activation data. Scatter plots of brain activation (regression coefficients) as a function of age (with linear trend line and 95% confidence interval), and bar plots with averages for the three age groups (± standard error of the mean). Abbreviations: IFG, inferior frontal gyrus; SMA, supplementary motor area.
Activation in the bilateral striatum [left striatum: F(1,59) = 0.48; r = −0.09; P = 0.49; right striatum: F(1,59) = 0.015; r = −0.016; P = 0.90], right IFG [F(1,59) = 0.05, P = 0.82], and SMA [F(1,59) = 0.49, P = 0.49] did not change over the course of adolescence. Post hoc t‐tests over the three age groups revealed that the oldest subjects (20–25 years) showed significantly more motor suppression than the youngest group (10–15 years) in both contrasts [t(49) = 2.03; P = 0.047, and t(49) = 2.64, P = 0.01, respectively].
We also investigated developmental changes in activation in the STN for both contrasts, as this region is commonly associated with response inhibition, but found no age‐related effects (Supporting Information Fig. S1).
We performed whole‐brain analyses for both contrasts to unravel potential age‐related effects outside the predefined regions. These analyses did not reveal significant clusters.
Functional connectivity
Results from the PPI analyses during reactive inhibition are presented in Supporting Information Figure S2. These analyses revealed no changes across age in functional connectivity between the right striatum and rIFG, SMA, or motor cortex.
Proactive Inhibition
Performance
Proactive inhibition data are presented in Figure 5. The amount of proactive inhibition, as calculated by the amount of response time slowing on go trials as function of stop‐signal probability, increased with age [F(1,59) = 11.25; r = 0.40; P = 0.001]. Post hoc t‐tests showed that the young adolescents (10–15 years) showed significantly less proactive inhibition than the young adults (20–25 years: t(46) = −3.25; P = 0.002). A repeated‐measures ANOVA with stop‐signal probability (five levels) as within and age group (three levels) as between‐subject variables yielded a significant interaction (stop‐signal probability × age group: F(4,57) = 2.15; P = 0.032), with the youngest group (10–15 years) showing the least amount of reaction time slowing with increasing stop‐signal probability.
Figure 5.
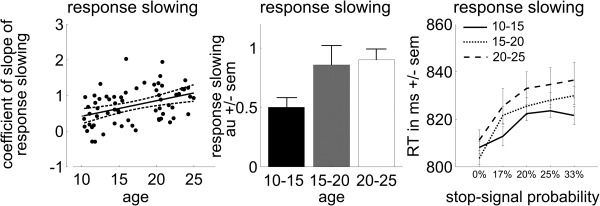
Proactive inhibition performance data. Scatter plot of the level of response slowing (regression coefficient of the slope of response slowing) as a function of age (with linear trend line and 95% confidence interval), bar plots with averages for the three age groups of the amount of response slowing (± standard error of the mean), and line plot of the reaction times (RT) for the various levels of stop‐signal probability split for the three age groups (± standard error of the mean).
Finally, regression analyses with the level of proactive inhibition as independent and the level of motor cortex deactivation as dependent variables were highly significant [contrast Successful stop versus Go baseline: F(1,59) = 11.30; r = −0.40; P = 0.001, contrast Successful versus failed stops: F(1,59) = 8.25; r = −0.36; P = 0.005], suggesting that those subjects showing a high degree of proactive inhibition also show the most motor cortex deactivation during actual inhibition.
Activation
Proactive inhibition activation data are presented in Figure 6. The analyses showed that across development, activation in the frontostriatal network increased. Specifically, the analysis revealed a positive age‐related effect in the right striatum [F(1,59) = 4.89; r = 0.28; P = 0.03] and ventral rIFG [F(1,59) = 9.22; r = 0.37; P = 0.004]. There was a trend toward an increase with age in activation in the dorsal rIFG [F(1,59) = 3.60; r = 0.24; P = 0.06] and the SMA [F(1,59) = 3.25; r = 0.23; P = 0.07]. Post hoc t‐tests between the three age groups revealed that the youngest group (10–15 years) showed less activation than the oldest group in the striatum [t(49) = −2.04; P = 0.047], ventral rIFG [t(49) = −2.79; P = 0.007], and dorsal rIFG [t(49) = −2.49; P = 0.016].
