Abstract
The tendency to worry is a facet of neuroticism that has been shown to mediate the relationship between neuroticism and symptoms of depression and anxiety. The aim of the current study was to investigate the neural correlates of state worry in association with neuroticism. One‐hundred twenty participants were selected from an initially recruited sample of 240 women based on their neuroticism score. First, participants completed a questionnaire to assess the excessiveness and uncontrollability of pathological worry. Second, we measured brain activation with functional magnetic resonance imaging (fMRI) while participants were randomly presented with 12 worry‐inducing sentences and 12 neutral sentences in a mood induction paradigm. Individuals scoring higher on neuroticism reported to worry more in daily life and to have generated more worry‐related thoughts after the presentation of a worry‐inducing sentence. Furthermore, imaging results showed the involvement of default mode and emotional brain areas during worry, previously associated with self‐related processing and emotion regulation. Specifically, cortical midline structures and the anterior insula showed more activation during worry, when individuals indicated to have generated more worry‐related thoughts. Activation in the retrosplenial and visual cortex was decreased in individuals scoring higher on neuroticism during worry, possibly suggesting reduced autobiographical specificity and visual mental imagery. In the literature, both these processes have been related to the cognitive avoidance of emotional distress. Excessive worry features in a number of emotional disorders and results from studies that elucidate its neural basis may help explain how and why neuroticism contributes to vulnerability for psychopathology. Hum Brain Mapp 35:4303–4315, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: autobiographical specificity, cognitive avoidance, default mode network, mood induction paradigm, retrosplenial and visual cortex, visual mental imagery
INTRODUCTION
Neuroticism is a robust personality trait [Costa and McCrae, 1989] that is characterized by a predisposition to express heightened emotional reactivity, especially to negative events [Canli, 2008] and to experience increased negative affect, such as feelings of depression and anxiety [Watson et al., 1994]. High scores on neuroticism are considered a powerful risk marker for a wide range of psychiatric disorders, in particular internalizing disorders [Lahey, 2009; Ormel et al., 2004, 2013]. Individuals scoring higher on neuroticism tend to appraise events as more threatening than others, which causes elevated levels of stress [Chan et al., 2007; Suls and Martin, 2005]. Occasionally, this may lead to the occurrence of mood spillovers as a result of applying maladaptive coping strategies [Suls and Martin, 2005].
Excessive worry is one of these maladaptive coping strategies and represents a form of repetitive negative thinking (RNT) [Roelofs et al., 2008], in which a cognitive attempt is made to anticipate and prepare for possible negative outcomes in the future [Borkovec et al., 1983, 2004; Roelofs et al., 2008]. Borkovec et al. [1983] have defined worry as “a chain of thoughts and images, negatively affect‐laden and relatively uncontrollable.” Prior studies have shown that trait worry varies continuously across the normal population and plays a role in a variety of psychiatric disorders [Hirsch and Mathews, 2012]. In particular, it is the main diagnostic criterion of Generalized Anxiety Disorder (GAD) in which worry is characterized as general, disproportionate, uncontrollable and irrational [Hirsch and Mathews, 2012].
A number of studies have related neuroticism to the construct of worry or to processes that negatively reinforce, initiate or continue the use of worry as a coping mechanism. First, the tendency to worry is a facet of neuroticism [Lahey, 2009; Watson et al., 1994] that has been shown to mediate the relationship between neuroticism and symptoms of depression and anxiety [Muris et al., 2005; Roelofs et al., 2008]. Specifically, Hale et al. [2010] demonstrated that neuroticism is strongly correlated with the GAD symptom of worry and that both constructs have strong predictive values for each other, but are not the same. Also, a recent paper, using a network approach to psychopathology, showed that worry is one of the most central nodes in the network of high neurotic individuals [Bringmann et al., 2013]. Furthermore, studies have revealed an association between neuroticism and meta‐worry (worry about worry) [Matthews et al., 2000]. Wells [1995] has proposed in his theory related to GAD that such dysfunctional meta‐cognitive beliefs about worry are involved in maintaining the worry cycle. Particularly, negative metacognitions are considered pathogenic and lead to the perpetuation of worry [Wells, 1995]. In addition, it has been argued that worry may be a consequence of the ineffective processing and regulation of emotions [Blair and Blair, 2012; Mennin et al., 2005]. In line with this theory of GAD, research has shown that high neurotic individuals are impaired in the processing of negative emotional stimuli and make less use of adaptive coping strategies to regulate their emotions, such as reappraisal [Chan et al., 2007; Gross and John, 2003]. Moreover, Borkovec's theory related to GAD [Borkovec et al., 2004] proposes that worry functions as a cognitive avoidance response in the face of future threat, that is, the abstract and verbal nature of worry reduces the experience of negative emotions normally provoked by visual mental imagery. Accordingly, high neurotic individuals have a tendency to rely on inefficient escape‐avoidance strategies [Lee‐Baggley et al., 2005; Watson and Hubbard, 1996].
Few functional magnetic resonance imaging (fMRI) studies have been conducted on the neural correlates of worry. One study demonstrated increased activation in brain areas related to self‐referential processing and introspection, such as the dorsomedial prefrontal cortex and anterior cingulate cortex, during experimentally induced worry in healthy individuals as well as GAD patients. Furthermore, GAD patients showed persistent activation in these same brain areas during the post‐worry rest period, possibly suggesting difficulties in the ability to terminate the worrying [Paulesu et al., 2010]. In contrast, more studies have investigated rumination—a different form of RNT—in the context of depression. Recent research has suggested that there are more similarities than differences between the two forms of RNT, with the only replicated difference being temporal orientation (worry is more directed to the future and rumination to the past) [McEvoy et al., 2010]. Studies on state as well as trait worry and rumination have shown the involvement of brain regions that are part of the default mode network (e.g., cortical midline structures) and limbic system (e.g., amygdala and insula) [Andreescu et al., 2011; Berman et al., 2011; Cooney et al., 2010; Hamilton et al., 2011; Marchetti et al., 2012; Paul et al., 2013; Paulesu et al., 2010; Schienle et al., 2009; Thomas et al., 2011; Whitfield‐Gabrieli and Ford, 2012; Zhu et al., 2012]. Activation in these brain areas has been related to self‐related processing, mental simulation, introspection, future planning and emotion processing/regulation [Buckner et al., 2008; Phillips et al., 2003; Sylvester et al., 2012].
