Abstract
The prefrontal cortex has a pivotal role in top‐down control of cognitive and sensory functions. In complex go‐nogo tasks, the right dorsolateral prefrontal cortex is considered to be important for guiding the response inhibition. However, little is known about the temporal dynamics and neurophysiological nature of this activity. To address this issue, we recorded magnetoencephalographic brain activity in 20 women during a visual go‐nogo task. The right dorsolateral prefrontal cortex showed an increase for the amplitude of the event‐related fields and an increase in induced alpha frequency band activity for nogo in comparison to go trials. The peak of this prefrontal activity preceded the mean reaction time of around 360 ms for go trials, and thus supports the proposed role of right dorsolateral prefrontal cortex in gating the response inhibition and further suggests that right prefrontal alpha band activity might be involved in this gating. However, the results in right dorsolateral prefrontal cortex were similar for both successful and unsuccessful response inhibition. In these conditions, we instead observed pre‐ and poststimulus differences in alpha band activity in occipital and central areas. Thus, successful response inhibition seemed to additionally depend on prestimulus anticipatory alpha desynchronization in sensory areas as it was reduced prior to unsuccessful response inhibition. In conclusion, we suggest a role for functional inhibition by alpha synchronization not only in sensory, but also in prefrontal areas. Hum Brain Mapp 35:5236–5248, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: event‐related fields, executive function, magnetoencephalography, prefrontal cortex, oscillatory activity
INTRODUCTION
Response inhibition is a crucial executive function that allows us to inhibit actions, thoughts, and impulses that are inappropriate in a given context. Response inhibition of motor actions can be investigated in go‐nogo tasks, in which subjects are required to perform speeded responses on go trials and to withhold responses on nogo trials. It is well established that the prefrontal cortex (PFC) has an important role for response inhibition (for reviews see: Aron et al. [2004] and Chikazoe et al. [2010]) and is essential for a variety of executive functions [Miller and D'Esposito, 2005]. In particular, a right‐lateralized network, which includes right dorsolateral prefrontal cortex (rDLPFC) and the right inferior parietal lobule near the temporo‐parietal junction, shows increased activation during complex go‐nogo tasks requiring higher‐level stimulus‐response associations [Simmonds et al., 2008]. This network appears to be necessary for maintaining representations of stimulus–response associations to guide the appropriate response selection with respect to the current stimulus [Hester et al., 2007]. The posterior cortical regions integrate cues and motor actions into stimulus–response associations and maintain these throughout the task [Liu et al., 2004], while rDLPFC mediates top‐down control to gate the selection of the required response given the current stimulus [Miller and Cohen, 2001]. Consequently, rDLPFC is considered to have a pivotal role in monitoring and guiding behavior in accordance to context dependent requirements.
To further delineate the functional involvement of a specific brain area during response inhibition, the timing of the neuronal processes is crucial. With the presentation of a stimulus during a go‐nogo trial a cascade of different processes is initiated, including stimulus classification, action selection, and subsequently performance or inhibition of an action. All of them require exact timing and coordination. Consequently, the temporal dynamics of rDLPFC activity can provide essential information regarding its proposed role for response inhibition and allow for a better understanding of the neurophysiological nature of its activity. Coordination and generation of the temporal structure of brain networks are essentially connected to oscillatory brain activity [Fries, 2005; Varela et al., 2001]. Recent evidence suggests a role for alpha band synchronization in gating information by functional inhibition (for reviews see: Jensen and Mazaheri [2010] and Klimesch et al. [2012; 2007a]).
So far, this active inhibitory mechanism of alpha was mainly observed in sensory areas. This raises the question whether this functional inhibition might generalize to operations in prefrontal control areas. Supporting evidence for a functional role of prefrontal alpha in selection processes is provided by a recent study by Buschman et al. [2012]. The authors showed that the deselection of a dominant rule led to an increased alpha synchrony in the prefrontal cortex of monkeys enabling the selection of the non‐dominant rule. As a go‐nogo task also includes one stronger (go) and one weaker response mode (nogo), we assumed that the selection of the nogo rule is associated with increased alpha activity.
To address these issues, we used a complex visual go‐nogo paradigm with category specific response inhibition during magnetoencephalographic (MEG) recordings. The stimulus material consisted of food and toy pictures, which was based on our long‐term goal to investigate food‐related control processes in individuals with eating disorders and observed loss of control over food intake. In the current study, however, we focused on general response inhibition aspects in healthy, lean adults.
At first, we addressed the question whether the involvement of rDLPFC in a complex go‐nogo task could be confirmed by magnetic evoked field analysis and whether the corresponding temporal dynamics could support the proposed function in gating the response inhibition. Second, we addressed the nature of the activity in rDLPFC. We hypothesized that the weaker tendency to withhold the response during nogo trials would be associated with increased alpha activity in right prefrontal cortex indicating a role for alpha band synchronization in functional inhibition. For behavioral significance in gating the response selection, changes in right prefrontal alpha band activity had to precede behavioral responses, e.g., prepotent response time during automated go trials, and more specifically, brain activity related to these behavioral responses. Accordingly, we analyzed the relative timing of beta band activity as a surrogate for the lack of behavioral responses during successful withholding of the response, because beta band desynchronization in motor areas is observed during movement preparation [Pfurtscheller et al., 1997; Zhang et al., 2008] and resynchronization after movement execution [Neuper and Pfurtscheller, 1996; Pfurtscheller et al., 1996]. Finally, we explored alpha band activity differences during errors, when a response was not successfully withheld.