Figure 6.
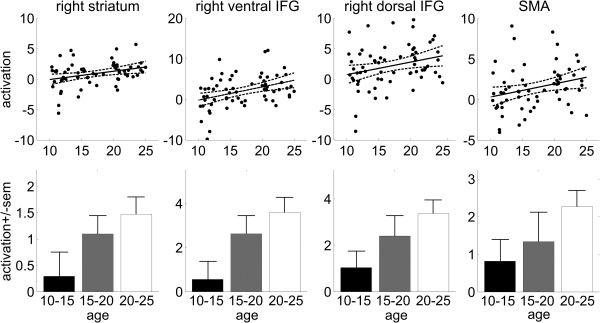
Proactive inhibition activation data. Scatter plots of brain activation (regression coefficients) as a function of age (with linear trend line and 95% confidence interval), and bar plots with averages for the three age groups (± standard error of the mean). Abbreviations: IFG, inferior frontal gyrus; SMA, supplementary motor area.
We performed a whole‐brain analysis to detect potential age‐related effects outside the predefined regions. This analysis did not reveal significant clusters.
Functional connectivity
Results from the PPI analyses are presented in Figure 7. Regression analyses showed that across development, activation in the striatum became more strongly connected with the ventral rIFG [F(1,59) = 4.99; r = 0.28; P = 0.03] but not the dorsal rIFG [F(1,59) = 0.07; r = 0.04; P = 0.789]. Post hoc t‐tests showed that for striatum–ventral rIFG connectivity, the young adolescents (10–15 years) showed significantly less functional connectivity than the older adolescents [15–20 years: t(34) = −3.45; P = 0.001] and young adults [20–25 years: t(46) = −2.45; P = 0.02]. Explorative post hoc analyses for striatum–dorsal rIFG connectivity revealed that young adolescents showed less connectivity than the older adolescents [t(34) = −2.77, P = 0.009]. The increase in connectivity with age between the striatum and SMA neared significance [F(1,57) = 3.09; r = 0.23; P = 0.08]. Explorative tests to investigate this trend showed that the youngest group displayed less connectivity than the oldest group [t(44) = −2.21; P = 0.03].
Figure 7.
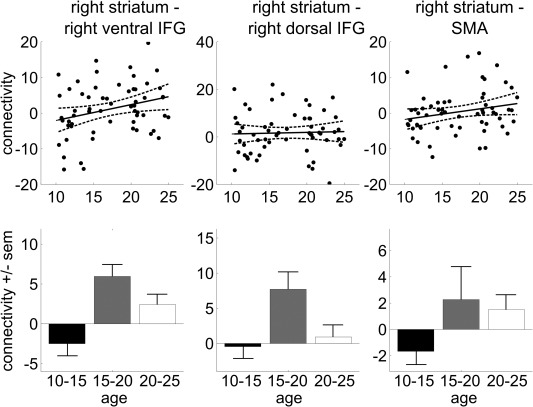
Proactive inhibition connectivity data. Scatter plots of the level of functional connectivity (regression coefficients) with the right striatum as a function of age (with linear trend line and 95% confidence interval), and bar plots with averages for the three age groups (± standard error of the mean). Abbreviations: IFG, inferior frontal gyrus; SMA, supplementary motor area.
DISCUSSION
Here, we present data on developmental changes in the frontostriatal network during both baseline response execution, reactive (i.e., outright stopping), and proactive inhibition (i.e., anticipation of stopping) in a cohort of 60 healthy volunteers aged 10–25 years.