Prior literature has stated that the tendency to worry is a facet of neuroticism [Lahey, 2009; Watson et al., 1994], however this relationship has not extensively been studied, specifically not with fMRI. Therefore, the aim of the current study was to investigate the neural correlates of state worry in association with neuroticism. For this purpose, we implemented an adapted form of the mood induction paradigm of Paulesu et al. [2010] in a sample of 120 women selected on the basis of their neuroticism score. We hypothesized increased worrying in individuals scoring higher on neuroticism in daily life (trait worry; questionnaire data) as well as during the mood induction paradigm (state worry; behavioral data). Second, we hypothesized enhanced activation in default mode and limbic brain regions during worry, specifically in individuals scoring higher on neuroticism. Third, we hypothesized that brain regions related to worry would show persistent activation during the post‐worry rest period in individuals scoring higher on neuroticism. Fourth, during worry, we investigated task‐dependent connectivity of brain regions associated with neuroticism to shed light on the neural networks involved [Burianova et al., 2010].
METHODS AND MATERIALS
Participants
Initially, 240 students from the University of Groningen filled in the NEO Five‐Factor Inventory (NEO‐FFI) (domains Neuroticism and Extraversion, 24 items). Individuals were included when they met the following selection criteria: (1) female gender, (2) age between 18 and 25 years, (3) Dutch as native language, (4) Caucasian descent, (5) right handed, (6) no use of contraceptive medication, except for oral contraceptive pills (21‐pill packet). Exclusion criteria were (1) a history of seizure or head injury, (2) a life time diagnosis of psychiatric and/or neurological disorders, (3) a life time diagnosis of psychiatric disorders in first degree relatives of the participant, (4) the use of medication that can influence test results, (5) visual or auditory problems that cannot be corrected, (6) MRI incompatible implants or tattoos, (7) claustrophobia, (8) suspected or confirmed pregnancy. From this sample, 120 individuals (mean age: 20.82 SD ± 1.99, age range: 18–25) were invited to participate in the experiment. To ensure sufficient numbers of participants with high levels of neuroticism, 60 individuals were selected from the highest quartile of neuroticism scores (NEO‐FFI score ≥ 32, range 32–47) and 60 individuals were randomly selected from the three lowest quartiles (NEO‐FFI < 32, range 17–31). Plots of normality (QQ‐plot and boxplot) showed that, in the selected 120 participants, neuroticism scores were normally distributed.
To reduce hormone‐related between‐subject variability, participants were invited for the experiment during the first 10 days of their menstrual cycle (early and mid‐follicular phase) or during the discontinuation week in case of oral contraceptive usage, which resembles the early and mid‐follicular phase in terms of ovarian hormonal levels [Cohen and Katz, 1979]. During these phases, ovarian hormonal levels are relatively low and menstrual cycle related changes in mood, stress sensitivity and neurocognitive function are minimal [Andreano and Cahill, 2010; Goldstein et al., 2010; Symonds et al., 2004].
On the day of the experiment, after explaining the procedure, participants gave written informed consent and completed the NEO personality inventory revised (NEO‐PI‐R) (domains Neuroticism, Extraversion and Conscientiousness, 144 items) and the Penn State Worry Questionnaire (PSWQ) to assess neuroticism and the excessiveness and uncontrollability of pathological worry, respectively. The study was approved by the Medical Ethical Committee of the University Medical Center Groningen and was conducted in accordance with the Declaration of Helsinki.
Experimental Design
The experimental paradigm had an event‐related design and consisted of two conditions; a worry inducing condition and a neutral condition [Paulesu et al., 2010]. Each trial started with an instruction of 2 s to either “Worry about” (worry trial) or “Think about” (neutral trial) the topic of the upcoming sentence. Worry inducing sentences were presented after the instruction “Worry about” and neutral sentences were presented after the instruction “Think about” (see Supporting Information Appendix A for a description of the pilot study conducted to identify the stimulus set and Supporting Information Appendix B for a list of the stimuli). The duration of the sentence presentation was determined by a self‐paced button press and lasted maximally 6 s. After having read the sentence, participants generated thoughts on the topic of the sentence for 15 s. Subsequently, an audible beep was presented for 2 s followed by a four‐point Likert scale (1 = no worry, 4 = excessive worry), which was shown for 6 s. On this scale, participants were able to rate how much of their generated thoughts were related to worry. Next, participants were presented with the instruction “Rest” for 2 s to discontinue the worry or thinking process and relax for 9 s. The experimental paradigm consisted of 12 worry trials and 12 neutral trials, which were presented randomly. The maximum duration of a trial was 42 s and the total maximum duration of the experimental paradigm was 16.8 min (see Fig. 1 for the task outline and Supporting Information Appendix C for an overview of the full fMRI session).
Figure 1.

Task outline. First, participants were presented with an instruction to either “Worry about” or “Think about” the topic of the upcoming sentence (2 s). Second, a worry‐inducing sentence was presented after the instruction “Worry about” and a neutral sentence was presented after the instruction “Think about” (see figure for examples) (6 s). Third, participants were able to generate thoughts on the topic of the sentence (15 s). Fourth, an audible beep was presented (2 s) and participants were instructed to rate how much of their generated thoughts were related to worry (6 s) (1 = no worry, 4 = excessive worry). Fifth, participants were instructed (2 s) to rest and relax (9 s), after which a new trial started.
Image Acquisition
A 3 Tesla Phillips Intera MRI scanner (Phillips Medical Systems, Best, the Netherlands), equipped with a 32‐channel SENSE head coil, was used to obtain the images. A high‐resolution T1‐weighted 3D structural image was obtained using fast‐field echo (FFE) for anatomical reference (170 slices; TR: 9 ms; TE: 8 ms; FOV: 256 × 231; 256 × 256 matrix; voxel size: 1 × 1 × 1 mm3). Functional images were acquired with T2*‐weighted gradient echo planar imaging (EPI) sequences. The experimental paradigm comprised 510 volumes of 39 axial‐slices (TR: 2,000 ms; TE: 30 ms; FOV: 224 × 224; 64 × 61 matrix; voxel size: 3.5 × 3.5 × 3.5 mm3). Slices were acquired in descending order without a gap. To prevent artifacts due to nasal cavities, images were tilted 10° to the AC‐PC transverse plane.
Statistical Analyses
Questionnaire and behavioral analyses
Questionnaire and behavioral analyses were performed using IBM SPSS Statistics Version 20 (IBM, SPSS, Chicago, IL). Pearson correlations were calculated between NEO‐PI‐R neuroticism scores, and scores on the PSWQ and subjective rating scale for worry inducing sentences and neutral sentences. Furthermore, a paired t‐test was performed to investigate differences in mean subjective rating score between the two sentence categories. Questionnaire as well as behavioral results with P‐values < 0.05 were considered significant.