MATERIAL AND METHODS
Participants
Twenty‐five healthy, female adults with no self‐reported eating disorder volunteered to participate in this study. Five participants had to be excluded from further analysis due to artifacts in the data or low performance. The remaining 20 participants had a mean age of 27 (range: 22–35) years and a mean body mass index of 21 (range: 18–24) kg m−2. All participants were right‐handed and had normal or corrected‐to‐normal vision (contact lenses, MEG compatible glasses). Each participant was asked to have a normal breakfast. Recordings started between 8 and 10 am. Before the recording, participants indicated their current hunger on a visual analogue scale from 0 to 10 (0: not hungry at all, 10: very hungry). All participants gave written consent. The study was approved by the Ethics Committee of the Medical Faculty and all recordings were conducted at the MEG‐Center of the University of Tübingen.
Study Design and Stimulus Material
The stimulus set consisted of 200 toy and 200 high caloric food pictures matched for spatial spectral power [Toepel et al., 2009] (Fig. 1B) and presented in a visual go‐nogo task. A single session included two experimental blocks, with each block lasting for about 15 min (800 trials in total with duration of 1,000–1,200 ms each). In one block food pictures were the go and toy pictures the nogo cues, and in the other block this was reversed. The blocks were presented in pseudo‐randomized order, 10 participants were assigned to first perform in the food‐go and then in the toys‐go block, and the remaining 10 were assigned to first perform in the toys‐go and then in the food‐go block. In each block 25% nogo and 75% go cues were presented. Pictures were presented for 400 ms each in randomized order with an interstimulus interval of 1,000–1,200 ms (400 ms stimulus presentation, 300 ms blank screen, 300–500 ms presentation of fixation cross) (Fig. 1A). Participants were asked to respond to go cues by button presses with their right index finger before the appearance of the fixation cross. Before each block, there was a training session of 100 trials to familiarize the participants with the task. Stimulus presentation was controlled with Presentation® (Neurobehavioural Systems, Albany, CA). Reaction time for correct responses during go trials (hits) and unsuccessful inhibition during nogo trials (unsuccessful withholds) were recorded. In addition, the percentage of correct responses during go trials and the percentage of successful inhibition of responses during nogo trials (successful withholds) were calculated. Participants with an accuracy level (percentage correct) lower than 50% for the nogo condition and 80% for the go condition were excluded from further analysis.
Figure 1.
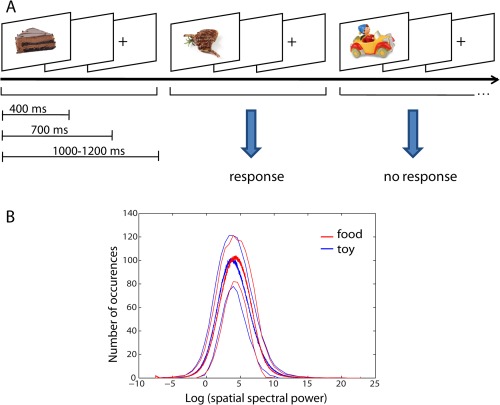
Illustration of the experimental design (A) and stimulus material (B). A The interstimulus time was between 1,000 and 1,200 ms and the stimulus presentation time was 400 ms. The requested response pattern for the block in which food was the go cue is shown. B Mean value of spatial frequency histogram for all food (red) and all toy (blue) images from the two image sets is highly similar between the two categories. Standard deviations of the mean values are indicated by thin lines. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Behavioral Statistical Analysis
Statistical analysis of behavioral data was performed with IBM SPSS Statistics 20.0 (IBM, Armonk, NY). All data are presented as unadjusted mean ± standard deviation. The dependent variables accuracy of response (percent correct) and reaction time were analyzed using a two‐way repeated measure analysis of variance (ANOVA) with two within factors, “task condition” (levels: go and nogo) and “stimulus category” (levels: food and toy), and post hoc paired t tests (two‐tailed). The significance level was P < 0.05.
MEG Data Acquisition
MEG signals were recorded using a 275‐sensor whole head system (VSM MedTech, Port Coquitlam, Canada). Three coils generating magnetic fields were attached at three fiducial points (nasion, preauricular point on each side of the participant's head). These coils were used for continuous recording of the head position in relation to the MEG sensor array. Only recordings with a maximum movement below 10 mm during the recording were analyzed further. MEG data and behavioral responses were recorded in a continuous mode with a sampling rate of 586 Hz.
Data Analysis
Source reconstruction of evoked activity
MEG data were analyzed for differences between hits and successful withholds. To limit the number of hit trials, only hit trials that preceded nogo trials were considered.
Continuous data were filtered with a 1 Hz high pass and a 40 Hz low pass filter and cut into trials of 700 ms (−100 ms to 600 ms) length according to the presented stimulus (hits‐food, successful withholds‐food, hits‐toy, successful withholds‐toy). All trials with eye movement artifacts (automatic detection of activity >2.5 pT in frontal channels) were excluded from further analysis. For each condition, the first 100 artifact free trials were selected to standardize the number of trials and averaged for each subject separately.