There were no effects of age on baseline response latency and motor activation, indicating adequate task performance in all subjects. In line with previous reports, we found that reactive inhibition improved with age: older subjects were more accurate and faster in inhibiting their responses than younger subjects (see Fig. 2). This was paralleled by increased deactivation of the motor cortex, whereas no effects of age were seen in the striatum, IFG, or STN (Fig. 4 and Supporting Information Fig. S1). In addition, there was no developmental change in functional connectivity between the striatum and frontal regions during reactive inhibition. Furthermore, we show here for the first time that proactive inhibition improves with age, with older subjects showing more response slowing in anticipation of having to stop their response (Fig. 3). This was coupled with an increase in brain activation in the frontostriatal network with age (Fig. 6). Moreover, as hypothesized, frontostriatal connectivity also increased with age during proactive inhibition (Fig. 7).
Taken together, these results suggest that young subjects are less effective in their inhibitory capabilities. As we will argue below, this may be caused by the fact that they are unable to proactively anticipate stopping. As proactive inhibition arises from the concerted actions of the frontal cortex and striatum [Aron, 2011; Chikazoe et al., 2009b; Zandbelt and Vink, 2010; Zandbelt et al., 2013b], the lack of proactive inhibitory control may reflect the relatively underdeveloped state of the frontal cortex and its connections with the striatum in younger subjects. With ongoing development, frontal control gradually increases leading to improvements in higher‐order cognitive functions such as proactive inhibition. Indeed, we found that older subjects do adjust their behavior in anticipation of stop‐signals, allowing them more time to cancel their responses when a stop‐signal appears. In the brain, this was paralleled by increased frontostriatal activation and enhanced connectivity.
Behavior
Basic response execution (Fig. 2) improved with age as evidenced by a decrease in response latency variability (standard deviation of go‐trials with a 0% stop‐probability). Response latencies on baseline go‐trials did not show a relationship with age, probably due to the fact that participants are asked to make timed rather than speeded responses. Reactive inhibition became more accurate and faster with age, and this finding is consistent with data from a large number of studies [Bedard et al., 2002; Durston et al., 2002; Rubia et al., 2007; Tamm et al., 2002; van de Laar et al., 2011; Velanova et al., 2009; Williams et al., 1999].
Proactive inhibition also improved with age. As we and others have shown previously, adults typically slow down responding when they anticipate having to stop their response [Chikazoe et al., 2009a,b; Jahfari et al., 2010; Logan and Burkell, 1986; Verbruggen and Logan, 2009; Vink et al., 2005, 2006; Zandbelt and Vink, 2010; Zandbelt et al., 2011]. Here, we replicate this effect in adults, but found that younger subjects showed less response slowing. This suggests that they do not anticipate upcoming stop‐signals, possibly because they fail to adequately process cues indicating the stop‐signal probability. To date, there are no other studies investigating developmental changes in proactive inhibition. However, our finding may be linked to that of reduced reward anticipation observed in young adolescents [Geier et al., 2010]. Similar to our task, in reward tasks, a cue is presented which signals a particular stimulus. Typically, adults speed up responding in anticipation of a reward associated with that stimulus, whereas young adolescents do not [Hoogendam et al., 2013]. Our finding may also be consistent with the notion that although young children perform above chance level on inhibition, they fail to exert their inhibitory control in a persistent manner throughout the task [see review by Luna, 2009]. We argue that this ability to exert reactive inhibitory control improves as adolescents learn to proactively engage inhibitory control in response to cues signaling potential inhibition. In summary, it may very well be concluded that reactive inhibition becomes more effective with the development of proactive inhibition capabilities.