Image analysis
Image processing and statistical analyses were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm), implemented in Matlab 7.8.0 (The Mathworks, Natick, MA). First, structural as well as functional images were reoriented parallel to the AC‐PC plane. Second, functional images were realigned to the first image using rigid body transformations and the mean EPI image, created during this step, was coregistered to the anatomical T1 image. Third, structural images were corrected for bias field inhomogeneities, registered using linear transformations and segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) (MNI template space). Fourth, we used DARTEL (diffeomorphic anatomical registration through exponentiated lie algebra toolbox) [Ashburner, 2007] to create a customized group template to increase the accuracy of inter‐subject alignment. Individual GM and WM tissue segments were iteratively aligned to the group template in order to acquire individual deformation flow fields. Fifth, the coregistered functional images were normalized to MNI space using the customized group template and individual deformation flow fields. Furthermore, images were resampled to 2 mm3 isotropic voxels and smoothed with a 8‐mm full‐width at half‐maximum (FWHM) Gaussian kernel. Three subjects were excluded from further analysis; two because of anatomical abnormalities and one because of task‐related movement. A total sample of 117 subjects remained for further analysis.
Hemodynamic changes for each condition were calculated using a General Linear Model (GLM). In the GLM, predictors were created for the different trial parts, i.e., sentence instruction, sentence presentation, thought generation, sound presentation, rating, rest instruction, and rest. Effects were modeled using a boxcar convolved with a canonical hemodynamic response function (HRF). Subsequent t‐contrasts were computed per subject: (worry < > neutral) and (rest worry < > rest neutral). In the latter contrast, we investigated whether brain regions related to worry showed persistent activation during the post‐worry rest period. Furthermore, subjective ratings of worry were included as single trial parametric weights for the worry condition. The reason for this is to examine which brain regions showed increased or decreased activation, when individuals indicated to have generated more worry‐related thoughts after the presentation of a worry‐inducing sentence. In addition, six rigid body head motion parameters and their first temporal derivatives were included as nuisance regressors. The resulting contrast images were entered in a second‐level random‐effect analysis. Neuroticism scores were centered and entered as a regressor of interest in the model. Main effects as well interactions with neuroticism (positive and negative correlations) were investigated with a series of one‐sample t‐tests. To correct for multiple comparisons, resulting brain images were thresholded on P < 0.05 FWE cluster level using an initial threshold of P < 0.001 uncorrected.
Furthermore, we used generalized psycho‐physiological interaction (gPPI) [McLaren et al., 2012] to investigate task‐dependent connectivity of brain regions associated with neuroticism during worry to shed light on the neural networks involved [Burianova et al., 2010]. First, the first eigenvariate was extracted from the time series of the voxels in the specific clusters for each subject. Second, hemodynamic deconvolution was performed on the extracted time series to remove the effects of the canonical HRF. Third, the resulting time series were multiplied by the task vectors and reconvolved with the HRF to obtain the PPI terms. Subsequently, these terms were entered as regressors at first level, along with the HRF convolved task vectors, the eigenvariate time course and a constant. Fourth, we calculated the PPI t‐contrast (worry > neutral) per subject and entered the resulting contrast images in a second‐level random‐effect analysis. Task‐dependent connectivity (positive and negative correlations) was investigated with a one‐sample t‐test. To correct for multiple comparisons, resulting brain images were thresholded on P < 0.05 FWE cluster level using an initial threshold of P < 0.001 uncorrected.
RESULTS
Questionnaire and Behavioral Data
The mean NEO‐PI‐R neuroticism score across the whole sample was 135.47 SD ± 18.92 (range: 94–195) and the mean PSWQ score was 48.58 SD ± 10.73 (range: 22–70). Furthermore, a significant correlation was found between scores on the NEO‐PI‐R neuroticism domain and PSWQ (r = 0.731, R 2 = 0.534, P < 0.0001) (see Fig. 2a). In addition, the mean subjective rating score for worry inducing sentences was significantly higher than the mean subjective rating score for neutral sentences (worry inducing, mean: 2.18, SD ± 0.443; neutral, mean: 1.19, SD ± 0.368, t(116) = 22.443, P < 0.0001). Moreover, a significant correlation was found between scores on the NEO‐PI‐R neuroticism domain and subjective rating scale for worry inducing sentences (r = 0.343, R 2 = 0.117, P < 0.0001) (see Fig. 2b). In contrast, NEO‐PI‐R neuroticism scores were not significantly correlated with scores on the subjective rating scale for neutral sentences (P > 0.05).
Figure 2.
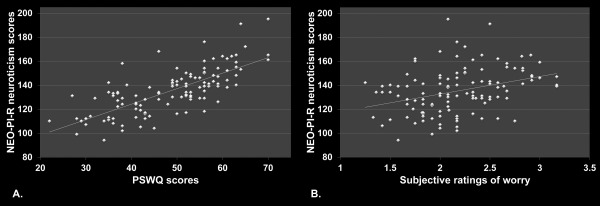
Questionnaire and behavioral results. A. Scores on the NEO‐PI‐R neuroticism domain correlated significantly with scores on the PSWQ (r = 0.731, R 2 = 0.534, P <0.0001). B. Scores on the NEO‐PI‐R neuroticism domain correlated significantly with scores on the subjective rating scale for worry‐inducing sentences (r = 0.343, R 2 = 0.117, P <0.0001).
Imaging Data
Main effects
First, brain regions were identified for the contrast (worry > neutral) (see Fig. 3a and Table 1 for the results). Several default mode brain areas were found to be more activated during worry compared to neutral, including the anterior cingulate gyrus, superior medial frontal gyrus, posterior cingulate gyrus, precuneus, inferior parietal gyrus and angular gyrus.
Figure 3.

Main effects and effects related to the subjective ratings of worry. A. Brain regions that showed more activation for the contrast (worry > neutral). B. Brain regions that showed more activation for the contrast (neutral > worry). C. Brain regions of which their activation levels correlated positively with the subjective ratings of worry for the contrast (worry > neutral) (yellow) overlayed on the activation pattern shown in Figure 3A (red). To correct for multiple comparisons, resulting brain images were thresholded on P < 0.05 FWE cluster level using an initial threshold of P < 0.001 uncorrected.
Table 1.