After preprocessing, neuronal sources were estimated during the 500‐ms period after stimulus presentation by a minimum norm algorithm implemented in spm8 (http://www.fil.ion.ucl.ac.uk/spm). A standard template cortical surface was transformed to match the fiducials of the MEG data [Mattout et al., 2007]. The sensor locations were registered to source space and a single shell head model was used to compute the gain matrix. The head model was adjusted to the individual head shape, which was obtained after the MEG measurement by Polhemus (3Space Fastrak, Polhemus Incorporated Colchester, VT). The source activation was estimated independently for all conditions and each participant using a spatial filter of 12 mm.
Statistical analysis of evoked activity
We calculated statistical parametrical maps in a full factorial design for the entire 500 ms after stimulus onset with two within factors, “task condition” (levels: hits and successful withholds) and “stimulus category” (levels: food and toy), in order to extract sources corresponding to inhibition of a prepotent motor response. We only report results significant at a level of P < 0.001 (uncorrected).
Time courses of evoked activity
To investigate the a priori hypothesis, we extracted time courses of the inverse solution for all conditions separately for the significant peak voxel in DLPFC. For each participant, the individual peak voxel was extracted in an area around the coordinates of the common peak voxel. The root mean square (RMS) values of voxels in the distance of 2 cm around this individual voxel were calculated and then averaged over all voxels. Finally, an average time course over all participants was calculated for each condition.
Preprocessing and sensor space of induced activity
In the frequency domain, MEG data were analyzed using the FieldTrip software package [Oostenveld et al., 2011]. Data were cut into trials according to stimulus onset and all trials with eye movement artifacts were excluded from further analysis. In addition, we combined the food and toy conditions as no significant behavioral differences and no significant difference in evoked activity in the prefrontal cortex were observed.
Planar gradients of the MEG data were calculated as described by Jokisch and Jensen [2007]. Time‐Frequency representations (TFRs) of power were calculated according to the procedure used in Mazaheri et al. [2009]. For each trial we calculated a TFR of power by using a taper approach which is applied to short sliding time windows [Percival and Walden, 1993]. For frequencies 4–40 Hz (in steps of 2 Hz), the data in each time window were multiplied with a Hanning taper with the length of the time window. The time window was adaptive with three cycles for each frequency (ΔT = 3/f). The power values were calculated for the horizontal and vertical components of the estimated planar gradient and summed. These power estimates were averaged over all trials for each condition separately. For source reconstruction, we used the data from the true axial sensors [Mazaheri et al., 2009].
Statistical analysis of induced activity
For the difference between hits and successful withholds on the sensor level, again only hit trials before nogo trials were selected and in total 200 trials of each task condition (100 food and 100 toy trials). We evaluated significant differences of the TFRs of power between successful withholds and hits over subjects by using the nonparametric cluster level randomization test in FieldTrip [Maris and Oostenveld, 2007]. A detailed description of the individual steps of the test is described in Jokisch and Jensen [2007]. We focused on analysis of the first 500 ms after stimulus onset in the alpha (8–12 Hz) and beta (13–30 Hz) frequency range. For unsuccessful withholds, all trials were selected as less trials were available. For the difference between unsuccessful and successful withholds, analysis was performed for a prestimulus interval between −300 and 0 ms and for the first 500 ms after stimulus onset.
For the time courses, we selected all channels showing significant differences in a right frontal area cluster and an occipital area cluster in the alpha band and in a left central area cluster in the beta band. An average TFR for these clusters was calculated between −300 and 700 ms at 10 and 20 Hz, respectively. For display, a mean over all participants was calculated for each condition.
Source reconstruction of induced activity
Source reconstruction in the frequency domain was performed using a frequency‐domain beam‐forming approach implemented in FieldTrip [Gross et al., 2001; Mazaheri et al., 2009]. However, instead of individual MRIs, a standard template was used for the single‐shell description. We estimated sources in the alpha and beta frequency band using 100–500 ms after stimulus onset (multi‐taper, center frequency: 10/20 Hz, smoothing: 3/8 Hz). For each participant, the difference in source activity between successful withholds and hits was calculated individually and then a grand average over all subjects was calculated. For analysis on the coordinate level (virtual channel), source reconstruction was performed similarly, however, we relied on the linear constrained minimum variance beamformer approach also implemented in FieldTrip [Van Veen et al., 1997]. With the virtual channel at the peak coordinate of interest (MNI coordinates: 40 40 10), time frequency analysis for the hits and successful withholds condition was performed [Levy et al., 2013].
RESULTS
Behavioral Results
As expected, participants stated low hunger levels (mean: 1.05 ± 1.07). Reaction time and accuracy of the go‐nogo task are shown in Table 1. There was a significant task condition effect for reaction time showing faster responses for unsuccessful withholds during nogo than for hits during go trials (F(1,19) = 134, P < 0.001). However, there was no effect of stimulus category and no interaction between stimulus category and task condition. For accuracy, a significant task condition effect revealed higher accuracy for go compared to nogo trials (F(1,19) = 102, P < 0.001). Similarly, there was no category effect and no interaction.
Table 1.
Behavioral results
| Food | Toys | |||
|---|---|---|---|---|
| Nogo | Go | Nogo | Go | |
| Reaction time (ms) | 332.02 ± 63.67a, b | 356.27 ± 44.93 | 322.30 ± 39.32b | 362.69 ± 43.84 |
| Accuracy (%) | 79.16 ± 12.39 | 99.07 ± 1.32 | 78.54 ± 9.94 | 99.09 ± 1.34 |
Data are presented as mean ± SD.