Neuroimaging
We investigated reactive inhibition by comparing brain activation during successful inhibition with failed inhibition (i.e., subjects do respond when they should have refrained from responding) and brain activation during successful inhibition versus baseline responding (go trials with a 0% stop‐signal probability). In these contrasts, the motor cortex is typically deactivated, indicating suppression of this region when a response is successfully inhibited [Chikazoe et al., 2009b; Vink et al., 2005; Zandbelt and Vink, 2010]. We found that this suppression of the motor cortex increases with age (Fig. 4), indicating more effective inhibition. On a behavioral level, this was paralleled by faster and more accurate stopping in older subjects.
In contrast to previous studies investigating reactive inhibition, we did not observe age‐related effects in the frontostriatal network. Casey et al. [1997] and Durston et al. [2002] reported a decrease in frontal activation across age during inhibition trials (no‐go trials) when compared with go trials. Rubia et al. [2007] found increased power of response in the right striatum and right inferior frontal cortex in adolescents when compared with adults. Velanova et al. [2009] tested 98 subjects aged 8–27 years on an antisaccade task, and they found transient activation trial locked to antisaccades to decrease from childhood to adolescence in regions implicated in inhibitory control. These studies all point to a decrease with age in activation during reactive inhibition in frontal and striatal regions. This may be caused by the fact that the task used [Casey et al., 1997; Durston et al., 2002; Rubia et al., 2007] did not include a baseline go‐signal condition (go trials with a 0% stop‐signal probability) in which it was clear to the participants that no stop‐signal (or no‐go stimulus) could occur. In contrast, stop trials were compared with go trials with a stop‐signal probability > 0%, which include proactive response strategies. As these strategies interact with age, they confound the results of these studies. Indeed, we have shown previously that, in adults, the striatum and right inferior frontal cortex already become activated during go trials in which a stop‐signal is anticipated [Vink et al., 2005; Zandbelt and Vink, 2010; Zandbelt et al., 2013b]. These regions are also activated during successful stop trials. Therefore, the contrast of successful stop trials versus go trials as used in these studies will not show activation in these regions specifically in adults, as they activate the network in both conditions. Moreover, younger subjects show less activation in the frontal cortex and the striatum during go trials in which a stop‐signal is anticipated, and thus, the contrast with stop trials will yield more activation. Indeed, these studies report less activation in these regions in adults when compared with children and adolescents. Taken together, these age‐related decreases in activation are likely not due merely to differences in reactive inhibition but also due to differences in proactive inhibition.
Our results show increases in activation with age during proactive inhibition in the right striatum, right inferior frontal cortex (rIFG), and SMA. These regions are well known for their involvement in inhibitory control [Aron, 2011], although their exact roles are still being debated. Although this is the first study to investigate developmental changes in proactive inhibition, there are two studies that show results which can be interpreted as being consistent with our findings. Stevens et al. [2007] showed that adolescents (n = 25, mean age 14.7 years) engaged the rIFG, right putamen, and thalamus to a lesser extent than adults (n = 25, mean age 25.1 years) during a Go/No‐Go task. A study by Velanova et al. [2009] reported that sustained activation increased from childhood to adulthood (children: n = 26, mean age, 10.5 years; adolescents: n = 25, mean age, 15.3 years; adults: n = 27, mean age, 20.7 years) in regions implicated in control, that is, the medial superior frontal gyrus, bilateral insula, inferior parietal lobule, and the middle temporal gyrus.
Finally, we found that functional connectivity between the striatum and the rIFG increased with age during proactive but not reactive inhibition. During proactive inhibition, young adolescents showed almost no functional connectivity between the striatum and frontal regions, whereas older adolescents (aged 15–20 years) and young adults (aged 20–25 years) did (Fig. 7). This finding of increased connectivity across age is consistent with findings of Hwang et al. [2010], who investigated the development of inhibitory control in 78 subjects aged 8–27 years [same groups as Velanova et al., 2009] using functional MRI and an antisaccade task. They showed that developmental improvements in inhibitory control may be supported by age‐related enhancements in top–down effective connectivity between frontal, oculomotor, and subcortical regions.