Peak activations of brain regions, which showed differential activation for the contrasts (worry > neutral), (neutral > worry) and (worry > neutral x neuroticism) and the parametric modulation (subjective ratings of worry)
| Cluster | Cluster size | T‐value | Z‐value | Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Worry sentences versus neutral sentences | ||||||
| Precuneus Middle cingulate gyrus Posterior cingulate gyrus Cuneus | 5525 | 11.39 | Inf | 8 | −66 | 36 |
| 11.22 | Inf | −4 | −68 | 36 | ||
| 10.29 | Inf | 12 | −50 | 30 | ||
| Angular gyrus Inferior parietal gyrus Supramarginal gyrus Middle temporal gyrus | 1109 | 8.23 | 7.28 | −58 | −58 | 30 |
| Angular gyrus Inferior parietal gyrus Supramarginal gyrus Superior temporal gyrus | 941 | 8.05 | 7.15 | 52 | −54 | 32 |
| 7.24 | 6.56 | 52 | −56 | 42 | ||
| Superior medial frontal gyrus Superior frontal gyrus Middle frontal gyrus Anterior cingulate gyrus | 3059 | 6.67 | 6.12 | −18 | 48 | 20 |
| 6.04 | 5.62 | −12 | 54 | 18 | ||
| 5.91 | 5.51 | 6 | 56 | 24 | ||
| Neutral sentences versus worry sentences | ||||||
| Inferior temporal gyrus Fusiform gyrus (Para)hippocampus/ amygdala Lingual gyrus Calcarine sulcus Cerebellum | 14284 | 13.95 | Inf | −24 | −38 | −20 |
| 13.38 | Inf | −54 | −52 | −12 | ||
| 9.44 | Inf | 56 | −44 | −8 | ||
| Inferior frontal triangularis Inferior frontal opercularis Precentral gyrus | 5358 | 13.71 | Inf | −40 | 2 | 28 |
| 12.24 | Inf | −40 | 28 | 18 | ||
| 9.84 | Inf | −38 | 16 | 24 | ||
| Middle occipital gyrus Superior occipital gyrus Inferior parietal gyrus Superior parietal gyrus | 1668 | 12.85 | Inf | −28 | −68 | 42 |
| Inferior frontal triangularis Inferior frontal opercularis Insula/ Rolandic operculum Heschl's gyrus Superior temporal gyrus Superior temporal pole | 2458 | 7.64 | 6.85 | 48 | 34 | 16 |
| 6.28 | 5.81 | 42 | 6 | 28 | ||
| 5.61 | 5.26 | 34 | −16 | 16 | ||
| Middle occipital gyrus Superior occipital gyrus Angular gyrus | 660 | 6.62 | 6.08 | 32 | −64 | 42 |
| Anterior cingulate gyrus | 246 | 6.19 | 5.74 | 6 | 4 | 28 |
| Middle cingulate gyrus | 5.66 | 5.30 | −6 | 4 | 28 | |
| Middle frontal gyrus | 230 | 4.39 | 4.21 | 26 | 8 | 56 |
| Superior frontal gyrus | 4.17 | 4.01 | 28 | 0 | 56 | |
| Middle temporal gyrus Superior temporal gyrus | 345 | 4.29 | 4.12 | −58 | −16 | 8 |
| 3.60 | 3.49 | −48 | −24 | 4 | ||
| 3.57 | 3.47 | −50 | −42 | 12 | ||
| Parametric modulation (subjective ratings of worry) | ||||||
| Anterior cingulate gyrus Superior medial frontal gyrus Superior frontal gyrus | 1423 | 5.60 | 5.25 | −10 | 50 | 10 |
| 5.03 | 4.77 | −10 | 46 | 24 | ||
| 4.96 | 4.71 | 0 | 54 | 4 | ||
| Posterior cingulate gyrus Precuneus | 587 | 5.19 | 4.91 | −8 | −50 | 34 |
| 4.38 | 4.21 | −4 | −60 | 26 | ||
| Middle frontal gyrus Superior frontal gyrus | 262 | 4.49 | 4.30 | −24 | 32 | 40 |
| Insula Inferior frontal triangularis Inferior frontal opercularis | 294 | 4.02 | 3.88 | −36 | 26 | 2 |
| 4.00 | 3.86 | −40 | 12 | −8 | ||
| 3.98 | 3.84 | −36 | 30 | −8 | ||
| Worry sentences versus neutral sentences, negative correlation with neuroticism | ||||||
| Retrosplenial cortex | 437 | 4.63 | 4.42 | 0 | −38 | 4 |
| 3.22 | 3.15 | 0 | −58 | 6 | ||
| Calcarine sulcus Cuneus | 436 | 4.15 | 4.00 | 6 | −92 | 0 |
| 3.76 | 3.65 | 16 | −94 | 0 | ||
| 3.68 | 3.57 | −12 | −84 | 8 | ||
Resulting brain images were thresholded on P < 0.05 FWE cluster level using an initial threshold of P < 0.001 uncorrected.
Second, brain regions were identified for the reverse contrast (neutral > worry) (see Fig. 3b and Table 1 for the results). We found more activation in the following brain areas during neutral contrasted to worry, including the temporal gyri, hippocampal‐parahippocampal complex, fusiform gyrus, inferior frontal gyrus, anterior/middle cingulate gyrus, superior/middle occipital gyrus and cerebellum. We note, however, that activation differences found for the contrasts (worry > neutral) and (neutral > worry), may be the result of less or more pronounced deactivation in the worry condition, respectively (see Supporting Information Appendix D and E for bar charts displaying activation differences in four key brain regions for the contrasts (worry > neutral) and (neutral > worry)).
No significant results were found for the post‐worry rest period; contrasts (rest worry > rest neutral) and (rest neutral > rest worry).
Effects related to the subjective ratings of worry (parametric modulation)
Brain regions were identified for which their activation levels correlated with scores on the subjective rating scale for worry‐inducing sentences during the generation of worry‐related thoughts (see Fig. 3c and Table 1 for the results). Subjective ratings of worry were associated with increased activation in default mode and emotional brain areas during worry, including the anterior cingulate gyrus, superior medial frontal gyrus, posterior cingulate gyrus, precuneus, superior/middle frontal gyrus, inferior frontal gyrus and anterior insula. No brain regions were found to be negatively correlated with the subjective ratings of worry during the generation of worry‐related thoughts.
Effects related to neuroticism
Brain regions were identified that correlated with neuroticism for the contrast (worry > neutral) (see Fig. 4a and Table 1 for the results). Neuroticism was associated with decreased brain activation in two clusters during worry compared to neutral, including the retrosplenial part of the cingulate gyrus and the cuneus/calcarine sulcus.