Reaction time for unsuccessful withholds.
Source Analysis of Evoked Activity
We observed an increase in amplitude in right dorsolateral prefrontal cortex, right temporal gyrus, right angular/parietal gyrus, and left superior medial frontal gyrus for successful withholds and an increase in amplitude in left postcentral and superior parietal gyrus for hits (Fig. 2A,B, Table 2). Increased activity in postcentral, somatosensory areas was also shown in an fMRI study and is considered to be associated with sensory feedback of the actual button press [Menon et al., 2001]. Furthermore, unsuccessful withholds in comparison to hits showed an increase in amplitude in similar areas as successful withholds, especially in right prefrontal cortex (see Supporting Information Fig. S1).
Figure 2.
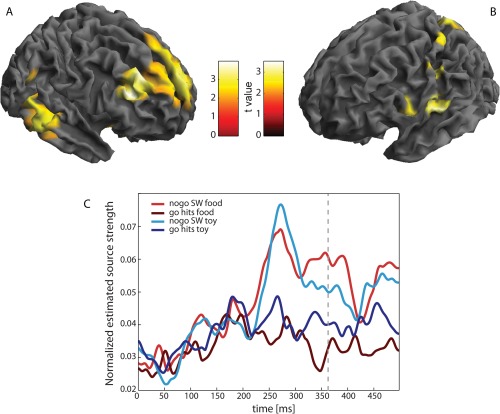
Evoked field analysis on source level. A and B show the main effect of go‐nogo for the period 0–500 ms. A Areas with stronger activity for successful withholds (SW); B Areas with stronger activity for hits. Cortical activity was rendered onto the surface of a standard anatomical brain volume (Montreal Neurological Institute). For display, significance threshold was lowered to P < 0.01 (uncorrected). C Time courses of activity of voxels surrounding the peak voxel of the most significant cluster for the contrast successful withholds versus hits (rDLPFC). Time courses are shown as mean over all subjects separately for all conditions. Dashed line: mean reaction time of hits. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Clusters of significant differences in evoked activity between successful withholds and hits (shown in Figure 2)
| Brain region | Side | Coordinates | Cluster size (in voxels) | Z | P (uncorr.) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Succesful withholds > Hits | |||||||
| Middle frontal gyrus | r | 40 | 36 | 20 | 586 | 3.76 | 0.000 |
| Middle temporal gyrus | r | 58 | −50 | 2 | 112 | 3.53 | 0.000 |
| Angular gyrus | r | 48 | −52 | 22 | 38 | 3.43 | 0.000 |
| Medial frontal gyrus | l | −8 | 56 | 24 | 153 | 3.26 | 0.001 |
| Hits > Successful withholds | |||||||
| Postcentral gyrus | l | −56 | −16 | 14 | 76 | 3.36 | 0.000 |
| Superior parietal lobule | l | −18 | −54 | s68 | 23 | 3.30 | 0.000 |
Extracted time courses of the source activity at the peak voxel in the rDLPFC during successful withholds in comparison to hits are shown in Figure 2C. Both hit conditions showed constant source activity throughout the entire time span, whereas the successful withhold conditions showed a prominent peak at around 270 ms with sustained increased amplitude for the rest of the investigated time span. A similar increase was also observed for unsuccessful withholds (see Supporting Information Fig. S1).
Induced Activity
Induced oscillatory activity in the alpha band showed a statistically significant difference between successful withholds and hits in a cluster of channels over the right frontal cortex. Alpha power in these channels was significantly higher for successful withhold trials between 250 and 400 ms compared to hit trials. Source reconstruction revealed a strong difference between successful withholds and hits in the rDLPFC (MNI peak coordinates: 40 40 10) (Fig. 3B). Time courses of the alpha power change in right frontal sensors for successful withholds and hits are shown in Figure 3C. Successful withhold as well as hit trials showed a small increase in alpha power after stimulus onset. Whereas the power slowly decreased at around 150 ms after stimulus onset in the hit condition, there was a further increase in the successful withhold condition with a peak at around 350 ms and an elevated level for the rest of the investigated time span.
Figure 3.
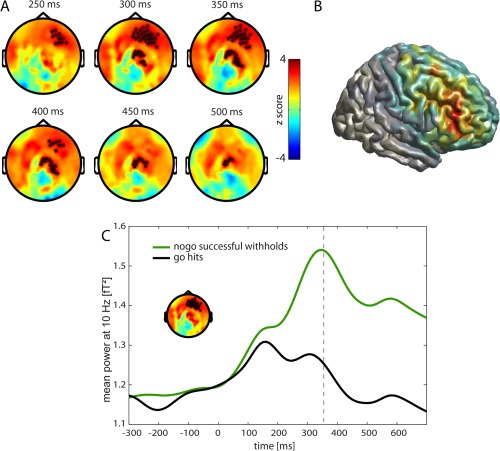
Activity within the alpha frequency band (8–12 Hz). A Go‐nogo difference of time frequency representation (TFR) in the alpha frequency band. Red indicates higher power for successful withholds and blue higher power for hits. Significant channels are displayed by stars (P < 0.01). B Source reconstruction of the difference in alpha band with DICS beamformer in the period of 100–500 ms, projected onto a surface. Areas with higher power for successful withholds than for hits are shown. The strongest activity difference was observed in right middle frontal gyrus (MNI coordinates: 40 40 10). C Mean power at 10 Hz of TFR in channels of right prefrontal cluster is shown. Black: go trials with correct response (hits), Green: nogo trials with successful withholding of the response, Dashed line: mean reaction time of hits. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4 shows the TFR in source space of the frontal peak coordinate (MNI: 40 40 10) (Fig. 3B). The contrast confirmed that in comparison to hits, successful withholds showed an increase in lower alpha activity between 250 and 400 ms (Fig. 4).