Our finding is also consistent with data from structural MRI studies. Brain development is associated not only with the local maturation of tissue, as evidenced by, for example, increases in gray matter [Brouwer et al., 2012], but also with strengthening of white matter connectivity [fiber‐tracts] which allow for the fine tuning of neural networks [Smit et al., 2012]. Specifically, evidence exists for progressive structural maturation of frontostriatal fiber tracts between the age range of 7–31 years, which correlated with inhibitory performance on a go/no‐go task [Liston et al., 2006].
The fact that we did not observe an increase in frontostriatal connectivity during reactive inhibition was not anticipated, but seems to be in line with our finding of no change in brain activation in the frontostriatal network during reactive inhibition. As such, this finding underlines the difference between reactive and proactive inhibition, the latter being more dependent on higher‐order cognitive functioning [Cools, 2011] and frontostriatal interactions [Vink et al., 2005; Zandbelt and Vink, 2010].
Reactive and Proactive Inhibition
Children at the beginning of adolescence are able to inhibit their responses and recruit frontostriatal regions to the same extent as adults. Nevertheless, young subjects are slower in inhibition, and they are less effective in suppressing the motor cortex. In itself, reactive inhibition is relatively simple as it is triggered by an external stop‐signal. Improving this process can potentially be achieved by preparing to stop in response to cues indicating stop‐signal probability. Therefore, we hypothesize that this poorer performance of younger participants on reactive inhibitory control may be related to the fact that they fail to anticipate stop‐signals to occur. This anticipation requires establishing higher‐order stimulus response associations, which involve frontal regions and their interconnections with subcortical regions such as the striatum [Vink et al., 2013]. Such interactions crucially depend on dopamine transmission [Cools, 2011]. For example, we found that patients with schizophrenia who are known to have frontal dopamine deficits show normal reactive inhibition capabilities, but fail specifically in proactive inhibition [Raemaekers et al., 2006; Vink et al., 2006; Zandbelt et al., 2011]. This parallels our finding in young adolescents, showing reduced levels of frontostriatal activation and connectivity when compared with adults during proactive but not reactive inhibition. These results are in line not only with functional and structural MRI data but also with the fact that the dopamine system, which play a key role in facilitating higher‐order cognitive functions [Cools, 2011], is underdeveloped in early adolescence, particularly in the frontal cortex [Garske et al., 2013]. Taken together, our data suggest that proactive inhibition may be a sensitive measure for probing the developmental (maturational) status of the frontostriatal network.
Limitations
A number of limitations need to be considered. First, we did not obtain information about pubertal state or hormonal states. We did include subjects within a wide age range so that pubertal stage and age are probably highly related [Blakemore et al., 2010]. However, although age‐related changes provide an indication for adolescent phases, our data do not allow investigation of specific pubertal effects [Crone and Dahl, 2012]. Second, although our study is the first to provide an indication of developmental changes in the frontostriatal network during adolescence, these findings should be replicated in a within‐subject longitudinal study. Third, inhibition accuracy for all subjects was below the predicted 50%, indicating that the online manipulation of task difficulty via varying the onset of the stop‐signal using a staircase procedure was suboptimal. In our task, the staircase procedure did not result in 50% inhibition accuracy (but rather reached 41%) as the initial stop‐signal onset was too close to the target response time (800 ms), and the number of trials for the staircase to converge to an inhibition ratio of 50% was too small.
SUMMARY AND CONCLUSION
In conclusion, the ability for reactive inhibition is already present at the beginning of adolescence and improves during development as frontal regions start to mature, allowing for higher‐order proactive response strategies. Indeed, although young people are primarily reacting to external stimuli [Gladwin et al., 2011; Hoogendam et al., 2013], such as stop‐signals, older subjects anticipate these upcoming events and are faster in reactive inhibition. This proactive response strategy is facilitated by developmental changes that are characterized by an increase in activation and in functional connectivity within the frontostriatal network.