Figure 4.
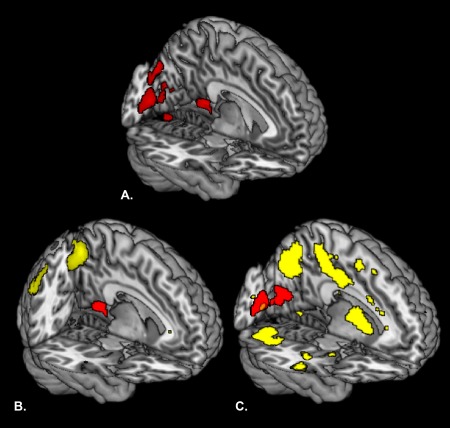
Effects related to neuroticism and functional connectivity results. A. Brain regions that correlated negatively with neuroticism for the contrast (worry > neutral). B. Brain regions (yellow) that showed a positive functional connection with the retrosplenial cortex (red; this area correlated negatively with neuroticism, see Fig. 4A) for the contrast (worry > neutral). C. Brain regions (yellow) that showed a positive functional connection with the visual cortex (red; this area correlated negatively with neuroticism, see Fig. 4A) for the contrast (worry > neutral). To correct for multiple comparisons, resulting brain images were thresholded on P < 0.05 FWE cluster level using an initial threshold of P < 0.001 uncorrected.
No brain regions were found to be positively or negatively correlated with neuroticism for the post‐worry rest period; contrasts (rest worry > rest neutral) and (rest neutral > rest worry).
Functional connectivity results
Brain regions were identified that were functionally connected to brain regions associated with neuroticism: the retrosplenial part of the cingulate gyrus (see Fig. 4b and Table 2 for the results) and the cuneus/calcarine sulcus (see Fig. 4c and Table 2 for the results) for the contrast (worry > neutral). We found that subsequent brain areas showed a positive correlation with the retrosplenial part of the cingulate gyrus during worry contrasted to neutral, including the posterior cingulate gyrus, precuneus, inferior parietal lobe, angular gyrus, hippocampus and thalamus. No brain regions were found to be negatively correlated with the retrosplenial part of the cingulate gyrus for the contrast (worry > neutral).
Table 2.
Peak activations of brain regions, which showed enhanced functional connectivity to the selected seed regions related to neuroticism for the contrast (worry > neutral)
| Seed region | Cluster | Cluster size | T‐value | Z‐value | Coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Worry sentences versus neutral sentences | |||||||
| Retrosplenial cortex | Precuneus | 701 | 4.89 | 4.65 | −8 | −64 | 50 |
| 4.42 | 4.24 | −2 | −66 | 44 | |||
| 3.67 | 3.56 | 0 | −50 | 50 | |||
| Retrosplenial cortex | Posterior cingulate gyrus Retrosplenial cortex Precuneus Hippocampus Thalamus | 362 | 4.66 | 4.45 | 6 | −40 | 8 |
| 4.61 | 4.41 | 2 | −16 | 10 | |||
| 4.16 | 4.00 | 6 | −30 | 10 | |||
| Retrosplenial cortex | Inferior parietal gyrus Angular gyrus | 282 | 3.98 | 3.85 | 44 | −56 | 48 |
| 3.84 | 3.72 | 50 | −68 | 36 | |||
| 3.33 | 3.25 | 56 | −46 | 46 | |||
| Calcarine sulcus Cuneus | Middle temporal gyrus Superior temporal gyrus | 865 | 6.13 | 5.69 | −62 | −22 | −2 |
| 5.47 | 5.15 | −62 | −30 | 0 | |||
| 4.78 | 4.55 | −52 | −24 | −2 | |||
| Calcarine sulcus Cuneus | Cuneus Occipital gyrus Precuneus Superior parietal gyrus Inferior temporal gyrus Middle temporal gyrus | 4620 | 5.88 | 5.48 | −16 | −72 | 34 |
| 5.78 | 5.40 | −36 | −74 | 0 | |||
| 5.75 | 5.38 | 16 | −74 | 34 | |||
| Calcarine sulcus Cuneus | Inferior frontal triangularis Inferior frontal opercularis Precentral gyrus Postcentral gyrus Caudate | 1142 | 5.51 | 5.18 | −38 | −2 | 44 |
| 4.24 | 4.08 | −38 | 14 | 20 | |||
| 4.23 | 4.07 | −20 | −2 | 22 | |||
| Calcarine sulcus Cuneus | Caudate Putamen Pallidum Thalamus | 1857 | 5.16 | 4.88 | 10 | 6 | 4 |
| 5.03 | 4.77 | −8 | −6 | 8 | |||
| 4.77 | 4.54 | −14 | 4 | 4 | |||
| Calcarine sulcus Cuneus | Cerebellum Fusiform gyrus | 1665 | 5.07 | 4.81 | 38 | −66 | −22 |
| 4.68 | 4.47 | 20 | −74 | −20 | |||
| 4.67 | 4.46 | 32 | −58 | −46 | |||
| Calcarine sulcus Cuneus | Middle cingulate gyrus Precuneus | 2372 | 5.03 | 4.77 | −6 | −18 | 42 |
| 4.83 | 4.60 | −6 | −36 | 52 | |||
| 4.61 | 4.40 | 6 | −42 | 52 | |||
| Calcarine sulcus Cuneus | Middle temporal gyrus Superior temporal gyrus Superior temporal pole Insula Inferior frontal opercularis | 438 | 4.78 | 4.55 | 60 | −8 | −10 |
| 4.42 | 4.24 | 50 | 14 | −8 | |||
| 3.84 | 3.72 | 58 | 8 | −8 | |||
| Calcarine sulcus Cuneus | Middle temporal gyrus Superior temporal gyrus Supramarginal gyrus Angular gyrus | 708 | 4.56 | 4.36 | 66 | −34 | 4 |
| 4.48 | 4.29 | 58 | −38 | 34 | |||
| 4.42 | 4.24 | 60 | −46 | 30 | |||
| Calcarine sulcus Cuneus | Cerebellum Fusiform gyrus | 242 | 4.49 | 4.30 | −38 | −64 | −26 |
| 3.80 | 3.68 | −40 | −64 | −34 | |||
| 3.69 | 3.58 | −30 | −70 | −20 | |||
| Calcarine sulcus Cuneus | Inferior temporal gyrus Middle temporal gyrus Hippocampus | 211 | 4.46 | 4.27 | 44 | −2 | −28 |
| 4.11 | 3.96 | 36 | −16 | −12 | |||
| 4.05 | 3.91 | 38 | −16 | −20 | |||
| Calcarine sulcus Cuneus | Inferior frontal triangularis Middle frontal gyrus | 227 | 4.12 | 3.97 | 32 | 26 | 24 |
| 4.05 | 3.91 | 34 | 40 | 34 | |||
| 3.81 | 3.69 | 40 | 30 | 24 | |||
| Calcarine sulcus Cuneus | Inferior temporal gyrus Middle temporal gyrus | 180 | 4.10 | 3.96 | −50 | −50 | −4 |
| 3.76 | 3.65 | −58 | −52 | 0 | |||
| 3.61 | 3.50 | −52 | −56 | 16 | |||
Resulting brain images were thresholded on P < 0.05 FWE cluster level using an initial threshold of P < 0.001 uncorrected.