Figure 4.
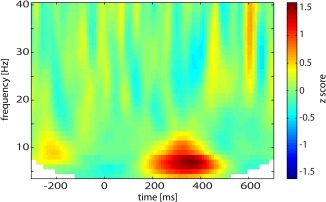
Virtual channel analysis. Time frequency representation of the activity filtered from the peak voxel of the source reconstruction in alpha band (MNI coordinates: 40 40 10) for the contrast successful withholds versus hits. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Furthermore, there was significantly more alpha power in a central cluster during successful withholds between 300 and 500 ms compared to hits (Fig. 3A). This difference was due to decreased alpha activity during hits in comparison to successful withhold trials with a source in areas related to motor preparation and execution (see Supporting Information Fig. S2).
In the beta frequency band, we observed a significantly smaller beta decrease during successful withholds in left central channels in comparison to hits (Fig. 5A) between 250 and 500 ms. This difference was mainly generated by a source in the left precentral gyrus (MNI peak coordinates: −30 −20 50) (Fig. 5B). Time courses of the power change in channels with significant differences are presented in Figure 5C. After stimulus onset, a decrease in beta power was observed for both conditions. However, beta desynchronization was stronger for hits compared to successful withholds. The desynchronization peak was reached at around 350 ms for successful withholds and about 100 ms later when a response was executed.
Figure 5.
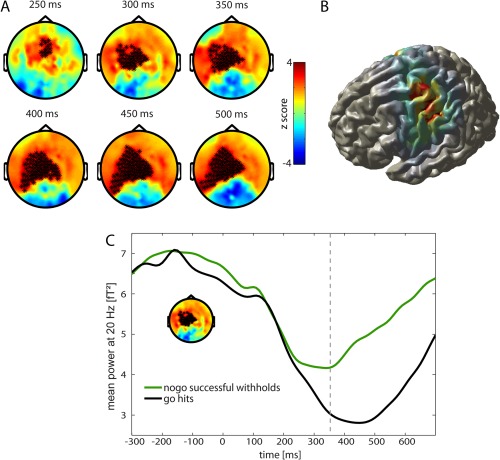
Activity within the beta frequency band (13–30 Hz). A Go‐nogo difference of time frequency representation (TFR) in the beta frequency band. Red indicates higher power for successful withholds and blue higher power for hits. Significant channels are displayed by stars (P < 0.01). B Source reconstruction of the difference in beta band with DICS beamformer in the period of 100–500 ms, projected onto a surface. Areas with higher power for successful withholds than for hits are shown. The strongest activity difference was observed in left precentral gyrus (MNI coordinates: −30 −20 50). C Mean power at 20 Hz of TFR in channels of left central cluster is shown. Black: go trials with correct response (hits), Green: nogo trials with successful withholding of the response, Dashed line: mean reaction time of hits. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 6 shows the difference between successful and unsuccessful withholds in the alpha and beta frequency band. For alpha band, we observed significant differences in three time periods: prestimulus, from 250 to 350, and after 500 ms. Differences were in occipital and central channels (Fig. 6A). Time courses of the significant cluster in occipital channels showed less alpha synchronization for successful withholds for the prestimulus period and between 250 and 350 ms and stronger desynchronization of alpha for successful withholds after 500 ms (Fig. 6B). For the beta band, we observed a similar difference as for the contrast successful withholds versus hits in a left central cluster which started at around 300 ms (Fig. 6C). Time courses revealed stronger desynchronization for unsuccessful withholds and a delayed resynchronization (also in comparison to hits, compare with Fig. 5C) (Fig. 6D).
Figure 6.
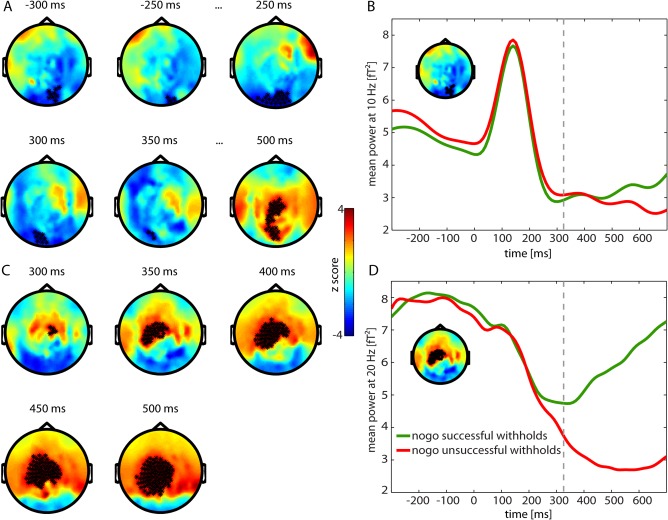
Activity differences for successful and unsuccessful withholds in alpha band (8–12 Hz) (A) and beta band (13–30 Hz) (C) of time frequency representation (TFR) with red showing higher power for successful withholds and blue higher power for unsuccessful withholds. Significant channels are displayed by stars (P < 0.01). B and D show mean power at 10 and 20 Hz of occipital and left central channels, respectively. Green: nogo trials with successful withholding of the response, Red: nogo trials with unsuccessful withholding of the response, Dashed line: mean reaction time of unsuccessful withholds. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
In this study, we showed that the temporal dynamics of rDLPFC supported its proposed role in response inhibition. Furthermore, results suggested a functional significance of right prefrontal alpha band activity in gating the selection of withholding the response. Right dorsolateral prefrontal cortex showed increased activity for nogo in comparison to go trials. Time courses of changes in evoked and induced activity revealed that the peak of right prefrontal cortex activity in nogo trials was reached at around 300 ms poststimulus. In addition, we observed pre‐ and poststimulus differences in alpha band activity between successful and unsuccessful withholds in occipital and central areas. For beta band activity, we replicated motor preparatory desynchronization after stimulus onset in left precentral gyrus. This was stronger for hits during go trials in comparison to successful withholds and strongest for unsuccessful withholds during nogo trials. For evoked activity, besides an increase in amplitude in rDLPFC, successful withholds also showed an increase in amplitude in right temporal and parietal regions. Hits were associated with an increased amplitude in left postcentral gyrus.