These data serve as a stepping stone to investigate abnormal development of the frontostriatal network. Psychiatric illnesses such as schizophrenia [Raemaekers et al., 2006; Vink et al., 2006; Zandbelt et al., 2011] and obsessive compulsive disorder [Figee et al., 2011, 2013] are characterized by impaired frontostriatal functioning and have their onset in early adolescence [Paus et al., 2008]. The interplay between neural maturation and acquiring control of behavior and cognition, in particular of inhibitory processes, may be an essential component of future models of the development of psychopathology.
Supporting information
Supplementary Information Figure 1.
Supplementary Information Figure 2.
Supplementary Information Table 1.
REFERENCES
- Aron AR (2011): From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006): Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard A, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. (2002): The development of selective inhibitory control across the life span. Developmental Neuropsychology 21(1), 93–111. doi:10.1207/S15326942DN2101_5 [DOI] [PubMed] [Google Scholar]
- Blakemore S‐J, Burnett S, Dahl RE (2010): The role of puberty in the developing adolescent brain. Hum Brain Mapp 31:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer RM, Mandl RCW, Schnack HG, van Soelen ILC, van Baal GC, Peper JS, Kahn RS, Boomsma DI, Hulshoff Pol HE (2012): White matter development in early puberty: A longitudinal volumetric and diffusion tensor imaging twin study. PLoS One 7:e32316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Caudle K (2013): The teenage brain: Self control. Curr Dir Psychol Sci 22:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Orendi J, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL (1997): A developmental functional MRI study of prefrontal activation during performance of a go‐no‐go task. J Cogn Neurosci 1997;9:835–847. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA (2008): The adolescent brain. Ann NY Acad Sci 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S (2009a): Functional dissociation in right inferior frontal cortex during performance of go/no‐go task. Cereb Cortex 19:146–152. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009b): Preparation to inhibit a response complements response inhibition during performance of a stop‐signal task. J Neurosci 29:15870–15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R (2011): Dopaminergic control of the striatum for high‐level cognition. Curr Opin Neurobiol 21:402–407. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD (2006): Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95:3371–3383. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE (2012): Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat Rev Neurosci 13:636–650. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, Casey B (2002): A neural basis for the development of inhibitory control. Dev Sci 5:F9–F16. [Google Scholar]
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D (2011): Dysfunctional reward circuitry in obsessive‐compulsive disorder. Biol Psychiatry 69:867–874. [DOI] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia‐Alfonso C‐E, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Vulink N, Levar N, Droge L, van den Munckhof P, Schuurman PR, Nederveen A, van den Brink W, Mazaheri A, Vink M, Denys D (2013): Deep brain stimulation restores frontostriatal network activity in obsessive‐compulsive disorder. Nat Neurosci 16:386–387. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Garske AK, Lawyer CR, Peterson BM, Illig KR (2013): Adolescent changes in dopamine d1 receptor expression in orbitofrontal cortex and piriform cortex accompany an associative learning deficit. PLoS One 8:e56191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B (2010): Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex 20:1613–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Figner B, Crone EA, Wiers RW (2011): Addiction, adolescence, and the integration of control and motivation. Dev Cogn Neurosci 1:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Kahn RS, Hillegers MHJ, van Buuren M, Vink M (2013): Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev Cogn Neurosci 6:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, & Luna B. (2010): Strengthening of Top‐Down Frontal Cognitive Control Networks Underlying the Development of Inhibitory Control: A Functional Magnetic Resonance Imaging Effective Connectivity Study. Journal of Neuroscience, 30(46), 15535–15545. doi:10.1523/JNEUROSCI.2825‐10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR (2010): Responding with restraint: What are the neurocognitive mechanisms? J Cogn Neurosci 22:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C‐SR, Yan P, Sinha R, Lee T‐W (2008): Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ (2006): Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex 16:553–560. [DOI] [PubMed] [Google Scholar]