Furthermore, the following brain areas showed a positive correlation with the cuneus/calcarine sulcus during worry contrasted to neutral, including the occipital gyri, precuneus, middle cingulate gyrus, middle temporal gyrus, inferior frontal gyrus, cerebellum, hippocampus, thalamus, caudate, putamen and pallidum. No brain regions were found to be negatively correlated with the cuneus/calcarine sulcus for the contrast (worry > neutral).
DISCUSSION
We implemented a mood induction paradigm [Paulesu et al., 2010] to investigate the neural correlates of state worry in association with neuroticism in women. As expected, individuals scoring higher on neuroticism reported to worry more in daily life than individuals scoring lower. This tendency was also observed in our task results of experimentally induced worry. High neurotic individuals indicated to have generated more worry‐related thoughts after the presentation of a worry‐inducing sentence than low neurotic individuals. Notably, the neuroimaging results implied the involvement of default mode and emotional brain areas during worry, which have consistently been associated with self‐relevant cognitive processes and the processing and regulation of negative affect [Buckner et al., 2008; Phillips et al., 2003; Sylvester et al., 2012]. In addition, activation in the retrosplenial and visual cortex was decreased in individuals scoring higher on neuroticism during worry, possibly suggesting reduced autobiographical specificity and visual mental imagery. No significant task or interaction effects were observed during the post‐worry rest period, indicating no persistent activation of brain regions related to worry during this period in high as well as low scoring neurotic individuals.
Effects Related to State Worry
We found that brain regions in the default mode network were more activated during worry, including the cortical midline structures and lateral parietal cortex. Specifically, the medial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, precuneus, dorsolateral prefrontal cortex, and anterior insula showed increased activation during worry, when individuals indicated to have generated more worry‐related thoughts. These findings are in line with the results of the study of Paulesu et al. [2010] and other studies demonstrating the involvement of the default mode network and brain regions related to emotion processing and regulation in state as well as trait worry and rumination [Andreescu et al., 2011; Berman et al., 2011; Cooney et al., 2010; Hamilton et al., 2011; Marchetti et al., 2012; Paul et al., 2013; Schienle et al., 2009; Thomas et al., 2011; Whitfield‐Gabrieli and Ford, 2012; Zhu et al., 2012].
The default mode network has been observed to be activated during passive experimental control conditions and is postulated to perform functions, such as autobiographical memory (memories of personally relevant experienced events), self‐reflection, envisioning the future, mental simulation, introspection and emotion regulation [Buckner et al., 2008; Schacter and Addis, 2007; Schacter et al., 2007, 2012; Spreng and Mar, 2012; Sylvester et al., 2012]. These functions may support cognitive processes related to worry, since worry is potentially used as a coping mechanism by individuals to prevent future negative outcomes, prepare for the worst, solve problems related to upcoming events and superstitiously lessen the probability of the occurrence of a negative event [Borkovec et al., 2004]. Furthermore, as mentioned above, we found the left anterior insula to be more activated during worry, when individuals indicated to have generated more worry‐related thoughts. Numerous studies have shown the involvement of the anterior insula in interoceptive awareness, subjective feelings and emotional arousal [Craig, 2009; Critchley et al., 2004]. Notably, trait rumination has been shown to be positively correlated with activation in the bilateral anterior insula after a stressful task [Paul et al., 2013].
In contrast, during thinking about neutral topics, we found brain regions that have been implicated in semantic and episodic memory, including the temporal gyri, hippocampal‐parahippocampal complex, fusiform gyrus and inferior frontal gyrus [Binder and Desai, 2011; Burianova and Grady, 2007; Burianova et al., 2010; Mion et al., 2010; Yang et al., 2012]. Semantic memory concerns an individuals' general and factual knowledge about the world, while episodic memory relates to memories of experienced events in the context they occurred [Binder and Desai, 2011; Burianova and Grady, 2007]. The observed activation differences between the worry and neutral condition are plausible, since neutral topics are inherently more abstract, conceptual and generalized and worry topics more self‐relevant and emotional [Binder and Desai, 2011; Burianova and Grady, 2007; Burianova et al., 2010; Yang et al., 2012].
Effects Related to Neuroticism
We found that individuals scoring higher on neuroticism demonstrated increased worrying, based on questionnaire, task and imaging results. The questionnaire results showed that, in daily life, high neurotic individuals rate their worry episodes as more excessive and uncontrollable in comparison to low neurotic individuals. Furthermore, neuroticism was correlated with having generated more worry‐related thoughts after the presentation of a worry‐inducing sentence in our experimental paradigm. These findings are in line with studies that show a link between the tendency to worry and neuroticism [Bringmann et al., 2013; Hale et al., 2010; Matthews et al., 2000; Muris et al., 2005; Roelofs et al., 2008; Watson et al., 1994].