The first goal of our study was to replicate the involvement of brain areas reported in previous go‐nogo studies and to investigate the time dynamics of right prefrontal cortex by evoked field analysis. As expected, successful withholds were associated with an increase in amplitude in prefrontal and temporal areas. Several areas of the prefrontal cortex have been suggested to be involved in response inhibition (Aron et al., 2004; Chikazoe et al., 2009). In complex go‐nogo tasks, right dorsolateral prefrontal cortex is considered to form a right‐lateralized network in combination with right inferior parietal lobule and gate the selection of the required response given the current stimulus (Simmonds et al., 2008). In the current task, the stimulus had to be allocated to its respective category and according to the task demand the appropriate response had to be selected. In accordance with the proposed rDLPFC function, we observed a strong amplitude increase at around 220 ms after stimulus onset, with a prominent peak at around 270 ms and prolonged elevated amplitude. Processes related to categorization are usually observed earlier in time (at around 160 ms [Stingl et al., 2010; Thorpe et al., 1996]) and motor responses on go trials occurred on average at about 360 ms after stimulus onset. Therefore, the increase in amplitude in rDLPFC occurred after the stimulus was categorized and before a response was made. A strong difference between nogo and go condition between 200 and 400 ms at frontal electrodes has also been observed in electroencephalographic (EEG) studies employing visual go‐nogo tasks and is considered to correspond to a monitoring component for response inhibition during infrequent nogo stimuli [Donkers and van Boxtel, 2004; Nieuwenhuis et al., 2003]. Interestingly, we observed in our study that not only successful, but also unsuccessful withholds in comparison to hits showed an increase in amplitude in rDLPFC. To sum up, these data suggest that the amplitude increase in rDLPFC is not a direct inhibitory mechanism and support the hypothesis that rDLPFC is involved in gating the selection of the weaker nogo stimulus‐response association over the stronger prepotent go response.
In a next step, we investigated the neurophysiological nature of the activity in rDLPFC. As mentioned in the introduction, alpha band synchronization is considered to play a significant role in selective gating and inhibition of sensory information. The question was whether functional inhibition by alpha band activity would generalize to prefrontal areas as described for monkeys during rule selection [Buschman et al., 2012]. Contrasting successful withholds and hits in the alpha band revealed an increase in activity for successful withholds in right lateral frontal regions and a decrease in activity in the alpha band for hits in central channels. The difference in central channels occurred later and lasted longer than in frontal channels and the source of this activity was localized in areas related to motor preparation and execution. Increased activity for successful withholds in comparison to hits is consistent with EEG studies that showed increased alpha band synchronization in electrodes overlying motor cortex during suppression of movements and alpha band desynchronization during movement execution [Hummel et al., 2002; Sauseng et al., 2013].
The time course of the frontal alpha difference revealed a strong increase in alpha activity for successful withholds peaking between 200 and 400 ms. Reconstructing the time frequency representation in the peak coordinate of the difference on source level in rDLPFC showed an increase in activity in frequencies around the lower alpha frequency band for successful withholds in comparison to hits between 250 and 400 ms. Comparison of successful and unsuccessful withholds showed that there was no significant difference in frontal channels between these two conditions, indicating that alpha synchronization also increased for unsuccessful withholds. This was in agreement with observations from the evoked field analysis. Reported results support the hypothesis that alpha synchronization has a functional role in gating information not only in sensory, but also in prefrontal regions. Interpretation in the context of the rule selection paradigm by Buschman et al. [2012] would suggest that alpha synchronization during the weaker nogo withhold response deselects the much stronger prepotent automated go response tendency and thereby enables the execution of the response inhibition. In the rule selection paradigm, monkeys were aware of the rule before the onset of the stimulus. Therefore, alpha synchronization was already observed prestimulus.