- Lo C‐C, Boucher L, Paré M, Schall JD, Wang X‐J (2009): Proactive inhibitory control and attractor dynamics in countermanding action: A spiking neural circuit model. J Neurosci 29:9059–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB (1984): On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev 91:295–327. [DOI] [PubMed] [Google Scholar]
- Logan GD, Burkell J. (1986): Dependence and independence in responding to double stimulation. A comparison of stop, change, and dual‐task paradigms. J Exp Psychol: Hum Percept Perform 12:549–563. [Google Scholar]
- Luna B (2009): Developmental changes in cognitive control through adolescence. Adv Child Dev Behav 37:233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (2001): Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13:786–793. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. (2008): Why do many psychiatric disorders emerge during adolescence? Nature Reviews. Neuroscience 9(12), 947–957. doi:10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE (2008): Region of interest template for the human basal ganglia: Comparing EPI and standardized space approaches. Neuroimage 39:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS (2006): Brain activation during antisaccades in unaffected relatives of schizophrenic patients. Biol Psychiatry 59:530–535. [DOI] [PubMed] [Google Scholar]
- Robbins TW (2007): Shifting and stopping: Fronto‐striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci 362:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M (2007): Linear age‐correlated functional development of right inferior fronto‐striato‐cerebellar networks during response inhibition and anterior cingulate during error‐related processes. Hum Brain Mapp 28:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJA, Boersma M, Schnack HG, Micheloyannis S, Boomsma DI, Hulshoff Pol HE, Stam CJ, de Geus EJC (2012): The brain matures with stronger functional connectivity and decreased randomness of its network. PLoS One 7:e36896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ (2011): Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci 23:2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD (2007): Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res 181:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL (2002): Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 41:1231–1238. [DOI] [PubMed] [Google Scholar]
- van de Laar MC, van den Wildenberg WPM, van Boxtel GJM, van der Molen MW (2011): Lifespan changes in global and selective stopping and performance adjustments. Front Psychol 2:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WPM, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T (2010): Mechanisms and dynamics of cortical motor inhibition in the stop‐signal paradigm: A TMS study. J Cogn Neurosci 22:225–239. [DOI] [PubMed] [Google Scholar]
- van Dijk KRA, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B (2009): The maturation of task set‐related activation supports late developmental improvements in inhibitory control. J Neurosci 29:12558–12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF (2005): Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp 25:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. (2009): Proactive adjustments of response strategies in the stop‐signal paradigm. J Exp Psychol Hum Percept Perform 35:835–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Ramsey NF, Raemaekers M, Kahn RS (2006): Striatal dysfunction in schizophrenia and unaffected relatives. Biol Psychiatry 60:32–39. [DOI] [PubMed] [Google Scholar]
- Vink M, Pas P, Bijleveld E, Custers R, Gladwin TE (2013): Ventral striatum is related to within‐subject learning performance. Neuroscience 250:408–416. [DOI] [PubMed] [Google Scholar]
- Williams BRR, Ponesse JSS, Schachar RJJ, Logan GDD, Tannock R (1999): Development of inhibitory control across the life span. Dev Psychol 35:205. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M (2010): On the role of the striatum in response inhibition. PLoS One 5:e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Gladwin TE, Raemaekers M, van Buuren M, Neggers SF, Kahn RS, Ramsey NF, Vink M (2008): Within‐subject variation in BOLD‐fMRI signal changes across repeated measurements: Quantification and implications for sample size. Neuroimage 42:196–206. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, van Buuren M, Kahn RS, Vink M (2011): Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biol Psychiatry 70:1151–1158. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Hoogendam JM, Kahn RS, Vink M (2013a): Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop‐signal response inhibition. J Cogn Neurosci 25:157–174. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M (2013b): Expectations and violations: Delineating the neural network of proactive inhibitory control. Hum Brain Mapp 34:2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figure 1.
Supplementary Information Figure 2.
Supplementary Information Table 1.