In addition, decreased activation in the retrosplenial and visual cortex was associated with neuroticism during worry. Two processes that have been related to these brain regions as well as to the cognitive avoidant function of worry [Borkovec et al., 2004] are (i) autobiographical specificity and (ii) visual mental imagery. Autobiographical specificity refers to differences in the amount of detail that is used to recollect a memory [Sumner, 2012; Williams et al., 2007]. Specifically, individuals with depression and trauma‐related anxiety disorders tend to recollect memories in an overgeneral way (e.g. memories that are summaries or classes of events, or last longer than a day) [Sumner, 2012; Williams et al., 2007]. Previous research has shown that the retrosplenial cortex is part of the core neural network underlying autobiographical memory [Svoboda et al., 2006] and is related to emotion processing [Maddock, 1999]. Furthermore, we found enhanced functional connectivity between the retrosplenial cortex and a number of brain areas during worry, including the posterior cingulate cortex, precuneus, inferior parietal lobe, angular gyrus, hippocampus and thalamus. Studies have revealed that these brain areas are collectively involved in processes related to memory access, such as the reactivation of distributed, stored memory traces and strategic retrieval search [Daselaar et al., 2008]. Therefore, the finding of decreased activation in the retrosplenial cortex during worry may suggest that individuals scoring higher on neuroticism “get stuck” in superficial memory processing (lacking detail and richness), resulting in overgeneral memories [Sumner, 2012; Williams et al., 2007]. Notably, rumination as well as functional avoidance (i.e., averting emotional distress) have been indicated as mechanisms underlying overgeneral autobiographical memory [Debeer et al., 2011, 2012; Geraerts et al., 2012; Sumner, 2012; Williams et al., 2007].
Visual mental imagery can be defined as the retrieval of perceptual information from memory in order to imagine a subjective event in the past or future [Ganis et al., 2004; Kosslyn et al., 2001]. Emotional disorders are often characterized by aversive mental images that intrude involuntarily and evoke a great deal of stress [Holmes and Mathews, 2010]. Visual mental images can have a pronounced effect on emotion because neural correlates underlying visual mental imagery are densely connected or show overlap with brain systems related to emotion, autobiographical memory and visual perception [Ganis et al., 2004; Holmes and Mathews, 2010; Kosslyn et al., 2001]. Borkovec et al. [2004] state in their theory of GAD that the abstract and verbal nature of worry reduces visual mental imagery. For this reason, it is proposed that individuals use worry as a cognitive avoidance mechanism to experience less negative emotions and somatic arousal [Borkovec et al., 2004]. In line with this, we found decreased activation in the visual cortex in individuals scoring higher on neuroticism during worry. Furthermore, increased functional connectivity was observed between the visual cortex and several brain areas during worry, including the occipital gyri, precuneus, middle temporal gyrus, inferior frontal gyrus, hippocampus and thalamus. This network of brain regions has been implicated in visualizing [Ganis et al., 2004], memory elaboration/reliving [Daselaar et al., 2008] and envisioning the future [Schacter et al., 2007]. Hence, our findings may indicate that individuals with higher levels of neuroticism use worry as a coping mechanism to reduce visual mental imagery and avoid negative feelings that are normally provoked by it. We note, however, that the processes of autobiographical specificity and visual mental imagery were not investigated in the current study; therefore, these interpretations should be regarded as speculative until verified in further research.
Persistence of Neural Effects
No significant differences were found between rest after a worry‐inducing sentence and rest after a neutral sentence. We expected that brain regions related to worry would show persistent activation during the post‐worry rest period in individuals scoring higher on neuroticism, because such an effect has been shown in patients with GAD. Paulesu et al. [2010] showed that brain regions found during worrying—dorsomedial prefrontal cortex and anterior cingulate cortex—were significantly more activated in GAD patients during the post‐worry rest period compared to healthy controls. Possibly, persistent activation of brain regions related to worry during the post‐worry rest period is more associated with pathological forms of worry.
CONCLUSION
Our findings reveal a relationship between neuroticism and increased worrying, shown in questionnaire, behavioral and imaging results. As expected, individuals scoring higher on neuroticism reported to worry more in daily life and to have generated more worry‐related thoughts after the presentation of a worry‐inducing sentence. Notably, high neurotic individuals showed decreased activation in brain regions that have been implicated in autobiographical specificity and visual mental imagery during worry. The findings may suggest that these individuals tend to recollect memories in an overgeneral way [Sumner, 2012; Svoboda et al., 2006] and reduce the visualization of emotional events [Holmes and Mathews, 2010] during worry [Borkovec et al., 2004]. In the literature, both processes have been related to the cognitive avoidance of emotional distress [Borkovec et al., 2004; Sumner, 2012]. Future studies should investigate whether our findings extend to male samples. Excessive worry features in a number of emotional disorders [Hirsch and Mathews, 2012] and results from studies that elucidate its neural basis may help explain how and why neuroticism contributes to vulnerability for psychopathology.
ACKNOWLEDGMENTS
The sponsor did not play a role in the study design; collection, analysis, and interpretation of the data; writing the report or the decision to submit the article for publication. Furthermore, the authors would like thank A. Sibeijn‐Kuiper and J. Streurman‐Werdekker for their support in the data acquisition.
Supporting information
Supporting Information
Footnotes
Note: In the current study, before the post‐worry rest period, participants were instructed to press one of the four buttons (1 = no worry, 4 = excessive worry) on the button box to rate how much of their generated thoughts were related to worry, while in the study of Paulesu et al. (2010), participants were instructed to press a button to indicate whether they had succeeded in generating thoughts on the topic of the sentence.