In our paradigm, withholding of a response could only be initiated after the stimulus was categorized. Accordingly, we observed an increase in alpha activity much later. With a peak at 350 ms, the alpha band activity increase seemed rather late with respect to mean reaction times of button responses between 320 and 360 ms. To further evaluate the timing of the activity, we analyzed beta band activity related to motor preparation and execution. We identified a region in left precentral gyrus which showed differential activity for successfully withheld and hit trials. Extracted time courses of the seed channels confirmed initial desynchronization followed by subsequent resynchronization of the beta band activity for the go condition. Resynchronization started at around 450 ms poststimulus, which was ∼100 ms later than the mean response time. For successful withholds, resynchronization already started at around 350 ms after stimulus onset. This is in line with a study by Zhang et al. [2008], who reported a beta rebound approximately at this point in time for nogo stimuli in motor areas of the macaque monkey. Thus, the motor response was prepared in both conditions. After stimulus discrimination, however, the motor system was restored back to its prestimulus state earlier in the nogo condition and the response was inhibited. Accordingly, during unsuccessful withhold trials, beta band desynchronization was stronger and lasted longer than for successful withholds or even hits. In this case, restoration of the motor system to the prestimulus state was not successful. We presume that desynchronization for unsuccessful withholds in comparison to hits lasts even longer due to realization of the error that has been made and possible compensatory movements to “retract” the wrong response.
Results suggest that frontal alpha band and movement related beta band activity have a very similar timing, with the difference in alpha band synchronization slightly preceding the difference in beta band resynchronization by some ten milliseconds (difference between unsuccessful and successful withholds only started to be significant at 300 ms). However, this comparison should be interpreted with caution as the temporal resolution between the alpha and the beta band is different due to the nature of the time‐frequency analysis. In general, it seems likely that the increase in right prefrontal alpha synchronization, rather than the peak itself, might affect the motor‐related activity by gating the selection of the response.
Beta desynchronization further confirmed that the go response is the prepotent, automated response mode that is always prepared. Consequently, this preparation has to be disrupted during nogo trials to withhold the response. To achieve this, it might not only be necessary that the signal for changing the response mode is generated at the appropriate time, but successful response inhibition might also depend on the current brain state. In this regard, alpha band activity has not only been associated with selective gating and timing of processes, but also with a general excitability of the cortex [Klimesch, 2012; Klimesch et al., 2007a]. It has been shown that visual and somatosensory perception is modulated by prestimulus oscillatory activity and errors can often already be predicted by alpha activity in prestimulus intervals [Hamm et al., 2012; Mazaheri et al., 2009; Thut et al., 2006; van Dijk et al., 2008]. In general, a large anticipatory event‐related alpha desynchronization in sensory areas required for the task is related to good discrimination performance. In contrast, in anticipation of a distractor during the retention interval of a working memory task, the power of alpha oscillations increased prior to the distractor to suppress its processing and predicted performance [Bonnefond and Jensen, 2012]. Klimesch [2012] describes this prestimulus alpha activity as a response to anticipatory attention in the absence of stimulation. For a visual go‐nogo task, Mazaheri et al. [2009] showed that increased prestimulus alpha activity in occipital, as well as left and right primary sensorimotor cortices predicted failures in response inhibition. We observed similar results in our study. Errors were predicted by prestimulus sensory alpha activity rather than by poststimulus differences in frontal alpha activity.
This might indicate that preparation of sensory modalities by desynchronization of alpha activity is essential for poststimulus modulation by top‐down processes. In nogo trials, the go response has to be deselected to change the response mode. This requires suppression of activity in regions related to the execution of the motor response and thus, suppression of the motor response itself. However, if the brain is not prepared by alpha desynchronization to process incoming information, the deselection of the dominant go rule by frontal alpha activity might occur too late and the automatic response mode will be carried out. Insufficient preparation for the upcoming trial might be related to inattention, which is suggested by reaction time differences. The mean reaction time of unsuccessful withholds was with around 325 ms significantly shorter than that of hits with around 360 ms. Shorter response latencies for unsuccessful response inhibition in comparison to prepotent, automated go responses have consistently been reported in the literature [Albert et al., 2013; Mazaheri et al., 2009; Menon et al., 2001]. This observation that errors are more likely for short RTs is discussed as temporary inattention as participants are drawn into an automated response mode [Mazaheri et al., 2009; Robertson et al., 1997].
In addition, we observed alpha desynchronization in occipital and central channels for unsuccessful withholds starting at around 500 ms. This has also been observed by Mazaheri et al. [2009] and is most likely related to error processing and optimized preparation of the brain for the upcoming stimulus.
A limitation of our study is that we didn't address how the response selection in rDLPFC is communicated to downstream areas in order to actually withhold the response execution. Dynamic modification of long‐range communication between regions according to task demands is associated with synchronization of oscillatory rhythms across cortical regions that link local neuronal populations [Fries, 2005; Varela et al., 2001]. Synchronization of oscillations across cortical regions has been suggested to be associated with phase locking of alpha oscillations [Palva and Palva, 2007] and/or beta band synchronization [Siegel et al., 2012]. However, these analyses demand a high signal to noise ratio which we did not achieve in our experimental design.
An interesting additional finding of our study was that the alpha peak was several ten milliseconds later and broader than the peak of the evoked activity in the prefrontal cortex. However, also for this comparison the potential differences in temporal smoothing between the two analyses have to be taken into account. Several recent papers put forward the idea that evoked potentials are generated by modulation of ongoing oscillations either by event‐related phase reorganization [Klimesch et al., 2007b] or nonsinusoidal properties of alpha oscillations [Nikulin et al., 2007; van Dijk et al., 2010]. With regard to our results, we suppose that these processes in the alpha band may have influenced the generation of the peak in the evoked activity. However, it is beyond the scope and experimental design of this study to resolve the issue whether the reported effects are dependent processes or not and to explain the observed differences.