REFERENCES
- Andreano JM, Cahill L (2010): Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage 53:1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, Aizenstein H (2011): Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety 28:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J (2011): Depression, rumination and the default network. Soc Cogn Affect Neurosci 6:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH (2011): The neurobiology of semantic memory. Trends Cogn Sci 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Blair RJR (2012): A cognitive neuroscience approach to generalized anxiety disorder and social phobia. Emotion Rev 4:133–138. [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA (1983): Preliminary exploration of worry: Some characteristics and processes. Behav Res Ther 21:9–16. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine O, Behar E (2004): Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York: Guilford Press; p 77–108. [Google Scholar]
- Bringmann LF, Vissers N, Wichers M, Geschwind N, Kuppens P, Peeters F, Borsboom D, Tuerlinckx F (2013): A network approach to psychopathology: New insights into clinical longitudinal data. PLoS One 8:e60188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna J, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. In: Kingstone A, Miller MB, editors. The Year in Cognitive Neuroscience 2008. Malden: Blackwell Publishing; p 1–38. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL (2007): Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cogn Neurosci 19:1520–1534. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL (2010): A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 49:865–874. [DOI] [PubMed] [Google Scholar]
- Canli T (2008): Toward a neurogenetic theory of neuroticism. Ann N Y Acad Sci 1129:153–174. [DOI] [PubMed] [Google Scholar]
- Chan SW, Goodwin GM, Harmer CJ (2007): Highly neurotic never‐depressed students have negative biases in information processing. Psychol Med 37:1281–1291. [DOI] [PubMed] [Google Scholar]
- Cohen BL, Katz M (1979): Pituitary and ovarian function in women receiving hormonal contraception. Contraception 20:475–487. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH (2010): Neural correlates of rumination in depression. Cogn Affect Behav Neurosci 10:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PTJ, McCrae RR (1989): The NEO‐PI/NEO‐FFI Manual Supplement. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Craig AD (2009): How do you feel—Now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC (2008): The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cereb Cortex 18:217–229. [DOI] [PubMed] [Google Scholar]
- Debeer E, Raes F, Williams JM, Hermans D (2011): Context‐dependent activation of reduced autobiographical memory specificity as an avoidant coping style. Emotion 11:1500–1506. [DOI] [PubMed] [Google Scholar]
- Debeer E, Raes F, Claes S, Vrieze E, Williams JM, Hermans D (2012): Relationship between cognitive avoidant coping and changes in overgeneral autobiographical memory retrieval following an acute stressor. J Behav Ther Exp Psychiatry 43 (Suppl 1):S37–S42. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM (2004): Brain areas underlying visual mental imagery and visual perception: An fMRI study. Brain Res Cogn Brain Res 20:226–241. [DOI] [PubMed] [Google Scholar]
- Geraerts E, Dritschel B, Kreplin U, Miyagawa L, Waddington J (2012): Reduced specificity of negative autobiographical memories in repressive coping. J Behav Ther Exp Psychiatry 43 (Suppl 1):S32–S36. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield‐Gabrieli S, Makris N (2010): Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP (2003): Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. J Pers Soc Psychol 85:348–362. [DOI] [PubMed] [Google Scholar]
- Hale WW III, Klimstra TA, Meeus WH (2010): Is the generalized anxiety disorder symptom of worry just another form of neuroticism? A 5‐year longitudinal study of adolescents from the general population. J Clin Psychiatry 71:942–948. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH (2011): Default‐mode and task‐positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry 70:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch CR, Mathews A (2012): A cognitive model of pathological worry. Behav Res Ther 50:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, Mathews A (2010): Mental imagery in emotion and emotional disorders. Clin Psychol Rev 30:349–362. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL (2001): Neural foundations of imagery. Nat Rev Neurosci 2:635–642. [DOI] [PubMed] [Google Scholar]
- Lahey BB (2009): Public health significance of neuroticism. Am Psychol 64:241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee‐Baggley D, Preece M, Delongis A (2005): Coping with interpersonal stress: Role of big five traits. J Pers 73:1141–1180. [DOI] [PubMed] [Google Scholar]
- Maddock RJ (1999): The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci 22:310–316. [DOI] [PubMed] [Google Scholar]
- Marchetti I, Koster EH, Sonuga‐Barke EJ, De Raedt R (2012): The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychol Rev 22:229–251. [DOI] [PubMed] [Google Scholar]
- Matthews G, Derryberry D, Siegle G (2000): Personality and emotion: Cognitive science perspectives. In: Hampton S, editor. Advances in Personality Psychology. Philadelphia: Psychology Press; p 199–237. [Google Scholar]
- McEvoy PM, Mahoney AE, Moulds ML (2010): Are worry, rumination, and post‐event processing one and the same? Development of the repetitive thinking questionnaire. J Anxiety Disord 24:509–519. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM (2005): Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther 43:1281–1310. [DOI] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta‐Cabronero J, Pengas G, Izquierdo‐Garcia D, Hong YT, Fryer TD, Williams GB, Hodges JR, Nestor PJ (2010): What the left and right anterior fusiform gyri tell us about semantic memory. Brain 133:3256–3268. [DOI] [PubMed] [Google Scholar]
- Muris P, Roelofs J, Rassin E, Franken I, Mayer B (2005): Mediating effects of rumination and worry on the links between neuroticism, anxiety and depression. Pers Individ Dif 39:1105–1111. [Google Scholar]
- Ormel J, Rosmalen J, Farmer A (2004): Neuroticism: A non‐informative marker of vulnerability to psychopathology. Soc Psychiatry Psychiatr Epidemiol 39:906–912. [DOI] [PubMed] [Google Scholar]
- Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, Rosmalen JG, Oldehinkel AJ (2013): Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clin Psychol Rev 33:686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L (2013): Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Soc Cogn Affect Neurosci 8:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, Sberna M, Ruggiero GM, Bottini G, Sassaroli S (2010): Neural correlates of worry in generalized anxiety disorder and in normal controls: A functional MRI study. Psychol Med 40:117–124. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003): Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54:504–514. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Huibers M, Peeters F, Arntz A, van Os J (2008): Rumination and worrying as possible mediators in the relation between neuroticism and symptoms of depression and anxiety in clinically depressed individuals. Behav Res Ther 46:1283–1289. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR (2007): The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci 362:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL (2007): Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci 8:657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: Remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Pignanelli R, Vaitl D (2009): Worry tendencies predict brain activation during aversive imagery. Neurosci Lett 461:289–292. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA (2012): I remember you: A role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Res 1428:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, Martin R (2005): The daily life of the garden‐variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. J Pers 73:1485–1510. [DOI] [PubMed] [Google Scholar]
- Sumner JA (2012): The mechanisms underlying overgeneral autobiographical memory: An evaluative review of evidence for the CaR‐FA‐X model. Clin Psychol Rev 32:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B (2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ (2012): Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 35:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds CS, Gallagher P, Thompson JM, Young AH (2004): Effects of the menstrual cycle on mood, neurocognitive and neuroendocrine function in healthy premenopausal women. Psychol Med 34:93–102. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, Deakin JF, Anderson IM (2011): Interaction between a history of depression and rumination on neural response to emotional faces. Psychol Med 41:1845–1855. [DOI] [PubMed] [Google Scholar]
- Watson D, Hubbard B (1996): Adaptational style and dispositional structure: Coping in the context of the five‐factor model. J Pers 64:737–774. [Google Scholar]
- Watson D, Clark LA, Harkness AR (1994): Structures of personality and their relevance to psychopathology. J Abnorm Psychol 103:18–31. [PubMed] [Google Scholar]
- Wells A (1995): Meta‐cognition and worry: A cognitive model of generalized anxiety disorder. Behav Res Ther 23:301–320. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Ford JM (2012): Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, Dalgleish T (2007): Autobiographical memory specificity and emotional disorder. Psychol Bull 133:122–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Bossmann J, Schiffhauer B, Jordan M, Immordino‐Yang MH (2012): Intrinsic default mode network connectivity predicts spontaneous verbal descriptions of autobiographical memories during social processing. Front Psychol 3:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S (2012): Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Biol Psychiatry 71:611–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