Finally, considering the late peak of the alpha band activity and the observation that the reconstructed TFR on source level indicated strong contributions of frequencies around the lower alpha frequency band, it can be argued that the observed synchronization is not involved in gating the fast response inhibition. It might alternatively reflect a different process like monitoring of the infrequent stimulus and alternate response mode and/or outcome evaluation.
In summary, we showed that the temporal dynamics of rDLPFC activity support its role in gating the selection of the response which is required in the current context. In the go‐nogo task this means gating the selection of withholding the response when a nogo cue is presented. Furthermore, results suggest that right prefrontal cortex alpha synchronization might be involved in this gating. For successful response inhibition, however, also anticipatory prestimulus alpha desynchronization seems to be required as it was reduced prior to response inhibition errors. This supports the notion that alpha activity has a functional role in controlling brain state and suggests an extension of the role for functional inhibition by alpha synchronization from sensory to prefrontal regions.
Supporting information
Supplementary Information Figure S1.
Supplementary Information Figure S2.
REFERENCES
- Albert J, Lopez‐Martin S, Hinojosa JA, Carretie L (2013): Spatiotemporal characterization of response inhibition. NeuroImage 76:272–281. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O (2012): Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol 22:1969–1974. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK (2012): Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron 76:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J (2010): Localizing performance of go/no‐go tasks to prefrontal cortical subregions. Curr Opin Psychiatry 23:267–272. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S (2009): Functional dissociation in right inferior frontal cortex during performance of go/no‐go task. Cereb Cortex 19:146–152. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ (2004): The N2 in go/no‐go tasks reflects conflict monitoring not response inhibition. Brain Cogn 56:165–176. [DOI] [PubMed] [Google Scholar]
- Fries P (2005): A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci 9:474–480. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R (2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Dyckman KA, McDowell JE, Clementz BA (2012): Pre‐cue fronto‐occipital alpha phase and distributed cortical oscillations predict failures of cognitive control. J Neurosci 32:7034–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, D'Esposito M, Cole MW, Garavan H (2007): Neural mechanisms for response selection: Comparing selection of responses and items from working memory. NeuroImage 34:446–454. [DOI] [PubMed] [Google Scholar]
- Hummel F, Andres F, Altenmuller E, Dichgans J, Gerloff C (2002): Inhibitory control of acquired motor programmes in the human brain. Brain 125 (Part 2):404–420. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010): Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front Hum Neurosci 4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O (2007): Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27:3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (2012): Alpha‐band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007a): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53:63–88. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R (2007b): Event‐related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev 31:1003–1016. [DOI] [PubMed] [Google Scholar]
- Levy J, Vidal JR, Oostenveld R, FitzPatrick I, Demonet JF, Fries P (2013): Alpha‐band suppression in the visual word form area as a functional bottleneck to consciousness. NeuroImage 78:33–45. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL (2004): Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event‐related fMRI. NeuroImage 22:1097–1106. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007): Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Mattout J, Henson RN, Friston KJ (2007): Canonical source reconstruction for MEG. Comput Intell Neurosci 2007:10. 67613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis IL, van Dijk H, Jensen O (2009): Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp 30:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M (2005): Searching for “the top” in top‐down control. Neuron 48:535–538. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G (1996): Post‐movement synchronization of beta rhythms in the EEG over the cortical foot area in man. Neurosci Lett 216:17–20. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR (2003): Electrophysiological correlates of anterior cingulate function in a go/no‐go task: Effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci 3:17–26. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Linkenkaer‐Hansen K, Nolte G, Lemm S, Muller KR, Ilmoniemi RJ, Curio G (2007): A novel mechanism for evoked responses in the human brain. Eur J Neurosci 25:3146–3154. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM (2007): New vistas for alpha‐frequency band oscillations. Trends Neurosci 30:150–158. [DOI] [PubMed] [Google Scholar]
- Percival D, Walden A, editors (1993): Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Pfurtscheller G, Stancak A Jr, Neuper C (1996): Post‐movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol 98:281–293. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G (1997): Foot and hand area mu rhythms. Int J Psychophysiol 26:121–135. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J (1997): “Oops!”: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 35:747–758. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Gerloff C, Hummel FC (2013): Two brakes are better than one: The neural bases of inhibitory control of motor memory traces. NeuroImage 65:52–58. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012): Spectral fingerprints of large‐scale neuronal interactions. Nat Rev Neurosci 13:121–134. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH (2008): Meta‐analysis of Go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia 46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl KT, Rogic M, Stingl K, Canova C, Tschritter O, Braun C, Fritsche A, Preissl H (2010): The temporal sequence of magnetic brain activity for food categorization and memorization—An exploratory study. NeuroImage 52:1584–1591. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C (1996): Speed of processing in the human visual system. Nature 381:520–522. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual‐Leone A (2006): Alpha‐band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26:9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepel U, Knebel JF, Hudry J, le Coutre J, Murray MM (2009): The brain tracks the energetic value in food images. NeuroImage 44:967–974. [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O (2008): Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O (2010): Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event‐related responses. Proc Natl Acad Sci USA 107:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A (1997): Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001): The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2:229–239. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M (2008): Response preparation and inhibition: The role of the cortical sensorimotor beta rhythm. Neuroscience 156:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figure S1.
Supplementary Information Figure S2.


